Label-Free Homogeneous Electrochemical Aptasensor Based on Size Exclusion/Charge-Selective Permeability of Nanochannel Arrays and 2D Nanorecognitive Probe for Sensitive Detection of Alpha-Fetoprotein
Abstract
:1. Introduction
2. Results and Discussion
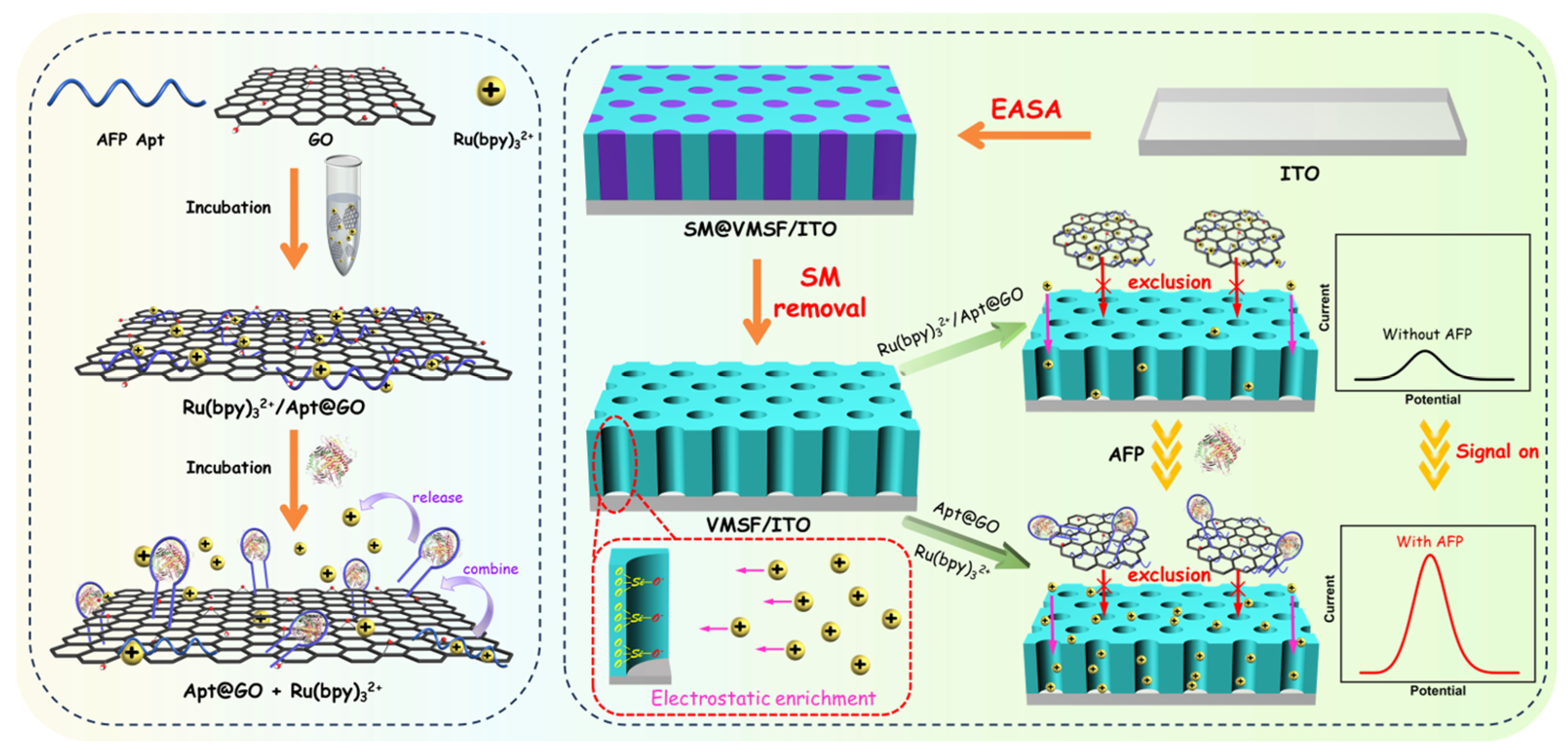
2.1. Strategy for the Construction of Labeling-Free and Immobilization-Free Homogeneous Electrochemical Aptasensor
2.2. Surface Morphology and Charge Selectivity of VMSF-Based Electrode
2.3. Synthesis and Characterization of 2D Nanoscale Recognition Probe
2.4. Feasibility of AFP Detection Using Homogeneous Aptamer Sensor
2.5. Electrochemical Determination of AFP Using the Fabricated Homogeneous Aptamer Sensor
2.6. Selectivity, Reproducibility, and Reuse of the Aptamer Sensor and Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Characteriaztions and Instrumentations
3.3. Synthesis of VMSF-Functionalized ITO Electrode
3.4. Preparation of Ru(bpy)32+/Apt@GO Nanocomposites
3.5. Electrochemical Detection of AFP and Ethical Approval
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018, 412, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Megha, G.K.; Zehra, A. Novel promising serum biomarkers for canine mammary tumors. Proc. Indian Natl. Sci. Acad. 2021, 87, 302–310. [Google Scholar] [CrossRef]
- Chang, Q.; Huang, J.; He, L.; Xi, F. Simple immunosensor for ultrasensitive electrochemical determination of biomarker of the bone metabolism in human serum. Front. Chem. 2022, 10, 940795. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Z.; Liu, C. CRISPR-powered biosensing platform for quantitative detection of alpha-fetoprotein by a personal glucose meter. Sens. Actuators B Chem. 2023, 390, 133994. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Zhou, H.; Wang, H. Recent advances in nanotechnology-enhanced biosensors for α-fetoprotein detection. Microchim. Acta 2022, 190, 3. [Google Scholar] [CrossRef]
- Kal-Koshvandi, A.T. Recent advances in optical biosensors for the detection of cancer biomarker α-fetoprotein (AFP). TrAC-Trend Anal. Chem. 2020, 128, 115920. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Cheng, H.; He, D.; He, X.; Wang, K.; Liu, Q.; Zhao, S.; Yang, X. A label-free homogeneous electrochemical sensing platform for protein kinase assay based on carboxypeptidase Y–assisted peptide cleavage and vertically ordered mesoporous silica films. Anal. Chem. 2017, 89, 9062–9068. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors targeting salivary biomarkers: A comprehensive review. TrAC-Trend Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J.-Y. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, L.; Yan, F.; Wang, K. Vertically-ordered mesoporous silica film based electrochemical aptasensor for highly sensitive detection of alpha-fetoprotein in human serum. Biosensors 2023, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Tsukakoshi, K.; Ikebukuro, K. Sensitive and homogeneous detection system with aptamer-based biosensor. Sens. Mater. 2016, 28, 1083–1089. [Google Scholar]
- Yang, W.; Zhang, G.; Ni, J.; Wang, Q.; Lin, Z. From signal amplification to restrained background: Magnetic graphene oxide assisted homogeneous electrochemiluminescence aptasensor for highly sensitive detection of okadaic acid. Sens. Actuators B Chem. 2021, 327, 128872. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J.-Y. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J.-J. Magnetic nanozyme based on loading nitrogen-doped carbon dots on mesoporous Fe3O4 nanoparticles for the colorimetric detection of glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef]
- Li, Y.; Gu, X.; Zhao, J.; Xi, F. Fabrication of a ratiometric fluorescence sensor based on carbon dots as both luminophores and nanozymes for the sensitive detection of hydrogen peroxide. Molecules 2022, 27, 7379. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J.-Y. Facile synthesis of iron and nitrogen co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef]
- Su, R.; Tang, H.; Xi, F. Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film. Front. Chem. 2022, 10, 954748. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef]
- Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-biofouling electrochemical sensor based on the binary nanocomposite of silica nanochannel array and graphene for doxorubicin detection in human serum and urine samples. Molecules 2022, 27, 8640. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Wang, T.; Jiang, X.; Qu, X.; Duan, W.; Xi, F.; He, Z.; Wu, J. Tissue imprinting on 2D nanoflakes-capped silicon nanowires for lipidomic mass spectrometry imaging and cancer diagnosis. ACS Nano 2022, 16, 6916–6928. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Jin, Y.; Cui, Y.; Xi, F.; Liu, X.; Wo, F.; Wu, J. A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 2021, 272, 120772. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Graphene quantum dot-decorated luminescent porous silicon dressing for theranostics of diabetic wounds. Acta Biomater. 2021, 131, 544–554. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, S.; Zhou, X.; Yan, F.; Hu, W. Silica nanochannel array on co-electrodeposited graphene-carbon nanotubes 3D composite film for antifouling detection of uric acid in human serum and urine samples. Microchem. J. 2023, 190, 108632. [Google Scholar] [CrossRef]
- Wei, X.; Luo, X.; Xu, S.; Xi, F.; Zhao, T. A Flexible electrochemiluminescence sensor equipped with vertically ordered mesoporous silica nanochannel film for sensitive detection of clindamycin. Front. Chem. 2022, 10, 872582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica nanochannel membranes for electrochemical analysis and molecular sieving: A comprehensive review. Crit. Rev. Anal. Chem. 2019, 50, 424–444. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hou, H.; Wei, H.; Yao, L.; Sun, L.; Yu, P.; Su, B.; Mao, L. In vivo monitoring of oxygen in rat brain by carbon fiber microelectrode modified with antifouling nanoporous membrane. Anal. Chem. 2019, 91, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.-J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Yu, G.; Liu, L.; Yan, F. Highly sensitive electrochemical detection of paraquat in environmental water samples using a vertically ordered mesoporous silica film and a nanocarbon composite. Nanomaterials 2022, 12, 3632. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, W.; Xu, L.; Yang, Q.; Su, B. Two orders-of-magnitude enhancement in the electrochemiluminescence of by vertically ordered silica mesochannels. Anal. Chim. Acta 2015, 886, 48–55. [Google Scholar] [CrossRef]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J.-Y. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450. [Google Scholar] [CrossRef]
- Yan, L.; Xu, S.; Xi, F. Disposal immunosensor for sensitive electrochemical detection of prostate-specific antigen based on amino-rich nanochannels array-modified patterned indium tin oxide electrode. Nanomaterials 2022, 12, 3810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-ordered mesoporous silica films for electrochemical detection of Hg(II) ion in pharmaceuticals and soil samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Wang, Y.; Xiao, Q.; Zhou, X.; Li, H. Conjugated polymer sensitized hyperbranched titanium dioxide based photoelectrochemical biosensor for detecting AFP in serum. Surf. Interfaces. 2021, 24, 101103. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, N.; Chen, H.; Bai, L.; Xu, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L.; Cheng, Z. Preparation of a novel sandwich-type electrochemical immunosensor for AFP detection based on an ATRP and click chemistry technique. Polym. Chem. 2020, 11, 900–908. [Google Scholar] [CrossRef]
- Shang, Z.; Su, T.; Jin, D.; Xu, Q.; Hu, X.; Shu, Y. An integrated and flexible PDMS/Au film-based electrochemical immunosensor via Fe-Co MOF as a signal amplifier for alpha fetoprotein detection. Biosens. Bioelectron. 2023, 230, 115245. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef]
- Qin, D.-M.; Jiang, X.; Mo, G.; Feng, J.; Deng, B. Boron nitride quantum dots as electrochemiluminescence coreactants of rGO@Au@Ru–SiO2 for label-free detection of AFP in human serum. Microchim. Acta 2020, 335, 135621. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Wang, J.; Wang, J. Dual-recognition immune-co-chemical ECL-sensor based on Ti,Mg@N-CDs-induced and novel signal-sensing units poly(DVB-co-PBA)-reported for alpha-fetoprotein detection. Sen. Actuators B Chem. 2021, 346, 130548. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]






| Electrode | Detection Method | Recognition Molecule | Linear Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|---|---|
| Ab/CP/TiO2/FTO | PEC | Antibody | 0.1–100 | 0.03 | [43] |
| P(VT-co-HEMA)-g-GO/Ab2/AFP/BSA/Ab1/AuNPs-rGO/GCE | EC | Antibody | 0.0025–50 | 1.83 × 10−4 | [44] |
| MOF-Ab/AFP/BSA/Apt-SH/Au/PDMS/GCE | EC | Aptamer | 0.01–300 | 0.01 | [45] |
| Ab/EDC-NHS/Fe3O4/MWCNTs-COOH/AuNPs/GCE | EC | Antibody | 10−3–104 | 1.09 × 10−3 | [46] |
| AFP/BSA/Ab/rGO@Au@Ru–SiO2/GCE | ECL | Antibody | 10−4–100 | 3 × 10−5 | [47] |
| Poly(DVB-Co-PBA)-AFP/Ab/Ti,Mg@N-CDs/GCE | ECL | Antibody | 2.25 × 10−6–2250 | 1.55 × 10−7 | [48] |
| VMSF/ITO | EC | Aptamer | 10−3–1000 | 0.82 × 10−4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, S.; Liu, J.; Qin, D. Label-Free Homogeneous Electrochemical Aptasensor Based on Size Exclusion/Charge-Selective Permeability of Nanochannel Arrays and 2D Nanorecognitive Probe for Sensitive Detection of Alpha-Fetoprotein. Molecules 2023, 28, 6935. https://doi.org/10.3390/molecules28196935
Zhang Y, Zhang S, Liu J, Qin D. Label-Free Homogeneous Electrochemical Aptasensor Based on Size Exclusion/Charge-Selective Permeability of Nanochannel Arrays and 2D Nanorecognitive Probe for Sensitive Detection of Alpha-Fetoprotein. Molecules. 2023; 28(19):6935. https://doi.org/10.3390/molecules28196935
Chicago/Turabian StyleZhang, Yue, Shiyue Zhang, Jiyang Liu, and Dongyuan Qin. 2023. "Label-Free Homogeneous Electrochemical Aptasensor Based on Size Exclusion/Charge-Selective Permeability of Nanochannel Arrays and 2D Nanorecognitive Probe for Sensitive Detection of Alpha-Fetoprotein" Molecules 28, no. 19: 6935. https://doi.org/10.3390/molecules28196935
APA StyleZhang, Y., Zhang, S., Liu, J., & Qin, D. (2023). Label-Free Homogeneous Electrochemical Aptasensor Based on Size Exclusion/Charge-Selective Permeability of Nanochannel Arrays and 2D Nanorecognitive Probe for Sensitive Detection of Alpha-Fetoprotein. Molecules, 28(19), 6935. https://doi.org/10.3390/molecules28196935







