Antioxidant Peptides from the Collagen of Antler Ossified Tissue and Their Protective Effects against H2O2-Induced Oxidative Damage toward HaCaT Cells
Abstract
:1. Introduction
2. Results and Discussion
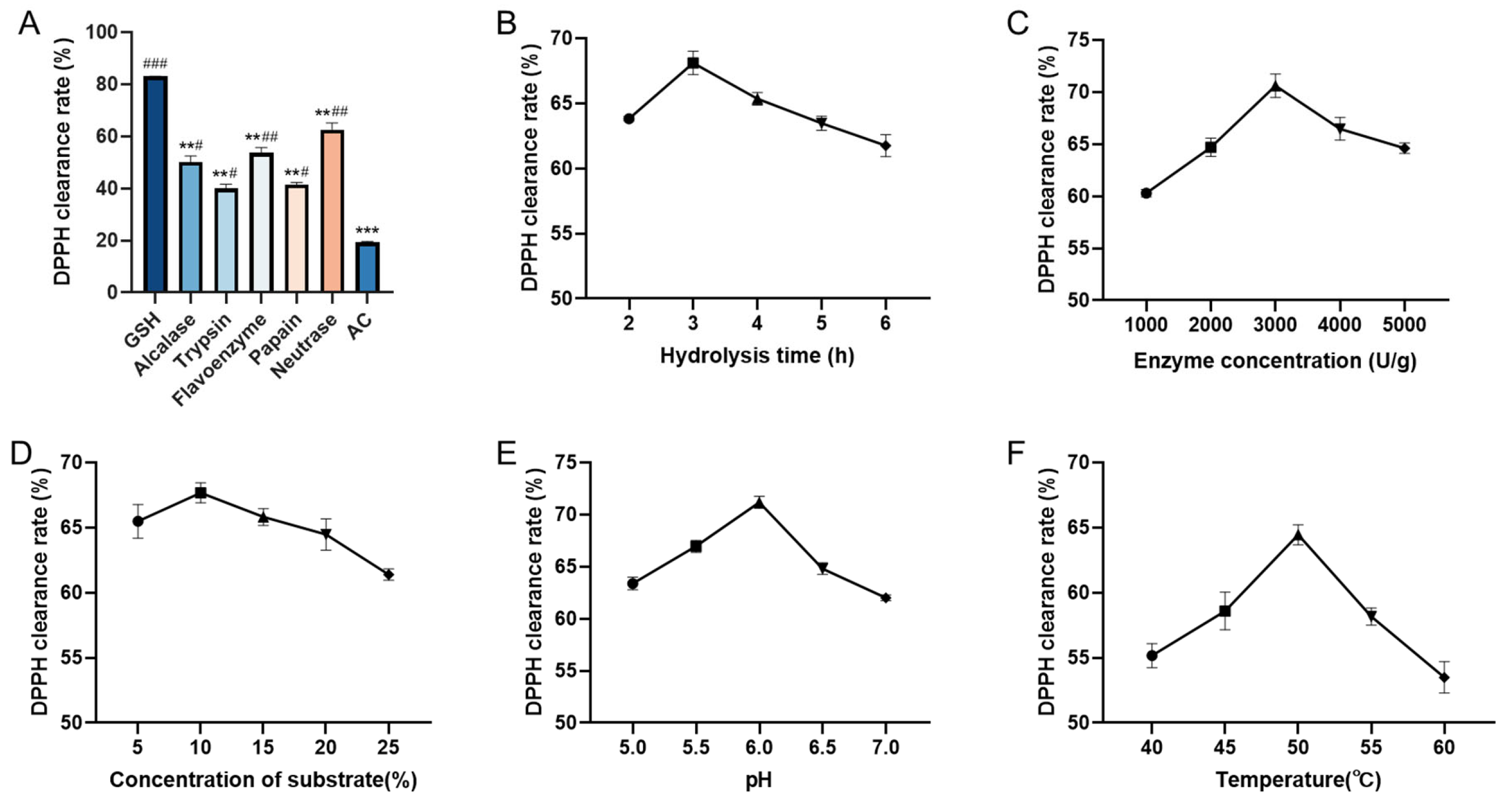
2.1. Protease Selection and Single-Factor Experiments
2.2. Optimization of Hydrolysis Conditions by Response Surface Methodology (RSM)
2.3. Molecular Weight Distribution of ACP
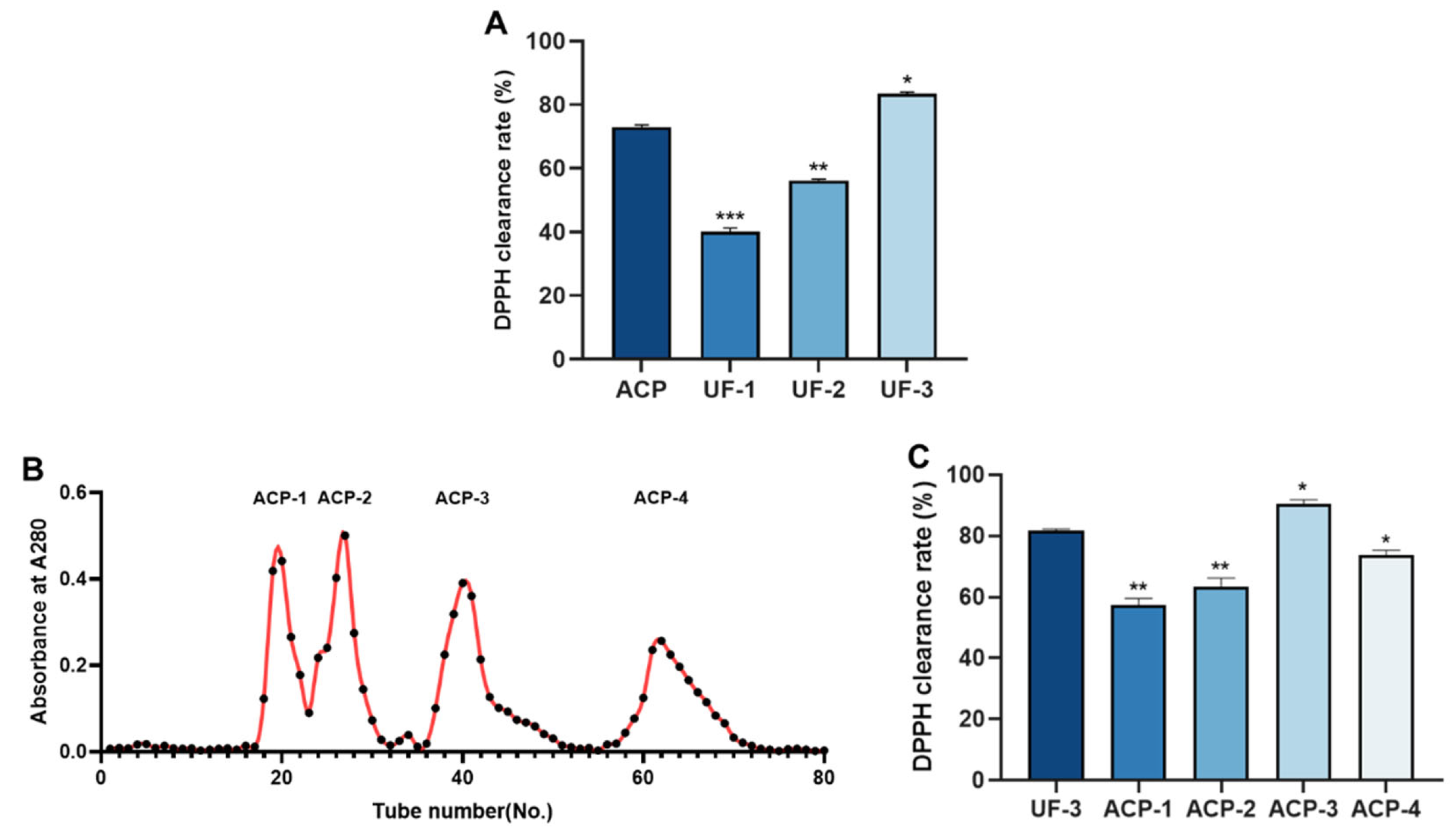
2.4. Isolation and Purification of ACP
2.5. Amino Acid Composition of ACP-3
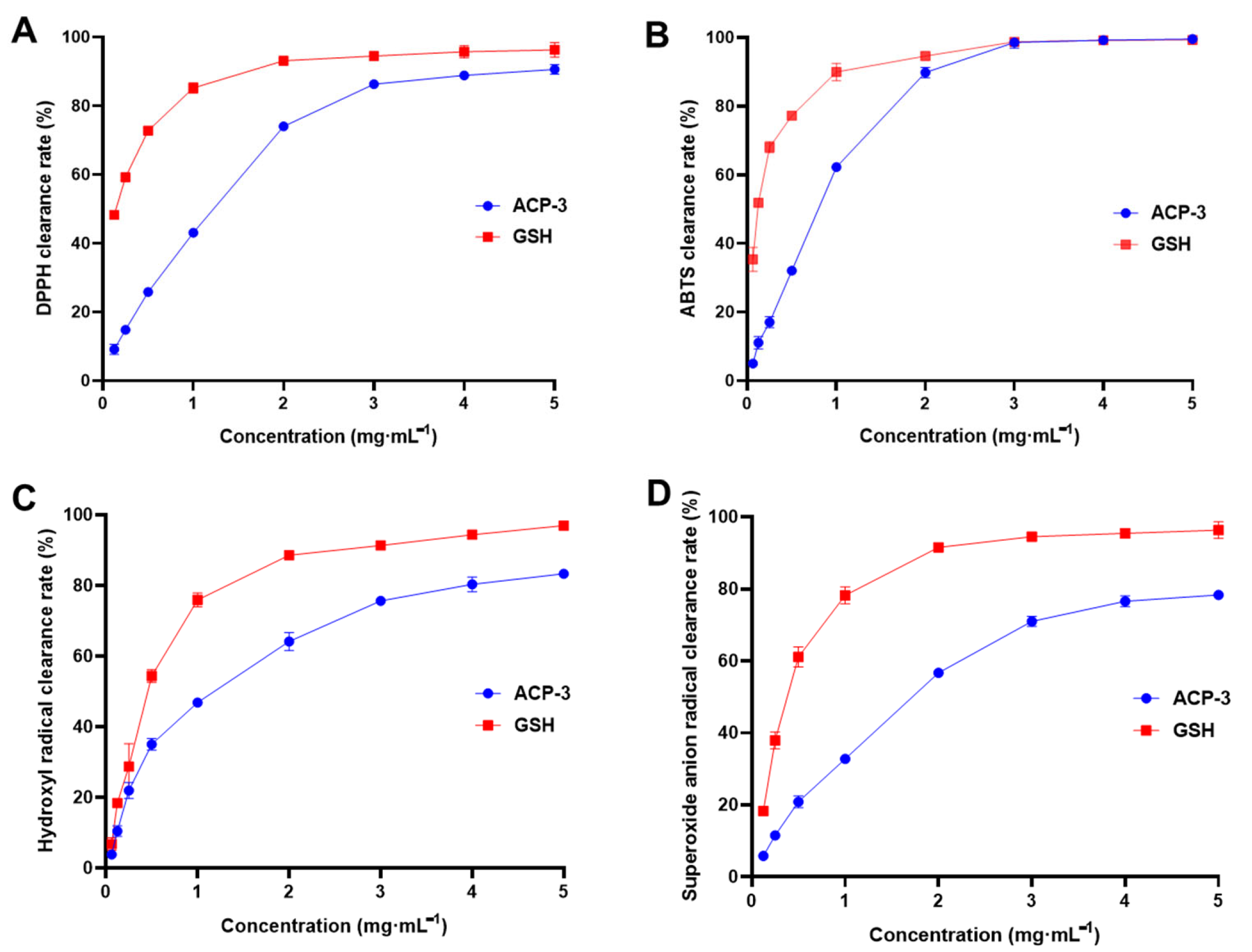
2.6. DPPH, ABTS, Hydroxyl Radical, and Superoxide Anion Clearance Rate of ACP-3
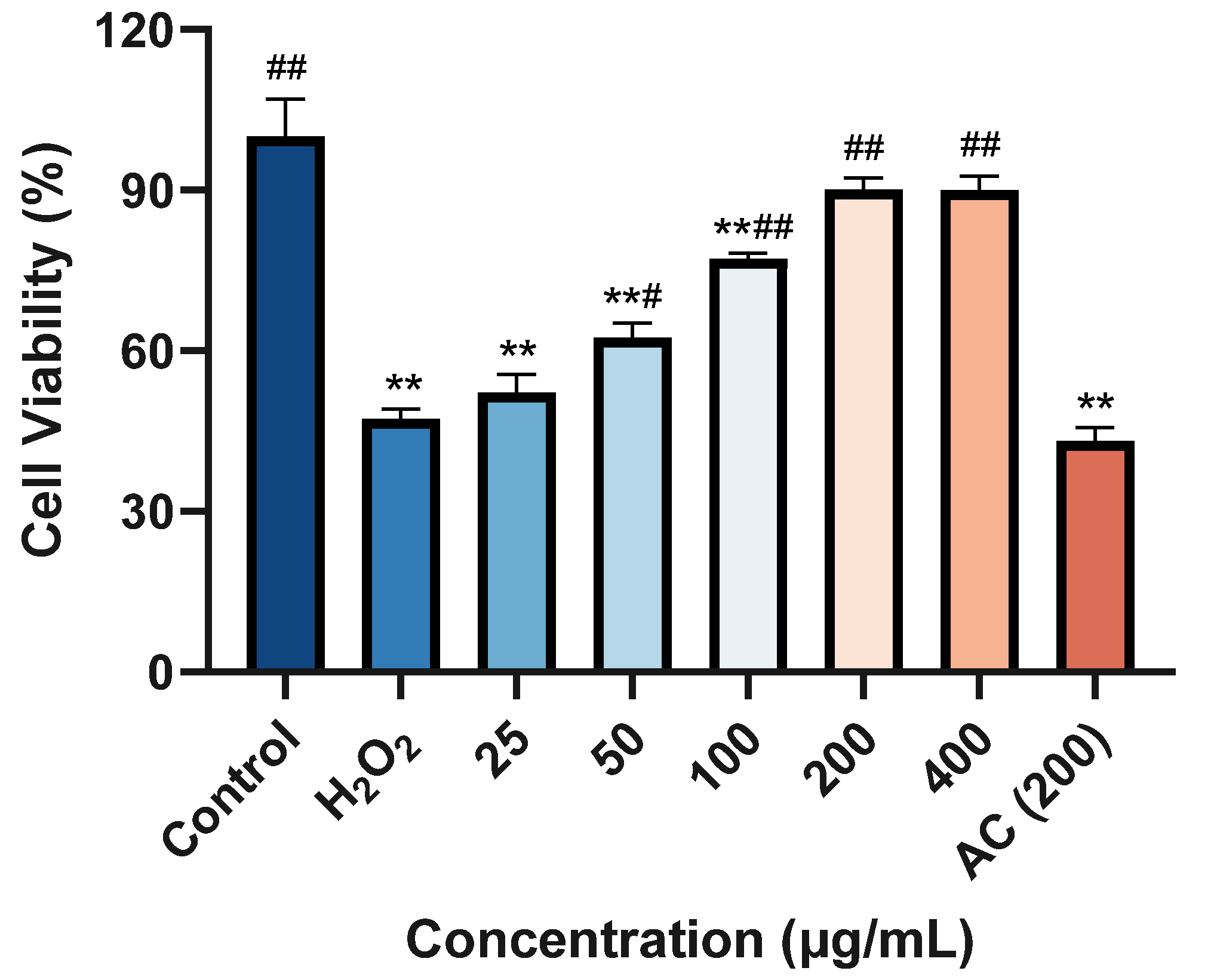
2.7. Cytotoxicity of ACP-3 at Different Concentrations on HaCaT Cells
2.8. Protective Effect against H2O2-Induced Damage on HaCaT Cells
2.9. Effect of ACP-3 on ROS Content with H2O2-Induced Damage on HaCaT Cells
2.10. Effects of ACP-3 on the Levels of SOD, CAT, GSH-Px, and MDA with H2O2-Induced Damage on HaCaT Cells
3. Materials and Methods
3.1. Materials
3.2. Preparation of Collagen
3.3. Optimization of Preparative Conditions of Collagen Hydrolysate from AC
3.4. Determination of the Mw Distribution of ACP
3.5. Fractionation of ACP
3.6. Amino Acid Composition Measurement of ACP-3
3.7. Antioxidant Activity of ACP-3
3.7.1. DPPH Clearance Rate
3.7.2. ABTS Clearance Rate
3.7.3. Hydroxyl Clearance Rate
3.7.4. Superoxide Anion Clearance Rate
3.8. Effects of ACP-3 on the H2O2-Induced HaCaT Cells
3.9. Determination of the Levels of ROS in H2O2-Induced HaCaT Cells
3.10. Determination of Antioxidant Enzyme and MDA Levels in H2O2-induced HaCaT Cells
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zheng, J.; Tian, X.; Xu, B.; Yuan, F.; Gong, J.; Yang, Z. Collagen Peptides from Swim Bladders of Giant Croaker (Nibea japonica) and Their Protective Effects against H2O2-Induced Oxidative Damage toward Human Umbilical Vein Endothelial Cells. Mar. Drugs 2020, 18, 430. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.W.; Prosser, B.L.; Lederer, W.J. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid. Redox Signal. 2014, 20, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F., Jr. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Daneault, A.; Prawitt, J.; Fabien Soule, V.; Coxam, V.; Wittrant, Y. Biological effect of hydrolyzed collagen on bone metabolism. Crit. Rev. Food Sci. Nutr. 2017, 57, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Song, W.; Wang, W.; Qian, G. Purification, optimization and physicochemical properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2016, 89, 344–352. [Google Scholar] [CrossRef]
- Zhao, W.H.; Chi, C.F.; Zhao, Y.Q.; Wang, B. Preparation, Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Swim Bladders of Miiuy Croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 161. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, Y.C.; Wang, C.T.; Ji, B.P. Antioxidant activity of protein hydrolysates from aqueous extract of velvet antler (Cervus elaphus) as influenced by molecular weight and enzymes. Nat. Prod. Commun. 2011, 6, 1683–1688. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, L.; An, M.; Wang, T.; Li, M.; Yu, Y. Heavy metals in marine food web from Laizhou Bay, China: Levels, trophic magnification, and health risk assessment. Sci. Total Environ. 2022, 841, 156818. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Pepłowska, K.; Wałecka, J.; Bąkowski, P.; Dzięgielewski, Z.; Dzięgielewski, D.; Grzywacz, K. Exploring dog antler chews as a novel source of collagen supplementation: Extraction and characterization of collagen from red deer antlers. BioTechnologia 2023, 104, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Hong, H.; Lodhi, G.; Cheong, S.H.; Park, P.J.; Hu, T.J.; Sangwoo, K.; Moon, S.H.; Jeon, B.T. Isolation and characterisation of collagen from elk antler velvet. Anim. Prod. Sci. 2014, 54, 1095–1101. [Google Scholar] [CrossRef]
- Yao, B.; Zhao, Y.; Zhang, H.; Zhang, M.; Liu, M.; Liu, H.; Li, J. Sequencing and de novo analysis of the Chinese Sika deer antler-tip transcriptome during the ossification stage using Illumina RNA-Seq technology. Biotechnol. Lett. 2012, 34, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrouso, M.; Lorenzo, J.M.; Landete-Castillejos, T.; Chonco, L.; Perez-Barberia, F.J.; Garcia, A.; Lopez-Garrido, M.P.; Franco, D. SWATH-MS Quantitative Proteomic Analysis of Deer Antler from Two Regenerating and Mineralizing Sections. Biology 2021, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.T.; Cheong, S.H.; Kim, D.H.; Park, J.H.; Park, P.J.; Sung, S.H.; Thomas, D.G.; Kim, K.H.; Moon, S.H. Effect of Antler Development Stage on the Chemical Composition of Velvet Antler in Elk (Cervus elaphus canadensis). Asian Austral. J. Anim. 2011, 24, 1303–1313. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, J.; Xu, B.; Ye, J.; Yang, Z.; Yuan, F. Optimization of Extraction of Bioactive Peptides from Monkfish (Lophius litulon) and Characterization of Their Role in H2O2-Induced Lesion. Mar. Drugs 2020, 18, 468. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Lin, S.J.; Yang, Z.S.; Jin, H.X. Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect against H2O2-Induced Damage of RAW264.7 Cells. Marine Drugs 2019, 17, 642. [Google Scholar] [CrossRef]
- Yang, F.; Jin, S.; Tang, Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, C.; Cao, J.; He, J.; Sun, Y.; Dang, Y.; Pan, D.; Xia, Q. Purification and Characterization of Novel Antioxidative Peptides From Duck Liver Protein Hydrolysate as Well as Their Cytoprotection against Oxidative Stress in HepG2 Cells. Front. Nutr. 2022, 9, 848289. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Shi, Z.; Xie, C.; Gong, W.; Hu, Z.; Peng, Y. A novel mechanism of Gamma-aminobutyric acid (GABA) protecting human umbilical vein endothelial cells (HUVECs) against H2O2-induced oxidative injury. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 217, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhuang, C.N.; Cui, J.J.; Wang, P.W.; Liu, D.G.; Yan, S.Q.; Zhou, L.T.; Ren, S.P. Effects of Combination of 1,25(OH)2D3 and TLR-4 Inhibitor on the Damage to HaCaT Cells Caused by UVB Irradiation. Biomed. Environ. Sci. 2022, 35, 1051–1062. [Google Scholar] [PubMed]
- Wu, X.; Cai, L.; Cao, A.; Wang, Y.; Li, T.; Li, J. Comparative study on acid-soluble and pepsin-soluble collagens from skin and swim bladder of grass carp (Ctenopharyngodon idella). J. Sci. Food Agric. 2016, 96, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhao, Y.Q.; Zhao, G.X.; Chi, C.F.; Wang, B. Antioxidant Peptides from Collagen Hydrolysate of Redlip Croaker (Pseudosciaena polyactis) Scales: Preparation, Characterization, and Cytoprotective Effects on H2O2-Damaged HepG2 Cells. Marine Drugs 2020, 18, 156. [Google Scholar] [CrossRef]
- Stella, S.P.; Ferrarezi, A.C.; dos Santos, K.O.; Monteiro, M. Antioxidant activity of commercial ready-to-drink orange juice and nectar. J. Food Sci. 2011, 76, C392–C397. [Google Scholar] [CrossRef]
- Cao, Y.; Chu, Q.; Ye, J. Determination of hydroxyl radical by capillary electrophoresis and studies on hydroxyl radical scavenging activities of Chinese herbs. Anal. Bioanal. Chem. 2003, 376, 691–695. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef]
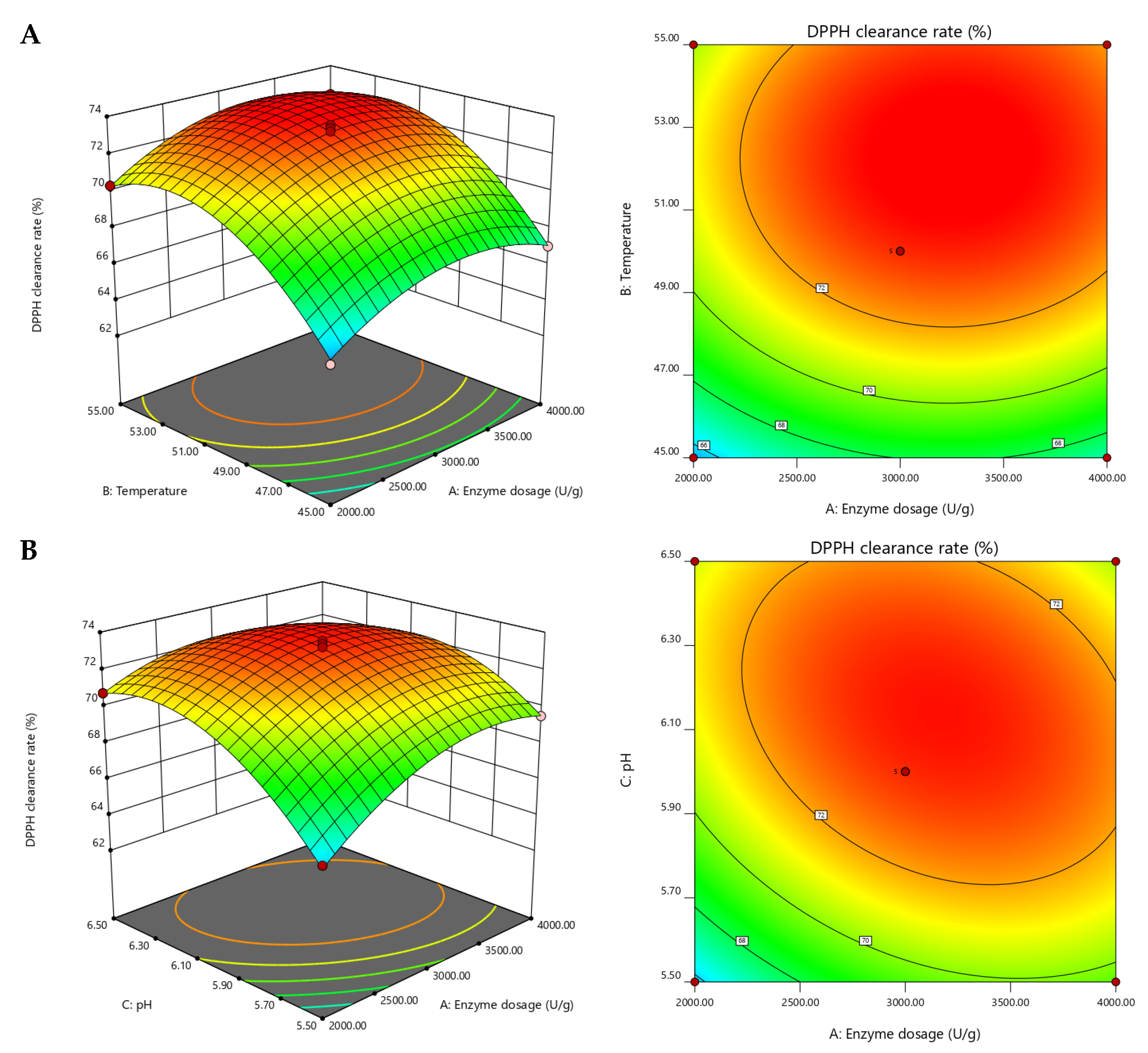





| Run Numbers | A Enzyme Concentration (U/g) | B Temperature (°C) | C pH | R (DPPH Clearance Eate) |
|---|---|---|---|---|
| 1 | 0 (3000) | −1 (45) | −1 (5.5) | 63.84 |
| 2 | −1 (2000) | 1 (55) | 0 (6.0) | 70.33 |
| 3 | 0 (3000) | 1 (55) | 1 (6.5) | 71.48 |
| 4 | 0 (3000) | −1 (45) | 1 (6.5) | 67.85 |
| 5 | 0 (3000) | 0 (50) | 0 (6.0) | 73.21 |
| 6 | 0 (3000) | 0 (50) | 0 (6.0) | 72.69 |
| 7 | 0 (3000) | 0 (50) | 0 (6.0) | 73.55 |
| 8 | 0 (3000) | 0 (50) | 0 (6.0) | 72.97 |
| 9 | −1 (2000) | 0 (50) | 1 (6.5) | 70.76 |
| 10 | 0 (3000) | 0 (50) | 0 (6.0) | 73.38 |
| 11 | 0 (3000) | 1 (55) | −1 (5.5) | 69.43 |
| 12 | −1 (2000) | −1 (45) | 0 (6.0) | 65.24 |
| 13 | 1 (4000) | 1 (55) | 0 (6.0) | 72.33 |
| 14 | 1 (4000) | −1 (45) | 0 (6.0) | 67.03 |
| 15 | 1 (4000) | 0 (50) | 1 (6.5) | 70.14 |
| 16 | −1 (2000) | 0 (50) | −1 (5.5) | 65.92 |
| 17 | 1 (4000) | 0 (50) | −1 (5.5) | 69.52 |
| Variables | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 149.27 | 9 | 16.59 | 152.99 | <0.0001 |
| A (Enzyme concentration) | 5.73 | 1 | 5.73 | 52.85 | 0.0002 |
| B (Temperature) | 48.02 | 1 | 48.02 | 442.96 | <0.0001 |
| C (pH) | 16.62 | 1 | 16.62 | 153.29 | <0.0001 |
| AB | 0.011 | 1 | 0.011 | 0.1 | 0.7591 |
| AC | 4.45 | 1 | 4.45 | 41.07 | 0.0004 |
| BC | 0.95 | 1 | 0.95 | 8.77 | 0.0211 |
| A2 | 12.84 | 1 | 12.84 | 118.4 | <0.0001 |
| B2 | 30.32 | 1 | 30.32 | 279.69 | <0.0001 |
| C2 | 22.88 | 1 | 22.88 | 211.04 | <0.0001 |
| Residual | 0.76 | 7 | 0.11 | ||
| Lack of Fit | 0.3 | 3 | 0.1 | 0.88 | 0.5211 |
| Pure Error | 0.46 | 4 | 0.11 | ||
| Cor Total | 150.03 | 16 | |||
| R2 = 0.9949 | Radj2 = 0.9884 | ||||
| Amino Acids | Proportion (%) |
|---|---|
| Aspartic acid (Asp) 2 | 6.609634551 |
| Threonine (Thr) * | 2.757641196 |
| Serine (Ser) | 3.718438538 |
| Glutamic (Glu) 2 | 10.82292359 |
| Glycine (Gly) | 14.81146179 |
| Alanine (Ala) 1 | 8.436710963 |
| Cystine (Cys) | 0.620431894 |
| Valine (Val) * | 2.993687708 |
| Methionine (Met) *, 1 | 2.101328904 |
| Isoleucine (Ile) *, 1 | 3.178571429 |
| Leucine (Leu) *, 1 | 5.138870432 |
| Tyrosine (Tyr) | 1.243853821 |
| Phenylalanine (Phe) *,1 | 2.56910299 |
| Histidine (His) 3 | 1.174916944 |
| Lysine (Lys) *,3 | 4.906146179 |
| Arginine (Arg) 3 | 8.487541528 |
| Proline (Pro) 1 | 20.48172757 |
| Protease | Temperature (°C) | pH | Time (h) | Enzyme Concentration (U/g) | Concentration of Substrate (%) |
|---|---|---|---|---|---|
| Neutrase | 50 | 6.5 | 4 | 2000 | 20 |
| Alcalase | 45 | 9.0 | 4 | 2000 | 20 |
| Papain | 55 | 6.0 | 4 | 2000 | 20 |
| Trypsin | 50 | 8.0 | 4 | 2000 | 20 |
| Flavourzyme | 50 | 7.5 | 4 | 2000 | 20 |
| Levels | Variables | ||
|---|---|---|---|
| A Enzyme Concentration (U/g) | B Temperature (°C) | C pH | |
| −1 | 2000 | 45 | 5.5 |
| 0 | 3000 | 50 | 6 |
| 1 | 4000 | 55 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xia, P.; Zheng, S.; Li, Y.; Fang, J.; Ma, Z.; Zhang, L.; Zhang, X.; Hao, L.; Zhang, H. Antioxidant Peptides from the Collagen of Antler Ossified Tissue and Their Protective Effects against H2O2-Induced Oxidative Damage toward HaCaT Cells. Molecules 2023, 28, 6887. https://doi.org/10.3390/molecules28196887
Chen X, Xia P, Zheng S, Li Y, Fang J, Ma Z, Zhang L, Zhang X, Hao L, Zhang H. Antioxidant Peptides from the Collagen of Antler Ossified Tissue and Their Protective Effects against H2O2-Induced Oxidative Damage toward HaCaT Cells. Molecules. 2023; 28(19):6887. https://doi.org/10.3390/molecules28196887
Chicago/Turabian StyleChen, Xi, Peijun Xia, Shuo Zheng, Yi Li, Jiayuan Fang, Ze Ma, Libo Zhang, Xunming Zhang, Linlin Hao, and Hong Zhang. 2023. "Antioxidant Peptides from the Collagen of Antler Ossified Tissue and Their Protective Effects against H2O2-Induced Oxidative Damage toward HaCaT Cells" Molecules 28, no. 19: 6887. https://doi.org/10.3390/molecules28196887
APA StyleChen, X., Xia, P., Zheng, S., Li, Y., Fang, J., Ma, Z., Zhang, L., Zhang, X., Hao, L., & Zhang, H. (2023). Antioxidant Peptides from the Collagen of Antler Ossified Tissue and Their Protective Effects against H2O2-Induced Oxidative Damage toward HaCaT Cells. Molecules, 28(19), 6887. https://doi.org/10.3390/molecules28196887