Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro
Abstract
:1. Introduction
2. Results
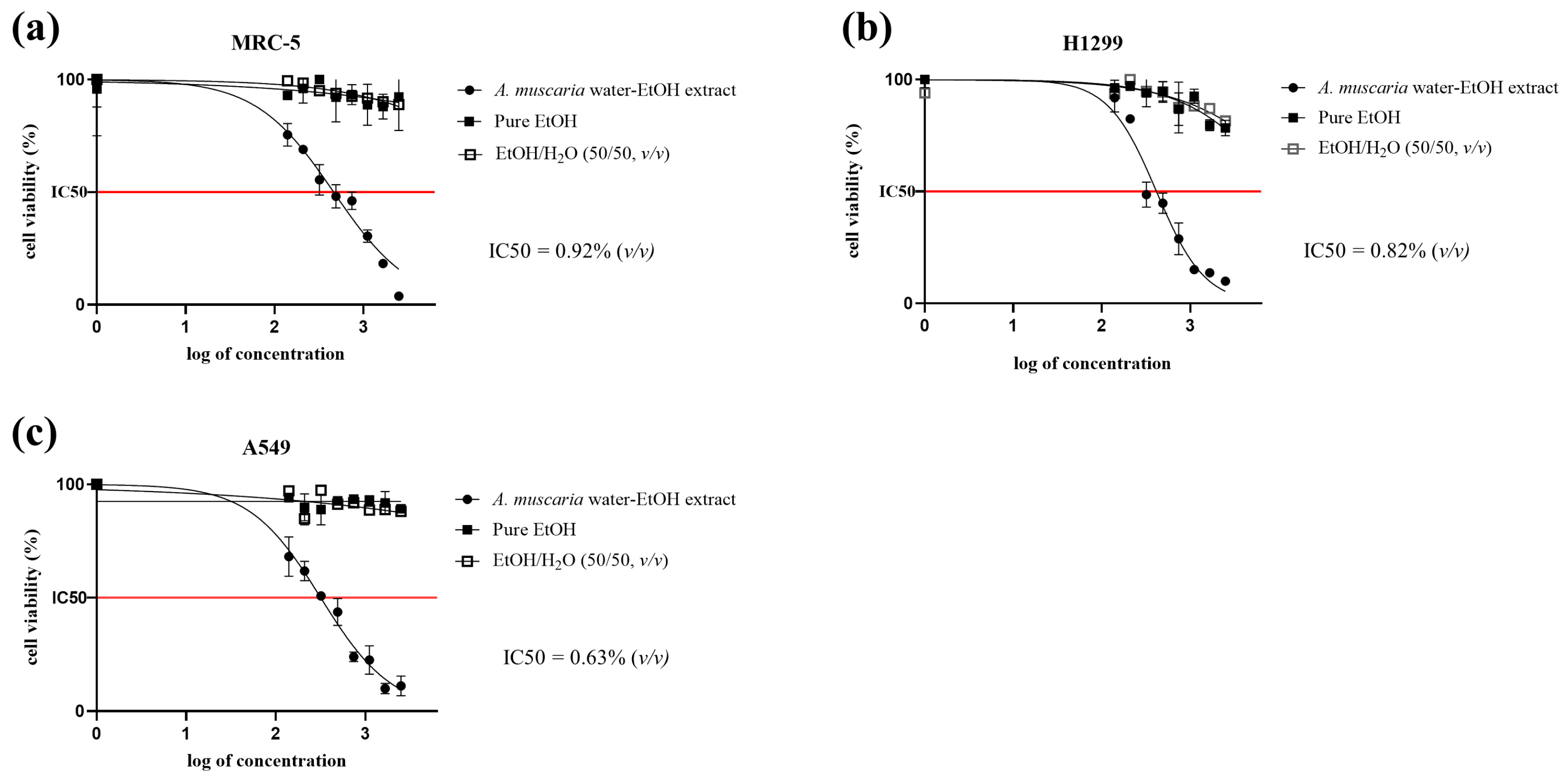
2.1. MTT Cytotoxicity Assay
2.2. HPLC Method Development and Optimization
2.3. Application of the Developed HPLC Methods to Amanita Muscaria Extracts and Fractions
2.4. Capillary Zone Electrophoresis with Contactless Conductivity Detection Analysis of the Ibotenic Acid and Muscimol Content of the Two A. muscaria Extracts
2.5. UHPLC-MS/MS Analysis of the Ibotenic Acid and Muscimol Content of the Two A. muscaria Extracts
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Lines and Media
4.3. Cell Line Cultivation
4.4. Amanita muscaria Ethanol Extracts
4.5. Liquid–Liquid Extraction Procedures
4.6. Preparation of Standardized Solutions of the Compounds of Interest
4.7. Samples and Sample Preparation
4.8. MTT Cytotoxicity Assay
4.9. Instrumentation and Experimental Procedures
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bosman, C.K.; Berman, L.; Isaacson, M.; Wolfowitz, B.; Parkes, J. Mushroom poisoning caused by Amanita pantherina. Report of 4 cases. S. Afr. Med. J. 1965, 39, 983–986. [Google Scholar] [PubMed]
- Isoxazole-Containing Mushrooms and Pantherina Syndrome (Amanita muscaria, Amanita pantherina). In Medical Toxicology of Natural Substances; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 298–302.
- Hatfield, G.M. Toxic mushrooms. In Toxic Plants; Columbia University Press: New York, NY, USA, 1979; pp. 7–58. [Google Scholar]
- Waser, P.G. The pharmacology of Amanita muscaria. Psychopharmacol. Bull. 1967, 4, 19–20. [Google Scholar] [PubMed]
- Lampe, K.F. Toxic fungi. Annu. Rev. Pharmacol. 1979, 19, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, M.; Harris, C. Poisoning by Amanita pantherina: A description of two cases. Cent. Afr. J. Med. 1982, 28, 159–163. [Google Scholar]
- Moss, M.J.; Hendrickson, R.G. Toxicity of muscimol and ibotenic acid containing mushrooms reported to a regional poison control center from 2002–2016. Clin. Toxicol. 2019, 57, 99–103. [Google Scholar] [CrossRef]
- Satora, L.; Pach, D.; Butryn, B.; Hydzik, P.; Balicka-Ślusarczyk, B. Fly agaric (Amanita muscaria) poisoning, case report and review. Toxicon 2005, 45, 941–943. [Google Scholar] [CrossRef]
- Yamaura, Y.; Komiyama, S.; Fukuhara, M.; Takabatake, E.; Hashimoto, T. Biochemical effects of Amanita muscaria extract in mice. Shokuhin Eiseigaku Zasshi 1983, 24, 459–464_1. [Google Scholar] [CrossRef]
- Faulstich, H.; Cochet-Meilhac, M. Amatoxins in edible mushrooms. FEBS lett. 1976, 64, 73–75. [Google Scholar] [CrossRef]
- Vargas, N.; Bernal, A.; Sarria, V.; Franco-Molano, A.; Restrepo, S. Amatoxin and phallotoxin composition in species of the genus Amanita in Colombia: A taxonomic perspective. Toxicon 2011, 58, 583–590. [Google Scholar] [CrossRef]
- Cochet-Meilhac, M.; Chambon, P. Animal DNA-dependent RNA polymerases 11. Mechanism of the inhibition of RNA polymerases B by amatoxins. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1974, 353, 160–184. [Google Scholar] [CrossRef]
- Faulstich, H. The amatoxins. In Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 1980; pp. 88–134. [Google Scholar]
- Santi, L.; Maggioli, C.; Mastroroberto, M.; Tufoni, M.; Napoli, L.; Caraceni, P. Acute liver failure caused by Amanita phalloides poisoning. Int. J. Hepatol. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Holzbauer, S.; Wanduragala, D.; Ivaskovic, A.; Spinosa, R.; Smith, K.; Corcoran, J.; Jensen, A. Notes from the Field: Acute Intoxications from Consumption of Amanita muscaria Mushrooms—Minnesota, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Bowden, K.; Drysdale, A.; Mogey, G. Constituents of Amanita muscaria. Nature 1965, 206, 1359–1360. [Google Scholar] [CrossRef] [PubMed]
- Bowden, K.; Drysdale, A.C. A novel constituent of Amanita muscaria. Tetrahedron Lett. 1965, 12, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Nakajima, T.; Yokobe, T. Structure of ibotenic acid. Yakugaku Zasshi 1964, 84, 1232–1233. [Google Scholar]
- Takemoto, T.; Nakajima, T.; Sakuma, R. Isolation of a flycidal constituent “ibotenic acid” from Amanita muscaria and A. pantherina. Yakugaku Zasshi 1964, 84, 1233–1234. [Google Scholar]
- Müller, G.; Eugster, C. Muscimol, ein pharmakodynamisch wirksamer Stoff aus Amanita muscaria. Helv. Chim. Acta 1965, 48, 910–926. [Google Scholar] [CrossRef]
- Patocka, J.; Kocandrlova, B. Pharmacologically and toxicologically relevant components of Amanita muscaria. Mil. Med. Sci. Lett. 2017, 86, 122–134. [Google Scholar] [CrossRef]
- APExBIO Technology LLC. Ibotenic Acid Product Page. Available online: https://www.apexbt.com/ibotenic-acid.html (accessed on 1 July 2023).
- Stromgaard, K.; Krogsgaard-Larsen, P.; Madsen, U. Textbook of Drug Design and Discovery, 3rd ed.; CRC Press: Hoboken, NJ, USA, 2002. [Google Scholar]
- Johnston, G.; Curtis, D.; De Groat, W.; Duggan, A. Central actions of ibotenic acid and muscimol. Biochem. Pharmacol. 1968, 17, 2488–2489. [Google Scholar] [CrossRef]
- Krogsgaard-Larsen, P.; Honore, T.; Hansen, J.; Curtis, D.; Lodge, D. New class of glutamate agonist structurally related to ibotenic acid. Nature 1980, 284, 64–66. [Google Scholar] [CrossRef]
- Ji, C.; Li, Q.; Aisa, H.; Yang, N.; Dong, Y.-L.; Liu, Y.-Y.; Wang, T.; Hao, Q.; Zhu, H.-B.; Zuo, P.-P. Gossypium herbaceam extracts attenuate ibotenic acid-induced excitotoxicity in rat hippocampus. J. Alzheimer’s Dis. 2009, 16, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Michelot, D.; Melendez-Howell, L.M. Amanita muscaria: Chemistry, biology, toxicology, and ethnomycology. Mycol. Res. 2003, 107, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, K.; Chilton, W.S.; Yamamura, H.I.; Enna, S.J. Muscimol binding in rat brain: Association with synaptic GABA receptors. Brain Res. 1978, 148, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, S.R. Use of 3H-muscimol for GABA receptor studies. Nature 1978, 273, 392–394. [Google Scholar] [CrossRef]
- Johnston, G.A.R. Muscimol as an Ionotropic GABA Receptor Agonist. Neurochem. Res. 2014, 39, 1942–1947. [Google Scholar] [CrossRef]
- Maciejczyk, E. Amanita muscaria chemistry: The mystery demystified? In Fly Agaric: A Compendium of History, Pharmacology, Mythology and Exploration; Feeney, K.M., Ed.; Fly Agaric Press: Washington, DC, USA, 2020; pp. 351–365. [Google Scholar]
- Johnston, G.A. Muscimol and the uptake of gamma-aminobutyric acid by rat brain slices. Psychopharmacologia 1971, 22, 230–233. [Google Scholar] [CrossRef]
- Schmied, A.; Amalric, M.; Dormont, J.F.; Farin, D. GABAergic mechanisms in the cat red nucleus: Effects of intracerebral microinjections of muscimol or bicuculline on a conditioned motor task. Exp. Brain Res. 1990, 81, 523–532. [Google Scholar] [CrossRef]
- Chilton, W.S.; Ott, J. Toxic metabolites of Amanita pantherina, A. cothurnata, A. muscaria and other Amanita species. Lloydia 1976, 39, 150–157. [Google Scholar]
- König-Bersin, P.; Waser, P.G.; Langemann, H.; Lichtensteiger, W. Monoamines in the brain under the influence of muscimol and ibotenic acid, two psychoactive principles of amanita muscaria. Psychopharmacologia 1970, 18, 1–10. [Google Scholar] [CrossRef]
- Catalfomo, P.; Eugster, C. L’Amanita muscaria: Connaissance actuelle de ses principes actifs. Bull. Stupef. 1970, 22, 35–43. [Google Scholar]
- Ott, J.; Wheaton, P.S.; Chilton, W.S. Fate of muscimol in the mouse. Physiol. Chem. Phys. 1975, 7, 381–384. [Google Scholar] [PubMed]
- Vazharov, V. Medicinal Fungi of Bulgaria, 3rd ed.; Biana: Sofia, Bulgaria, 2021. [Google Scholar]
- Dushkov, A.; Petrova, M.; Todorova, J.; Gospodinov, A.; Ugrinova, I. Natural medicine: An evaluation of the in vitro cytotoxic effect of several Bulgarian fungal species on two panels of cancer cell lines. Bulg. Chem. Commun. 2021, 53, 35–41. [Google Scholar]
- Yoshida, I.; Kiho, T.; Usui, S.; Sakushima, M.; Ukai, S. Polysaccharides in Fungi. XXXVII. Immunomodulating Activities of Carboxymethylated Derivatives of Linear (1→3)-α-D-Glucans Extracted from the Fruiting Bodies of Agrocybe cylindracea and Amanita muscaria. Biol. Pharm. Bull. 1996, 19, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zavadinack, M.; de Lima Bellan, D.; da Rocha Bertage, J.L.; da Silva Milhorini, S.; da Silva Trindade, E.; Simas, F.F.; Sassaki, G.L.; Cordeiro, L.M.C.; Iacomini, M. An α-D-galactan and a β-D-glucan from the mushroom Amanita muscaria: Structural characterization and antitumor activity against melanoma. Carbohydr. Polym. 2021, 274, 118647. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, B.-Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget 2015, 6, 17832. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jang, J.-E.; Mishra, S.K.; Lee, H.-J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef]
- Pustovalova, M.; Alhaddad, L.; Smetanina, N.; Chigasova, A.; Blokhina, T.; Chuprov-Netochin, R.; Osipov, A.N.; Leonov, S. The p53-53BP1-Related Survival of A549 and H1299 Human Lung Cancer Cells after Multifractionated Radiotherapy Demonstrated Different Response to Additional Acute X-ray Exposure. Int. J. Mol. Sci. 2020, 21, 3342. [Google Scholar] [CrossRef]
- Wagner, A.; Pehar, M.; Yan, Z.; Kulka, M. Amanita muscaria extract potentiates production of proinflammatory cytokines by dsRNA-activated human microglia. Front. Pharmacol. 2023, 14, 1102465. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Geml, J.; Laursen, G.A.; O’Neill, K.; Nusbaum, H.C.; Taylor, D.L. Beringian origins and cryptic speciation events in the fly agaric (Amanita muscaria). Mol. Ecol. 2006, 15, 225–239. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [PubMed]




| Method Used | Concentration in Extract with Rye Vodka—mg/mL | Concentration in Extract with dH2O/EtOH (50/50 v/v)—mg/mL | ||
|---|---|---|---|---|
| IBO | MUS | IBO | MUS | |
| CZE/CCD | 0.02 | 0.07 | ND * | 0.02 |
| LC-MS | 0.04 | 0.02 | ND * | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushkov, A.; Vosáhlová, Z.; Tzintzarov, A.; Kalíková, K.; Křížek, T.; Ugrinova, I. Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro. Molecules 2023, 28, 6824. https://doi.org/10.3390/molecules28196824
Dushkov A, Vosáhlová Z, Tzintzarov A, Kalíková K, Křížek T, Ugrinova I. Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro. Molecules. 2023; 28(19):6824. https://doi.org/10.3390/molecules28196824
Chicago/Turabian StyleDushkov, Alexander, Zuzana Vosáhlová, Alexander Tzintzarov, Květa Kalíková, Tomáš Křížek, and Iva Ugrinova. 2023. "Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro" Molecules 28, no. 19: 6824. https://doi.org/10.3390/molecules28196824
APA StyleDushkov, A., Vosáhlová, Z., Tzintzarov, A., Kalíková, K., Křížek, T., & Ugrinova, I. (2023). Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro. Molecules, 28(19), 6824. https://doi.org/10.3390/molecules28196824







