Therapeutic Potential of Natural Products in the Treatment of Schistosomiasis
Abstract
:1. Introduction
2. Plant-Derived Compounds
2.1. In Vitro Studies
2.2. In Vivo Studies
2.3. Essential Oils and Their Components in Use against Schistosoma Mansoni
3. Useful Tools for the Screening of New Drugs in Schistosomiasis
4. Strategies Employing Functional Genomics Approaches and New Perspectives
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lo, N.C.; Bezerra, F.S.M.; Colley, D.G.; Fleming, F.M.; Homeida, M.; Kabatereine, M.; Kabole, F.M.; King, C.H.; Mafe, M.A.; Midzi, M.; et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect Dis. 2022, 22, e327–e335. [Google Scholar] [CrossRef]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From immunopathology to vacines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef]
- LoVerde, P.T. Schistosomiasis. Adv. Exp. Med. Biol. 2019, 1154, 45–70. [Google Scholar] [CrossRef]
- Wu, G.Y.; Halim, M.H. Schistosomiasis: Progress and problems. World J. Gastroenterol. 2000, 6, 12–19. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Whitfield, P.J.; Bartlett, A.; Khammo, N.; Clothier, R.H. Age-dependent survival and infectivity of Schistosoma mansoni cercariae. Parasitology 2003, 127 Pt 1, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Andrade, C.H.; Cravo, P.V. Natural products as leads in schistosome drug discovery. Molecules 2015, 20, 1872–1903. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef]
- Siqueira, L.D.P.; Fontes, D.A.F.; Aguilera, C.S.B.; Timóteo, T.R.R.; Ângelos, M.A.; Silva, L.C.P.B.B.; de Melo, C.G.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Schistosomiasis: Drugs used and treatment strategies. Acta Trop. 2017, 176, 179–187. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.G.; Bartley, P.B.; Sleigh, A.C.; Olds, G.R.; Li, Y.; Williams, G.M.; McManus, D.P. Schistosomiasis. N. Engl. J. Med. 2002, 346, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Thétiot-Laurent, S.A.; Boissier, J.; Robert, A.; Meunier, B. Schistosomiasis chemotherapy. Angew. Chem. Int. Ed. Engl. 2013, 52, 7936–7956. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/schistosomiasis/strategy/en/ (accessed on 15 January 2023).
- Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Machado, L.F.; Bortoleti, B.T.S.; Sahd, C.S.; Chagas, A.F.; Assolini, J.P.; Oliveira, F.J.A.; Pavanelli, W.R.; Conchon-Costa, I.; et al. Nanotechnology as a potential therapeutic alternative for schistosomiasis. Acta Trop. 2017, 174, 64–71. [Google Scholar] [CrossRef]
- Cioli, D. Chemotherapy of Schistosomiasis: An Update. Parasitol. Today 1998, 14, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, C.R. Schistosomiasis and its treatment. Future Med. Chem. 2015, 7, 675–676. [Google Scholar] [CrossRef]
- Mäder, P.; Rennar, G.A.; Ventura, A.M.P.; Grevelding, C.G.; Schlitzer, M. Chemotherapy for Fighting Schistosomiasis: Past, Present and Future. Chem. Med. Chem. 2018, 13, 2374–2389. [Google Scholar] [CrossRef]
- Adegboye, O.; Field, M.A.; Kupz, A.; Pai, S.; Sharma, D.; Smout, M.J.; Wangchuk, P.; Wong, Y.; Loiseau, C. Natural-Product-Based Solutions for Tropical Infectious Diseases. Clin. Microbiol. Rev. 2021, 34, e0034820. [Google Scholar] [CrossRef]
- Eze, A.A.; Ogugofor, M.O.; Ossai, E.C. Plant-Derived Compounds for the Treatment of Schistosomiasis: Improving Efficacy Via Nano-Drug Delivery. Niger. J. Clin. Pract. 2022, 25, 747–764. [Google Scholar] [CrossRef]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420–451. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Cunha, N.L.; Uchôa, C.J.D.M.; Cintra, L.S.; Souza, H.C.D.; Peixoto, J.A.; Silva, C.P.; Magalhães, L.G.; Gimenez, V.M.M.; Groppo, M.; Rodrigues, V.; et al. In vitro Schistosomicidal Activity of Some Brazilian Cerrado Species and Their Isolated Compounds. Evid.-Based Complement. Altern. Med. 2012, 2012, 173614. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Nascimento, C.; Yamaguchi, L.F.; Kato, M.J.; Nakano, E. Schistosoma Mansoni: In vitro Schistosomicidal Activity and Tegumental Alterations Induced by Piplartine on Schistosomula. Exp. Parasitol. 2012, 132, 222–227. [Google Scholar] [CrossRef]
- Matos, J.L.; da Silva, K.R.; de Lima Paula, L.A.; Cunha, W.R.; Ramos, S.B.; Rodrigues, V.; Cabral, F.J.; Magalhães, L.G. Molluscicidal and Cercaricidal Activities of Curcumin on Biomphalaria glabrata and Schistosoma mansoni Cercariae. Pest. Manag. Sci. 2020, 76, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Martorell, M.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Anti-Schistosoma mansoni effects of essential oils and their components. Phytother. Res. 2020, 34, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.N.; Oliveira, G.S.L.; de Carvalho, R.B.F.; de Sousa, D.P.; Freitas, R.M.; Pinto, P.L.S.; de Moraes, J. Antischistosomal Activity of the Terpene Nerolidol. Molecules 2014, 19, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, C.; Magalhães, L.; Rodrigues, V.; Mulhovo, S.; Da Silva Filho, A.A.; Ferreira, M.J.U. In vitro Schistosomicidal Activity of Balsaminol F and Karavilagenin, C. Planta Med. 2012, 78, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.P.; Brissow, E.; Kellner Filho, L.C.; Senabio, J.; de Siqueira, K.A.; Vandresen Filho, S.; Damasceno, J.L.; Mendes, S.A.; Tavares, D.C.; Magalhães, L.G.; et al. Bioactive Compounds of Aspergillus Terreus—F7, an Endophytic Fungus from Hyptis suaveolens (L.) Poit. World J. Microbiol. Biotechnol. 2017, 33, 62. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Nascimento, C.; Miura, L.M.C.V.; Leite, J.R.S.A.; Nakano, E.; Kawano, T. Evaluation of the in vitro Activity of Dermaseptin 01, a Cationic Antimicrobial Peptide, against Schistosoma mansoni. Chem. Biodivers. 2011, 8, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Almeida, A.A.C.; Brito, M.R.M.; Marques, T.H.C.; Lima, T.C.; De Sousa, D.P.; Nakano, E.; Mendonça, R.Z.; Freitas, R.M. Anthelmintic Activity of the Natural Compound (+)-Limonene Epoxide against Schistosoma mansoni. Planta Med. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Carrara, V.S.; Vieira, S.C.H.; De Paula, R.G.; Rodrigues, V.; Magalhães, L.G.; Cortez, D.A.G.; Da Silva Filho, A.A. In vitro Schistosomicidal Effects of Aqueous and Dichloromethane Fractions from Leaves and Stems of Piper species and the Isolation of an Active Amide from P. Amalago L. (Piperaceae). J. Helminthol. 2014, 88, 321–326. [Google Scholar] [CrossRef]
- Santos, A.F.; Fonseca, S.A.; César, F.A.; De Azevedo Albuquerque, M.C.P.; Santana, J.V.; Santana, A.E.G. A Penta-Substituted Pyridine Alkaloid from the Rhizome of Jatropha elliptica (Pohl) Muell. Arg. Is Active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol. Res. 2014, 113, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Aleixo de Carvalho, L.S.; Geraldo, R.B.; de Moraes, J.; Silva Pinto, P.L.; de Faria Pinto, P.; Pereira, O.d.S.; Da Silva Filho, A.A. Schistosomicidal Activity and Docking of Schistosoma mansoni ATPDase 1 with Licoflavone B Isolated from Glycyrrhiza inflata (Fabaceae). Exp. Parasitol. 2015, 159, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, T.A.; De Oliveira, P.F.; De Souza, J.M.; Tavares, D.C.; Andrade, E.; Silva, M.L.; Cunha, W.R.; Groppo, M.; Januário, A.H.; Magalhães, L.G.; et al. Schistosomicidal Activity of Alkyl-Phenols from the Cashew Anacardium occidentale against Schistosoma mansoni Adult Worms. J. Agric. Food Chem. 2016, 64, 8821–8827. [Google Scholar] [CrossRef] [PubMed]
- Eraky, M.A.; Aly, N.S.M.; Selem, R.F.; El-Kholy, A.A.E.M.; Rashed, G.A.E.R. In vitro Schistosomicidal Activity of Phytol and Tegumental Alterations Induced in Juvenile and Adult Stages of Schistosoma haematobium. Korean J. Parasitol. 2016, 54, 477–484. [Google Scholar] [CrossRef]
- Pereira, V.R.D.; Junior, I.J.A.; da Silveira, L.S.; Geraldo, R.B.; Pinto, P.d.F.; Teixeira, F.S.; Salvadori, M.C.; Silva, M.P.; Alves, L.A.; Capriles, P.V.S.Z.; et al. In vitro and in vivo Antischistosomal Activities of Chalcones. Chem. Biodivers. 2018, 15, e1800398. [Google Scholar] [CrossRef]
- Parreira, R.L.T.; Costa, E.S.; Heleno, V.C.G.; Magalhães, L.G.; Souza, J.M.; Pauletti, P.M.; Cunha, W.R.; Januário, A.H.; Símaro, G.V.; Bastos, J.K.; et al. Evaluation of Lignans from Piper Cubeba against Schistosoma mansoni Adult Worms: A Combined Experimental and Theoretical Study. Chem. Biodivers. 2019, 16, e1800305. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.; Saoud, M.; Rennert, R.; Fotso, G.W.; Andrae-Marobela, K.; Imming, P.; Häberli, C.; Keiser, J.; Arnold, N. Anthelmintic Activity and Cytotoxic Effects of Compounds Isolated from the Fruits of Ozoroa insignis Del. (Anacardiaceae). Biomolecules 2021, 11, 1893. [Google Scholar] [CrossRef] [PubMed]
- Sirak, B.; Asres, K.; Hailu, A.; Dube, M.; Arnold, N.; Häberli, C.; Keiser, J.; Imming, P. In vitro Antileishmanial and Antischistosomal Activities of Anemonin Isolated from the Fresh Leaves of Ranunculus multifidus Forsk. Molecules 2021, 26, 7473. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free. Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Castellano, A.; Díaz-Quintana, A.; Pérez-Mejías, G.; Elena-Real, C.A.; González-Arzola, K.; García-Mauriño, S.M.; De la Rosa, M.A.; Díaz-Moreno, I. Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Biophys. Comput. Biol. 2018, 115, 7955–7960. [Google Scholar] [CrossRef]
- Allam, G.; Abuelsaad, A.S.A. In vitro and in vivo Effects of Hesperidin Treatment on Adult Worms of Schistosoma mansoni. J. Helminthol. 2014, 88, 362–370. [Google Scholar] [CrossRef]
- Moraes, J.; de Oliveira, R.N.; Costa, J.P.; Junior, A.L.G.; de Sousa, D.P.; Freitas, R.M.; Allegretti, S.M.; Pinto, P.L.S. Phytol, a Diterpene Alcohol from Chlorophyll, as a Drug against Neglected Tropical Disease Schistosomiasis Mansoni. PLoS Negl. Trop. Dis. 2014, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Reimer, A.; Blohm, A.; Quack, T.; Grevelding, C.G.; Kozjak-Pavlovic, V.; Rudel, T.; Hentschel, U.; Abdelmohsen, U.R. Inhibitory Activities of the Marine Streptomycete-Derived Compound SF2446A2 against Chlamydia Trachomatis and Schistosoma mansoni. J. Antibiot. 2015, 68, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Mafud, A.C.; Silva, M.P.N.; Monteiro, D.C.; Oliveira, M.F.; Resende, J.G.; Coelho, M.L.; De Sousa, D.P.; Mendonça, R.Z.; Pinto, P.L.S.; Freitas, R.M.; et al. Structural Parameters, Molecular Properties, and Biological Evaluation of Some Terpenes Targeting Schistosoma mansoni Parasite. Chem. Biol. Interact. 2016, 244, 129–139. [Google Scholar] [CrossRef]
- Martins, M.C.B.; Silva, M.C.; Silva, H.A.M.F.; Silva, L.R.S.; De Azevedo Albuquerque, M.C.P.; Aires, A.L.; Da Silva Falcão, E.P.; Pereira, E.C.; De Melo, A.M.M.A.; De Silva, N.H. Barbatic Acid Offers a New Possibility for Control of Biomphalaria glabrata and Schistosomiasis. Molecules 2017, 22, 568. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.L.; Gonçalves, U.O.; Badoco, F.R.; de Souza Galvão, L.; Santos, R.A.d.; de Carvalho, P.H.D.; de Carvalho, L.S.A.; da Silva Filho, A.A.; Veneziani, R.C.S.; Rodrigues, V.; et al. Licochalcone A Induces Morphological and Biochemical Alterations in Schistosoma mansoni Adult Worms. Biomed. Pharmacother. 2017, 96, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lago, E.M.; Xavier, R.P.; Teixeira, T.R.; Silva, L.M.; da Silva Filho, A.A.; de Moraes, J. Antischistosomal Agents: State of art and perspectives. Future Med. Chem. 2018, 10, 89–120. [Google Scholar] [CrossRef]
- Allam, G. Immunomodulatory Effects of Curcumin Treatment on Murine Schistosomiasis Mansoni. Immunobiology 2009, 214, 712–727. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S.; Shebl, A.M.; Said, S.A. Prevention and Treatment of Schistosoma mansoni-Induced Liver Fibrosis in Mice. Inflammopharmacology 2011, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Keiser, J.; Vargas, M.; Gubaidullin, R.R.; Nedopekina, D.A.; Shakurova, E.R.; Khalitova, R.R.; Odinokov, V.N. Synthesis and Activity of New Triphenylphosphonium Derivatives of Betulin and Betulinic Acid against Schistosoma mansoni in vitro and in vivo. Bioorg. Med. Chem. 2014, 22, 6297–6304. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.A.; de Oliveira, R.N.; Véras, L.M.C.; Lima, D.F.; Campelo, Y.D.M.; Campos, S.A.; Kuckelhaus, S.A.S.; Pinto, P.L.S.; Eaton, P.; Mafud, A.C.; et al. Anthelmintic Activity In vivo of Epiisopiloturine against Juvenile and Adult Worms of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015, 9, e0003656. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; de Oliveira, R.N.; Mengarda, A.C.; Roquini, D.B.; Allegretti, S.M.; Salvadori, M.C.; Teixeira, F.S.; de Sousa, D.P.; Pinto, P.L.S.; da Silva Filho, A.A.; et al. Antiparasitic Activity of Nerolidol in a Mouse Model of Schistosomiasis. Int. J. Antimicrob. Agents 2017, 50, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aal, N.F.; Hamza, R.S.; Harb, O. Paeoniflorin Targets Apoptosis and Ameliorates Fibrosis in Murine Schistosomiasis Mansoni: A Novel Insight. Exp. Parasitol. 2017, 183, 23–32. [Google Scholar] [CrossRef]
- Castro, A.P.; Kawano, T.; Spelta, L.E.W.; de Castro, A.T.; Pereira, N.A.; Couto, F.F.B.; dos Santos, M.H.; Boralli, V.B.; Marques, M.J. In vivo Schistosomicidal Activity of 7-Epiclusianone and Its Quantification in the Plasma of Healthy and Schistosoma mansoni Infected Mice Using UPLC-MS/MS. Phytomedicine 2018, 38, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Metwally, D.M.; Al-Olayan, E.M.; Alanazi, M.; Alzahrany, S.B.; Semlali, A. Antischistosomal and Anti-Inflammatory Activity of Garlic and Allicin Compared with That of Praziquantel in vivo. BMC Complement. Altern. Med. 2018, 18, 135. [Google Scholar] [CrossRef]
- Guimarães, M.A.; de Oliveira, R.N.; de Almeida, R.L.; Mafud, A.C.; Sarkis, A.L.V.; Ganassin, R.; da Silva, M.P.; Roquini, D.B.; Veras, L.M.; Sawada, T.C.H.; et al. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. PLoS ONE. 2018, 13, e0196667. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Mendonça, P.S.; Morais, C.S.; Cogo, R.M.; Mazloum, S.F.; Salvadori, M.C.; Teixeira, F.S.; Morais, T.R.; Antar, G.M.; Lago, J.H.G.; et al. Antiparasitic Activity of Piplartine (Piperlongumine) in a Mouse Model of Schistosomiasis. Acta Trop. 2020, 205, 105350. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Koch, V.; Deckers, A.; Cheung, H.T.A.; Jung, N.; Bräse, S. Naturally Occurring Cardenolides Affecting Schistosoma mansoni. ACS Infect. Dis. 2020, 6, 1922–1927. [Google Scholar] [CrossRef]
- Matos-Rocha, T.J.; Cavalcanti, M.G.S.; Veras, D.L.; Santos, A.F.; Freitas, C.F.; Suassuna, A.S.C.L.; Melo, E.S.; Barbosa-Filho, J.M.; Alves, L.C.; Santos, F.A.B.D. In vivo effect of essential oil of Mentha x villosa and its active compound against Schistosoma mansoni (Sambon, 1907). Braz J. Biol. 2020, 80, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Marconato, D.G.; da Silva, M.P.N.; Raposo, N.R.B.; Facchini, G.d.F.S.; Macedo, G.C.; Teixeira, F.D.S.; Salvadori, M.C.B.d.S.; Pinto, P.D.F.; de Moraes, J.; et al. Licochalcone A-Loaded Solid Lipid Nanoparticles Improve Antischistosomal Activity in vitro and in vivo. Nanomedicine 2021, 16, 1641–1655. [Google Scholar] [CrossRef]
- Silva, C.B.; Mengarda, A.C.; Rodrigues, V.C.; Cajas, R.A.; Carnaúba, P.U.; Espírito-Santo, C.; Bezerra-Filho, C.S.M.; Souza, D.P.; Moraes, J. Efficacy of Caracryl Acetate in vitro and Following Oral Administration to Mice Harboring either Prepatent or Patent Shistosoma mansoni Infections. Parasitol. Res. 2021, 120, 3837–3844. [Google Scholar] [CrossRef]
- Carvalho, L.S.A.; Silva, L.M.; de Souza, V.C.; da Silva, M.P.N.; Capriles, P.V.S.Z.; de Faria Pinto, P.; de Moraes, J.; Da Silva Filho, A.A. Cardamonin Presents in vivo Activity against Schistosoma mansoni and Inhibits Potato Apyrase. Chem. Biodivers. 2021, 18, e2100604. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.S.A.; de Souza, V.C.; Rodrigues, V.C.; Ribeiro, A.C.; Nascimento, J.W.L.; Capriles, P.V.S.Z.; Pinto, P.F.; de Moraes, J.; da Silva Filho, A.A. Identification of Asiaticoside from Centella erecta (Apiaceae) as Potential Apyrase Inhibitor by UF-UHPLC-MS and Its In vivo Antischistosomal Activity. Pharmaceutics 2022, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Bakery, H.H.; Allam, G.A.; Abuelsaad, A.S.A.; Abdel-Latif, M.; Elkenawy, A.E.; Khalil, R.G. Anti-inflammatory, Antioxidant, Anti-fibrotic and Schistosomicidal Properties of Plumbagin in Murine Schistosomiasis. Parasite Immunol. 2022, 44, e12945. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.G.; Ibrahim, A.M.; Bakery, H.H. Juglone: “A Novel Immunomodulatory, Antifibrotic, and Schistosomicidal Agent to Ameliorate Liver Damage in Murine schistosomiasis Mansoni”. Int. Immunopharmacol. 2022, 113, 109415. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Razi, B.; Aslani, S.; Abbasifard, M.; Imani, D.; Sathyapalan, T.; Sahebkar, A. Effect of curcumin on proinflammatory cytokines: A meta-analysis of randomized controlled trials. Cytokine 2021, 143, 155541. [Google Scholar] [CrossRef]
- Schramm, G.; Haas, H. Th2 Immune Response against Schistosoma mansoni Infection. Microbes Infect. 2010, 12, 881–888. [Google Scholar] [CrossRef]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; de Moura, R.O.; Mendonca, F.J.B. Active essential oils and their components in use against neglected diseases and Arboviruses. Oxidative Med. Cell. Longev. 2019, 2019, 6587150–6587152. [Google Scholar] [CrossRef] [PubMed]
- Mendes, N.M.; Araújo, N.; de Souza, C.P.; Pereira, J.P.; Katz, N. Molluscacide and cercariacide activity of different species of Eucalyptus. Rev. Soc. Bras. Med. Trop. 1990, 23, 197–199. [Google Scholar] [CrossRef]
- de Carvalho Augusto, R.; Merad, N.; Rognon, A.; Gourbal, B.; Bertrand, C.; Djabou, N.; Duval, D. Molluscicidal and parasiticidal activities of Eryngium triquetrum essential oil on Schistosoma mansoni and its intermediate snail host Biomphalaria Glabrata, a double impact. Parasit. Vectors 2020, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.C.G.; Leite, J.A.C.; Luz, T.R.S.A.; Silveira, D.P.B.; Bezerra, S.A.; Frazão, G.C.C.G.; Pereira, L.P.L.A.; Guimarães Dos Santos, E.G.; Ribeiro Filho, P.R.C.F.; Soares, A.M.S.; et al. Molluscicidal activity of monoterpenes and their effects on inhibition of acetylcholinesterase activity on Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Acta Trop. 2021, 223, 106089. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.P.; Albuquerque, R.D.D.G.; Rangel, L.D.S.; Caldas, G.R.; Tietbohl, L.A.C.; Santos, M.G.; Ricci-Júnior, E.; Thiengo, S.; Fernandez, M.A.; Santos, J.A.A.D.; et al. Nanoemulsion containing essential oil from Xylopia ochrantha Mart. produces molluscicidal effects against different species of Biomphalaria (Schistosoma hosts). Mem. Inst. Oswaldo Cruz 2019, 114, e180489. [Google Scholar] [CrossRef]
- Santos-Filho, D.; Sarti, S.J.; Katz, N.; Araújo, N.; Rocha Filho, P.A.; Abreu, J.E.; Bortolin, M.E. Chemoprophylactic activity of soaps containing essential oil from the fruit of Pterodon pubescens in schistosomiasis mansoni. Mem. Inst. Oswaldo Cruz 1987, 82 (Suppl. S4), 343–345. [Google Scholar]
- Saleh, M.M.; Zwaving, J.H.; Malingré, T.M.; Bos, R. The essential oil of Apium graveolens var. secalinum and its cercaricidal activity. Pharm. Weekbl. Sci. 1985, 7, 277–279. [Google Scholar] [CrossRef]
- Naples, J.M.; Shiff, C.J.; Rosler, K.H. Schistosoma mansoni: Cercaricidal effects of Cedarwood oil and various of its components. J. Trop. Med. Hyg. 1992, 95, 390–396. [Google Scholar] [PubMed]
- Parreira, N.A.; Magalhães, L.G.; Morais, D.R.; Caixeta, S.C.; de Sousa, J.P.; Bastos, J.K.; Cunha, W.R.; Silva, M.L.; Nanayakkara, N.P.; Rodrigues, V.; et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem. Biodivers. 2010, 7, 993–1001. [Google Scholar] [CrossRef]
- Caixeta, S.C.; Magalhães, L.G.; de Melo, N.I.; Wakabayashi, K.A.; Aguiar Gde, P.; Mantovani, A.L.; Alves, J.M.; Oliveira, P.F.; Tavares, D.C.; Groppo, M.; et al. Chemical composition and in vitro schistosomicidal activity of the essential oil of Plectranthus neochilus grown in Southeast Brazil. Chem. Biodivers. 2011, 8, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- de Melo, N.I.; Magalhaes, L.G.; de Carvalho, C.E.; Wakabayashi, K.A.; de P Aguiar, G.; Ramos, R.C.; Mantovani, A.L.; Turatti, I.C.; Rodrigues, V.; Groppo, M.; et al. Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against adult Schistosoma mansoni worms. Molecules 2011, 16, 762–773. [Google Scholar] [CrossRef] [PubMed]
- de Melo, N.I.; Mantovani, A.L.; de Oliveira, P.F.; Groppo, M.; Filho, A.A.; Rodrigues, V.; Cunha, W.R.; Tavares, D.C.; Magalhães, L.G.; Crottii, A.E. Antischistosomal and Cytotoxic Effects of the Essential Oil of Tetradenia riparia (Lamiaceae). Nat. Prod. Commun. 2015, 10, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Matos-Rocha, T.J.; dos Santos Cavalcanti, M.G.; Barbosa-Filho, J.M.; Lúcio, A.S.; Veras, D.L.; Feitosa, A.P.; de Siqueira Júnior, J.P.; de Almeida, R.N.; Marques, M.O.; Alves, L.C.; et al. In vitro evaluation of schistosomicidal activity of essential oil of Mentha x villosa and some of its chemical constituents in adult worms of Schistosoma mansoni. Planta Med. 2013, 79, 1307–1312. [Google Scholar] [CrossRef]
- de Oliveira, R.N.; Rehder, V.L.; Santos Oliveira, A.S.; Júnior, Í.M.; de Carvalho, J.E.; de Ruiz, A.L.; Jeraldo, V.d.L.; Linhares, A.X.; Allegretti, S.M. Schistosoma mansoni: In vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp. Parasitol. 2012, 132, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Matos-Rocha, T.J.; Cavalcanti, M.G.; Veras, D.L.; Feitosa, A.P.; Gonçalves, G.G.; Portela-Junior, N.C.; Lúcio, A.S.; Silva, A.L.; Padilha, R.J.; Marques, M.O.; et al. Ultrastructural changes in Schistosoma mansoni male worms after in vitro incubation with the essential oil of Mentha x villosa Huds. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.A.; de Melo, N.I.; Aguiar, D.P.; de Oliveira, P.F.; Groppo, M.; da Silva Filho, A.A.; Rodrigues, V.; Cunha, W.R.; Tavares, D.C.; Magalhães, L.G.; et al. Anthelmintic effects of the essential oil of fennel (Foeniculum vulgare Mill., Apiaceae) against Schistosoma mansoni. Chem. Biodivers. 2015, 12, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.H.; Fracarolli, L.; Vieira, T.M.; Dias, H.J.; Cruz, M.G.; Deus, C.C.; Nicolella, H.D.; Stefani, R.; Rodrigues, V.; Tavares, D.C.; et al. Schistosomicidal Effects of the Essential Oils of Citrus limonia and Citrus reticulata against Schistosoma mansoni. Chem. Biodivers. 2017, 14, e1600194. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.H.; Dias, H.J.; Vieira, T.M.; de Souza, M.G.M.; Cruz, A.F.F.; Badoco, F.R.; Nicolella, H.D.; Cunha, W.R.; Groppo, M.; Martins, C.H.G.; et al. Chemical Composition, Antibacterial, Schistosomicidal, and Cytotoxic Activities of the Essential Oil of Dysphania ambrosioides (L.) Mosyakin & Clemants (Chenopodiaceae). Chem. Biodivers. 2017, 14, e1700149. [Google Scholar] [CrossRef]
- Rizk, M.; Ibrahim, N.; El-Rigal, N. Comparative in vivo antioxidant levels in Schistosoma mansoni infected mice treated with praziquantel or the essential oil of Melaleuca armillaris leaves. Pak. J. Biol. Sci. 2012, 15, 971–978. [Google Scholar] [CrossRef]
- Mafud, A.C.; Ferreira, L.G.; Mascarenhas, Y.P.; Andricopulo, A.D.; de Moraes, J. Discovery of novel antischistosomal agents by molecular modeling approaches. Trends Parasitol. 2016, 32, 874–886. [Google Scholar] [CrossRef]
- Moreira-Filho, J.T.; Silva, A.C.; Dantas, R.F.; Gomes, B.F.; Souza Neto, L.R.; Brandao-Neto, J.; Owens, R.J.; Furnham, N.; Neves, B.J.; Silva-Junior, F.P.; et al. Schistosomiasis drug discovery in the era of automation and artificial intelligence. Front. Immunol. 2021, 12, 642383. [Google Scholar] [CrossRef]
- Yu, W.; Weber, D.J.; MackKerell, A.D., Jr. Computer-Aided Drug Design: An update. Methods Mol. Biol. 2023, 2601, 123–152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Bae, H.; Jo, J.; Yoon, S. Comprehensive ensemble in QSAR prediction for drug discovery. BMC Bioinform. 2019, 20, 521. [Google Scholar] [CrossRef]
- Berriman, M.; Haas, B.J.; LoVerde, P.T.; Wilson, R.A.; Dillon, G.P.; Cerqueira, G.C.; Mashiyama, S.T.; Al-Lazikani, B.; Andrade, L.F.; Ashton, P.D.; et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009, 460, 352–358. [Google Scholar] [CrossRef]
- Buddenborg, S.K.; Tracey, A.; Berger, D.J.; Lu, Z.; Doyle, S.R.; Fu, B.; Yang, F.; Reid, A.J.; Rodgers, F.H.; Rinaldi, G.; et al. Assembled chromosomes of the blood fluke Schistosoma mansoni provide insight into the evolution of its ZW sex-determination system. BioRxiv 2021, 13, 456314. [Google Scholar] [CrossRef]
- Lund, A.J.; Wade, K.J.; Nikolakis, Z.L.; Ivey, K.N.; Perry, B.W.; Pike, H.N.C.; Paull, S.H.; Liu, Y.; Castoe, T.A.; Pollock, D.D.; et al. Integrating genomic and epidemiologic data to accelerate progress toward schistosomiasis elimination. Elife 2022, 11, e79320. [Google Scholar] [CrossRef] [PubMed]
- Wangwiwatsin, A.; Protasio, A.V.; Wilson, S.; Owusu, C.; Holroyd, N.E.; Sanders, M.J.; Keane, J.; Doenhoff, M.J.; Rinaldi, G.; Berriman, M. Transcriptome of the parasitic flatworm Schistosoma mansoni during intra-mammalian development. PLoS Negl. Trop. Dis. 2020, 14, e0007743. [Google Scholar] [CrossRef]
- Cheuka, P.M. Drug discovery and target identification against schistosomiasis: A reality check on progress and future prospects. Curr. Top. Med. Chem. 2022, 22, 1595–1610. [Google Scholar] [CrossRef]
- Ali, Z.; Hayat, M.F.; Shaukat, K.; Alam, T.M.; Hameed, I.A.; Luo, S.; Basheer, S.; Ayadi, M.; Ksibi, A. A proposed framework for early prediction of schistosomiasis. Diagnostics 2022, 12, 3138. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Akachukwu, I.; Olubiyi, O.O.; Kosisochukwu, A.; John, M.C.; Justina, N.N. Structure-based study of natural products with anti-schistosoma activity. Curr. Comput. Aided Drug Des. 2017, 13, 91–100. [Google Scholar] [CrossRef]
- de Menezes, R.P.B.; Viana, D.O.; Muratov, E.; Scotti, L.; Scotti, M.T. Computer-assisted discovery of alkaloids with schistosomicidal activity. Curr. Issues Mol. Biol. 2022, 44, 383–408. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Dantas, R.F.; Senger, M.R.; Melo-Filho, C.C.; Valente, W.C.G.; de Almeida, A.C.M.; Rezende-Neto, J.M.; Lima, E.F.C.; Paveley, R.; Furnham, N.; et al. Discovery of new anti-schistosomal hits by integration of QSAR- based virtual screening and high content screening. J. Med. Chem. 2016, 59, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- Gallinger, T.L.; Aboagye, S.Y.; Obermann, W.; Weiss, M.; Grünweller, A.; Unverzagt, C.; Williams, D.L.; Schlitzer, M.; Haeberlein, S. First in silico screening of insect molecules for identification of novel anti-parasitic compounds. Pharmaceuticals 2022, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.P.; Weber, M.H.W.; Haeberlein, S.; Mokosch, A.S.; Spengler, B.; Grevelding, C.G.; Falcone, F.H. Drug repurposing and de novo drug discovery of protein kinase inhibitors as new drugs against schistosomiasis. Molecules 2022, 27, 1414. [Google Scholar] [CrossRef]
- Marcellino, C.; Gut, J.; Lim, K.C.; Singh, R.; Mckerrow, J.; Sakanari, J. WormAssay: A novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Negl. Trop. Dis. 2012, 6, e1494. [Google Scholar] [CrossRef]
- Mathavan, I.; Liu, L.J.; Robinson, S.W.; El-Sakkary, N.; Elatico, A.J.J.; Gomez, D.; Nellas, R.; Owens, R.J.; Zuercher, W.; Navratilova, I.; et al. Identification of inhibitors of the Schistosoma mansoni VKR2 kinase domain. ACS Med. Chem. Lett. 2022, 13, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Simoben, C.V.; Robaa, D.; Chakrabarti, A.; Schmidtkunz, K.; Marek, M.; Lancelot, J.; Kannan, S.; Melesina, J.; Shaik, T.B.; Pierce, R.J.; et al. A novel class of Schistosoma mansoni histone deacetylase 8 (HDAC8) inhibitors identified by structure-based virtual screening and in vitro testing. Molecules 2018, 23, 566. [Google Scholar] [CrossRef]
- Berger, D.J.; Crellen, T.; Lamberton, P.H.L.; Allan, F.; Tracey, A.; Noonan, J.D.; Kabatereine, N.B.; Tukahebwa, E.M.; Adriko, M.; Holroyd, N.; et al. Whole-Genome Sequencing of Schistosoma mansoni Reveals Extensive Diversity with Limited Selection despite Mass Drug Administration. Nat. Commun. 2021, 12, 4776. [Google Scholar] [CrossRef] [PubMed]
- Vianney, T.J.; Berger, D.J.; Doyle, S.R.; Sankaranarayanan, G.; Serubanja, J.; Nakawungu, P.K.; Besigye, F.; Sanya, R.E.; Holroyd, N.; Allan, F.; et al. Genome-Wide Analysis of Schistosoma mansoni Reveals Limited Population Structure and Possible Praziquantel Drug Selection Pressure within Ugandan Hot-Spot Communities. PLoS Negl. Trop. Dis. 2022, 16, e0010188. [Google Scholar] [CrossRef] [PubMed]
- Kenney, E.T.; Mann, V.H.; Ittiprasert, W.; Rosa, B.A.; Mitreva, M.; Bracken, B.K.; Loukas, A.; Brindley, P.J.; Sotillo, J. Differential Excretory/Secretory Proteome of the Adult Female and Male Stages of the Human Blood Fluke, Schistosoma mansoni. Front. Parasitol. 2022, 1, 950744. [Google Scholar] [CrossRef]
- Abou-El-Naga, I.F.; Amer, E.I.; Boulos, L.M.; El-Faham, M.H.; Abou Seada, N.M.; Younis, S.S. Biological and Proteomic Studies of Schistosoma mansoni with Decreased Sensitivity to Praziquantel. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101341. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Almeida, A.; Mendes, T.M.F.; Ferreira, P.; Abecasis, A.B.; Belo, S.; Anibal, F.F.; Allegretti, S.M.; Galinaro, C.A.; Carrilho, E.; Afonso, A. A Comparative Proteomic Analysis of Praziquantel-Susceptible and Praziquantel-Resistant Schistosoma mansoni Reveals Distinct Response between Male and Female Animals. Front. Trop. Dis. 2021, 2, 664642. [Google Scholar] [CrossRef]
- Protasio, A.V.; Tsai, I.J.; Babbage, A.; Nichol, S.; Hunt, M.; Aslett, M.A.; De Silva, N.; Velarde, G.S.; Anderson, T.J.C.; Clark, R.C.; et al. A Systematically Improved High Quality Genome and Transcriptome of the Human Blood Fluke Schistosoma mansoni. PLoS Negl. Trop. Dis. 2012, 6, e1455. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Cupit, P.M.; Bu, L.; Cunningham, C. Transcriptomic Analysis of Reduced Sensitivity to Praziquantel in Schistosoma mansoni. Mol. Biochem. Parasitol. 2019, 228, 6–15. [Google Scholar] [CrossRef]
- Wendt, G.R.; Reese, M.L.; Collins, J.J., 3rd. SchistoCyte Atlas: A Single-Cell Transcriptome Resource for Adult Schistosomes. Trends Parasitol. 2021, 37, 585–587. [Google Scholar] [CrossRef]
- Soria, C.L.D.; Attenborough, T.; Lu, Z.; Graham, J.; Hall, C.; Thompson, S.; Andrews, T.G.R.; Rawlinson, K.A.; Berriman, M.; Rinaldi, G. Single Cell Transcriptomics of the Human Parasite Schistosoma mansoni First Intra-Molluscan Stage Reveals Tentative Tegumental and Stem Cell Regulators. bioRxiv 2023. [Google Scholar] [CrossRef]
- Padalino, G.; Ferla, S.; Brancale, A.; Chalmers, I.W.; Hoffmann, K.F. Combining Bioinformatics, Cheminformatics, Functional Genomics and Whole Organism Approaches for Identifying Epigenetic Drug Targets in Schistosoma mansoni. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 559–570. [Google Scholar] [CrossRef]
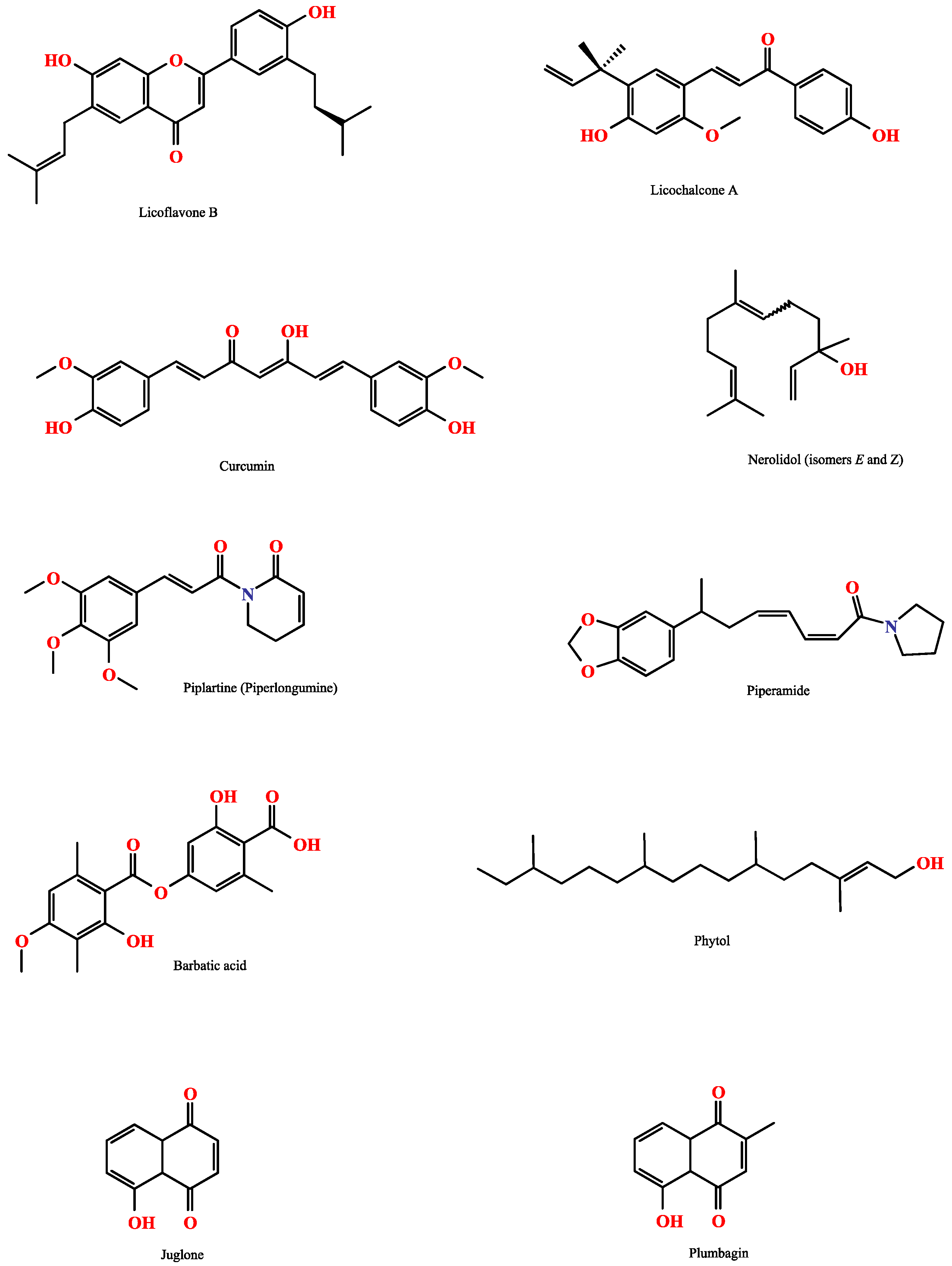

| Molecules | Concentrations | Main Results | References |
|---|---|---|---|
| Dermaseptin 01 | 25, 50, 75, 100, 150, and 200 µg/mL | Dermaseptin 01 reduced motility and induced death in adult worms of S. mansoni at concentrations between 50 and 200 µg/mL. In addition, Dermaseptin 01 reduced the egg output of paired female worms and induced morphological alterations in the tegument of S. mansoni | [29] |
| Betulin, Oleanolic acid, Ursolic acid, Quercetin 3-O-β-d-rhamnoside, Quercetin 3-O-β-d-glucoside, Quercetin 3-O-β-d-glucopyranosyl-(1-2)- α-l-rhamnopyranoside, and Isorhamnetin 3-O-β-d-glucopyranosyl-(1-2)-α-l-rhamnopyranoside | 50, 100, and 200 µM | Natural products reduced motor activity and caused death in adult S. mansoni worms | [22] |
| Pirplatine | 7.5, 15, 30, and 60 µM | Piplartine treatment resulted in the death of all schistosomula in a concentration- and time-dependent manner. Microscopic observation revealed extensive tegumental destruction, including blebbing, granularity, and shortened S. mansoni schistosomula body length. | [23] |
| Balsaminol F and Karavilagenin C | 10, 25, 50, and 100 µM | Balsaminol F and Karavilagenin presented LC50 values of 14.7 and 28.9 µM, respectively, against 56-day-old adult S. mansoni. In addition, at 10–50 µM, both compounds caused significantly reduced worm motor activity and significantly decreased egg production. At 10–100 µM, both triterpenes separated adult worm pairs into males and females after 24 h | [27] |
| (+)-limonene epoxide | 12.5, 25, 50, and 75 µg/mL | Treatment with compound reduced motility and induced death in adult S. mansoni worms at concentrations ≥25 µg/mL. Microscopic analysis revealed (+)-limonene epoxide mediated worm killing in association with tegumental destruction | [30] |
| Hesperidin | 50, 100, and 200 µg/mL | Hesperidin, at 200 µg/mL, caused 100% mortality in 56-day-old adult worms within 72 h, with partial tegumental alterations observed in 10% of worms | [43] |
| N-[7-(30,40-methylenedioxyphenyl)-2(Z),4(Z)-heptadienoyl] pyrrolidine | 10, 25, 50, and 100 µM | The isolated compound N-[7-(3′,4′-methylenedioxyphenyl)-2(Z),4(Z)-heptadienoyl] pyrrolidine promoted death of all adult worms of S. mansoni at 100 µM after 24 h of treatment | [31] |
| Phytol | 12.5, 25, 50, 75, and 100 µg/mL | Treatment with phytol reduced worm motor activity and caused death. Confocal laser scanning microscopy analysis revealed extensive tegumental alterations in a concentration-dependent manner (50 to 100 µg/mL). Additionally, sublethal doses of phytol (25 µg/mL) reduced numbers of Schistosoma mansoni eggs | [44] |
| Diethyl 4-phenyl-2,6-dimethyl-3,5-pyridinedicarboxylate | 1, 10, and 100 µg/mL | The alkaloid promoted the inhibition of movement and death in S. mansoni adult worms, accompanied by the formation of vesicles and vacuolization. In addition, the alkaloid exhibited a potent cercaricidal activity (LC100 = 2 μg/mL) as well as activity against adult snails (LC90 = 36.43 μg/ mL) | [32] |
| Nerolidol | 15.6, 31.2, 62.5, 125, and 250 µM | Nerolidol reduced motor activity and caused death in adult S. mansoni worms. In addition, morphological alterations were observed in the tegument of worms (disintegration, sloughing, and surface erosion) | [26] |
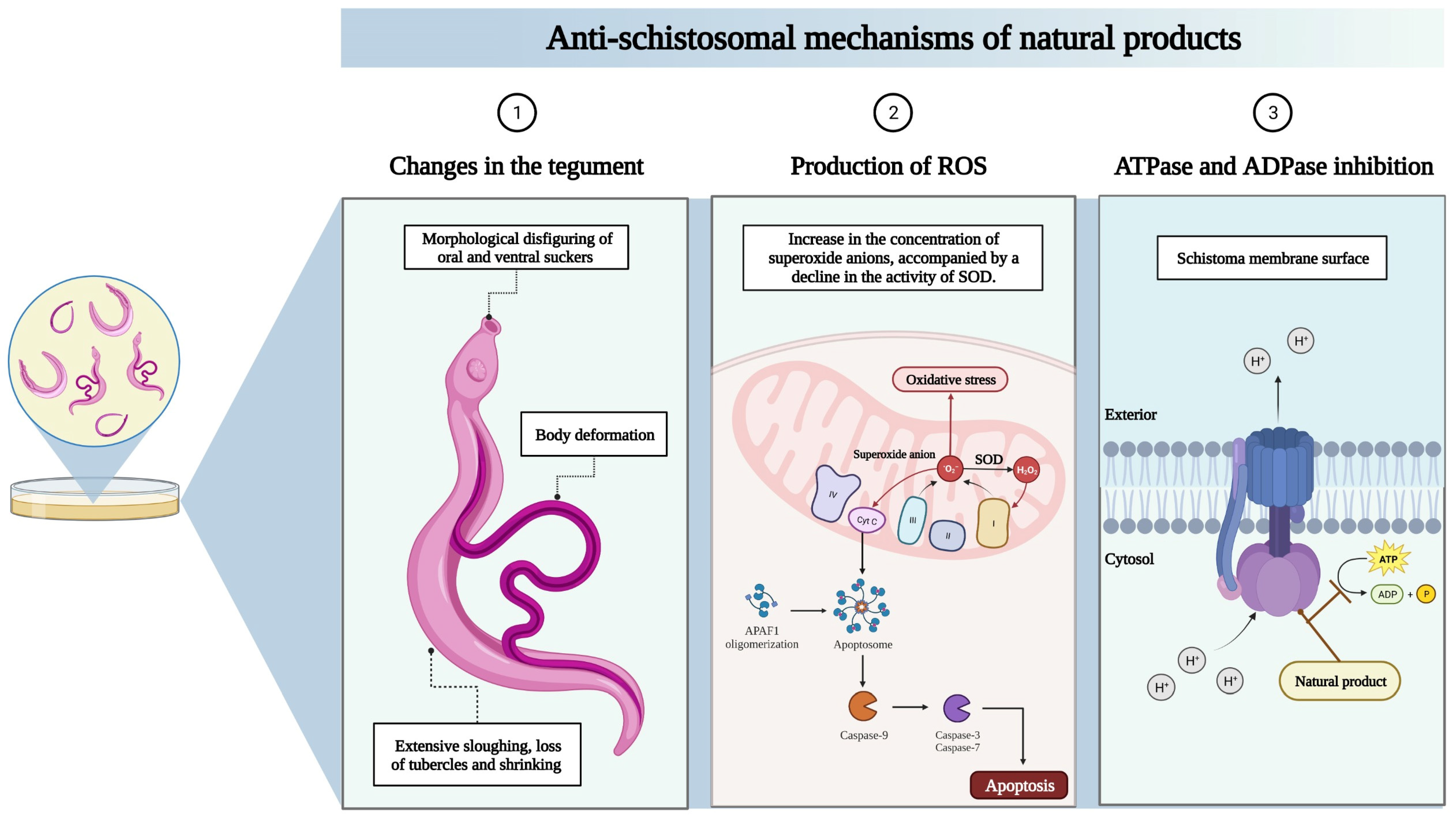
| Licoflavone B | 5, 10, 25, 50, and 100 µM | Licoflavone B (25 to 100 µM) caused 100% mortality, tegumental alterations, and reduced oviposition and motor activity in all adult worms, without affecting mammalian Vero cells. Licoflavone B also highly inhibited S. mansoni ATPase (IC50 of 23.78 µM) and ADPase (IC50 of 31.50 µM) activity | [33] |
| Streptomycete-derived compound SF2446A2 | 0.5–10 µM | Treatment with 100 µM of SF2446A2 affected the gonads by impairing oogenesis and spermatogenesis. In addition, SF2446A2 caused disruptive effects on the tegument surface of S. mansoni | [45] |
| Cardol triene, Cardol diene, Anacardic acid triene, Cardol monoene, Anacardic acid diene, 2-methylcardol triene, and 2-methylcardol diene | 12.5, 25, 50, 100, and 200 µM | Compounds Cardol diene and 2-methylcardol diene showed activity against S. mansoni adult worms, with LC50 values of 32.2 and 14.5 μM and selectivity indices of 6.1 and 21.2, respectively. Transmission electron microscopy revealed alterations in the tegument and mitochondrial membrane. | [34] |
| Phytol | 25, 50, 75, 100, 125, and 150 µg/mL | Phytol reduced motility and induced death in adult S. mansoni worms at 150 μg/mL, with male worms more susceptible to treatment. On an ultrastructural level, phytol induced tegumental peeling, disintegration of tubercles and spines, as well as morphological disfiguring of oral and ventral suckers | [35] |
| Series of 38 terpenes | 10, 20, 40, 80, 100, and 160 µM | Only dihydrocitronellol at 100 µM presented schistosomicidal activity after the maximal screening time of 120 h. Confocal laser scanning microscopy revealed severe tegumental damage induced by dihydrocitronellol in adult schistosomes | [46] |
| Barbatic acid | 0.25, 0.5, 1, 10, 25, and 100 µg/mL | Barbatic acid exhibited molluscicidal activity against snails, especially at 25 µg/mL, with 100% lethality. In addition, barbatic acid presented cercaricidal activity, completely eliminating cercariae at concentrations between 1 and 100 µg/mL | [47] |
| Terrein, Butyrolactone I, and butyrolactone V | 25–1297.3 µM | All compounds reduced motility and induced death in adult S. mansoni worms at concentrations between 235.6 and 454.1 µM | [28] |
| Licochalcone A | 3.125, 6,25, 12,5, 25, 50, 100, and 200 µM | Licochalcone A reduced the number of S. mansoni eggs and affected egg development in adult worms. Drastic changes in the tegument of S. mansoni adult worms and alterations in mitochondria and chromatin condensation were related to increased superoxide anion levels and decreased superoxide dismutase activity in adult S. mansoni worms | [48] |
| A series of 15 chalcones | 10, 50, and 100 µM | Chalcones, especially 1 and 3, induced adult worm death, reduced motility, and caused changes in the tegument of adult S. mansoni worms | [36] |
| (-) Hinoquinin, (-)-Cubebin, Yatein, 5-Methoxyyatein, Dihydrocubebin, and Dihydroclusin. | 10, 25, 50, and 100 µM | (-) Hinoquinin, (-)-Cubebin, Yatein, and 5-Methoxyyatein decreased motor activity in adult S. mansoni worms. All compounds, except Dihydrocubebin, were found to separate adult worm pairs and reduce egg numbers after 24 h of treatment | [37] |
| Curcumin | 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 µg/mL | Curcumin presented LC50 values <10 µg/mL against cercariae. Treatment with curcumin affected egg-laying capacity and egg hatchability, causing death in newborns, embryos, and adult B. globrata snails. | [24] |
| 6-[8(Z)-pentadecenyl] anacardic, 6-[10(Z)-heptadecenyl] anacardic acid, and 3-[7(Z)-pentadecenyl] phenol | 1, 10, and 100 µM | All compounds presented activity against S. mansoni, killing 100% of adult S. mansoni worms at 100 µM | [38] |
| Anemonin | 1 and 10 µM | Anemonin demonstrated activity against adult S. mansoni and newly transformed schistosomules (49% activity against adult S. mansoni at 10 µM and 41% activity against newly transformed schistosomules at 1 µM) | [39] |
| Molecules | Route | Dose | Main Results | References |
|---|---|---|---|---|
| Curcumin | Intraperitoneal | 400 mg/kg/day | Curcumin reduced worm and tissue egg burden, hepatic granuloma volume, and liver collagen content by 44.4%, 30.9%, 79%, and 38.6%, respectively | [50] |
| Curcumin | Oral | 300 mg/kg/day | Curcumin treatment exerted antifibrotic effects in S. mansoni-infected mice | [51] |
| Phytol | Oral | 40 mg/kg/day | A single dose of phytol (40 mg/kg) resulted in total and female worm burden reductions of 51.2% and 70.3%, respectively. Also, reduced numbers of eggs were found in feces (76.6%), with a lower frequency of immature eggs | [44] |
| Hesperidin | Intraperitoneal | 100 mg/kg/day | Reductions of 50, 45.2, 50, and 47.5% in males, females, worm pairs, and total worm burden, respectively. In addition, respective reductions, based on the number of eggs/g of tissue, of 41.5, 63.7, and 58.6% were observed in the liver, intestine, and liver/intestinal tissue combined | [43] |
| Triphenylphosphonium | Oral | 400 mg/kg/day | Triphenylphosphonium salts 10 and 11 resulted in low worm burden reductions against S. mansoni of 21.9% and 22.2%, respectively. Both compounds were well-tolerated by mice | [52] |
| Epiisopiloturine | Oral | 40, 100, and 300 mg/Kg/day | Treatment with epiisopiloturine at 40 mg/kg reduced total worm burden by 50.2%, as well as hepatosplenomegaly, egg burden in feces, and granuloma diameter. Electron microscopy revealed a loss of important features in the parasite tegument | [53] |
| Nerolidol | Oral | 100, 200, and 400 mg/kg/day | Nerolidol (100, 200, or 400 mg/kg) reduced worm burden and egg production in mice infected with adult schistosomes. Treatment with the highest concentration reduced total worms by 70.06% and immature eggs by 84.6%. Microscopic observations revealed that nerolidol-mediated worm killing was associated with tegumental damage | [54] |
| Paeoniflorin | Oral | 50 mg/kg/day | Paeoniflorin treatment decreased worm burden, as well as immature and mature eggs, with reductions in hepatic granuloma size and fibrotic areas | [55] |
| 7-epiclusianone | Oral | 100 or 300 mg/kg/day | 7-epiclusianone showed significant schistosomicidal in vivo activity following treatment with 300 mg/kg for 5 days | [56] |
| Allicin | Oral | 0.5 μM/mouse | Prophylactic administration of allicin in infected mice significantly reduced worm burden. Serum concentrations of liver fibrosis markers and proinflammatory cytokines were also reduced | [57] |
| Series of 15 chalcones | Oral | 400 mg/Kg/day | Chalcones 1 and 3 demonstrated moderate schistosomicidal activity with total worm burden significantly reduced by 32.8% and 31.8%, respectively, at a single oral dose (400 mg/kg) | [36] |
| Epiisopilosine alkaloid | Oral | 100 or 400 mg/Kg/day | A single dose of epiisopilosine significantly decreased total worm load by 57.78 and 60.61% at doses of 400 and 100 mg/Kg, respectively. In addition, epiisopilosine significantly reduced eggs number and decreased hepatosplenomegaly | [58] |
| Piplartine | Oral | 100, 200 or 400 mg/kg/day | Treatment with the highest piplartine dose (400 mg/kg) caused a significant (60.4%) reduction in total worm burden in mice harboring adult parasites. Microscopy revealed substantial tegumental alterations in parasites recovered from mice | [59] |
| Gomphoside monoacetate and Uscharin | Oral | 10 mg/kg/day | Only gomphoside monoacetate (10 mg/kg) demonstrated activity against S. mansoni, with a low worm burden reduction of 38% | [60] |
| Rotundifolone | Oral | 35.9, 70.9 and 141.9 mg/Kg/day | Rotundifolone (141.9 mg/kg) significantly reduced fluke burden by 74.48%. Marked reductions in liver, intestinal, and fecal fluke burden, together with changes in the oogram pattern were observed. Treatment affected the viability of both mature and immature eggs | [61] |
| Licochalcone A | Oral; intraperitoneal | 1.5 or 2.5 mg/kg/day (oral); 25 mg/kg/day (intraperitoneal) | Oral treatment with L-SLNs decreased worm burden. However, under intraperitoneal administration, both free licochalcone A and L-SLNs significantly decreased worm burden and intestinal egg load | [62] |
| Carvacryl acetate | Oral | 100, 200, or 400 mg/kg/day | Carvacryl acetate (400 mg/kg) showed moderate efficacy against S. mansoni, with slightly reduced worm burden (32–40%). Egg production was markedly reduced (70–80%) | [63] |
| Cardamonin | Oral | 400 mg/kg/day | Oral treatment with cardamonin (400 mg/kg) demonstrated efficacy against S. mansoni, with decreased total worm load in 46.8% of mice and a 54.5% reduction in egg numbers | [64] |
| Asiaticoside | Oral | 400 mg/kg/day | A single oral dose (400 mg/kg) of asiaticoside presented significant in vivo antischistosomal efficacy, markedly decreasing total worm and egg burden | [65] |
| Plumbagin | Intraperitoneal | 20 mg/kg/day | Mice treated with plumbagin (20 mg/kg) showed reductions of 64.28% and 59.88% in male and female worms, respectively. Plumbagin treatment also alleviated schistosome-induced hepatosplenomegaly and reduced hepatic granuloma and liver collagen content | [66] |
| Juglone | Intraperitoneal | 2 mg/Kg/day | Treatment with the compound reduced male and female worms by 63.1% and 52.1%, respectively. The number of eggs/g of tissue in the liver and intestine were also reduced. Juglone decreased hepatic granuloma size and collagen fiber deposition. Mice treated with juglone presented significantly lower levels of IL-4, IL-13, IL-37, TNF-α, TGF-β, and IFN-γ than PZQ mice | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, C.M.; Meira, C.S.; da Silva, J.W.; Moura, D.M.N.; de Oliveira, S.A.; da Costa, C.J.; Santos, E.d.S.; Soares, M.B.P. Therapeutic Potential of Natural Products in the Treatment of Schistosomiasis. Molecules 2023, 28, 6807. https://doi.org/10.3390/molecules28196807
Azevedo CM, Meira CS, da Silva JW, Moura DMN, de Oliveira SA, da Costa CJ, Santos EdS, Soares MBP. Therapeutic Potential of Natural Products in the Treatment of Schistosomiasis. Molecules. 2023; 28(19):6807. https://doi.org/10.3390/molecules28196807
Chicago/Turabian StyleAzevedo, Carine Machado, Cássio Santana Meira, Jaqueline Wang da Silva, Danielle Maria Nascimento Moura, Sheilla Andrade de Oliveira, Cícero Jádson da Costa, Emanuelle de Souza Santos, and Milena Botelho Pereira Soares. 2023. "Therapeutic Potential of Natural Products in the Treatment of Schistosomiasis" Molecules 28, no. 19: 6807. https://doi.org/10.3390/molecules28196807
APA StyleAzevedo, C. M., Meira, C. S., da Silva, J. W., Moura, D. M. N., de Oliveira, S. A., da Costa, C. J., Santos, E. d. S., & Soares, M. B. P. (2023). Therapeutic Potential of Natural Products in the Treatment of Schistosomiasis. Molecules, 28(19), 6807. https://doi.org/10.3390/molecules28196807






