Preparation and Molecular Structural Characterization of Fulvic Acid Extracted from Different Types of Peat
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Content of Fulvic Acid from Different Types of Peat
2.2. UV–Vis Spectra of Fulvic Acid Extracted from Different Types of Peat
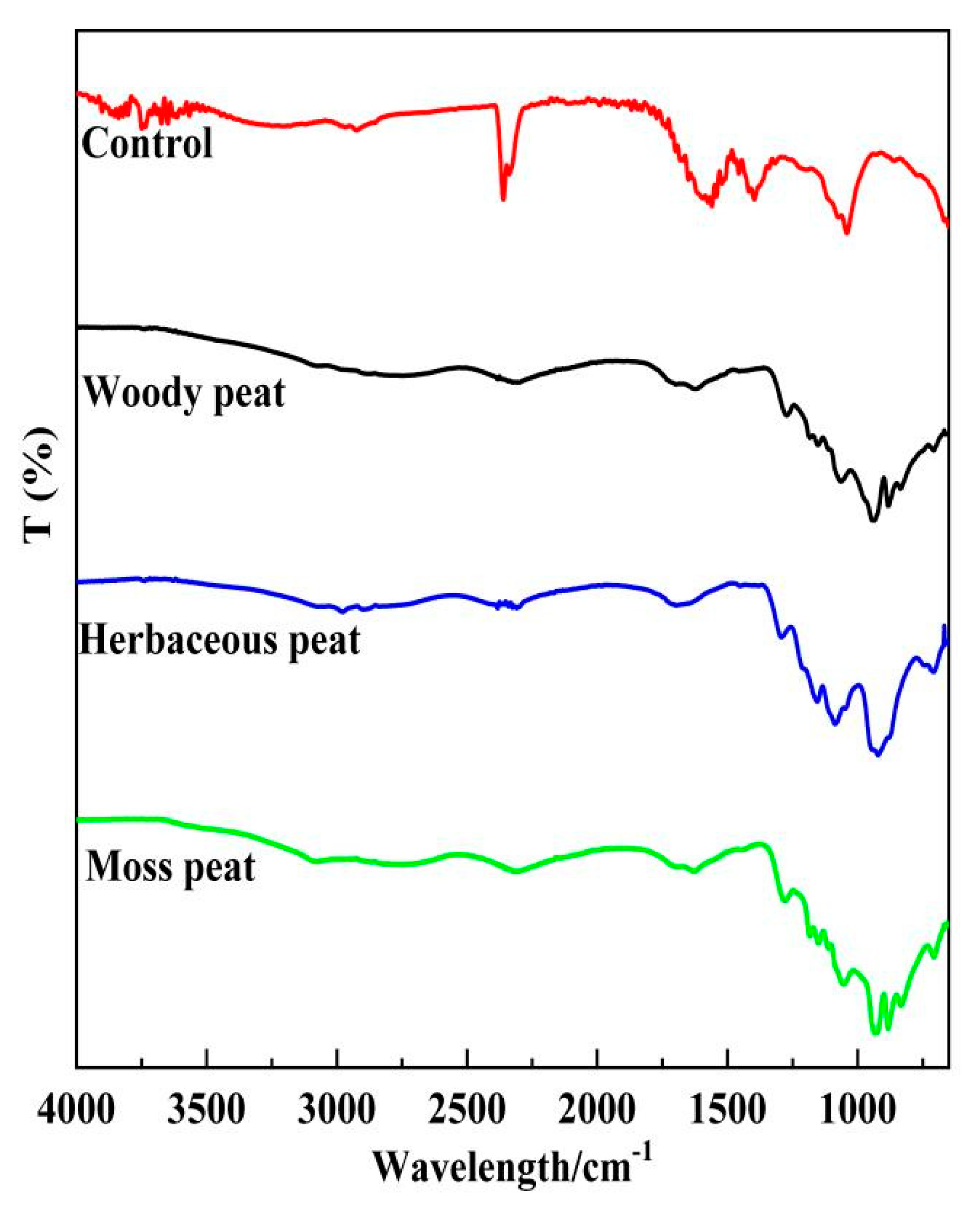
2.3. Infrared and Visible Spectra of Different Types of Peat Fulvic Acids
2.4. 13C Spectra of Different Types of Peat Fulvic Acids
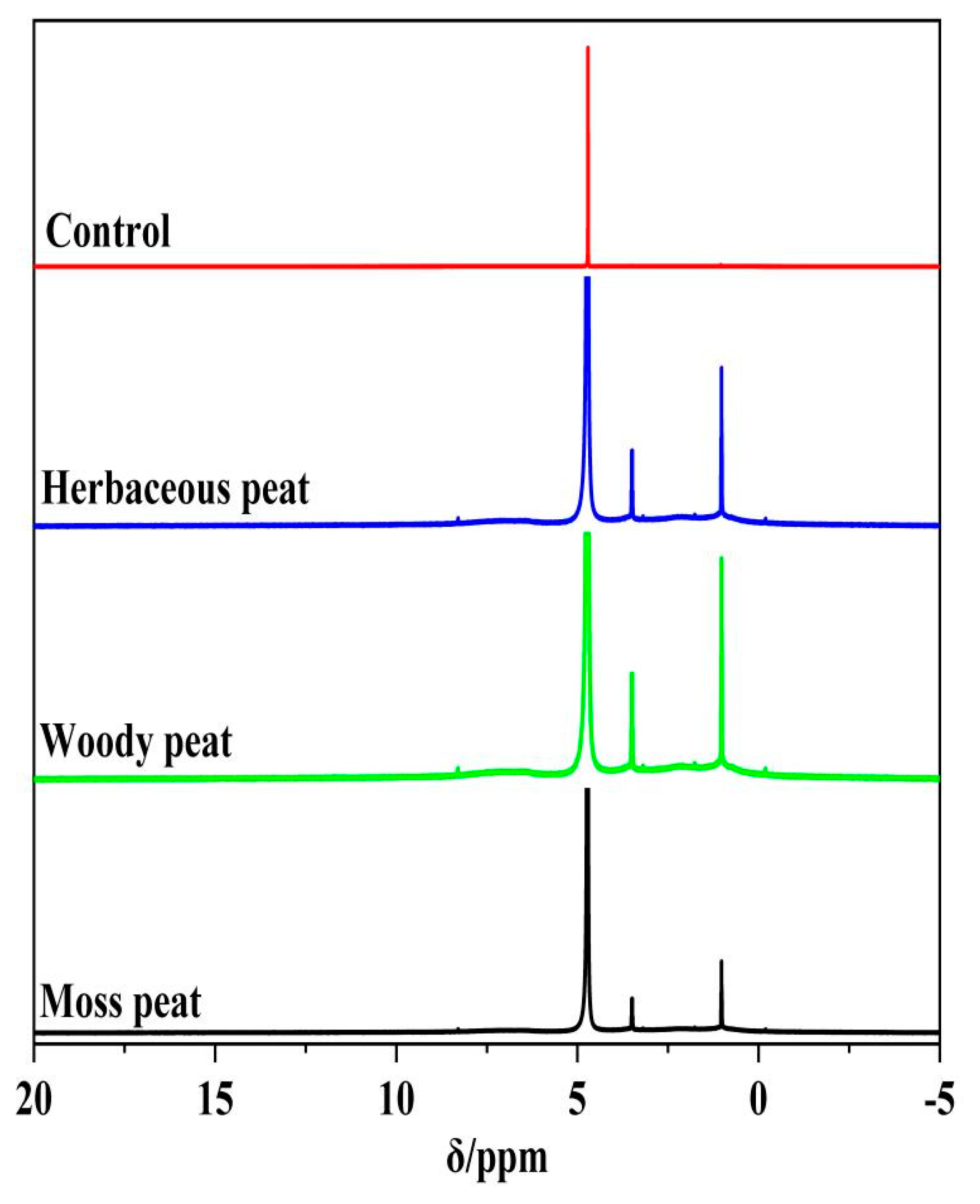
2.5. 1H Spectra of Different Types of Peat Fulvic Acid
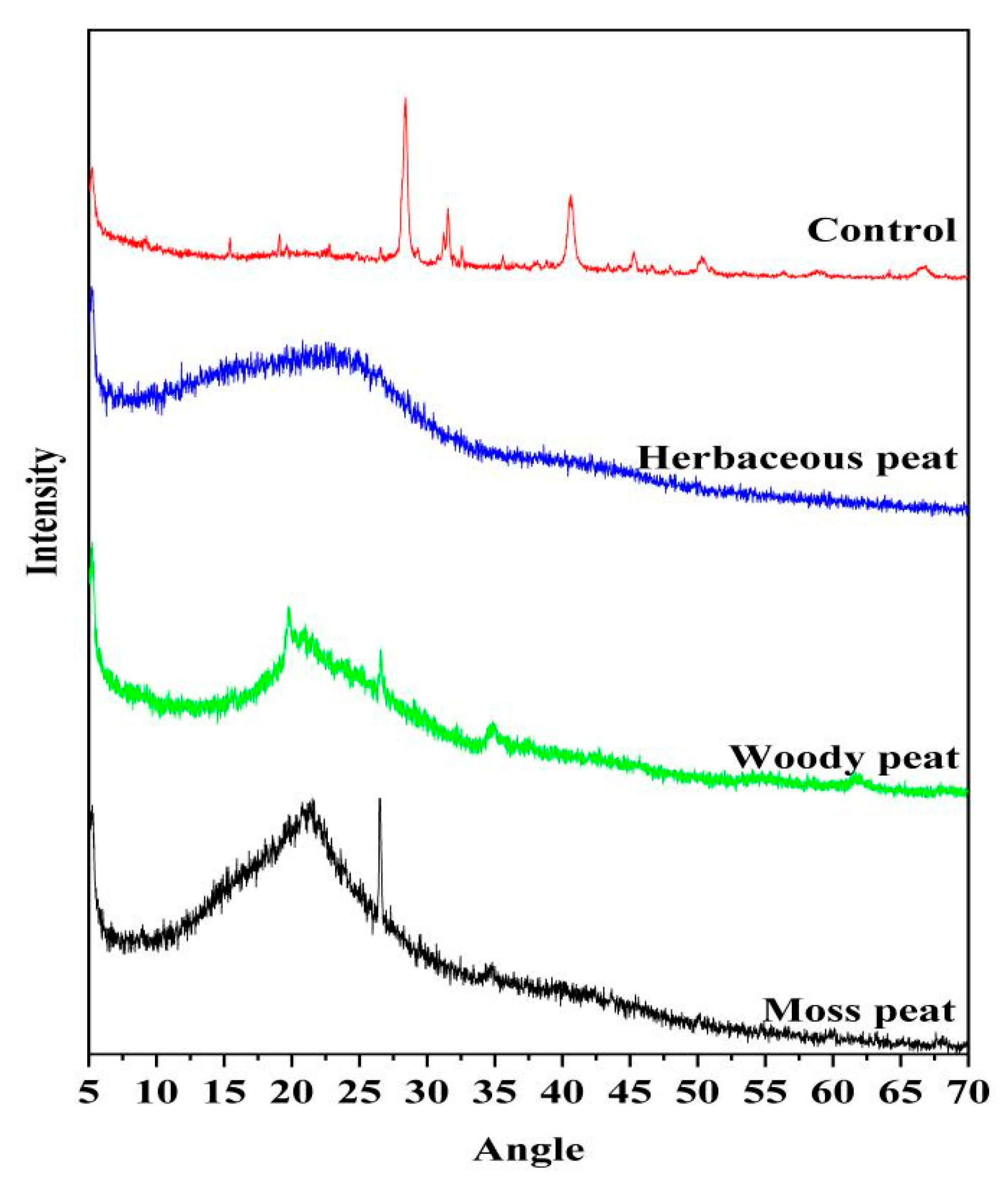
2.6. XRD Spectra of Fulvic Acid in Different Types of Peat
2.7. XPS Spectra of Different Types of Peat Fulvic Acid
3. Materials and Methods
3.1. Materials
3.2. Experimental Methods
3.3. Characterization Methods
3.3.1. FTIR Spectroscopy
3.3.2. UV–Vis Light Analysis
3.3.3. XPS Analysis
3.3.4. XRD Analysis
3.3.5. 13C-NMR Analysis
3.3.6. 1H-NMR Analysis
4. Conclusions
- Herbaceous peat had the highest fulvic acid yield, whereas the woody peat had the highest fulvic acid content. Therefore, herbaceous peat is the most suitable source for extracting fulvic acid.
- According to all the characterization methods in this experiment, the molecular differences in obtaining fulvic acid from the three different types of peat manifested in the content, not the types, of functional groups. In addition, the fulvic acid obtained from the three different types of peat comprised Aromatic and aliphatic carbon with a disordered molecular arrangement and no inorganic oxygen. Of the three peat fulvic acids, woody peat contained more oxygen in the forms of C=O and C–O, whereas mossy peat contained more oxygen in the form of carboxyl groups.
- Based on the various characterization methods mentioned above, the following conclusions can also be drawn. Herbaceous peat fulvic acid contained significant amounts of carbonyl, amino, methylene, carboxyl, and phenolic hydroxyl groups and ether bonds. Woody peat fulvic acid contains carbonyl and methoxy groups, benzene rings, aromatic carbons, aromatic ethers, and phenols. Mossy peat contained fulvic acid with the lowest molecular weight and simplest structure, and high quantities of methyl and methylene.
- This article studied the yield and content of humic acid extracted from peat, analyzed the molecular structure of three types of peat humic acid using different characterization methods, and reached the above conclusion. The research in this article has increased our understanding of humic acid extraction and can also provide reference for future scholars to study peat.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, S.; Bechtel, A.; Eble, C.F.; Flores, R.M.; French, D.; Graham, I.T.; Hood, M.M.; Hower, J.C.; Korasidis, V.A.; Moore, T.A.; et al. Recognition of peat depositional environments in coal. Int. J. Coal Geol. 2020, 219, 103383. [Google Scholar] [CrossRef]
- Hinzke, T.; Li, G.; Tanneberger, F.; Seeber, E.; Aggenbach, C.; Lange, J.; Kozub, Ł.; Knorr, K.H.; Kreyling, J.; Kotowski, W. Potentially peat-forming biomass of fen sedges increases with increasing nutrient levels. Funct. Ecol. 2021, 35, 1579–1595. [Google Scholar] [CrossRef]
- Zhao, B.; Zhuang, Q. Peatlands and their carbon dynamics in northern high latitudes from 1990 to 2300: A process-based biogeochemistry model analysis. Biogeosciences 2023, 20, 251–270. [Google Scholar] [CrossRef]
- Ayala, L.P.; Barois, I. Development of the earthworm Pontoscolex corethrurus in soils amended with a peat-based plant growing medium. Appl. Soil Ecol. 2016, 104, 131–137. [Google Scholar] [CrossRef]
- Fedoseeva, E.V.; Tereshina, V.M.; Danilova, O.A.; Ianutsevich, E.A.; Yakimenko, O.S.; Terekhova, V.A. Effect of humic acid on the composition of osmolytes and lipids in a melanin-containing phytopathogenic fungus Alternaria alternate. Environ. Res. 2020, 193, 110395. [Google Scholar] [CrossRef]
- Zou, D.Y. Further exploration of the concept of unifying humic acid and fulvic acid. Fertil. Ind. 2014, 41, 5–8. [Google Scholar]
- Ghosh, K.; Schnitzer, M. Macromolecular structures of humic substances. Soil Sci. 1980, 129, 266–276. [Google Scholar] [CrossRef]
- Chen, M.; Jin, X.; Wang, Y.; Wang, X.; Cai, Z.; Sun, X. Enhanced removal of humic substances in effluent organic matter from a leachate treatment system via biological upgradation of molecular structure. Environ. Technol. 2020, 43, 3620–3630. [Google Scholar] [CrossRef]
- Cui, T.; Li Wang, S. Effects of in-situ straw decomposition on composition of humus and structure of humic acid at different soil depths. Soils Sediments 2017, 17, 2391–2399. [Google Scholar] [CrossRef]
- Machado, W.; Franchini, J.C.; de Fátima Guimarães, M.; Tavares Filho, J. Spectroscopic characterization of humic and fulvic acids in soil aggregates, Brazil. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, F.; Wei, D. Impacts of Long-term Fertilization on the Molecular Structure of Humic Acid and Organic Carbon Content in Soil Aggregates in Black Soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yuan, Q.; Yuan, H. Changes of chemical properties of humic acids from crude and fungal transformed lignite. Fuel 2006, 85, 402–407. [Google Scholar] [CrossRef]
- Tate, R.L. Humic substances in soil and crop sciences. Soil Sci. 1991, 152, 237. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Env. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Jarošová, M.; Bi, K.; Kováčik, J.; Babula, P.; Hedbavny, J. Humic acid protects barley against salinity. Acta Physiol. Plant. 2006, 38, 161. [Google Scholar] [CrossRef]
- Dinler, B.S.; Gunduzer, E.; Tekinay, T. Pre-treatment of fulvic acid plays a stimulant role in protection of soybean (Glycine max L.) leaves against heat and salt stress. Acta Biol. Cracoviensia S Bot. 2016, 58, 21–41. [Google Scholar] [CrossRef]
- Shu, R.; Tianrong, H.; Xian, Z.; Deliang, Y. Effects of fulvic acid and humic acid from different sources on Hg methylation in soil and accumulation in rice. J. Environ. Sci. 2022, 119, 93–105. [Google Scholar]
- Dominik, N.; Marta, H.; Magdalena, B. Ultrasound-Assisted Extraction of Humic Substances from Peat: Assessment of Process Efficiency and Products’ Quality. Molecules 2022, 27, 3413. [Google Scholar]
- Huculak-Mączka, M.; Kaniewski, M.; Marecka, K.; Biegun, M.; Tymoszewicz, M.; Klem-Marciniak, E.; Nieweś, D.; Hoffmann, K. Evaluation of the simplified method of fulvic fractions extraction from peat and lignite. J. Therm. Anal. Calorim. 2023, 3, 12444. [Google Scholar] [CrossRef]
- Ma, L.T.; Lu, Y.N.; Wang, Y.X. Effects of methane fermentation on spectral properties of fulvic acid extracted from peat through liquid acid precipitation. J. Chem. 2020, 2, 5084508. [Google Scholar] [CrossRef]
- Laurynas, J.; Liudas, I.; Giedre, K. Determination of Organic Compounds, Fulvic Acid, Humic Acid, and Humin in Peat and Sapropel Alkaline Extracts. Molecules 2021, 26, 995. [Google Scholar]
- Lu, Y.N.; Ma, L.T.; Jun, L. Effects of sulfuric acid pretreatment and liquid acids precipitation on the structure of fulvic acid extracted from peat. Spectrosc. Lett. 2020, 10, 89–98. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V. Binding of atrazine to humic substances from soil, peat and coal related to their structure. Environ. Sci. Technol. 2002, 36, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Báez, M.E.; Fuentes, E.; Espinoza, J. Characterization of the Atrazine Sorption Process on Andisol and Ultisol Volcanic Ash-Derived Soils: Kinetic Parameters and the Contribution of Humic Fractions. J. Agric. Food Chem. 2013, 61, 6150–6160. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, B.; Zhang, X.; Ma, Y.X.; Wang, P.; Lu, Z.; Lu, A.G.; Li, B. Study on Peaty Soil and Its Utilization in the Shanxi foothills of Liupan Mountain. China Coal Geol. 2023, 35, 37–42. [Google Scholar]
- Ma, L.T.; Li, L.P.; Lu, Y.N.; Dai, B. Study on the effect of alkaline solution on the extraction of fulvic acid from lignite. Coal Convers. 2023, 46, 1–9. [Google Scholar]
- Zalba, P.; Amiottin, M.; Galantinij, A.; Pistola, S. Soilhumic and fulvic acids from different land-use system sevaluated by E4/E6 ratios. Commun. Soil Sci. Plant Anal. 2016, 47, 1675–1679. [Google Scholar] [CrossRef]
- Niu, Y.H.; Han, X.X.; Huang, L.X. Student on the purification process of humic acid from linite. Appl. Chem. Ind. 2021, 50, 407–411. [Google Scholar]
- Shi, H.P.; Wu, R.F.; Zhang, J.G.; Shi, L.; Li, J.Y. Extraction and characterization of humic acids with different molecular weights and types in Baotou tailings storage area. Jiangsu Agric. Sci. 2016, 44, 451–454. [Google Scholar]
- Aranganathan, L.; Radhika, S.R.; Rajasree, B.; Govindaraju, K. Spectral and microscopic analysis of fulvic acids isolated from marine fish waste and sugarcane bagasse co–compost. Biocatal. Agric. Biotechnol. 2020, 29, 101762. [Google Scholar] [CrossRef]
- Zherebtsov, S.I.; Malyshenko, N.V.; Bryukhovetskaya, L.V.; Lyrshchikov, S.Y.; Nikitin, A.P.; Ismagilov, Z.R. Influence of Chemical Modification on the Structure, Composition, and Properties of Lignite Humic Acids. Coke Chem. 2018, 61, 396–400. [Google Scholar] [CrossRef]
- Aldmour, S.T.; Burke, I.T.; Bray, A.W.; Baker, D.L.; Ross, A.B.; Gill, F.L.; Cibin, G.; Ries, M.E.; Stewart, D.I. Abiotic reduction of Cr(VI) by humic acids derived from peat and lignite: Kinetics and removal mechanism. Environ. Sci. Pollut. Res. 2019, 26, 4717–4729. [Google Scholar] [CrossRef]
- Li, Y.H. Study on the Structural Characteristics of Typical Lignite in Yunnan and Physicochemical Properties of Humic Acid. Master’s Thesis, Taiyuan University of Technology Learning, Taiyuan, China, 2019. [Google Scholar]
- Liu, Z.G.; Ma, X.H.; Li, X.Y.; Mou, X.J.; Yuan, Y.X.; Lu, X.R. Comparison of two methods for determining organic matter content in peat. Wetl. Sci. 2023, 21, 465–469. [Google Scholar]
- Gong, G.; Xu, L.; Zhang, Y.; Liu, W.; Wang, M.; Zhao, Y.; Yuan, X.; Li, Y. Extraction of Fulvic Acid from Lignite and Characterization of Its Functional Groups. ACS Omega 2020, 5, 27953–27961. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.N. Study on the Extraction of Humic Acid and Fulvic Acid From Peat. Master’s Thesis, Inner Mongolia University of Science and Technology, Baotou, China, 2022. [Google Scholar]

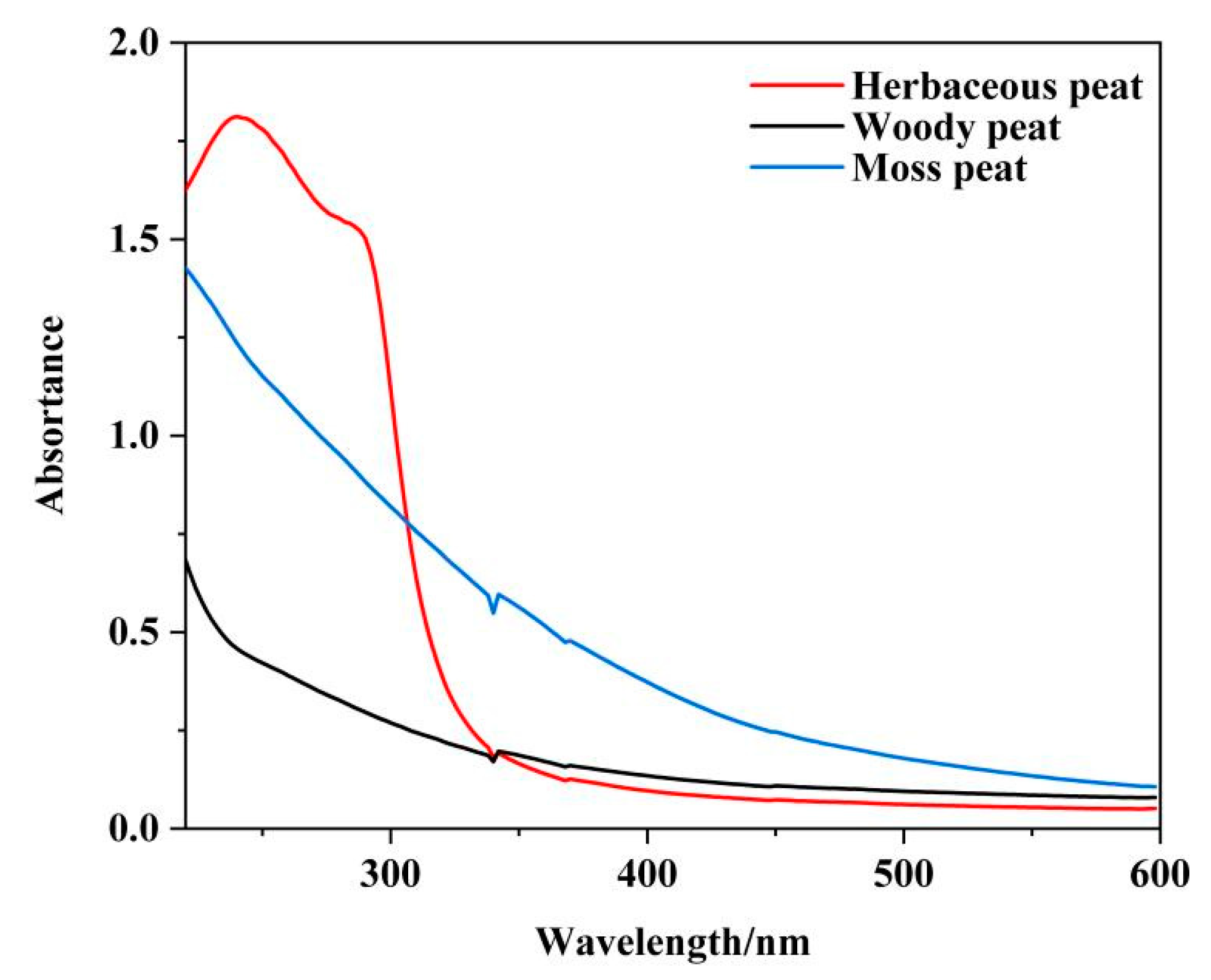


| Herbaceous Peat | Woody Peat | Mossy Peat | |
|---|---|---|---|
| E4 | 0.070 | 0.069 | 0.198 |
| E6 | 0.040 | 0.042 | 0.062 |
| E4/E6 | 1.750 | 1.643 | 3.129 |
| ΔlogK | 0.275 | 0.230 | 0.545 |
| Peak Position | Functional Groups | Absorption Type |
|---|---|---|
| 3350 cm−1 | –OH | extensional vibration |
| 2870 cm−1 | –CH3 | stretch vibration |
| 2850 cm−1 | –CH2- | stretch vibration |
| 1710 cm−1 | –C=O | extensional vibration |
| 1560–1450 cm−1 | C–C bond on benzene | extensional vibration |
| 1033 cm−1 | C–O on alcohols, phenols and ethers | extensional vibration |
| 15–24 Methyl Carbon | 32–42 Methylene Carbon | 65–90 Methoxy Group Carbon | 90–140 Aromatic Charcoal | 140–160 Aromatic C–O | 160–190 Carboxyl Carbon | Aromaticity Index | |
|---|---|---|---|---|---|---|---|
| Herbaceous peat fulvic acid | 1.19 | 0.78 | 12.10 | 0.99 | 0.15 | 0.41 | 1.14 |
| Woody peat fulvic acid | 0.09 | 1.00 | 33.00 | 1.24 | 0.89 | / | 2.13 |
| Mossy peat fulvic acid | 1.68 | 11.98 | 3.46 | 0.17 | 0.63 | / | 0.80 |
| 0.7–1.4 Methyl Methylene Hydrogen | 3.5–40 Methoxy Group Hydrogen | 4.5–6.5 Carbon–Carbon Double Bond Hydrogen | 6.4–7.2 Benzene Ring Hydrogen | 8.3–9 Phenolic Hydroxyl Hydrogen | |
|---|---|---|---|---|---|
| Herbaceous peat fulvic acid | 1.06 | 0.91 | 8.03 | 0.09 | 0.15 |
| Woody peat fulvic acid | 1.14 | 1.26 | 21.06 | 4.20 | 0.08 |
| Mossy peat fulvic acid | 5.56 | 1.01 | 23.99 | 2.42 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Lu, Y.; Ma, L.; Cheng, J.; Wang, X. Preparation and Molecular Structural Characterization of Fulvic Acid Extracted from Different Types of Peat. Molecules 2023, 28, 6780. https://doi.org/10.3390/molecules28196780
Wu D, Lu Y, Ma L, Cheng J, Wang X. Preparation and Molecular Structural Characterization of Fulvic Acid Extracted from Different Types of Peat. Molecules. 2023; 28(19):6780. https://doi.org/10.3390/molecules28196780
Chicago/Turabian StyleWu, Di, Yanan Lu, Litong Ma, Jianguo Cheng, and Xiaoxia Wang. 2023. "Preparation and Molecular Structural Characterization of Fulvic Acid Extracted from Different Types of Peat" Molecules 28, no. 19: 6780. https://doi.org/10.3390/molecules28196780
APA StyleWu, D., Lu, Y., Ma, L., Cheng, J., & Wang, X. (2023). Preparation and Molecular Structural Characterization of Fulvic Acid Extracted from Different Types of Peat. Molecules, 28(19), 6780. https://doi.org/10.3390/molecules28196780





