Antioxidant Properties and Secondary Metabolites Profile of Hyptis colombiana at Various Phenological Stages
Abstract
:1. Introduction
2. Results
2.1. Distillation and Extraction Yields
2.2. Analysis of EOs from H. colombiana Plants Collected at Different Phenological Stages
2.3. Analysis of H. colombiana Hydroethanolic Extracts Using UHPLC-ESI+/−-Orbitrap-MS
2.4. Antioxidant Activity of H. colombiana EOs and Extracts
3. Discussion
3.1. Chemical Composition of H. colombiana EOs
3.2. Chemical Composition of H. colombiana Extracts
3.3. Antioxidant Activity of H. colombiana EOs and Extracts
4. Materials and Methods
4.1. Chemical Substances and Reagents
4.2. Vegetal Materials
4.3. Distillation of Essential Oils
4.4. Solvent Extraction
4.5. Chromatographic Analysis
4.5.1. GC/MS/FID Essential Oil Analysis
4.5.2. LC/MS Extract Analysis
4.6. Evaluation of Antioxidant Activity
4.6.1. Decoloration of the ABTS+• Cation–Radical Assay
4.6.2. Evaluation of the Oxygen Radical Absorption Capacity
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ABTS | 2,2-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt assay |
| AD | After distillation |
| AT | Agilent Technologies |
| BD | Before distillation |
| df | Film thickness |
| i.d. | Internal Diameter |
| EIC | Extracted Ion Chromatogram |
| EO(s) | Essential oil(s) |
| ESI | Electrospray ionization |
| eV | Electron volt |
| FID | Flame ionization detection |
| GC | Gas chromatography or gas chromatogram |
| HCD | Higher-Energy Collision Dissociation Cell |
| HRMS | High-Resolution Mass Spectrometry |
| I, % | Intensity (Abundance) |
| LC | Liquid Chromatography |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LRI | Linear Retention Index |
| MS | Mass Spectrometry or Mass Spectrum |
| MSD | Mass Spectrometric Detector |
| m/z | Mass-to-Charge Ratio |
| MWHD | Microwave-Assisted Hydrodistillation |
| ORAC | Oxygen Radical Absorption Capacity Assay |
| tR | Retention time (min) |
| UHPLC | Ultrahigh Performance Liquid Chromatography |
References
- U.S. Departament of Health & Human Services. U.S. Food & Drug. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172 (accessed on 18 April 2023).
- European Commission. European Federation of Essential Oils (E.F.E.O.). Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12975-Revision-of-EU-legislation-on-hazard-classification-labelling-and-packaging-of-chemicals/F2333084_en (accessed on 13 April 2023).
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Karla, A.; Kumar, S.; Katiyar, N.; Bahl, J.R.; Bansal, R.P.; Chauhan, H.S.; Prasad, A.; Pandey, R.; Dhawan, O.P.; Krishna, A.; et al. Method for the Faster Multiplication of Earthworms, and Production of Vermicompost from the Distillation Waste of Industrial Aromatic Crops. U.S. Patent 6,488,733, 2 January 2001. [Google Scholar]
- Ventura-Canseco, L.M.; Mendoza, J.; Abud, M.; Oliva, M.; Dendooven, L.; Gutiérrez-Miceli, F.A. Sugarcane molasse and whey as additives in the silage of lemongrass (Cymbopogon citratus [DC.] Stapf) leaves. Chil. J. Agric. Res. 2012, 72, 87–91. [Google Scholar] [CrossRef]
- Singh, A.; Singh, M.; Singh, K. Productivity and economic viability of a palmarosa–pigeonpea intercropping system in the subtropical climate of north India. J. Agric. Sci. 1998, 130, 149–154. [Google Scholar] [CrossRef]
- Alfa, M.I.; Adie, D.B.; Iorhemen, O.T.; Okafor, C.C.; Ajayi, S.A.; Danhunsi, S.O.; Akali, D.M. Assessment of mesophilic co-digestion of cow dung with lemon grass for biogas production. Niger. J. Technol. 2013, 32, 478–484. [Google Scholar]
- Gavarić, N.; Kladar, N.; Mišan, A.; Nikolić, A.; Samojlik, I.; Mimica-Dukić, N.; Božin, B. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crop. Prod. 2015, 74, 457–464. [Google Scholar] [CrossRef]
- Cid-Pérez, T.; Ávila-Sosa, R.; Ochoa-Velasco, C.; Rivera-Chavira, B.; Nevárez-Moorillón, G. Antioxidant and antimicrobial activity of mexican oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef]
- GlobeNewswire. Essential Oils Market Size to Worth Around US$ 34.6 Billion by 2030. Available online: https://www.globenewswire.com/en/news-release/2022/06/08/2458521/0/en/Essential-Oils-Market-Size-to-Worth-Around-US-34-6-Bn-by-2030.html (accessed on 29 March 2023).
- Observatory of Economic Complexity (OEC). Colombia, Essential Oils. Available online: https://oec.world/en/profile/bilateral-product/essential-oils/reporter/col (accessed on 18 April 2023).
- Epling, C. Revisión del género Hyptis (Labiatae). Botánica 1949, 7, 193–394. [Google Scholar]
- Harley, R.M.; Pastore, J. A generic revision and new combinations in the Hyptidinae (Lamiaceae), based on molecular and morphological evidence. Phytotaxa 2012, 58, 1–55. [Google Scholar] [CrossRef]
- Fernández, J.; Rivera, O. El Libro Rojo de Las Plantas de Colombia, de LAS Labiadas (Familia Labiatae), 1st ed.; Editorial Santillana: Bogotá, Colombia, 2006; Volume 3, pp. 387–684. [Google Scholar]
- Fernández-Alonso, J.L. Hyptis. In Catálogo de Plantas y Líquenes de Colombia; Bernal, S.R., Gradstein, R., Celis, M., Eds.; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, Colombia, 2015; Available online: http://catalogoplantasdecolombia.unal.edu.co (accessed on 31 May 2023).
- Fernández-Alonso, J.L.; Chacón, M.I. Especies aromáticas de la Provincia de Sumapaz y la Cuenca del río Chicamocha en Colombia. In Programa de Aprovechamiento de Aceites Esenciales de Especies Nativas y Foráneas Promisorias de Uso Agrícola en Colombia; Facultad de Agronomía, Universidad Nacional de Colombia: Bogotá, Colombia, 2012; p. 189. ISBN 978-958-761-226-4. [Google Scholar]
- Pastore, J.F.; Antar, G.M.; Soares, A.S.; Forest, S.; Harley, R.M. A new and expanded phylogenetic analysis of Hyptidinae (Ocimeae-Lamiaceae). Syst. Bot. 2021, 46, 1086–1094. [Google Scholar] [CrossRef]
- García-Barriga, H. Flora medicinal de Colombia. In Botánica Médica; Universidad Nacional de Colombia: Bogotá, Colombia, 1975; Volume 3, p. 538. [Google Scholar]
- Flores, M.; Rojas, L.; Aparicio, R.; Lucena, M.; Usubillaga, A. Essential oil composition and antibacterial activity of Hyptis colombiana from the Venezuelan Andes. Nat. Prod. Commun. 2015, 10, 1751–1752. [Google Scholar] [CrossRef]
- McNeil, M.; Facey, P.; Porter, R. Essential oils from the Hyptis genus. A review (1909–2009). Nat. Prod. Commun. 2011, 11, 1775–1796. [Google Scholar] [CrossRef]
- Peerzada, N. Chemical composition of the essential oil of Hyptis suaveolens. Molecules 1997, 2, 165–168. [Google Scholar] [CrossRef]
- Moreira, A.; Lima, E.; Wanderley, P.; Carmo, E.; Souza, E. Chemical composition and antifungal activity of Hyptis suaveolens (L.) poit leaves essential oil against Aspergillus species. Braz. J. Microbiol. 2010, 41, 28–33. [Google Scholar] [CrossRef]
- Noudogbessi, J.P.; Agbangnan, P.; Yehouenou, B.; Adjalian, E.; Nonviho, G.; Osseni, M.A.; Sohounhloue, D. Physico-chemical properties of Hyptis suaveolens essential oil. J. Med. Aromat. Plants. 2013, 3, 191–199. [Google Scholar]
- Falcao, R.; Nascimiento, P.; Souza, S.; Da Silva, V. Antileishmanial phenylpropanoids from the leaves of Hyptis pectinata (L.) poit. Evid. Based Complement. Altern. Med. 2013, 2013, 460613. [Google Scholar] [CrossRef]
- Bezerra, J.; Costa, A.; Da Silva, M.; Rocha, M. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) poiteau (Lamiaceae) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017, 113, 442–447. [Google Scholar] [CrossRef]
- Tang, G.; Liu, X.; Gong, X.; Lin, X.; Lai, X.; Wang, D. Studies on the chemical compositions of Hyptis suaveolens (L.) poit. J. Serb. Chem. Soc. 2019, 84, 245–252. [Google Scholar] [CrossRef]
- Pereda, R.; Delgado, G. Triterpenoids and flavonoids from Hyptis albida. J. Nat. Prod. 1990, 53, 182–185. [Google Scholar] [CrossRef]
- Fernández-Alonso, J.L.; Delgado-Ávila, W.A.; Chacón-Sanchez, M.I. Especies Aromáticas de la Provincia de Sumapaz y de la Cuenca del río Chicamocha en Colombia, y Composición Química de Sus Aceites Esenciales. In Programa de Aprovechamiento de Aceites Esenciales de Especies Nativas y Foráneas Promisorias de Uso Agrícola en Colombia; Facultad de Agronomía, Universidad Nacional de Colombia: Bogotá, Colombia, 2012; p. 151. ISBN 978-958-761-324-7. [Google Scholar]
- Adesanya, E.O.; Oyesiku, O.O.; Adesanya, O.O.; Ogunlakin, A.D.; Odugbemi, A.I.; Egieyeh, S.A. Phytochemical components and GC–MS analysis of Petiveria alliaceae L. fractions and volatile oils. Phys. Sci. Rev. 2023. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Nasruddin; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical profiling, antioxidant, antimicrobial and cholinesterase inhibitory effects of essential oils isolated from the leaves of Artemisia scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Llauradó, G.; Méndez, D.; Hendrix, S.; Escalona, J.; Fung, Y.; Pacheco, A.; Cuypers, A. Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef]
- Mandal, S.; Mondal, K.; Dey, S.; Pati, B. Antimicrobial activity of the leaf extracts of Hyptis suaveolens (L.) poit. Indian J. Pharm. Sci. 2007, 69, 568–569. [Google Scholar]
- Medoatinsa, S.; Agbangnan, C.; Viwami, F.; Noudogbessi, J.; Lagnika, H.; Ahissou, H.; Sohounhloue, D. In vitro antiplasmodial and antioxidant activities of ethanolic and hydroethanolic extracts of Hyptis suaveolens. World. J. Pharm. Pharm. Sci. 2015, 4, 101–112. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–809. [Google Scholar]
- Babushok, V.; Linstrom, P.; Zenkevich, I. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011, 40, 043101. [Google Scholar] [CrossRef]
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Pala-Paul, J.; Copeland, L.; Brophy, J.; Goldsack, R. Essential oil composition of two variants of Prostanthera lasianthos Labill. Biochem. Syst. Ecol. 2006, 34, 48–55. [Google Scholar] [CrossRef]
- Choi, H. Characteristic odor components of kumquat (Fortunella japonica Swingle) peel oil. J. Agric. Food Chem. 2005, 53, 1642–1647. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, A.-F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 6, 479–487. [Google Scholar] [CrossRef]
- Perraudin, F.; Popovici, J.; Bertrand, C. Analysis of headspace-solid microextracts from flowers of Maxillaria tenuifolia Lindl, by GC-MS. Electronic J. Nat. Subst. 2006, 1, 1–5. [Google Scholar]
- Bell, W.-M. Examination of Aroma Volatiles Formed from Thermal Processing of Florida Reconstituted Grapefruit Juice. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2004. [Google Scholar]
- Delort, E.; Jaquier, A. Novel terpenyl esters from Australian finger lime (Citrus australasica) peel extract. Flavour Fragr. J. 2009, 24, 123–132. [Google Scholar] [CrossRef]
- Sonboli, A.; Azizian, D.; Yousefzadi, M.; Kanani, M.; Mehrabian, A.R. Volatile constituents and antimicrobial activity of the essential oil of Tetrataenium lasiopetalum (Apiaceae) from Iran. Flavour Fragr. J. 2007, 1, 119–122. [Google Scholar] [CrossRef]
- Chen, C.; HO, C. Gas chromatographic analysis of volatile components of ginger oil (Zingiber officinale Roscoe) extracted with liquid carbon dioxide. J. Agric. Food Chem. 1988, 36, 322–328. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Venskutonis, R.; Demyttenaere, J. Sensory and instrumental evaluation of sweet marjoram (Origanum majorana L.) aroma. Flavour Fragr. J. 2005, 20, 492–500. [Google Scholar] [CrossRef]
- Boalino, D.; Connolly, J.; McLean, S.; Reynolds, W.; Tinto, W. α-Pyrones and a 2 (5H)-furanone from Hyptis pectinata. Phytochemistry. 2003, 64, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Pereda, R.; Rosas, D.; Fragoso, M.; Cerda, C.; Da Silva, A.; Leitão, S. Structure elucidation, conformation, and configuration of cytotoxic 6-heptyl-5,6-dihydro-2 H-pyran-2-ones from Hyptis species and their molecular docking to α-tubulin. J. Nat. Prod. 2019, 82, 520–531. [Google Scholar] [CrossRef]
- Kollner, T.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.; Gershenzon, J.; Degenhardt, J. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008, 20, 482–494. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Rastogi, S.; Lepoittevin, J.; Johansen, J.; Frosch, P.; Menné, T.; Bruze, M.; White, I. Fragrances and other materials in deodorants: Search for potentially sensitizing molecules using combined GC-MS and structure activity relationship (SAR) analysis. Contact. Derm. 1998, 39, 293–303. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Santana, F.; Luna, L.; Antunes, V.; Tormena, C.; Cominetti, M.; Duarte, M.; Da Silva, J. Evaluation of the cytotoxicity on breast cancer cell of extracts and compounds isolated from Hyptis pectinata (L.) poit. Nat. Prod. Res. 2020, 34, 102–109. [Google Scholar] [CrossRef]
- Litvinenko, V. “Tannins” and derivatives of hydroxycinnamic acid in Labiatae. Planta Med. 1975, 27, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Petersena, M.; Simmonds, M. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, T.; Rokaya, M.; Maršík, P.; Rezek, J.; Skuhrovec, J.; Pavela, R.; Münzbergová, Z. Trade-off among different anti-herbivore defence strategies along an altitudinal gradient. AoB Plants 2016, 8, plw026. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.; Perumal, S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef]
- Adomako-Bonsu, A.; Chan, S.; Pratten, M.; Fry, J. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Gamaro, G.; Suyenaga, E.; Borsoi, M.; Lermen, J.; Pereira, P.; Ardenghi, P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol. 2011, 2011, 451682. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C. LC-MS identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef]
- Aditivos Alimentarios. Available online: https://www.aditivos-alimentarios.com/2016/01/E392.html (accessed on 11 July 2022).
- Raadt, P.; Wirtz, S.; Vos, E.; Verhagen, H. Short review of extracts of rosemary as a food additive. Eur. J. Nutr. Food Saf. 2015, 5, 126–137. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef]
- Kevers, C.; Sipel, A.; Pincemail, J.; Dommes, J. Antioxidant capacity of hydrophilic food matrices: Optimization and validation of ORAC assay. Food Anal. Methods 2014, 7, 409–416. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Tafurt-García, G.; Jiménez, L.; Calvo, A. Capacidad antioxidante y contenido de fenoles totales de Hyptis spp., P. heptaphyllum, T. panamensis, T. rhoifolia, y Ocotea sp. Rev. Colomb. Quim. 2015, 44, 28–33. [Google Scholar] [CrossRef]
- Dos Santos, K.; Sedano, M.; Sala, W.; Loureiro, B.; Da Silva, L.; Furlan, C. Biological activity of Hyptis Jacq. (Lamiaceae) is determined by the environment. Ind. Crops. Prod. 2018, 112, 705–715. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- MassBank. Available online: www.massbank.eu/MassBank/ (accessed on 15 March 2023).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decoloration assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Arias, G. Estudio de la Composición Química y la Actividad Antioxidante de Aceites Esenciales y Extractos Aislados de Salvia officinalis, Rosmarinus officinalis, Thymus vulgaris y Lippia citriodora, Cultivadas en el Municipio de Sucre (Santander). Tesis de Maestría en Química, Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia, 2012; pp. 66–70. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food. Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
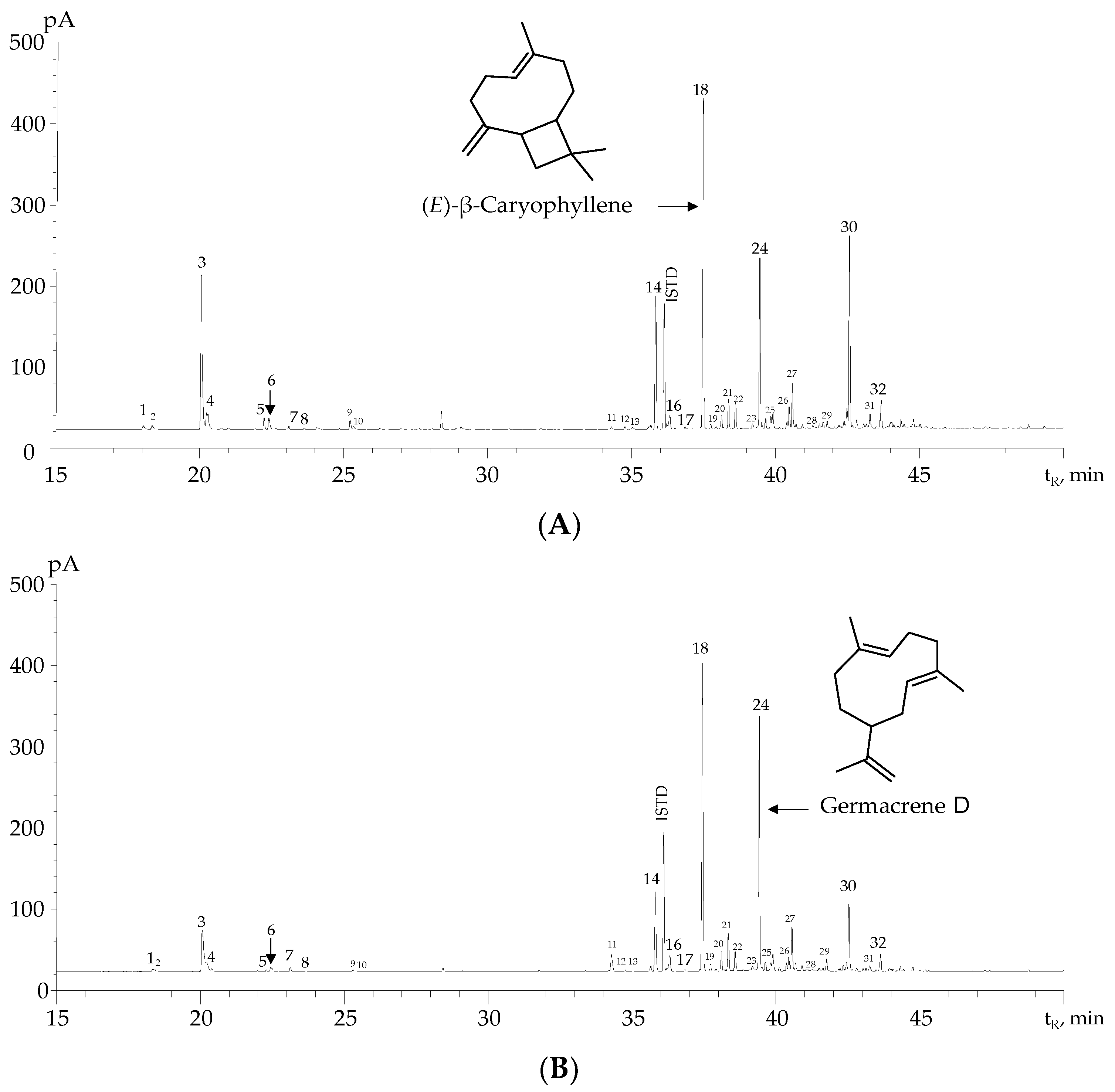




| Phenological Stage | Harvest Date | Total Number of Replicates, n | Yield, % (w/w) ± s | ||
|---|---|---|---|---|---|
| Essential Oil | Solvent Extraction | ||||
| Before Distillation | After Distillation | ||||
| Vegetative | 1 October 2021 28 June 2022 12 January 2023 | 9 | 0.1 ± 0.1 | 10 ± 3 | 6 ± 3 |
| Flowering | 30 March 2022 24 November 2022 12 January 2023 | 9 | 0.10 ± 0.05 | 10 ± 3 | 10 ± 3 |
| Post-flowering | 21 January 2022 12 January 2023 | 6 | 0.05 ± 0.03 | 10 ± 2 | 10 ± 1 |
| N° Figure 1 | Compound | Type | Linear Retention Indices | GC/FID Peak Area, % ± s | Identification Criteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DB-5MS | DB-WAX | |||||||||
| Plant Materials Stages | ||||||||||
| LRIexp | LRIlit | LRIexp | LRIlit | Vegetative (n = 9) | Flowering (n = 9) | Post-Flowering (n = 6) | ||||
| 1 | α-Thujene | MH | 928 | 928 [35] | 1027 | 1027 [35] | 0.3 ± 0.1 | 0.30 ± 0.07 | 0.20 ± 0.09 | a, b |
| 2 | α-Pinene | MH | 936 | 936 [35] | 1023 | 1025 [35] | 0.4 ± 0.1 | 2 ± 1 | 0.9 ± 0.8 | a, b, c |
| 3 | Sabinene | MH | 976 | 973 [35] | 1124 | 1122 [35] | 10 ± 2 | 10 ± 3 | 4 ± 3 | a, b, c |
| 4 | β-Pinene | MH | 981 | 981 [36] | 1111 | 1110 [35] | 2 ± 1 | 2.0 ± 0.9 | 1.0 ± 0.9 | a, b |
| 5 | p-Cymene | MH | 1028 | 1024 [35] | 1274 | 1274 [35] | 0.5 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.1 | a, b, c |
| 6 | Limonene | MH | 1032 | 1030 [36] | 1202 | 1198 [35] | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.4 ± 0.3 | a, b, c |
| 7 | (E)-β-Ocimene | MH | 1047 | 1044 [34] | 1253 | 1250 [35] | 0.30 ± 0.07 | 1.0 ± 0.7 | 0.4 ± 0.1 | a, b |
| 8 | γ-Terpinene | MH | 1061 | 1059 [35] | 1249 | 1245 [35] | 0.10 ± 0.05 | 0.16 ± 0.07 | 0.10 ± 0.04 | a, b, c |
| 9 | Linalool | OM | 1100 | 1099 [35] | 1550 | 1549 [37] | 0.4 ± 0.3 | 0.40 ± 0.04 | 0.4 ± 0.1 | a, b, c |
| 10 | (E)-Sabinene hydrate | OM | 1105 | 1098 [35] | 1558 | 1549 [35] | 0.10 ± 0.05 | 0.20 ± 0.04 | 0.2 ± 0.1 | a, b |
| 11 | δ-Elemene | SH | 1341 | 1337 [35] | 1473 | 1469 [36] | 1 ± 1 | 3 ± 3 | 4 ± 1 | a, b |
| 12 | α-Cubebene | SH | 1351 | 1448 [34] | 1465 | 1460 [35] | 0.20 ± 0.01 | 0.15 ± 0.02 | 0.16 ± 0.03 | a, b |
| 13 | α-Ylangene | SH | 1378 | 1376 [34] | 1493 | 1492 [38] | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.03 | a, b |
| 14 | α-Copaene | SH | 1385 | 1378 [36] | 1503 | 1496 [39] | 8 ± 1 | 6 ± 1 | 5.0 ± 0.9 | a, b |
| 15 | β-Bourbonene | SH | 1394 | 1390 [36] | 1530 | 1526 [36] | 0.3 ± 0.1 | 0.40 ± 0.01 | 1.0 ± 0.3 | a, b |
| 16 | β-Elemene | SH | 1397 | 1390 [35] | 1600 | 1595 [38] | 2 ± 1 | 3 ± 1 | 3 ± 1 | a, b |
| 17 | C15H24 (Figure S1) | SH | 1416 | - | 1536 | - | 0.25 ± 0.02 | 0.20 ± 0.04 | 0.2 ± 0.1 | a, b |
| 18 | (E)-β-Caryophyllene | SH | 1435 | 1428 [36] | 1613 | 1617 [36] | 30 ± 4 | 24 ± 4 | 30 ± 5 | a, b, c |
| 19 | γ-Elemene | SH | 1438 | 1436 [35] | 1645 | 1639 [35] | 1 ± 1 | 3 ± 2 | 2 ± 2 | a, b |
| 20 | 6,9-Guaiadiene | SH | 1452 | 1442 [34] | 1617 | - | 1.5 ± 0.2 | 1.5 ± 0.1 | 2.0 ± 0.1 | a, b |
| 21 | C15H24 (Figure S2) | SH | 1459 | - | 1630 | - | 1 ± 1 | 2 ± 1 | 2 ± 2 | a, b |
| 22 | α-Humulene | SH | 1469 | 1465 [36] | 1682 | 1681 [38] | 2.0 ± 0.2 | 2.0 ± 0.2 | 2.00 ± 0.01 | a, b, c |
| 23 | γ-Muurolene | SH | 1484 | 1478 [35] | 1696 | 1690 [37] | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 | a, b |
| 24 | Germacrene D | SH | 1495 | 1493 [40] | 1722 | 1708 [35] | 20 ± 6 | 20 ± 6 | 22 ± 1 | a, b, c |
| 25 | β-Selinene | SH | 1499 | 1490 [34] | 1726 | 1717 [35] | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | a, b |
| 26 | γ-Cadinene | SH | 1523 | 1529 [41] | 1767 | 1766 [36] | 2.0 ± 0.6 | 1.0 ± 0.3 | 0.80 ± 0.03 | a, b |
| 27 | δ-Cadinene | SH | 1527 | 1524 [36] | 1763 | 1761 [36] | 3.0 ± 0.4 | 3.0 ± 0.4 | 2.0 ± 0.5 | a, b |
| 28 | C15H24O (Figure S3) | OS | 1566 | - | 1979 | - | 0.5 ± 0.3 | 0.4 ± 0.1 | 0.9 ± 0.1 | a, b |
| 29 | Germacrene B | OS | 1574 | 1572 [42] | 1719 | 1708 [35] | 0.5 ± 0.2 | 0.7 ± 0.4 | 1.0 ± 0.6 | a, b |
| 30 | Caryophyllene oxide | OS | 1599 | 1595 [43] | 1996 | 1993 [44] | 9 ± 7 | 6 ± 2 | 10 ± 2 | a, b, c |
| 31 | Viridiflorol | OS | 1609 | 1600 [45] | 2093 | 2089 [35] | 0.3 ± 0.2 | 0.20 ± 0.08 | 0.3 ± 0.1 | a, b |
| 32 | C15H24O (Figure S4) | OS | 1640 | - | 2255 | - | 2.0 ± 0.3 | 1 ± 1 | 2.0 ± 0.2 | a, b |
| Monoterpene hydrocarbons (MH), % | 16 | 18 | 6 | |||||||
| Oxygenated monoterpenes (OM), % | 0.6 | 0.6 | 0.6 | |||||||
| Sesquiterpene hydrocarbons (SH), % | 70 | 74 | 75 | |||||||
| Oxygenated sesquiterpenes (OS), % | 12 | 9 | 17 | |||||||
| Phenological Stages | n * | μmol Trolox®/g Sample, ± s | |||||
|---|---|---|---|---|---|---|---|
| ABTS+• | ORAC | ||||||
| Essential Oil | Extract * | Essential Oil | Extract * | ||||
| Before Distillation | After Distillation | Before Distillation | After Distillation | ||||
| Vegetative | 9 | 292 ± 106 | 1990 ± 144 | 2260 ± 440 | 610 ± 69 | 4644 ± 1142 | 5393 ± 1992 |
| Flowering | 9 | 390 ± 114 | 1780 ± 191 | 1940 ± 97 | 632 ± 11 | 4670 ± 514 | 4274 ± 1047 |
| Post-flowering | 6 | 904 ± 2 | 2274 ± 83 | 2180 ± 255 | 540 ± 42 | 7690 ± 4345 | 4950 ± 1260 |
| Standard Compounds | n | ABTS+• | ORAC | ||||
| Rosmarinic acid | 3 | 3260 ± 116 | 32030 ± 327 | ||||
| Caffeic acid | 4510 ± 92 | 23400 ± 80 | |||||
| Rutin | 1190 ± 37 | 8210 ± 11 | |||||
| Sabinene | 25.0 ± 0.5 | 350 ± 13 | |||||
| (E)-β-Caryophyllene | ** | 440 ± 27 | |||||
| Caryophyllene oxide | ** | 540 ± 34 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán, S.B.; Sierra, L.J.; Fernández-Alonso, J.L.; Romero, A.K.; Martínez, J.R.; Stashenko, E.E. Antioxidant Properties and Secondary Metabolites Profile of Hyptis colombiana at Various Phenological Stages. Molecules 2023, 28, 6767. https://doi.org/10.3390/molecules28196767
Beltrán SB, Sierra LJ, Fernández-Alonso JL, Romero AK, Martínez JR, Stashenko EE. Antioxidant Properties and Secondary Metabolites Profile of Hyptis colombiana at Various Phenological Stages. Molecules. 2023; 28(19):6767. https://doi.org/10.3390/molecules28196767
Chicago/Turabian StyleBeltrán, Sheila B., Lady J. Sierra, José L. Fernández-Alonso, Angie K. Romero, Jairo R. Martínez, and Elena E. Stashenko. 2023. "Antioxidant Properties and Secondary Metabolites Profile of Hyptis colombiana at Various Phenological Stages" Molecules 28, no. 19: 6767. https://doi.org/10.3390/molecules28196767
APA StyleBeltrán, S. B., Sierra, L. J., Fernández-Alonso, J. L., Romero, A. K., Martínez, J. R., & Stashenko, E. E. (2023). Antioxidant Properties and Secondary Metabolites Profile of Hyptis colombiana at Various Phenological Stages. Molecules, 28(19), 6767. https://doi.org/10.3390/molecules28196767








