Compatibility of Nucleobases Containing Pt(II) Complexes with Red Blood Cells for Possible Drug Delivery Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Investigation of Biocompatibility and Incorporation of Platinum Complexes in Human Red Blood Cells (RBCs)
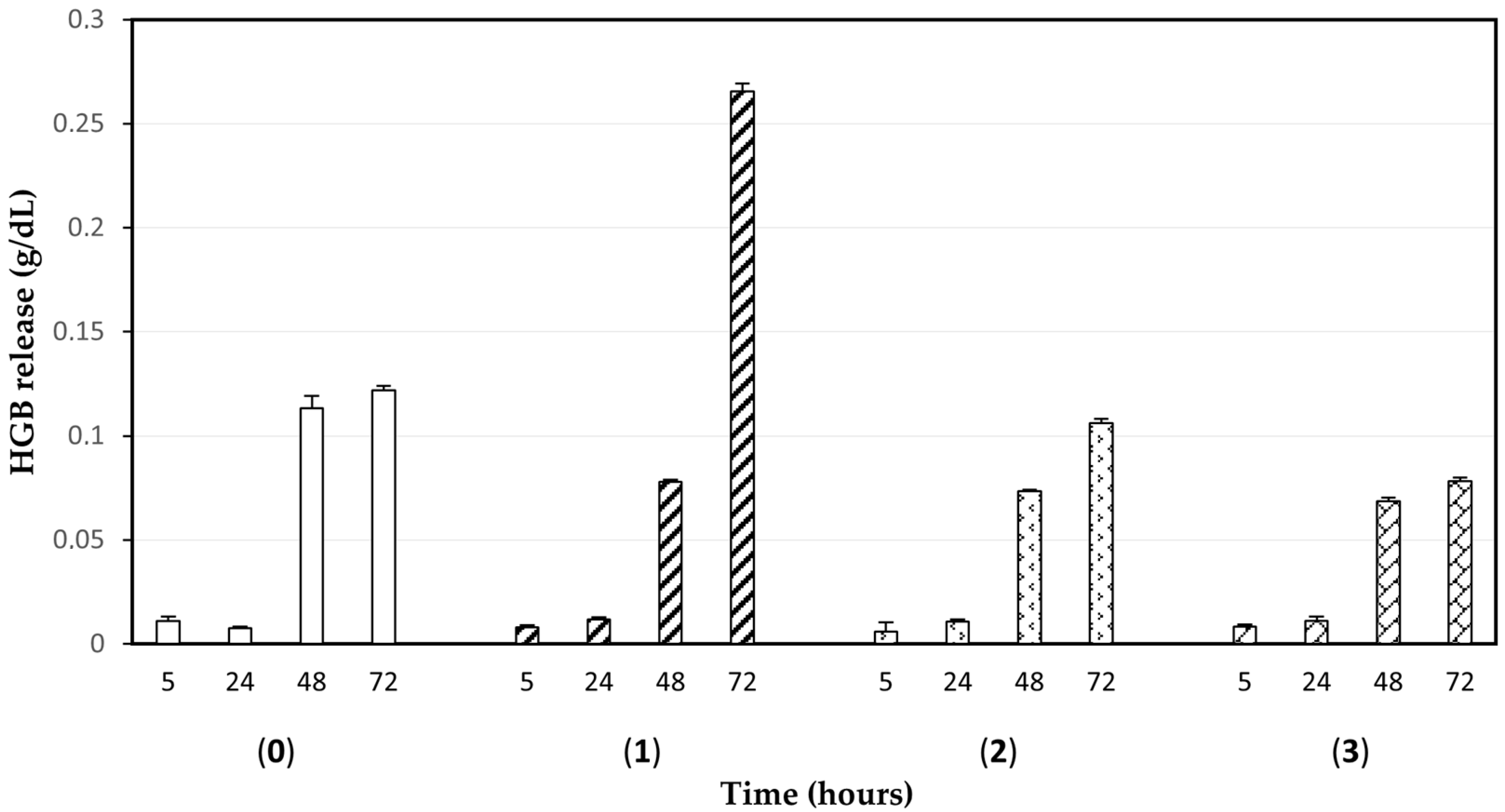
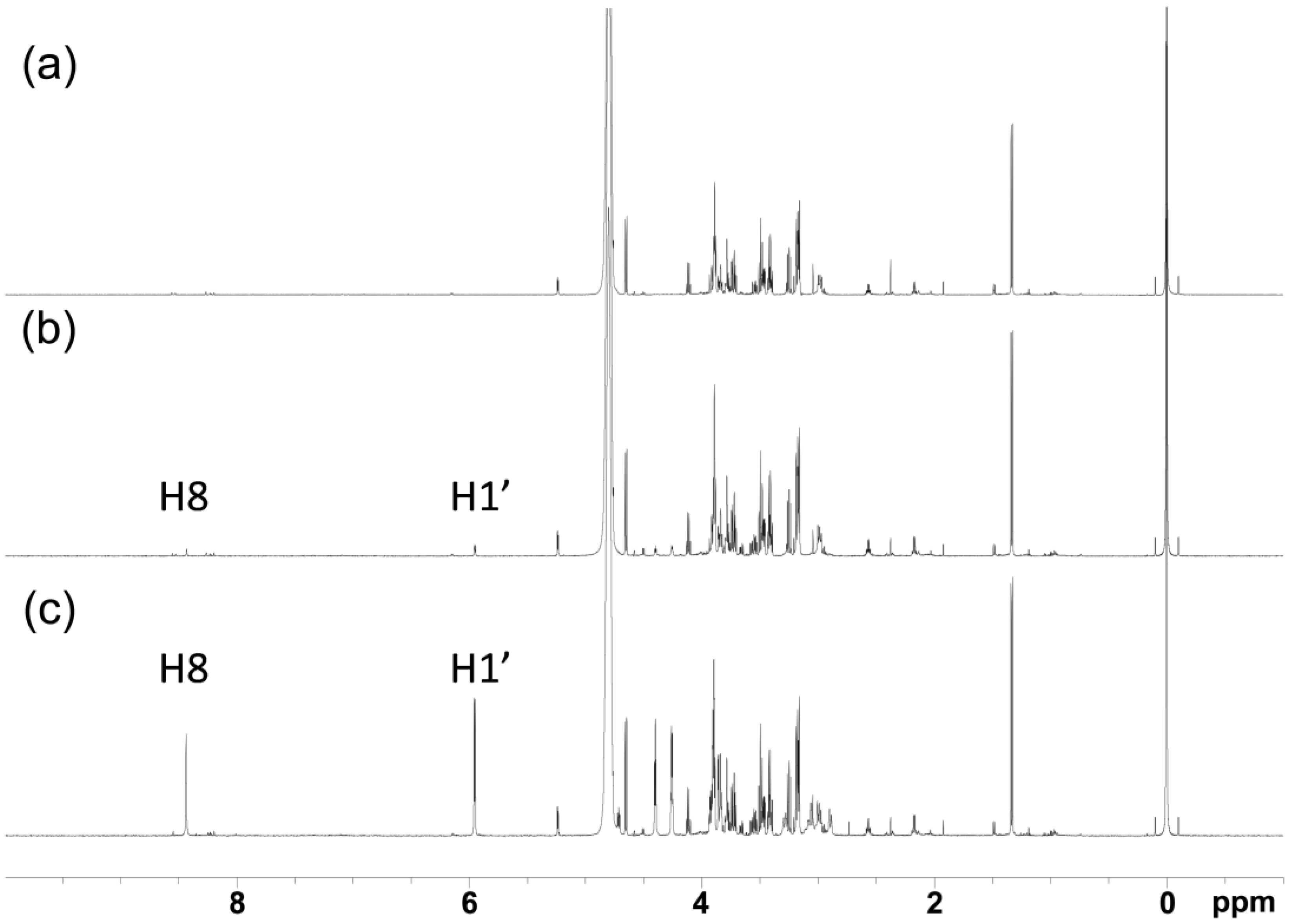
2.2. NMR Spectroscopy Determination of Loading Efficiency of Platinated Nucleos(t)ides in RBCs
2.3. Cell Integrity Check of Loaded RBCs and Drug Biocompatibility Remarks
3. Materials and Methods
3.1. Reagents and Methods
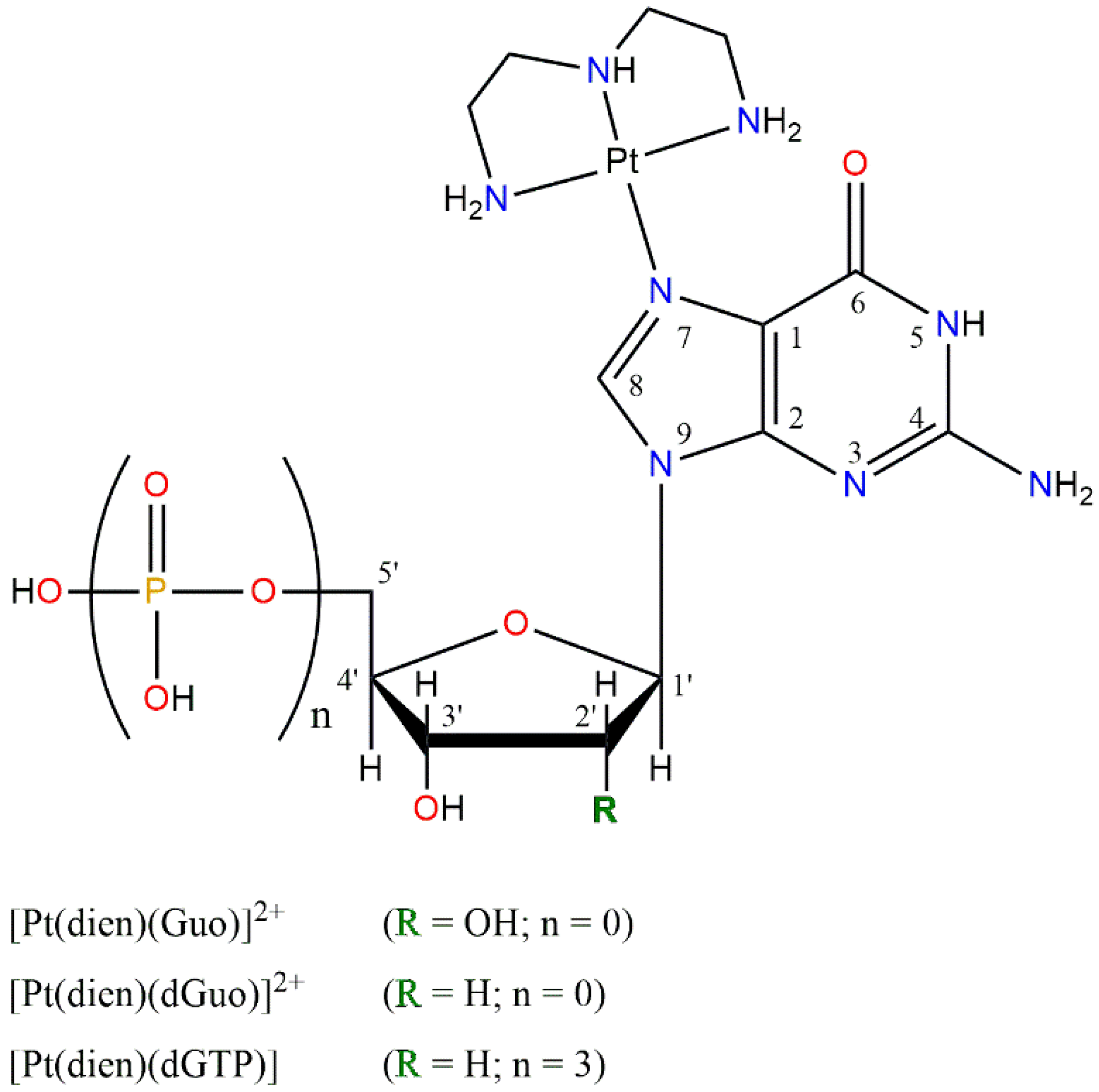
3.2. Synthesis of [Pt(dien)(N7-G)] Complexes
3.3. Evaluation of the Biocompatibility of [Pt(dien)(Guo)]2+ (1), [Pt(dien)(dGuo)]2+ (2), and [Pt(dien)(dGTP)] (3) Complexes with Red Blood Cells (RBCs)
3.4. Incorporation of Platinum-Nucleos(t)ide Complexes in Human RBCs
3.5. Annexin V Binding Assay
3.6. ICP-AES Measurements
3.7. NMR Measurements for Determining the Content of Platinum Complexes 1–3
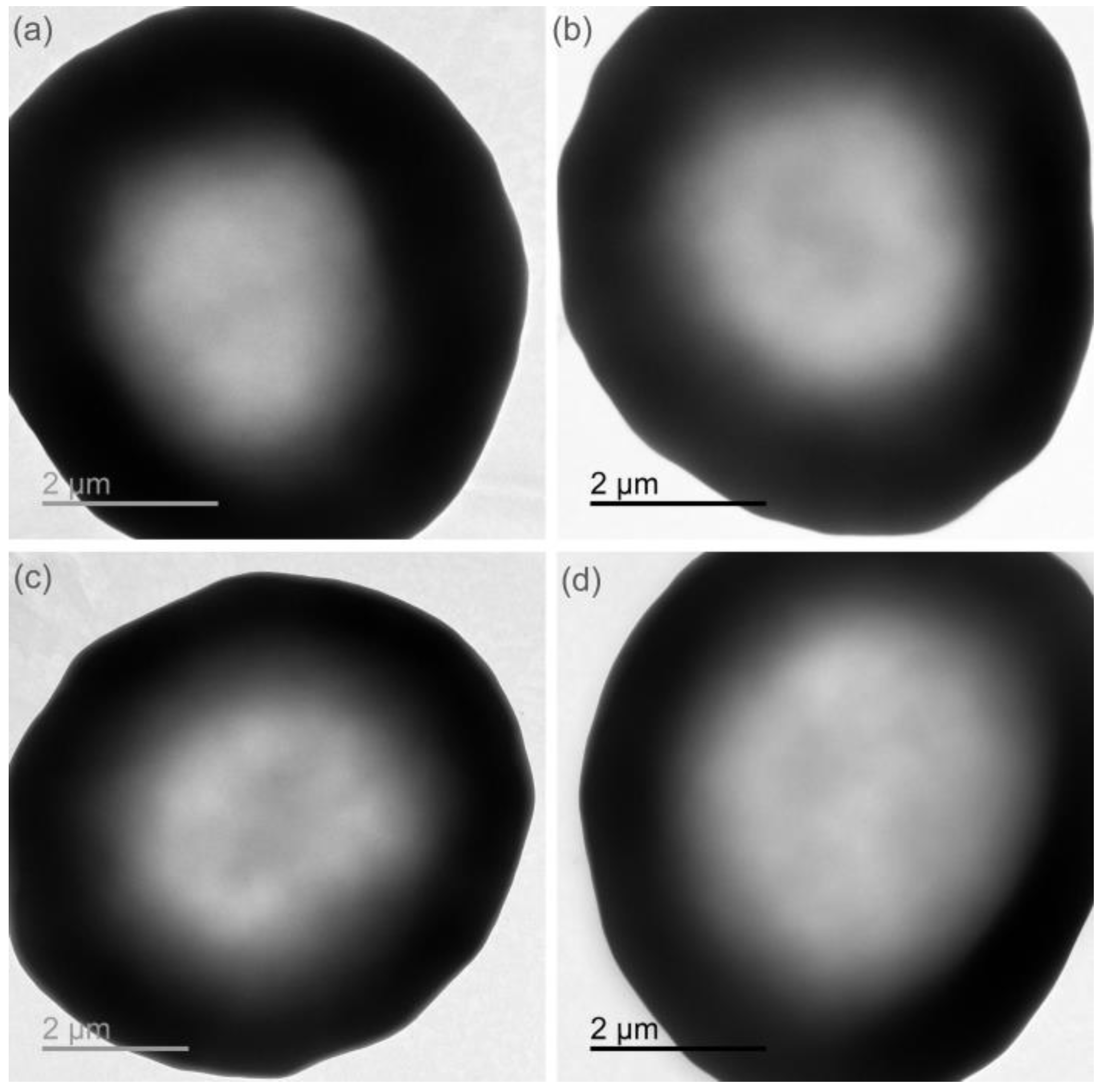
3.8. TEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pierigè, F.; Bigini, N.; Rossi, L.; Magnani, M. Reengineering Red Blood Cells for Cellular Therapeutics and Diagnostics. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1454. [Google Scholar] [CrossRef]
- Serafini, S.; Rossi, L.; Antonelli, A.; Fraternale, A.; Cerasi, A.; Crinelli, R.; Chiarantini, L.; Schiavano, G.F.; Magnani, M. Drug Delivery through Phagocytosis of Red Blood Cells. Transfus. Med. Hemother. 2004, 31, 92–101. [Google Scholar] [CrossRef]
- Slavu, L.M.; Antonelli, A.; Scarpa, E.S.; Abdalla, P.; Wilhelm, C.; Silvestri, N.; Pellegrino, T.; Scheffler, K.; Magnani, M.; Rinaldi, R.; et al. Optimization of Magnetic Nanoparticles for Engineering Erythrocytes as Theranostic Agents. Biomater. Sci. 2023, 11, 3252–3268. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Magnani, M. Red Blood Cells as Carriers of Iron Oxide-Based Contrast Agents for Diagnostic Applications. J. Biomed. Nanotechnol. 2014, 10, 1732–1750. [Google Scholar] [CrossRef] [PubMed]
- Magnani, M.; Serafini, S.; Fraternale, A.; Antonelli, A.; Biagiotti, S.; Pierigè, F.; Sfara, C.; Rossi, L. Red Blood Cell-Based Deliveryof Drugs and Nanomaterials for Therapeu Tic and Diagnostic Applications. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2011; Volume 22, ISBN 978-1-58883-159-0. [Google Scholar]
- Yao, H.; Zhu, G. Blood Components as Carriers for Small-Molecule Platinum Anticancer Drugs. ChemMedChem 2022, 17, e202200482. [Google Scholar] [CrossRef]
- Yan, J.; Yu, J.; Wang, C.; Gu, Z. Red Blood Cells for Drug Delivery. Small Methods 2017, 1, 1700270. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Yu, D.-G.; Tran, C.H.; Wang, M.; Varyambath, A.; Kim, J.; Kim, I. Efficient Synthesis of Folate-Conjugated Hollow Polymeric Capsules for Accurate Drug Delivery to Cancer Cells. Biomacromolecules 2021, 22, 732–742. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Xu, Y.; Zhou, Z.; Li, Y.; Ling, W.; Song, W. Bio-Inspired Drug Delivery Systems: From Synthetic Polypeptide Vesicles to Outer Membrane Vesicles. Pharmaceutics 2023, 15, 368. [Google Scholar] [CrossRef]
- Mambrini, G.; Mandolini, M.; Rossi, L.; Pierigè, F.; Capogrossi, G.; Salvati, P.; Serafini, S.; Benatti, L.; Magnani, M. Ex Vivo Encapsulation of Dexamethasone Sodium Phosphate into Human Autologous Erythrocytes Using Fully Automated Biomedical Equipment. Int. J. Pharm. 2017, 517, 175–184. [Google Scholar] [CrossRef]
- Nguyen, P.H.D.; Jayasinghe, M.K.; Le, A.H.; Peng, B.; Le, M.T.N. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano 2023, 17, 5187–5210. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; Fraternale, A.; Bianchi, M.; Antonelli, A.; Crinelli, R.; Chiarantini, L. Erythrocyte-Mediated Delivery of Drugs, Peptides and Modified Oligonucleotides. Gene Ther. 2002, 9, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Pierigè, F.; Antonelli, A.; Bigini, N.; Gabucci, C.; Peiretti, E.; Magnani, M. Engineering Erythrocytes for the Modulation of Drugs’ and Contrasting Agents’ Pharmacokinetics and Biodistribution. Adv. Drug Deliv. Rev. 2016, 106, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Muzykantov, V.R. Drug Delivery by Red Blood Cells: Vascular Carriers Designed by Mother Nature. Expert Opin. Drug Deliv. 2010, 7, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Zarrin, A.; Foroozesh, M.; Mohammadi-Samani, S. Applications of Carrier Erythrocytes in Delivery of Biopharmaceuticals. J. Control. Release 2007, 118, 145–160. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; Fraternale, A.; Casabianca, A.; Brandi, G.; Benatti, U.; De Flora, A. Targeting Antiviral Nucleotide Analogues to Macrophages. J. Leukoc. Biol. 1997, 62, 133–137. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; Casabianca, A.; Fraternale, A.; Schiavano, G.; Brandi, G.; Mannello, F.; Piedimonte, G. Red Blood Cells as Advanced Drug Delivery Systems for Antiviral Nucleoside Analogues. In The Use of Resealed Erythrocytes as Carriers and Bioreactors; Magnani, M., DeLoach, J.R., Eds.; Springer: Boston, MA, USA, 1992; pp. 239–245. ISBN 978-1-4613-6321-7. [Google Scholar]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef]
- Antonelli, A.; Pacifico, S.; Sfara, C.; Tamma, M.; Magnani, M. Ferucarbotran-Loaded Red Blood Cells as Long Circulating MRI Contrast Agents: First in Vivo Results in Mice. Nanomedicine 2018, 13, 675–687. [Google Scholar] [CrossRef]
- Villa, C.H.; Cines, D.B.; Siegel, D.L.; Muzykantov, V. Erythrocytes as Carriers for Drug Delivery in Blood Transfusion and Beyond. Transfus. Med. Rev. 2017, 31, 26–35. [Google Scholar] [CrossRef]
- Antonelli, A.; Szwargulski, P.; Scarpa, E.-S.; Thieben, F.; Cordula, G.; Ambrosi, G.; Guidi, L.; Ludewig, P.; Knopp, T.; Magnani, M. Development of Long Circulating Magnetic Particle Imaging Tracers: Use of Novel Magnetic Nanoparticles and Entrapment into Human Erythrocytes. Nanomedicine 2020, 15, 739–753. [Google Scholar] [CrossRef]
- Boni, A.; Ceratti, D.; Antonelli, A.; Sfara, C.; Magnani, M.; Manuali, E.; Salamida, S.; Gozzi, A.; Bifone, A. USPIO-Loaded Red Blood Cells as a Biomimetic MR Contrast Agent: A Relaxometric Study. Contrast Media Mol. Imaging 2014, 9, 229–236. [Google Scholar] [CrossRef]
- Johnson, K.M.; Tao, J.Z.; Kennan, R.P.; Gore, J.C. Gadolinium-Bearing Red Cells as Blood Pool MRI Contrast Agents. Magn. Reson. Med. 1998, 40, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, E.; Ferrauto, G.; Gianolio, E.; Lanzardo, S.; Carrera, C.; Fedeli, F.; Aime, S. An MRI Method To Map Tumor Hypoxia Using Red Blood Cells Loaded with a pO2-Responsive Gd-Agent. ACS Nano 2015, 9, 8239–8248. [Google Scholar] [CrossRef] [PubMed]
- Ferrauto, G.; Delli Castelli, D.; Di Gregorio, E.; Langereis, S.; Burdinski, D.; Grüll, H.; Terreno, E.; Aime, S. Lanthanide-Loaded Erythrocytes As Highly Sensitive Chemical Exchange Saturation Transfer MRI Contrast Agents. J. Am. Chem. Soc. 2014, 136, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z. Influenza A Virus Polymerase: An Attractive Target for next-Generation Anti-Influenza Therapeutics. Drug Discov. Today 2018, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Deng, Z.; Zhu, Q.; Zhao, J.; Xie, K.; Shi, P.; Wang, Z.; Chen, X.; Wang, F.; Shi, J.; et al. An Erythrocyte-Delivered Photoactivatable Oxaliplatin Nanoprodrug for Enhanced Antitumor Efficacy and Immune Response. Chem. Sci. 2021, 12, 14353–14362. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or Cell Membrane-Based Drug Delivery Systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef]
- Millán, C.G.; Marinero, M.L.S.; Castañeda, A.Z.; Lanao, J.M. Drug, Enzyme and Peptide Delivery Using Erythrocytes as Carriers. J. Control. Release 2004, 95, 27–49. [Google Scholar] [CrossRef]
- Tonetti, M.; Gasparini, A.; Giovine, M.; Bini, D.; Mazzucotelli, A.; de Paz, F.; Benatti, U.; de Flora, A. Interaction of Carboplatin with Carrier Human Erythrocytes. Biotechnol. Appl. Biochem. 1992, 15, 267–277. [Google Scholar] [CrossRef]
- Tonetti, M.; Gasparini, A.; Giovine, M.; Benatti, U. Enhanced intracellular activation of carboplatin in hemoglobin-synthesizing versus undifferentiated erythroleukemic cells. Biochem. Biophys. Res. Commun. 1992, 182, 760–766. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Z.; Wang, N.; Deng, Z.; Liu, G.; Zhou, J.; Chen, S.; Shi, J.; Zhu, G. Enhancing Circulation and Tumor Accumulation of Carboplatin via an Erythrocyte-Anchored Prodrug Strategy. Angew. Chem. 2022, 134, e202203838. [Google Scholar] [CrossRef]
- Wadhwa, R.; Aggarwal, T.; Thapliyal, N.; Kumar, A.; Priya; Yadav, P.; Kumari, V.; Reddy, B.S.C.; Chandra, P.; Maurya, P.K. Red Blood Cells as an Efficient in Vitro Model for Evaluating the Efficacy of Metallic Nanoparticles. 3 Biotech 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, M.; Gao, Y.; Cheng, X.; Liu, X.; Tang, S.; Peng, Y.; Wang, N.; Hu, D.; Peng, H.; et al. Biomimetic Erythrocytes Engineered Drug Delivery for Cancer Therapy. Chem. Eng. J. 2022, 433, 133498. [Google Scholar] [CrossRef]
- Chen, M.; Leng, Y.; He, C.; Li, X.; Zhao, L.; Qu, Y.; Wu, Y. Red Blood Cells: A Potential Delivery System. J. Nanobiotechnol. 2023, 21, 288. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Stefàno, E.; De Castro, F.; De Luca, E.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. Synthesis and Comparative Evaluation of the Cytotoxic Activity of Cationic Organometallic Complexes of the Type [Pt(η1-CH2-CH2-OR)(DMSO)(Phen)]+ (R = Me, Et, Pr, Bu). Inorg. Chim. Acta 2023, 546, 121321. [Google Scholar] [CrossRef]
- Benedetti, M.; Antonucci, D.; Girelli, C.R.; Capitelli, F.; Fanizzi, F.P. Reactivity of [PtCl(η2-C2H4)(N-N)]+, N-N = Diimine Ligand, with Phenol Derivatives and First Comparison between Single Crystal X-Ray Structures of Syn- and Anti-[Pt(N-N)(Phenolate)2] Rotamers in the Solid State. Inorg. Chim. Acta 2014, 409, 427–432. [Google Scholar] [CrossRef]
- De Castro, F.; Stefàno, E.; De Luca, E.; Benedetti, M.; Fanizzi, F.P. Platinum-Nucleos(t)Ide Compounds as Possible Antimetabolites for Antitumor/Antiviral Therapy: Properties and Perspectives. Pharmaceutics 2023, 15, 941. [Google Scholar] [CrossRef]
- De Castro, F.; De Luca, E.; Benedetti, M.; Fanizzi, F.P. Platinum Compounds as Potential Antiviral Agents. Coord. Chem. Rev. 2022, 451, 214276. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The Status of Platinum Anticancer Drugs in the Clinic and in Clinical Trials. Dalton Trans. Camb. Engl. 2003 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Sarkisyan, Z.M.; Shkutina, I.V.; Srago, I.A.; Kabanov, A.V. Relevance of Using Platinum-Containing Antitumor Compounds (A Review). Pharm. Chem. J. 2022, 56, 729–735. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Maeda, M.; Abiko, N.; Uchida, H.; Sasaki, T. Synthesis and Antitumor Activity of Cis-Dichloroplatinum (II)-N-Aminated Nucleoside Complexes. J. Med. Chem. 1984, 27, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Hiller, W.G.; Lippert, B. Regarding the Diamagnetic Components in Rosenberg’s “Platinum Pyrimidine Blues”: Species in the Cis-Pt(NH3)2-1-Methyluracil System. Inorg. Chim. Acta 2019, 494, 168–180. [Google Scholar] [CrossRef]
- Ali, M.S.; Ali Khan, S.R.; Ojima, H.; Guzman, I.Y.; Whitmire, K.H.; Siddik, Z.H.; Khokhar, A.R. Model Platinum Nucleobase and Nucleoside Complexes and Antitumor Activity: X-ray Crystal Structure of [PtIV(Trans-1R,2R-Diaminocyclohexane)Trans-(Acetate)2(9-Ethylguanine)Cl]NO3·H2O. J. Inorg. Biochem. 2005, 99, 795–804. [Google Scholar] [CrossRef]
- De Castro, F.; De Luca, E.; Girelli, C.R.; Barca, A.; Romano, A.; Migoni, D.; Verri, T.; Benedetti, M.; Fanizzi, F.P. First Evidence for N7-Platinated Guanosine Derivatives Cell Uptake Mediated by Plasma Membrane Transport Processes. J. Inorg. Biochem. 2022, 226, 111660. [Google Scholar] [CrossRef]
- Carrisi, C.; Antonucci, D.; Lunetti, P.; Migoni, D.; Girelli, C.R.; Dolce, V.; Fanizzi, F.P.; Benedetti, M.; Capobianco, L. Transport of Platinum Bonded Nucleotides into Proteoliposomes, Mediated by Drosophila Melanogaster Thiamine Pyrophosphate Carrier Protein (DmTpc1). J. Inorg. Biochem. 2014, 130, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Ducani, C.; Migoni, D.; Antonucci, D.; Vecchio, V.M.; Romano, A.; Verri, T.; Fanizzi, F.P. Possible Incorporation of Free N7-Platinated Guanines in DNA by DNA Polymerases, Relevance for the Cisplatin Mechanism of Action. In Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy; Bonetti, A., Leone, R., Muggia, F.M., Howell, S.B., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 125–132. ISBN 978-1-60327-458-6. [Google Scholar]
- Benedetti, M.; Romano, A.; De Castro, F.; Girelli, C.R.; Antonucci, D.; Migoni, D.; Verri, T.; Fanizzi, F.P. N7-Platinated Ribonucleotides Are Not Incorporated by RNA Polymerases. New Perspectives for a Rational Design of Platinum Antitumor Drugs. J. Inorg. Biochem. 2016, 163, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Antonucci, D.; De Castro, F.; Girelli, C.R.; Lelli, M.; Roveri, N.; Fanizzi, F.P. Metalated Nucleotide Chemisorption on Hydroxyapatite. J. Inorg. Biochem. 2015, 153, 279–283. [Google Scholar] [CrossRef]
- Benedetti, M.; Ducani, C.; Migoni, D.; Antonucci, D.; Vecchio, V.M.; Ciccarese, A.; Romano, A.; Verri, T.; Ciccarella, G.; Fanizzi, F.P. Experimental Evidence That a DNA Polymerase Can Incorporate N7-Platinated Guanines to Give Platinated DNA. Angew. Chem. Int. Ed. Engl. 2008, 47, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Lunetti, P.; Romano, A.; Carrisi, C.; Antonucci, D.; Verri, T.; De Benedetto, G.E.; Dolce, V.; Fanizzi, F.P.; Benedetti, M.; Capobianco, L. Platinated Nucleotides Are Substrates for the Human Mitochondrial Deoxynucleotide Carrier (DNC) and DNA Polymerase γ: Relevance for the Development of New Platinum-Based Drugs. ChemistrySelect 2016, 1, 4633–4637. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; Chiarantini, L.; Fraternale, A.; Casabianca, A. Red Blood Cells as Carriers of Drugs Against Retroviruses. In Targeting of Drugs 4; Gregoriadis, G., McCormack, B., Poste, G., Eds.; Springer: Boston, MA, USA, 1994; pp. 147–152. ISBN 978-1-4899-1209-1. [Google Scholar]
- Nagana Gowda, G.A.; Gowda, Y.N.; Raftery, D. Expanding the Limits of Human Blood Metabolite Quantitation Using NMR Spectroscopy. Anal. Chem. 2015, 87, 706–715. [Google Scholar] [CrossRef] [PubMed]
- De Castro, F.; Benedetti, M.; Del Coco, L.; Fanizzi, F.P. NMR-Based Metabolomics in Metal-Based Drug Research. Molecules 2019, 24, 2240. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR Spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Del Coco, L.; Marisi, G.; Conti, F.; Rovesti, G.; Ulivi, P.; Canale, M.; Frassineti, G.L.; Foschi, F.G.; Longo, S.; et al. 1H-NMR Based Serum Metabolomics Highlights Different Specific Biomarkers between Early and Advanced Hepatocellular Carcinoma Stages. Cancers 2020, 12, 241. [Google Scholar] [CrossRef]
- Strauss, D.; de Verdier, C.D. Preservation of Red Blood Cells with Purines and Nucleosides. III. Synthesis of Adenine, Guanine, and Hypoxanthine Nucleotides. Folia Haematol. Leipz. Ger. 1928 1980, 107, 434–453. [Google Scholar]
- Annibale, G.; Brandolisio, M.; Pitteri, B. New Routes for the Synthesis of Chloro(Diethylenetriamine) Platinum(II) Chloride and Chloro(2,2′: 6′,2″-Terpyridine) Platinum(II) Chloride Dihydrate. Polyhedron 1995, 14, 451–453. [Google Scholar] [CrossRef]
- Brunskill, S.; Thomas, S.; Whitmore, E.; McDonald, C.P.; Dorée, C.; Hopewell, S.; Staves, J.; Cardigan, R.; Murphy, M.F. What Is the Maximum Time That a Unit of Red Blood Cells Can Be Safely Left Out of Controlled Temperature Storage? Transfus. Med. Rev. 2012, 26, 209–223.e3. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Pierigè, F.; Aliano, M.P.; Magnani, M. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs 2020, 34, 265–272. [Google Scholar] [CrossRef]
- De Castro, F.; Ciardullo, G.; Fanizzi, F.P.; Prejanò, M.; Benedetti, M.; Marino, T. Incorporation of N7-Platinated Guanines into Thermus Aquaticus (Taq) DNA Polymerase: Atomistic Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2023, 24, 9849. [Google Scholar] [CrossRef]


| 5 h | RBCs 106/μl | HGB g/dL | HCT % | MCV fl | MCH pg | MCHC g/dL |
|---|---|---|---|---|---|---|
| (0) 500 µL RBCs (NT) | 5.30 ± 0.1 | 15.2 ± 0.55 | 44.6 ± 045 | 87 ± 1.0 | 31.6 ± 0.47 | 33.9 ± 1.4 |
| (1) 500 µL RBCs + 1 | 4.87 ± 0.25 | 15.8 ± 0.30 | 44.3 ± 0.47 | 87 ± 1.0 | 30.9 ± 0.4 | 35.3 ± 0.32 |
| (2) 500 µL RBCs + 2 | 4.67 ± 0.26 | 13.5 ± 0.4 | 39.7 ± 0.3 | 85 ± 0.58 | 30.7 ± 0.9 | 35.2 ± 0.55 |
| (3) 500 µL RBCs + 3 | 4.85 ± 0.12 | 14.4 ± 0.4 | 40.7 ± 0.83 | 87 ± 0.5 | 29.9 ± 0.15 | 34.7 ± 0.46 |
| 24 h | ||||||
| (0) 500 µL RBCs (NT) | 5.11 ± 0.08 | 15.4 ± 0.1 | 44.3 ± 0.47 | 87 ± 1.0 | 30.7 ± 0.5 | 35.1 ± 0.1 |
| (1) 500 µL RBCs + 1 | 4.88 ± 0.07 | 14.2 ± 0.25 | 44.5 ± 0.4 | 85 ± 0.57 | 30.3 ± 0.61 | 35.3 ± 0.3 |
| (2) 500 µL RBCs + 2 | 4.64 ± 0.07 | 13.9 ± 0.4 | 39.7 ± 0.37 | 85 ± 1.0 | 29.8 ± 0.1 | 34.7 ± 0.55 |
| (3) 500 µL RBCs + 3 | 4.41 ± 0.06 | 13.3 ± 0.3 | 37.7 ± 0.32 | 86 ± 0.57 | 29.5 ± 0.15 | 36.8 ± 0.36 |
| 48 h | ||||||
| (0) 500 µL RBCs (NT) | 5.58 ± 0.09 | 16.9 ± 0.2 | 46.6 ± 1.25 | 85 ± 0.57 | 30.5 ± 0.45 | 35.1 ± 0.8 |
| (1) 500 µL RBCs + 1 | 4.72 ± 0.16 | 14.6 ± 0.38 | 38.9 ± 0.15 | 83 ± 0.57 | 30.5 ± 0.58 | 36.4 ± 0.25 |
| (2) 500 µL RBCs + 2 | 4.69 ± 0.1 | 13.5 ± 0.3 | 39.5 ± 0.23 | 83 ± 0.57 | 29.4 ± 0.15 | 35.1 ± 0.3 |
| (3) 500 µL RBCs + 3 | 3.87 ± 0.13 | 11.5 ± 0.34 | 31.7 ± 0.45 | 83 ± 0.58 | 30.7 ± 0.35 | 36.8 ± 0.36 |
| 72 h | ||||||
| (0) 500 µL RBCs (NT) | 4.35 ± 0.61 | 12.7 ± 0.45 | 32.6 ± 0.77 | 79 ± 1.00 | 30.7 ± 0.25 | 37.4 ± 0.81 |
| (1) 500 µL RBCs + 1 | 3.85 ± 0.10 | 13.4 ± 0.21 | 26.9 ± 0.83 | 70 ± 0.58 | 34.7 ± 0.5 | 49.7 ± 0.8 |
| (2) 500 µL RBCs + 2 | 3.64 ± 0.12 | 10.6 ± 0.2 | 32.1 ± 0.1 | 66 ± 1.00 | 32.3 ± 0.3 | 47.7 ± 1.6 |
| (3) 500 µL RBCs + 3 | 1.38 ± 0.06 | 3.93 ± 0.25 | 9.7 ± 0.4 | 71 ± 1.00 | 29.8 ± 0.2 | 41.9 ± 0.25 |
| Samples | RBCs 106/μL | HGB g/dL | HCT % | MCV fl | MCH pg | MCHC g/dL | Cell Recovery % |
|---|---|---|---|---|---|---|---|
| ND (Not dialyzed RBCs) | 5.84 ± 0.1 | 15.8 ± 0.3 | 41.1 ± 1.2 | 82 ± 0.5 | 27.0 ± 0.1 | 38.3 ± 0.21 | / |
| (0) Unloaded RBCs | 6.46 ± 0.22 | 14.3 ± 0.5 | 41.6 ± 1.3 | 72 ± 0.6 | 22.2 ± 0.3 | 33.9 ± 0.80 | 68 ± 1.5 |
| (1) RBCs loaded by 1 | 6.69 ± 0.10 | 14.7 ± 0.4 | 44.6 ± 0.15 | 69 ± 1.0 | 22.1 ± 0.2 | 32.1 ± 0.51 | 64 ± 1.3 |
| (2) RBCs loaded by 2 | 6.87 ± 0.04 | 14.6 ± 0.1 | 44.2 ± 0.31 | 64 ± 0.6 | 21.5 ± 0.4 | 33.3 ± 0.57 | 64 ± 0.58 |
| (3) RBCs loaded by 3 | 6.45 ± 0.02 | 13.4 ± 0.2 | 42.9 ± 0.85 | 66 ± 1.7 | 20.8 ± 0.3 | 31.4 ± 1.0 | 65 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Castro, F.; Stefàno, E.; Fanizzi, F.P.; Di Corato, R.; Abdalla, P.; Luchetti, F.; Nasoni, M.G.; Rinaldi, R.; Magnani, M.; Benedetti, M.; et al. Compatibility of Nucleobases Containing Pt(II) Complexes with Red Blood Cells for Possible Drug Delivery Applications. Molecules 2023, 28, 6760. https://doi.org/10.3390/molecules28196760
De Castro F, Stefàno E, Fanizzi FP, Di Corato R, Abdalla P, Luchetti F, Nasoni MG, Rinaldi R, Magnani M, Benedetti M, et al. Compatibility of Nucleobases Containing Pt(II) Complexes with Red Blood Cells for Possible Drug Delivery Applications. Molecules. 2023; 28(19):6760. https://doi.org/10.3390/molecules28196760
Chicago/Turabian StyleDe Castro, Federica, Erika Stefàno, Francesco Paolo Fanizzi, Riccardo Di Corato, Pasant Abdalla, Francesca Luchetti, Maria Gemma Nasoni, Rosaria Rinaldi, Mauro Magnani, Michele Benedetti, and et al. 2023. "Compatibility of Nucleobases Containing Pt(II) Complexes with Red Blood Cells for Possible Drug Delivery Applications" Molecules 28, no. 19: 6760. https://doi.org/10.3390/molecules28196760
APA StyleDe Castro, F., Stefàno, E., Fanizzi, F. P., Di Corato, R., Abdalla, P., Luchetti, F., Nasoni, M. G., Rinaldi, R., Magnani, M., Benedetti, M., & Antonelli, A. (2023). Compatibility of Nucleobases Containing Pt(II) Complexes with Red Blood Cells for Possible Drug Delivery Applications. Molecules, 28(19), 6760. https://doi.org/10.3390/molecules28196760











