Abstract
Sulfides poisoning of metallic Ni is an important issue in catalyst deactivation. SO2, similar to H2S and other sulfides, is an impurity presented in reactants or during the regeneration steps. Herein, spin-polarized density functional theory calculations were used to study the adsorption and decomposition of SO2 on a pristine and metal-doped Ni(111) surface. The adsorption energy, transition state energy, and partial density of state (PDOS) were calculated. On the pristine Ni(111) surface, ten different configurations were considered, and three typical ones were selected for transition state searching. It was found that the reaction barrier of the first S-O bond dissociation was much higher than that of the second one. Doping the top layer with a second metal could strongly change the adsorption and decomposition behavior. Doping with 3/9ML Co slightly increases the adsorption energy of SO2 for most configurations and decreases the reaction barriers of the SO2-tht-2 decomposition, while the others decrease the adsorption ability and increase the barriers. The order of adsorption energy for the most stable configurations is Co > Ni > Cu > Rh > Pd. The order of the first S-O bond dissociation reaction barriers is Pd > Rh > Cu = Ni > Co, and the order of the second bond dissociation barrier is Rh > Pd > Cu > Ni > Co.
1. Introduction
SO2 is an important raw material that produces bulk chemicals, and it is also a major air pollutant, which kills lives [1], erodes metals [2,3,4], and poisons catalysts [5,6,7]. The interaction of SO2 with metal is drawing researchers’ attention because it plays an important role in the environment and industry [8].
One of the main sources of SO2 is the burning of fossil fuels. Desulfurization is a widely used strategy to decrease SO2 emissions from fossil fuels [9,10]. The S Zorb process of SINOPEC Corp., as a typical reactive adsorption desulfurization (RADS), shows its distinguished advantages [11,12,13,14,15]. It is carried out in a circulating fluidized bed reactor using a sorbent with Ni and ZnO as the main active phases. In the reactor, sulfur is transferred to the sorbent under a H2 atmosphere to form ZnS. The sorbent with a particular sulfur loading is then regenerated using air to convert ZnS back to ZnO with SO2. Although numerous efforts on revealing the chemistry of desulfurization reaction have been made [16,17,18], the behavior of nickel in the SO2-rich gas during regeneration is still unclear. A deep understanding of the SO2 reaction on metal surfaces would provide insights that would help develop more efficient catalysts and processes.
Despite the sulfur-removing strategies, SO2 still remains a question for catalyst scientists. Using density functional theory (DFT), Benjamin D. Gould et al. studied the effect of different sulfides on the Pt of a fuel cell electrode and found that SO2 had a poisoning ability similar to H2S and COS [19]. This is due to the strong adsorption energy of S on Pt’s surface [20,21], which is a similar case for sulfides deactivating Ni [21]. The three-way automotive catalysts could also be poisoned by SO2 in the tail gas [22].
Based on those issues, understanding SO2 adsorption and conversion over a metal surface could shed light on developing new catalysts. A few groups have devoted themselves to studying the adsorption of SO2 on different transition metal surfaces using first-principles calculations, such as Ni [23,24,25], Cu [26,27], Pt [28,29,30,31], and others [32]. Xi Lin reported 20 different configurations of SO2 on the Pt (111) surface and figured out the most energetically stable configurations [28]. Markus Happel et al. performed a combined experiment and density functional study on the adsorption of SO2 on clean and oxygen-precovered Pt(111), and also found that there were parallel or perpendicular geometries [30]. M. J. Ungerer et al. compared the interaction of SO2 with the Pt’s different surfaces and concluded that the order of adsorption energy was (001) > (011) > (111) [31]. Yoshiko Sakai et al. used cluster models with 4 and 15 Ni atoms and found that the most stable configuration was a molecular plane nearly parallel to the surface, with all the S and O atoms on bridge sites [23]. M.J. Harrison et al. used a slab model and concluded that the parallel–hollow geometry was the more preferred adsorption site on Ni [24]. Xin Wei et al. noticed that the existence of atomic O strengthened the adsorption capacity of SO2 on a pristine Ni(111) surface [25].
The conversion of SO2 was also studied. Chen-Hao Yeh studied the oxidation of S on Pt(111), Ni(111), and the core–shell structures [33]. They considered four absorbed structures and found that S could be oxidized with either atomic O or O2 on a Ni surface to form SO, continuing to be oxidized to SO2 of the parallel bridge orientation. The core–shell structure could change both the adsorption energy and the reaction barrier for SO2 formation. H. N. Sharma et al. used DFT calculations coupled with microkinetic modeling to examine the oxidation of SOx to SO4 on Pd(111) and Pt(111) surfaces, in which three different orientations were considered [34]. Natasha M. Galea studied removing sulfur from Ni by O2 using a p(2 × 2) three-layer model and found that atomic sulfur could be removed up to an initial coverage of 50% at high temperatures [35].
In this study, we used DFT to investigate the adsorption of SO2 on the Ni(111) surface with different configurations and studied the decomposition pathways of the three most stable ones. The effect of doping metals, namely Cu/Co/Pd/Rh, on whether a second metal would improve the tolerance of SO2 relative to pure Ni was further modeled.
2. Results
2.1. Absorption of S and O on Different Metal Surfaces
Understanding the nature of sulfur and oxygen bonding with different metals is decisive for investigating the decomposition of SO2. The adsorption energy (Figure 1) and bond length (Figure S1) of O and S on three typical sites of different metal surfaces were calculated. The results show that the sulfur and oxygen over these metals’ surfaces are strongly chemisorbed. The Fcc site is the most stable for both atoms adsorbed on all the calculated metals except for S on Rh(111) and O on Co(111), of which the hcp site is more stable, while the top site has the weakest bind energy. For the adsorption energy of O, Ni shows the highest value, and rest are in the following order: Co > Rh > Cu > Pd. S is a little different from O, the adsorption of which is in the order of Rh > Ni > Pd > Cu > Co.
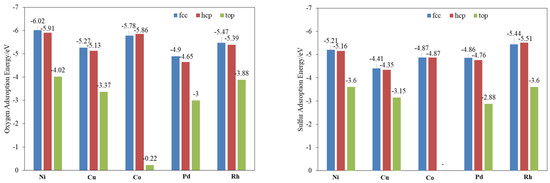
Figure 1.
Binding energies of O and S on different metal (111) surfaces.
2.2. SO2 on Ni(111)
2.2.1. Configurations of SO2 on Ni(111) Surface
The binding energetic and geometric data of the stable configurations are presented in Figure 2 and Table 1. The S-O bond length of gaseous SO2 is 1.449 Å, and the bond angle of ∠O-S-O is 119.5°. There are ten different configurations identified, as shown in Figure 2, which could be categorized into four groups: (1) binding with two O on the Ni top site and the molecular plane perpendicular to the Ni surface (a: SO2-tnt); (2) binding with only S and the molecular plane perpendicular to the Ni surface (b: SO2-ntn/c: SO2-ntn-2/d: SO2-nbn). SO2-ntn and SO2-ntn-2’s S are on Ni’s top site while SO2-nbn’s is on the bridge site; (3) S on the bridge site, and one O on the top site (e: SO2-tbn/ f: SO2-nbt); (4) all atoms of SO2 binding to the metal surfaces and the molecular plane parallel to the metal surface (g: SO2-tht/h: SO2-ttt/i: SO2-btt/j: SO2-ttb). The difference between SO2-tht) and SO2-ttt) is that two O atoms of the SO2-tht) are on the top site of two adjacent Ni atoms and those of SO2-ttt) are on the top site of two Ni surface, which are on the long diagonal of the equilateral diamond composed of four nickel atoms.
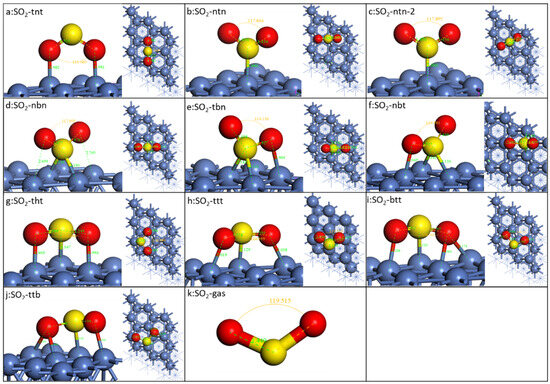
Figure 2.
Optimized geometries of SO2 on Ni(111) surface. The blue, red, and yellow spheres represent Ni, O, and S atoms, respectively.

Table 1.
Binding energies, bond lengths, and angles of SO2 on Ni(111).
It can be found In Table 1 that when adsorbed on the Ni(111) surface, both the bond length and the angle of SO2 decrease. For all the configurations, SO2-tnt shows the lowest binding energy, even lower than SO2-ntn and SO2-ntn-2, although O has a higher adsorption energy than S on the top site of Ni. SO2-ntn-2 is the rotation about the S-metal bond of SO2-ntn, which has a small effect on the energy, meaning that SO2 of this type could rotate almost freely on the surface. SO2-nbn has a much higher adsorption energy than SO2-ntn and SO2-ntn-2 because its S is on the bridge site. SO2-tbn and SO2-nbt are quite similar with S on the bridge site and O on the top site. They show the highest binding energy, −1.22 eV and −1.23 eV, respectively, which might be due to the fact that S on the bridge site is much more stable than the top site. A higher result of −1.40 eV is obtained using a different function [24]. The similar configuration of SO2 on Pt(111) is also the most stable SO2 on Pt(111) [28,31] and Ir(111) [32]. SO2-tht’s binding energy is higher than SO2-ttt, SO2-btt, and SO2-ttb. The same configuration as the reported was also identified with a slightly higher adsorption energy (−1.11 eV vs. −1.06 eV) [33].
The length of the S-O bond of all adsorbed SO2 is longer than the gaseous SO2, indicating that adsorption could stretch the bond, which would decrease the bond energy. The angles are smaller than the gas phase, decreasing to the range of between 109.0° and 117.8°. The smallest angle is SO2-btt, which is caused by two O binding to two adjacent nickel atoms. However, there is no simple numerical relationship between the binding energy and the bond length or angle.
2.2.2. SO2 Decomposition on Ni(111)
As types SO2-tbn, SO2-nbt, and SO2-tht have the highest binding energy, they would be the dominant configurations on the surface. To obtain the difference of decomposition pathways, we chose SO2-tbn, SO2-tht, and SO2-ttt to study the decomposition behavior of SO2 on the pristine Ni surface.
- Pathway1: SO2-tbn
When searching for the TS, IS as well as FS must be recognized beforehand. For the dissociation of the first S-O bond, the final state is shown in Figure 3b. The dissociated O is on the fcc site, while the left SO group is vertical to the nickel surface, and the S is on the nearby fcc site. This step is energetically favorable, with an energy change of −0.44 eV. During the first S-O dissociation, O on the top site moves to the nearest nearby fcc site, and the left SO group moves to the fcc site with the S-O bond vertical to the Ni(111) surface. The distance of S and O increases from 1.459 Å to 2.975 Å, indicating the breakdown of this S-O bond (Table 2). However, the left S-O bond length decreases from 1.527 Å to 1.475 Å. The transition state is similar to the final state. The reaction barrier of this step is 0.79 eV.
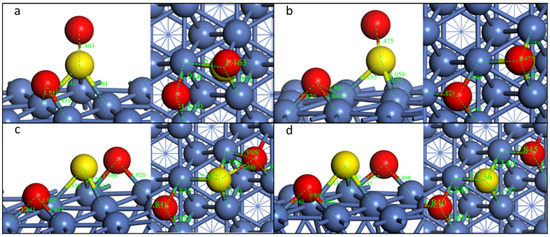
Figure 3.
Geometric structures of the dissociation of SO2-b-4 on Ni(111) surface. ((a,b): TS and FS of the first S-O dissociation; (b–d)): IS, TS, and FS of the second S-O dissociation).

Table 2.
Bond lengths and energy change in IS, TS, and FS for the dissociation of SO2-tbn on Ni(111) surface.
For the dissociation of the second S-O bond, the FS is shown in Figure 3c. The second detached O is also on the fcc site, while the left S is on its original site. The second S-O dissociation takes place when the O moves to the fcc site and the distance increases from 1.475 Å to 2.579 Å, meaning a total decomposition of SO2 to S and O adsorbed on Ni(111). This step is almost energetic neutral, while the reaction barrier is 0.39 eV, which is much lower than the first step. The overall decomposition is exothermic.
- Pathway2: SO2-tht
For the decomposition of SO2-tht, the FS of the two steps is shown in Figure 4. Similar to the Pathway1, for the first S-O dissociation, the O on the top site moves to the nearby fcc site. Meanwhile, the S of the left SO group migrates to the bridge site, while the O is almost unmoved. The S-O bond is nearly parallel to the Ni(111) surface. The distance of S and O increases from 1.544 Å to 2.758 Å (Table 3), indicating the breakdown of this S-O bond. For the other S-O bond, it increases from 1.544 Å to 1.574 Å, which shows a different tendency from Pathway1. This is because for Pathway2, both atoms of SO bind to the Ni surface, stretching the S-O bond.
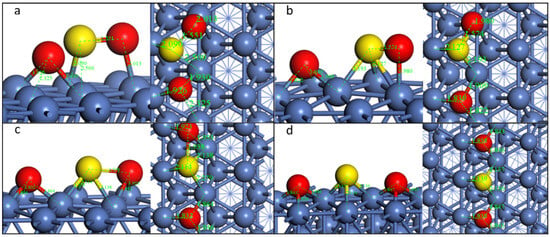
Figure 4.
Geometric structures of the dissociation of SO2-tht on Ni(111) surface. ((a,b): TS and FS of the first S-O dissociation; (b–d)): IS, TS, and FS of the second S-O dissociation).

Table 3.
Bond lengths and energy change of the IS, TS, and FS for the dissociation of SO2-tht on Ni(111) surface.
The second S-O dissociation takes place when the O moves from the top site to the nearby fcc site and the distance increases from 1.574 Å to 2.573 Å. The reaction barriers of the two steps for this configuration are smaller than the first path, especially for the second step, which decreases from 0.39 eV to 0.17 eV.
- Pathway3: SO2-ttt
The first S-O dissociation of Pathway3 is similar to Pathway2, and the second step of decomposition is almost the same (Figure 5). The reaction barriers are the lowest among the three pathways (Table 4).
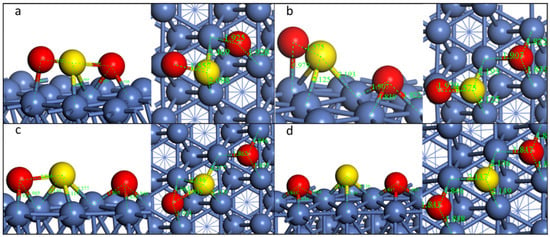
Figure 5.
Geometric structures of the dissociation of SO2-ttt on Ni(111) surface. ((a,b): TS and FS of the first S-O dissociation; (b–d)): IS, TS, and FS of the second S-O dissociation).

Table 4.
Bond lengths and energy change of the IS, TS, and FS for the dissociation of SO2-ttt on Ni(111) surface.
Figure 6 presents a detailed energy profile for the dissociation of the three different adsorbed SO2 configurations. It must be noted that for all three pathways, the overall decomposition processes are exothermic. The first step has a higher energy barrier compared with the second one. The higher barriers for the SO2-tbn type is attributed to the fact that the dissociated O does not bond with the nickel surface, which cannot decrease the bond energy as in the other two cases.
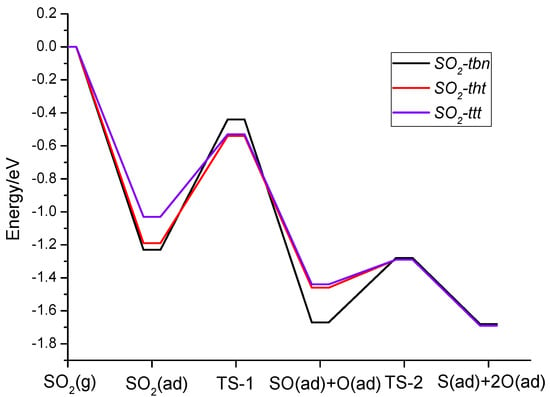
Figure 6.
Calculated possible potential energy diagram of electronic energy for SO2 decomposition on Ni(111).
2.3. SO2 on Doped Ni(111)
2.3.1. Doping Effect
Doping with a second element usually changes a catalyst’s property [36,37,38]. Nishith K. Das studied the effect of Cr doping on the adsorption of S, O, SO. and SO2 over the Ni(111) surface and found that Cr doping of the Ni(111) surface increased their adsorption energies [39]. Chen-Hao Yeh studied the core–shell structure of Ni@Pt and Pt@Ni and found that the Pt@Ni(111) surface exhibited less affinity for SOx or S than pure Pt(111), while Ni@Pt showed the opposite results [33]. We herein studied the doping effect of Co/Cu/Rh/Pd on Ni(111). Three Ni atoms on the first layer were replaced by the doping metal. Three topologies were considered (Figure S3). The dispersion of Co and Cu had a minor effect on the energy, while the uniformly dispersed Rh or Pd showed the lowest energy. Thus, we chose the uniformly dispersed surfaces to study.
2.3.2. Adsorption
The adsorption of SO2 on different doped Ni(111) is compared. As there are too many different configurations if all the possibilities were considered, we chose two similar ones with the most stable configurations as on the pristine Ni(111) surface, namely SO2-nbt, SO2-tht, and SO2-ttt. On the doped Ni(111), there are seven different configurations. Taking Cu-doped Ni(111), for example (Figure 7), for the SO2-tht types, when S is on Cu’s top site, the two O atoms are on Ni’s top site. And when the S is on Ni’s top site, one O is on Ni’s top site and the other is on Cu’s top site. For the SO2-nbt and SO2-ttt types, there are three and two different configurations, respectively. This is similar for all other doped surfaces.
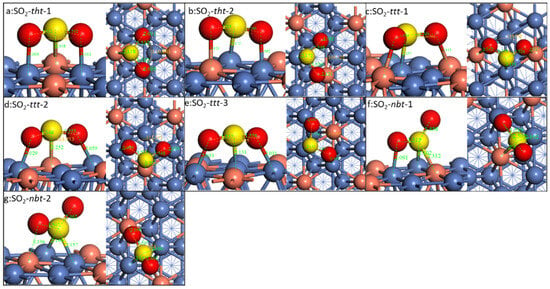
Figure 7.
Configurations of SO2 on Cu-doped surface. The blue, red, yellow, and brass spheres represent Ni, O, S, and Cu atoms, respectively.
Table 5 shows the binding energies on different surfaces. The change in binding energies is quite different. On the Cu-doped Ni(111) surface, the SO2-tht and SO2-bnt types’ binding energies all decreased compared with the pristine Ni(111) surface. It must be noted that when S binds to Cu, the binding energy is weaker than the binding energy when O binds Cu. Thus, SO2-tht-1 has a lower binding energy than SO2-tht-2. SO2-ttt-3 has even higher energy than on the pristine Ni(111) surface (−1.09 eV vs. −1.03 eV). This thus concludes that SO2-ttt-3, SO2-nbt-2, and SO2-tht-2 would be the most abundant configurations on the Cu-doped Ni surface.

Table 5.
The adsorption energy of SO2 on different metal-doped Ni(111) surfaces.
The Co-doped Ni surface is a bit different from the Cu-doped surface. The SO2-tht-1 has higher bind energy than that on the pristine Ni, while SO2-tht-2 is a little lower. For the SO2-ttt types, all the configurations have higher binding energy than that on the pristine Ni. SO2-nbt-2 shows the highest binding energy among all the configurations.
Rh and Pd doping have a similar trend. All the configurations have a much lower binding energy than that on the pristine Ni. Meanwhile, the most abundant configurations are SO2-tht-2 and SO2-nbt-2.
2.3.3. Decomposition on Doped Metal Surfaces
For the doped Ni surface, we selected SO2-tht-2 to study the decomposition pathway. The transition state and final state are illustrated in Figure 8 and Figure S4.
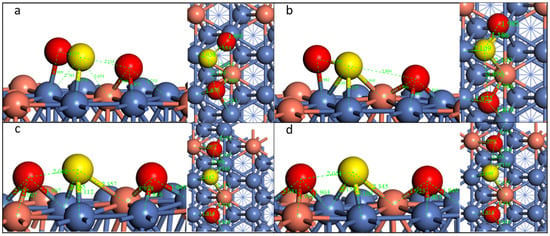
Figure 8.
Geometric structures of the dissociation of SO2-tht-2 on Cu-doped Ni(111) surface. ((a,b): TS and FS of the first S-O dissociation; (b–d)): IS, TS, and FS of the second S-O dissociation.
On the Cu-, Co-, and Rh-doped surfaces, the reaction pathways are similar; thus, we still used Cu as the example for a detailed examination. When the first S-O bond dissociates, the O migrates to the nearby fcc site, and the S of the second SO moves to the fcc site underneath the previous S-O bond. However, the dissociated O is not in the center of the fcc site, leaning toward the bridge site of Ni-Ni, and the left SO deviates from Cu, not alike on the pristine Ni surface, which is along the median of the fcc site. This phenomenon originates from the fact that both O and S have a higher adsorption energy with Ni compared with Cu (Figure 1). The second S-O bond dissociates when the O moves to the nearby the fcc site. All S and O atoms are close to the fcc sites.
The configuration is a little different on the Pd-doped surface (Figure S5-3). Although the initial configuration is similar to that on the pristine Ni surface, when the first S-O bond dissociates, the leaving O migrates from the top site to the bridge site, and the left SO group lies across the Ni-Ni bond. When the second S-O dissociates, the S atom is on the bridge site at the transition state, and the O also moves to the bridge site. After the total dissociation of SO2, the first O is on the site nearby the fcc site, the S is on the bridge site, and the second O is on the hcp site. This result is due to the fact that Pd has a much lower affiliation ability with both O and S compared to Ni.
Although the adsorption energy also decreases for the same configuration on the Co-doped surface compared with the pristine surface as on other doped surfaces, the reaction energy for both dissociation steps increases and the reaction barriers all decrease (Table 6). The opposite phenomenon could be observed on other doped surfaces, among which the Pd-doped surface shows the highest reaction barriers for the determining step (first S-O dissociation) and becomes an endothermic process.

Table 6.
The adsorption energy (ΔEads), reaction barriers (Ea), and reaction energies (ΔEr) of SO2-tht-2 on various surfaces.
2.4. Analysis
The interactions of SO2 and different Ni surfaces were investigated in detail by the projected density of states (PDOSs). The results of Ni were chosen for the illustration (Figure 9). In the gas phase, the overlapping of the p orbit of the S and O atoms expresses the S-O bonds in SO2. For SO2, the interactions between the S and O atoms of the SO2 and Ni atoms of nickel surface can be explained by the p-DOS of S, O of SO2, and d-DOS of Ni. The redistribution of p-DOS of S and O indicates the bond formations of S and O atoms of SO2 with Ni. Similar cases are observed on doped surfaces (Figure S6).
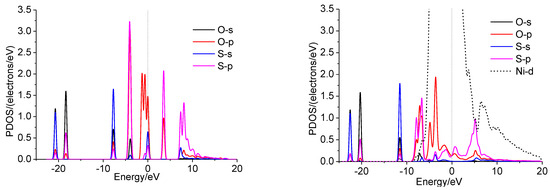
Figure 9.
PDOS of SO2 (g) and SO2 adsorbed on Ni(111) surface.
3. Methods
Density functional theory (DFT) was performed on the CASTEP (Cambridge Sequential Total Energy Package) code implemented in Material Studio of Accelrys Inc., Cambridge, UK [40]. The electron exchange correlation energy was modeled with ultrasoft pseudopotentials and Perdew–Burke–Ernzerh (PBE) exchange–correlation functional based on the generalized gradient approximation (GGA). A wave function energy cutoff of 400 eV was used according to the literature [35,41]. A Fermi smearing of 0.1 eV was utilized. The spin polarization calculation was used when Ni was included in the model. The convergence criteria for the structure optimization and energy calculation were set to (a) an SCF tolerance of 1.0 × 10−6 eV/atom, (b) an energy tolerance of 1.0 × 10−5 eV/atom, (c) a maximum force tolerance of 0.03 eV/Å, and (d) a maximum displacement tolerance of 1.0 × 10−3/Å. For the adsorption of O and S on different metals, 2 × 2 supercell of a four-layer slab and the 6 × 6 × 1 k-point Monkhorst-Pack mesh were used. The SO2 adsorption, transition structures, and products of the reactions over the pristine and doped Ni(111) surface were modeled using periodic 3 × 3 supercell of a four-layer slab, and the 5 × 5 × 1 k-point Monkhorst-Pack mesh was used. For both cases, the slabs were separated with a vacuum spacing of 15 Å to minimize interactions between the slabs. All the metals have a face-centered cubic lattice. The calculated lattice parameters of the pure metals are 3.54 Å for bulk Ni for both 2 × 2 and 3 × 3 supercells, which are in satisfactory agreement with experimental observations of 3.52 Å. The lattice parameters of Co, Cu, Rh, and Pd were 3.46, 3.64, 3.89, and 3.93 Å, respectively. The corresponding experimental values were 3.54, 3.61, 3.80, and 3.89 Å, respectively. All of the calculated results were within 3% of the measured values. During geometry optimization, the bottom two layers were fixed, whereas the top two layers were allowed to relax.
The dissociation pathway was sequential abstraction of O atom from SO2. The transition states (TSs) were searched using the complete LST/QST method [42], and the convergence criterion of root-mean-square forces on atoms tolerance of was set to 0.05 eV/Å. The adsorption energy ΔEads, reaction energy (ΔEr), and activation barrier Ea was defined as follows:
where EA-S is the energy of the slab together with the adsorbate, and Eadsorbate, Eslab, EFS, ETS, and EIS are the total energy of the free adsorbate, bare slab, final state (FS), transition state (TS), and initial state (IS).
ΔEads = EA-S − (Eslab + Eadsorbate)
ΔEr = EFS − EIS
Ea = ETS − EIS
4. Conclusions
In summary, the periodic spin-polarized DFT calculation was exploited to gain a detailed understanding of the adsorption and decomposition of SO2 on pristine and metal-doped Ni(111) surfaces. The adsorption energy, reaction barrier, and partial density of state (PDOS) were calculated. On the pristine Ni surface, ten different configurations were considered, and three typical ones were selected to search for the transition states in order to reveal the reaction barriers. Among the three types, it was found that the barrier of the first S-O bond was much higher than the second one, and a lower reaction barrier was associated with weaker adsorption energy. Doping the top layer with a second metal could strongly change the adsorption and decomposition behavior. The order of adsorption energy for the most stable configurations is Co > Ni > Cu > Rh > Pd. The order of the first S-O bond dissociation reaction barriers is Pd > Rh > Cu = Ni > Co, and the order of the second bond dissociation barrier is Rh > Pd > Cu > Ni > Co. Overall, Pd is the best choice among the studied metals for decreasing the poisoning effect of Ni from SO2 because it decreases the adsorption energy of SO2 and increases the determining step of the decomposition reaction barriers the most.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28186739/s1.
Author Contributions
L.L.: Conceptualization, Supervision, Investigation, Review and Editing. C.Z.: Formal Analysis, Data Curation, Writing. W.W.: Formal Analysis, Data Curation. G.L.: Formal Analysis, Data Curation. B.Z.: Give Valuable Advice. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by research grants from Sinopec (Fund No. 114009, 116004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Aas, W.; Mortier, A.; Bowersox, V.; Cherian, R.; Faluvegi, G.; Fagerli, H.; Hand, J.; Klimont, Z.; Galy-Lacaux, C.; Lehmann, C.M.B.; et al. Global and regional trends of atmospheric sulfur. Sci. Rep. 2019, 9, 953. [Google Scholar] [CrossRef]
- Grabke, H.J.; Reese, E.; Spiegel, M. The effects of chlorides, hydrogen chloride, and sulfur dioxide in the oxidation of steels below deposits. Corros. Sci. 1995, 37, 1023. [Google Scholar] [CrossRef]
- Oesch, S.; Faller, M. Environmental effects on materials: The effect of the air pollutants SO2, NO2, NO and O3 on the corrosion of copper, zinc and aluminum. A short literature survey and results of laboratory exposures. Corros. Sci. 1997, 39, 1505–1530. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011.
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Ma, X.; Sprague, M.; Song, C. Deep Desulfurization of Gasoline by Selective Adsorption over Nickel-Based Adsorbent for Fuel Cell Applications. Ind. Eng. Chem. Res. 2005, 44, 5768–5775. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Bezverkhyy, I.; Bellat, J. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step. Appl. Catal. B Environ. 2008, 84, 766–772. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Tian, S.; Chai, Y.; Liu, C. Reactive adsorption of thiophene on Ni/ZnO adsorbent: Effect of ZnO textural structure on the desulfurization activity. J. Nat. Gas Chem. 2010, 19, 327–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Han, H.; Yang, M.; Wang, L.; Zhang, Y.; Jiang, Z.; Li, C. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance. Appl. Catal. B Environ. 2012, 119–120, 13–19. [Google Scholar] [CrossRef]
- Meng, X.; Huang, H.; Shi, L. Reactive Mechanism and Regeneration Performance of NiZnO/Al2O3-Diatomite Adsorbent by Reactive Adsorption Desulfurization. Ind. Eng. Chem. Res. 2013, 52, 6092–6100. [Google Scholar] [CrossRef]
- Qiu, L.; Xiang, Y.; Xin, M.; Zou, K.; Zheng, A.; Xu, G. Structural verification of nickel sulfide on spent S Zorb adsorbent as studied by HRTEM and XPS. J. Mol. Struct. 2020, 1202, 127215. [Google Scholar] [CrossRef]
- Peng, B.; Zou, K.; Song, Y.; Xin, M.; Yang, X.; Lu, L.; Liu, J.; Lin, W. Development of Ni/ZnO desulfurization adsorbent with high stability: Formation of Zn2SiO4 and the impact from substrate. Chem. Eng. J. 2021, 409, 127374. [Google Scholar] [CrossRef]
- Song, Y.; Peng, B.; Yang, X.; Jiang, Q.; Liu, J.; Lin, W. Trail of sulfur during the desulfurization via reactive adsorption on Ni/ZnO. Green Energy Environ. 2021, 6, 597–606. [Google Scholar] [CrossRef]
- Gould, B.D.; Baturina, O.A.; Swider-Lyons, K.E. Deactivation of Pt/VC proton exchange membrane fuel cell cathodes by SO2, H2S and COS. J. Power Sources 2009, 188, 89–95. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, R.; Rodriguez, J.A. First-principles study of the adsorption of sulfur on Pt(111). Score-level shifts and the nature of the Pt-S bond. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 65, 155409/1. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M. Surface regeneration of sulfur-poisoned Ni surfaces under SOFC operation conditions predicted by first-principles-based thermodynamic calculations. J. Power Sources 2008, 176, 23–30. [Google Scholar] [CrossRef]
- Turner, M.D.; Laurence, R.L.; Yngvesson, K.; Conner, W.C. The effect of microwave energy on three-way automotive catalysts poisoned by SO2. Catal. Lett. 2001, 71, 133–138. [Google Scholar] [CrossRef]
- Sakai, Y.; Koyanagi, M.; Mogi, K.; Miyoshi, E. Theoretical study of adsorption of SO2 on Ni(111) and Cu(111) surfaces. Surf. Sci. 2002, 513, 272–282. [Google Scholar] [CrossRef]
- Harrison, M.J.; Woodruff, D.P.; Robinson, J. Density functional theory investigation of the structure of SO2 and SO3 on Cu(111) and Ni(111). Surf. Sci. 2006, 600, 1827–1836. [Google Scholar] [CrossRef]
- Wei, X.; Dong, C.; Chen, Z.; Xiao, K.; Li, X. Density functional theory study of SO2-adsorbed Ni(1 1 1) and hydroxylated NiO(1 1 1) surface. Appl. Surf. Sci. 2015, 355, 429–435. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, P.; Hrbek, J.; Rodriguez, J. The chemisorption of SO2 on the Cu/Au(111) surface: Interplay between ensemble and electronic effects. Surf. Sci. 2005, 592, 25–36. [Google Scholar] [CrossRef]
- Mozo, R.; Agusta, M.K.; Rahman, M.; Diño, W.A.; Rodulfo, E.T.; Kasai, H. Pathways for SO2 dissociation on Cu(100): Density functional theory. J. Phys. Condens. Matter. 2007, 19, 365244. [Google Scholar] [CrossRef]
- Lin, X.; Hass, K.C.; Schneider, W.F.; Trout, B.L. Chemistry of Sulfur Oxides on Transition Metals I: Configurations, Energetics, Orbital Analyses, and Surface Coverage Effects of SO2 on Pt(111). J. Phys. Chem. B 2002, 106, 12575–12583. [Google Scholar] [CrossRef]
- Tang, H.; Trout, B.L. Electronic Composition−Property Relationship Applied to SO2 Chemisorption on Pt(111) Surfaces, Alloys, and Overlayers. J. Phys. Chem. B 2005, 109, 6948–6951. [Google Scholar] [CrossRef]
- Happel, M.; Luckas, N.; Viñes, F.; Sobota, M.; Laurin, M.; Görling, A.; Libuda, J. SO2 adsorption on Pt(111) and oxygen precovered Pt(111). A combined infrared reflection absorption spectroscopy and density functional study. J. Phys. Chem. C 2011, 115, 479–491. [Google Scholar] [CrossRef]
- Ungerer, M.J.; Santos-Carballal, D.; Santos-Carballal, D.; Santos-Carballal, D.; de Leeuw, N.H. Interaction of SO2 with the Platinum (001), (011), and (111) Surfaces: A DFT Study. Catalysts 2020, 10, 558. [Google Scholar] [CrossRef]
- Jiang, R.; Guo, W.; Li, M.; Zhu, H.; Li, J.; Zhao, L.; Fu, D.; Shan, H. Density Functional Study of the Reaction of SO2 on Ir(111). J. Phys. Chem. C 2009, 113, 18223–18232. [Google Scholar] [CrossRef]
- Yeh, C.; Ho, J. A First-Principle Calculation of Sulfur Oxidation on Metallic Ni(111) and Pt(111), and Bimetallic Ni@Pt(111) and Pt@Ni(111) Surfaces. ChemPhysChem 2012, 13, 3194–3203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.N.; Sharma, V.; Hamzehlouyan, T.; Epling, W.; Mhadeshwar, A.B.; Ramprasad, R. SOx Oxidation Kinetics on Pt(111) and Pd(111): First-Principles Computations Meet Microkinetic Modeling. J. Phys. Chem. C 2014, 118, 6934–6940. [Google Scholar] [CrossRef]
- Galea, N.M.; Lo, J.M.H.; Ziegler, T. A DFT study on the removal of adsorbed sulfur from a nickel(111) surface: Reducing anode poisoning. J. Catal. 2009, 263, 380–389. [Google Scholar] [CrossRef]
- Niu, J.; Ran, J.; Du, X.; Qi, W.; Zhang, P.; Yang, L. Effect of Pt addition on resistance to carbon formation of Ni catalysts in methane dehydrogenation over Ni-Pt bimetallic surfaces: A density functional theory study. Mol. Catal. 2017, 434, 206–218. [Google Scholar] [CrossRef]
- Qiu, H.; Ran, J.; Niu, J.; Guo, F.; Ou, Z. Effect of different doping ratios of Cu on the carbon formation and the elimination on Ni (111) surface: A DFT study. Mol. Catal. 2021, 502, 111360. [Google Scholar] [CrossRef]
- Jing, W.; Shen, H.; Qin, R.; Wu, Q.; Liu, K.; Zheng, N. Surface and Interface Coordination Chemistry Learned from Model Heterogeneous Metal Nanocatalysts: From Atomically Dispersed Catalysts to Atomically Precise Clusters. Chem. Rev. 2023, 123, 5948–6002. [Google Scholar] [CrossRef]
- Das, N.K.; Saidi, W.A. Effects of Cr-doping on the adsorption and dissociation of S, SO, and SO2 on Ni(111) surfaces. J. Chem. Phys. 2017, 146, 154701. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Wang, P.; Shi, X.R.; Zhang, Y.; Wei, M. The influence of Co nucleation in Co Ni single atom alloy on low-temperature methane dry reforming with DFT simulations and microkinetic modeling. Mol. Catal. 2023, 549, 113515. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).