GeO2 Nanoparticles Decorated in Amorphous Carbon Nanofiber Framework as Highly Reversible Lithium Storage Anode
Abstract
:1. Introduction
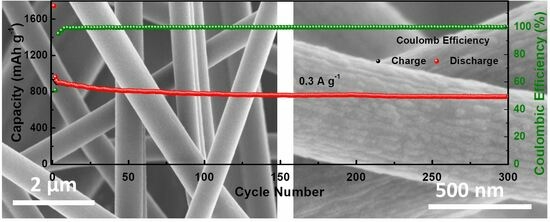
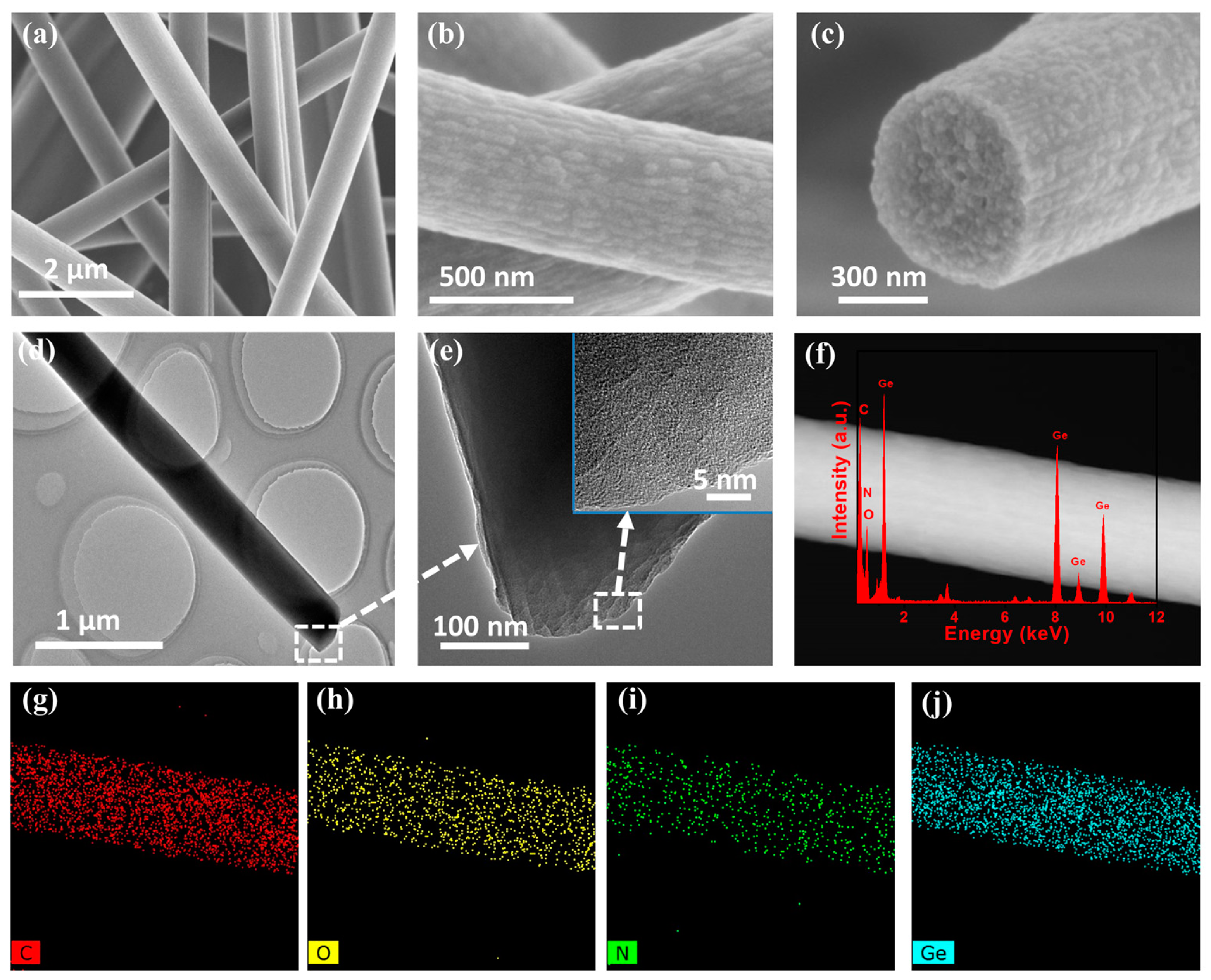
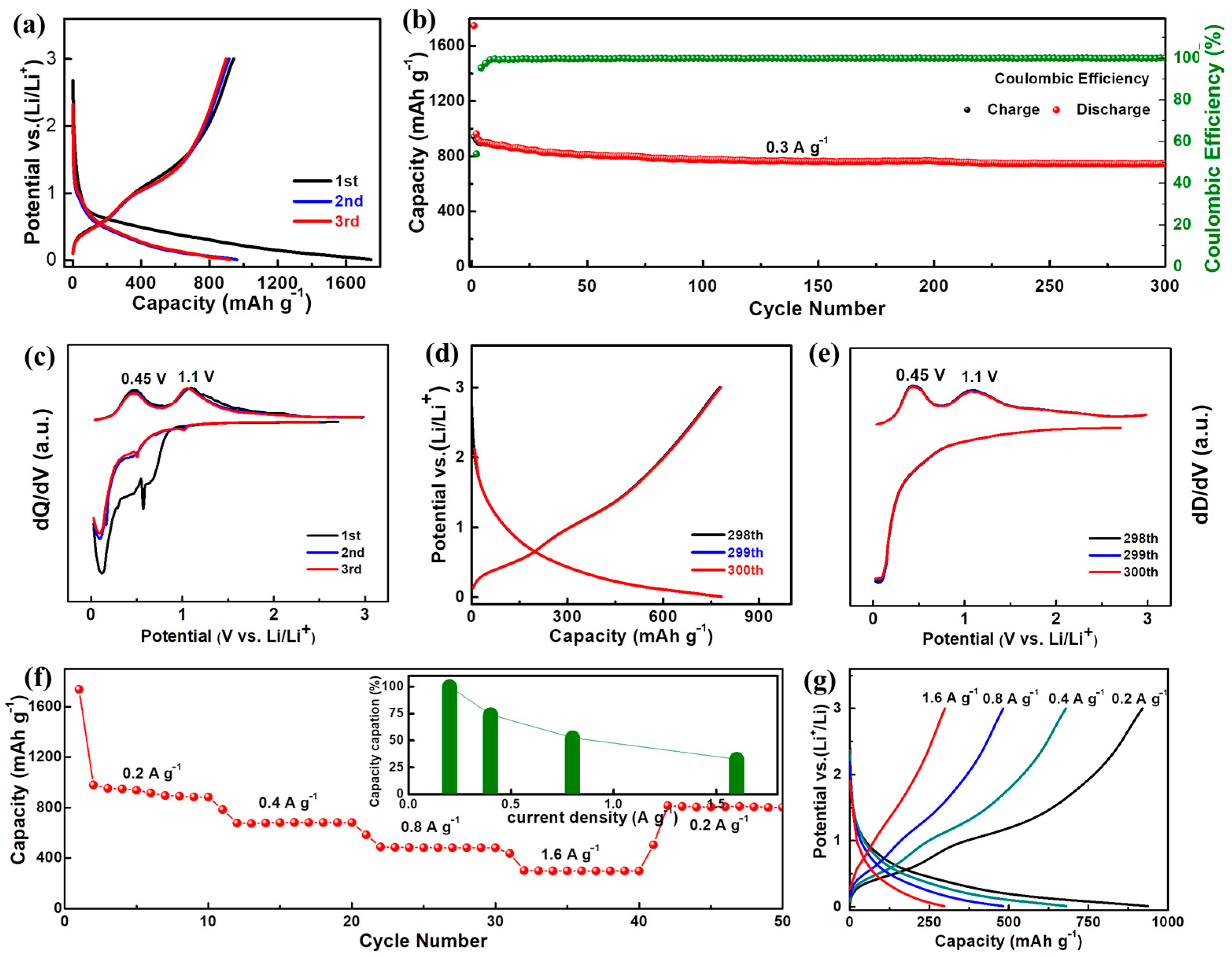
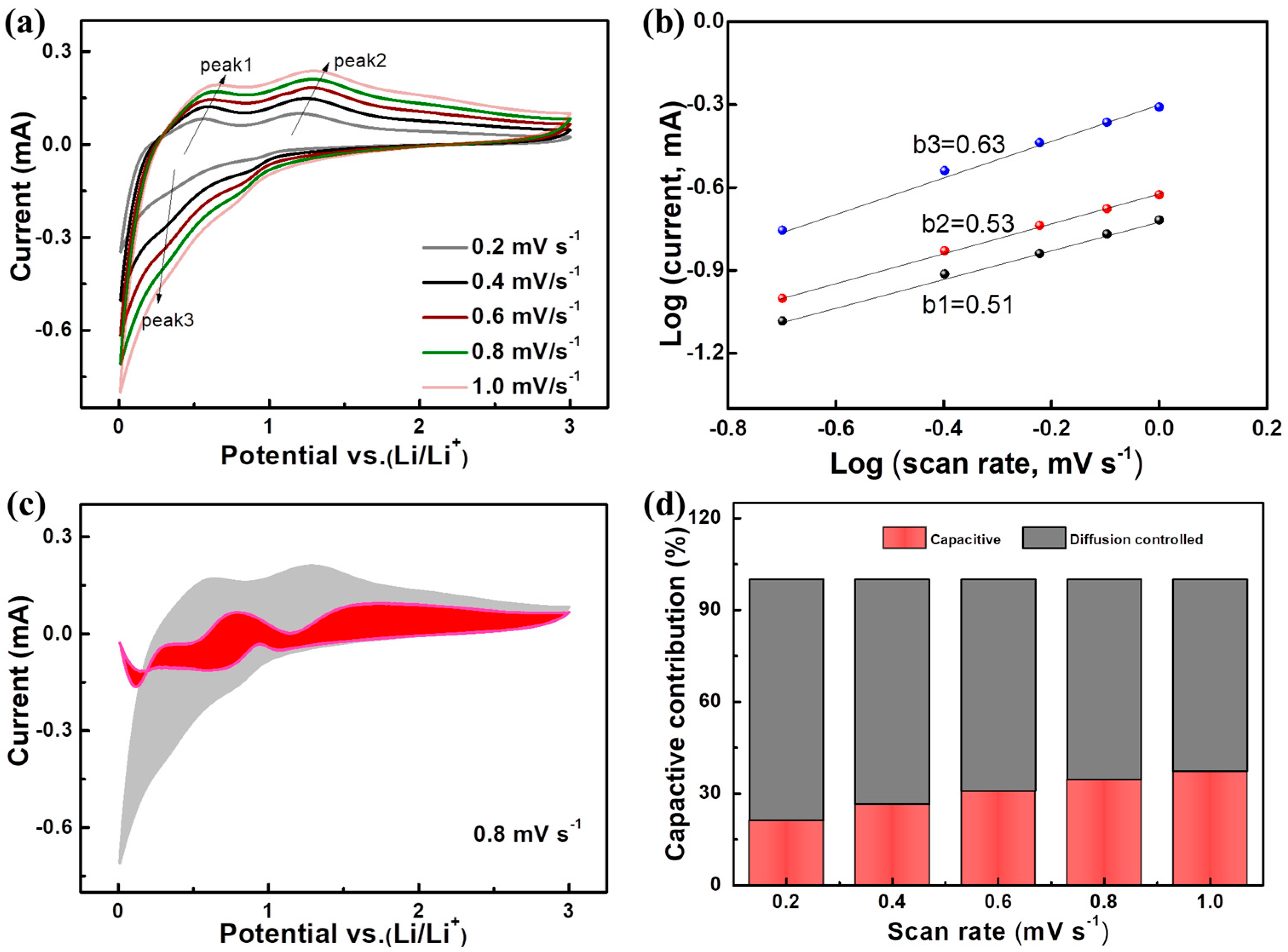
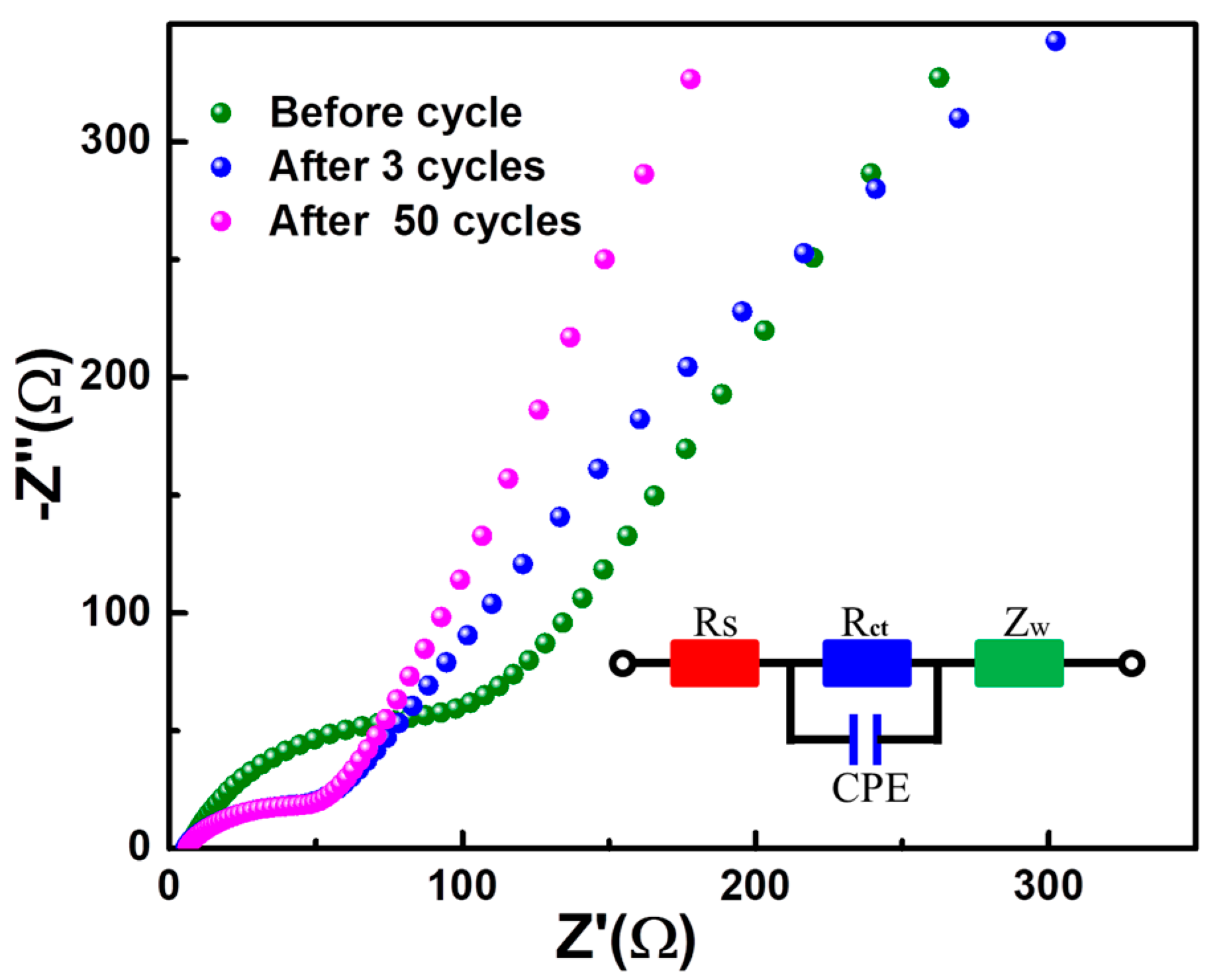
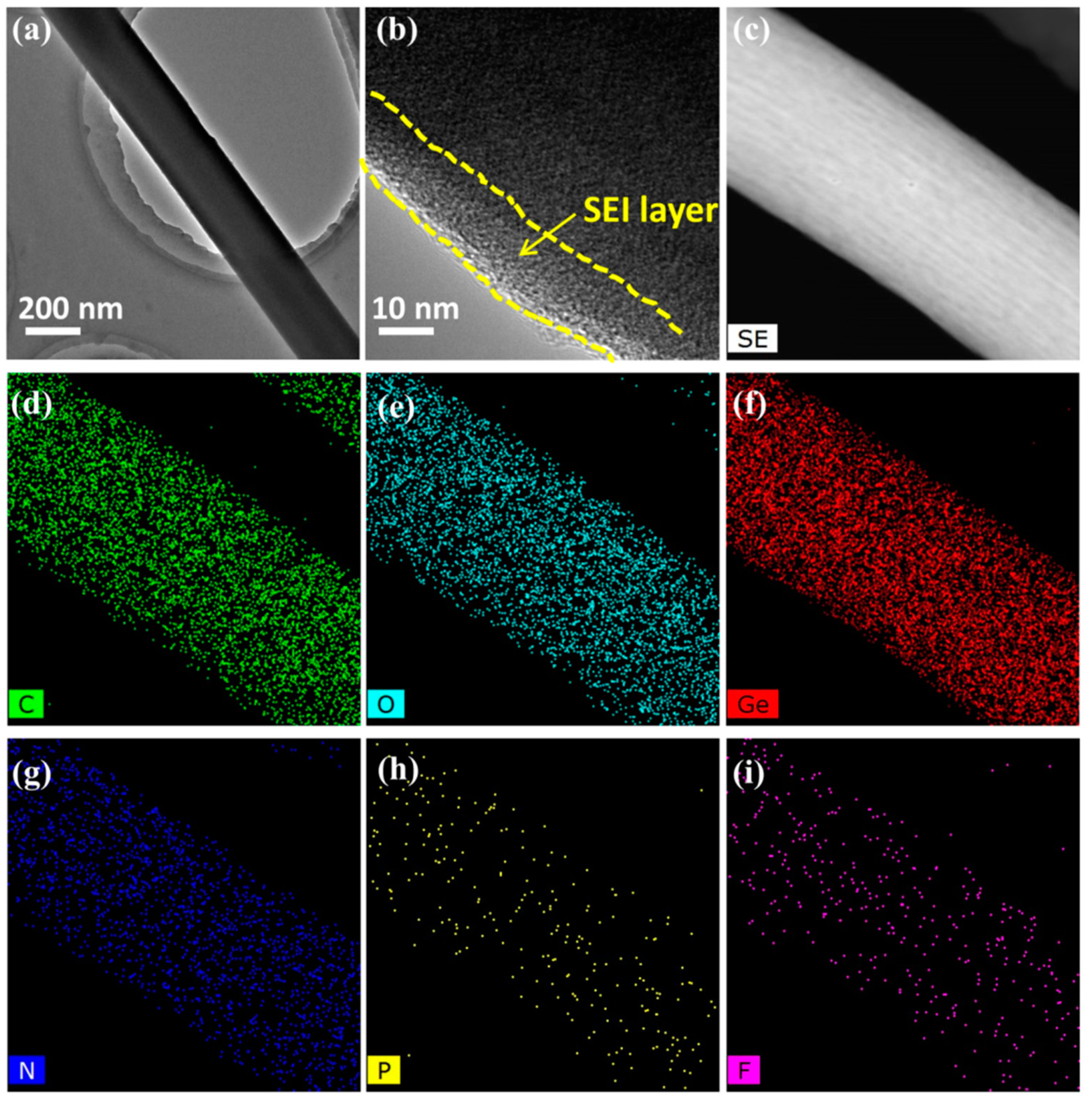
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Sample
3.2. Materials Characterization
3.3. Synthesis of Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peng, T.; Guo, W.; Zhang, Q.; Zhang, Y.; Chen, M.; Wang, Y.; Yan, H.; Lu, Y.; Luo, Y. Uniform coaxial CNT@Li2MnSiO4@C as advanced cathode material for lithium-ion battery. Electrochim. Acta 2018, 291, 1–8. [Google Scholar] [CrossRef]
- Bai, X.; Li, D.; Zhang, D.; Yang, S.; Pei, C.; Sun, B.; Ni, S. Boosting high-rate lithium storage in Li3VO4 via a honeycomb structure design and electrochemical reconstruction. J. Mater. Chem. A 2023, 11, 12164–12175. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Liu, C.; Fang, X.; Peng, H.; Li, Y.; Yang, Y. Fabrication of Composite Gel Electrolyte and F-Doping Carbon/Silica Anode from Electro-Spun P(VDF-HFP)/Silica Composite Nanofiber Film for Advanced Lithium-Ion Batteries. Molecules 2023, 28, 5304. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, J.; Wang, X.; Li, W.; Deng, L.; Ma, S.; Zhang, H.; Guan, C.; Huang, W. MOF-Derived Bifunctional Co0.85Se Nanoparticles Embedded in N-Doped Carbon Nanosheet Arrays as Efficient Sulfur Hosts for Lithium–Sulfur Batteries. Nano Lett. 2021, 21, 8579–8586. [Google Scholar] [CrossRef]
- Peng, T.; Liu, C.; Hou, X.; Zhang, Z.; Wang, C.; Yan, H.; Lu, Y.; Liu, X.; Luo, Y. Control Growth of Mesoporous Nickel Tungstate Nanofiber and Its Application as Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2017, 224, 460–467. [Google Scholar] [CrossRef]
- Wang, C.; Han, Q.; Xie, R.; Wang, B.; He, T.; Xie, W.; Tang, Q.; Li, Y.; Xu, J.; Yu, B. Fabrication of petal-like Ni3S2 nanosheets on 3D carbon nanotube foams as high-performance anode materials for Li-ion batteries. Electrochim. Acta 2020, 331, 135383. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, Y.; Zhang, D.; Wang, Y.; Guo, Y.; Yan, H.; Chu, P.K.; Luo, Y. Carbon-encapsulated nanosphere-assembled MoS2 nanosheets with large interlayer distance for flexible lithium-ion batteries. J. Solid State Electrochem. 2021, 25, 1657–1665. [Google Scholar] [CrossRef]
- Sun, X.; Xie, W.; Luo, F. Nanoarchitectonics of multilayered NiO submicron flakes for ultrafast and stable lithium storage. J. Alloys Compd. 2023, 936, 168259. [Google Scholar] [CrossRef]
- Cao, K.; Jia, Y.; Wang, S.; Huang, K.-J.; Liu, H. Mn3O4 nanoparticles anchored on carbon nanotubes as anode material with enhanced lithium storage. J. Alloys Compd. 2021, 854, 157179. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, D.; Bai, Z.; Yang, Y.; Wang, Y.; Cheng, J.; Chu, P.K.; Luo, Y. MXene nanofibers confining MnOx nanoparticles: A flexible anode for high-speed lithium ion storage networks. Dalton Trans. 2022, 51, 1423–1433. [Google Scholar] [CrossRef]
- Sun, X.; Jing, M.; Dong, H.; Xie, W.; Luo, F. CuO-ZnO submicroflakes with nanolayered Al2O3 coatings as high performance anode materials in lithium-ion batteries. J. Alloys Compd. 2023, 953, 170137. [Google Scholar] [CrossRef]
- Chen, M.; Liu, F.-M.; Zhao, H.; Chen, S.-S.; Qian, X.; Yuan, Z.-Y.; Wan, R. In situ encapsulation of iron oxide nanoparticles into nitrogen-doped carbon nanotubes as anodic electrode materials of lithium ion batteries. Phys. Chem. Chem. Phys. 2022, 24, 27114–27120. [Google Scholar] [CrossRef]
- Wang, K.; Ye, W.; Yin, W.; Chai, W.; Tang, B.; Rui, Y. One-step synthesis of MOF-derived Ga/Ga2O3@C dodecahedra as an anode material for high-performance lithium-ion batteries. Dalton Trans. 2019, 48, 12386–12390. [Google Scholar] [CrossRef]
- Qu, J.; Xiao, J.; Wang, T.; Legut, D.; Zhang, Q. High Rate Transfer Mechanism of Lithium Ions in Lithium–Tin and Lithium–Indium Alloys for Lithium Batteries. J. Phys. Chem. C 2020, 124, 24644–24652. [Google Scholar] [CrossRef]
- Lang, W.; Yue, C.; Dang, M.; Wang, G.; Chen, Y.; Hu, F.; Liu, Z.; Shu, J. Germanium decorated on three dimensional graphene networks as binder-free anode for Li-ion batteries. J. Power Sources 2023, 560, 232706. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Kong, D.; Yang, Y.; Wang, Y.; Guo, Y.; Lu, Y.; Kim, J.-K.; Luo, Y. In situ growth of Sn nanoparticles confined carbon-based TiO2/TiN composite with long-term cycling stability for sodium-ion batteries. Electrochim. Acta 2021, 367, 137450. [Google Scholar] [CrossRef]
- Shen, D.; Jia, M.; Li, M.; Fu, X.; Liu, Y.; Dong, W.; Yang, S. High Coulomb Efficiency Sn–Co Alloy/rGO Composite Anode Material for Li ion Battery with Long Cycle Life. Molecules 2023, 28, 3923. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Cao, K.; Wang, S.; Jiang, Y.; Liu, X.; Huang, K.-J.; Jing, Q.-S.; Jiao, L. Stimulating the Reversibility of Sb2S3 Anode for High-Performance Potassium-Ion Batteries. Small 2021, 17, 2008133. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.-Y.; Chang, B.; Wang, K.-X. Recent progress on germanium-based anodes for lithium ion batteries: Efficient lithiation strategies and mechanisms. Energy Storage Mater. 2020, 30, 146–169. [Google Scholar] [CrossRef]
- Zhao, Y.; Manthiram, A. High-Capacity, High-Rate Bi–Sb Alloy Anodes for Lithium-Ion and Sodium-Ion Batteries. Chem. Mater. 2015, 27, 3096–3101. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Shen, G.; Guo, Y.; Zhao, X.; Xia, Y.; Sun, H.; Hou, P.; Xie, W.; Xu, X. Large-scale carbon framework microbelts anchoring ultrafine SnO2 nanoparticles with enhanced lithium storage properties. Electrochim. Acta 2019, 297, 879–887. [Google Scholar] [CrossRef]
- Han, L.; Wei, Q.; Chen, H.; Tang, J.; Wei, M. Open-framework germanates derived GeO2/C nanocomposite as a long-life and high-capacity anode for lithium-ion batteries. J. Alloys Compd. 2021, 881, 160533. [Google Scholar] [CrossRef]
- Eisenmann, T.; Birrozzi, A.; Mullaliu, A.; Asenbauer, J.; Giuli, G.; Trapananti, A.; Olivi, L.; Moon, H.; Geiger, D.; Kaiser, U.; et al. Impact of Crystal Density on the Electrochemical Behavior of Lithium-Ion Anode Materials: Exemplary Investigation of (Fe-Doped) GeO2. J. Phys. Chem. C 2021, 125, 8947–8958. [Google Scholar] [CrossRef]
- McNulty, D.; Geaney, H.; Buckley, D.; O’Dwyer, C. High capacity binder-free nanocrystalline GeO2 inverse opal anodes for Li-ion batteries with long cycle life and stable cell voltage. Nano Energy 2018, 43, 11–21. [Google Scholar] [CrossRef]
- Cao, K.; Zheng, R.; Wang, S.; Shu, J.; Liu, X.; Liu, H.; Huang, K.-J.; Jing, Q.-S.; Jiao, L. Boosting Coulombic Efficiency of Conversion-Reaction Anodes for Potassium-Ion Batteries via Confinement Effect. Adv. Funct. Mater. 2020, 30, 2007712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Yang, Y.; Guo, Y.; Bai, Z.; Chu, P.K.; Luo, Y. Three-dimensional nano/micro-structured porous MoP/CNTs microspheres as high-capacity anode for lithium-ion batteries. J. Alloys Compd. 2021, 872, 159608. [Google Scholar] [CrossRef]
- Yan, S.; Song, H.; Lin, S.; Wu, H.; Shi, Y.; Yao, J. GeO2 Encapsulated Ge Nanostructure with Enhanced Lithium-Storage Properties. Adv. Funct. Mater. 2019, 29, 1807946. [Google Scholar] [CrossRef]
- Wu, J.; Luo, N.; Huang, S.; Yang, W.; Wei, M. Reversible conversion reaction of GeO2 boosts lithium-ion storage via Fe doping. J. Mater. Chem. A 2019, 7, 4574–4580. [Google Scholar] [CrossRef]
- Hohn, N.; Wang, X.; Giebel, M.A.; Yin, S.; Müller, D.; Hetzenecker, A.E.; Bießmann, L.; Kreuzer, L.P.; Möhl, G.E.; Yu, H.; et al. Mesoporous GeOx/Ge/C as a Highly Reversible Anode Material with High Specific Capacity for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 47002–47009. [Google Scholar] [CrossRef]
- Wu, J.; Tang, A.; Wang, K.; Huang, S.; Wei, M. Self-Optimizing Effect in Lithium Storage of GeO2 Induced by Heterointerface Regulation. Small 2022, 18, 2106067. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.G.; Mikhaylov, A.A.; Grishanov, D.A.; Yu, D.Y.W.; Gun, J.; Sladkevich, S.; Lev, O.; Prikhodchenko, P.V. GeO2 Thin Film Deposition on Graphene Oxide by the Hydrogen Peroxide Route: Evaluation for Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2017, 9, 9152–9160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Wang, Y.; Zhang, D.; Lu, Y.; Luo, R.; Wang, Y.; Liu, X.; Kim, J.-K.; Luo, Y. Vertically aligned ultrathin MoS2 nanosheets grown on graphene-wrapped hollow carbon microtubes derived from loofah sponge as advanced anodes for highly reversible lithium storage. Electrochim. Acta 2019, 296, 989–998. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, H.; Sun, W.; Lv, L.-P.; Wang, Y. Metal-organic frameworks derived germanium oxide nanosheets for large reversible Li-ion storage. Electrochem. Commun. 2017, 84, 80–85. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Wang, Q.; Cui, D.; Gao, G.; Wang, C.; Hu, J.; Yao, Y.; Li, Y. Three-dimensional honeycomb-like porous carbon embedded with Ge nanoparticles anode composites for ultrastable lithium storage. J. Alloys Compd. 2023, 939, 168759. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Shi, J.; Lin, J.; Zhou, W.; Pan, A. A new strategy to prepare Ge/GeO2-reduced graphene oxide microcubes for high-performance lithium-ion batteries. Electrochim. Acta 2019, 318, 314–321. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Kudryashova, Y.O.; Kuz’mina, A.A.; Kulova, T.L.; Skundin, A.M.; Emets, V.V.; Volkov, R.L.; Dronov, A.A.; Borgardt, N.I.; Gavrilov, S.A. High-rate and low-temperature performance of germanium nanowires anode for lithium-ion batteries. J. Electroanal. Chem. 2021, 888, 115209. [Google Scholar] [CrossRef]
- Koo, J.-H.; Paek, S.-M. Microwave-Assisted Synthesis of Ge/GeO2-Reduced Graphene Oxide Nanocomposite with Enhanced Discharge Capacity for Lithium-Ion Batteries. Nanomaterials 2021, 11, 319. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, K.Y.; Kang, Y.C. Amorphous GeOx-Coated Reduced Graphene Oxide Balls with Sandwich Structure for Long-Life Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 13952–13959. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Hou, Z.; Zhu, Y.; Wang, Y.; Qian, Y. Coordination complex pyrolyzation for the synthesis of nanostructured GeO2 with high lithium storage properties. Chem. Commun. 2014, 50, 13956–13959. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Yan, Y.; Zhang, Y.; Wang, Y.; Qin, C.; Bakenov, Z. Nanoporous GeO2/Cu/Cu2O network synthesized by dealloying method for stable Li-ion storage. Electrochim. Acta 2019, 300, 363–372. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Zhang, Y.; Qin, C.; Yu, H.; Bakenov, Z.; Wang, Z. Sn modified nanoporous Ge for improved lithium storage performance. J. Colloid Interface Sci. 2021, 602, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, W.; Li, H.; Cai, W.; Wang, J.; Zhang, H.; Jia, H.; Wang, X.; Cheng, S. A Hierarchical Copper Oxide–Germanium Hybrid Film for High Areal Capacity Lithium Ion Batteries. Front. Chem. 2020, 7, 869. [Google Scholar] [CrossRef]
- Wei, X.; Li, W.; Zeng, L.; Yu, Y. Influence of Carbon Matrix Dimensions on the Electrochemical Performance of Germanium Oxide in Lithium-Ion Batteries. Part. Part. Syst. Charact. 2016, 33, 524–530. [Google Scholar] [CrossRef]
- He, X.; Hu, Y.; Shen, Z.; Chen, R.; Wu, K.; Cheng, Z.; Zhang, X.W.; Pan, P. Channelized carbon nanofiber with uniform-dispersed GeO2 as anode for long-lifespan lithium-ion batteries. J. Alloys Compd. 2017, 729, 313–322. [Google Scholar] [CrossRef]
- Sun, X.; Luo, F. Sodium Storage Properties of Carbonaceous Flowers. Molecules 2023, 28, 4753. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, T.; Yu, J.-Y.; Ma, J.-X. Electrospinning of GeO2–C fibers and electrochemical application in lithium-ion batteries. Chin. Chem. Lett. 2016, 27, 412–416. [Google Scholar] [CrossRef]
- Han, L.; Tang, J.; Yang, R.; Wei, Q.; Wei, M. Stable Li-ion storage in Ge/N-doped carbon microsphere anodes. Nanoscale 2021, 13, 5307–5315. [Google Scholar] [CrossRef]
- Lin, Y.; Zhong, K.; Zheng, J.; Liang, M.; Xu, G.; Feng, Q.; Li, J.; Huang, Z. Fluorine-Doped GeO2@C Composite with Abundant Oxygen Vacancies for High-Capacity Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 9848–9857. [Google Scholar] [CrossRef]
- Youn, D.H.; Patterson, N.A.; Park, H.; Heller, A.; Mullins, C.B. Facile Synthesis of Ge/N-Doped Carbon Spheres with Varying Nitrogen Content for Lithium Ion Battery Anodes. ACS Appl. Mater. Interfaces 2016, 8, 27788–27794. [Google Scholar] [CrossRef]
- Xie, Y.; Ao, J.; Zhang, L.; Shao, Y.; Zhang, H.; Cheng, S.; Wang, X. Multi-functional bilayer carbon structures with micrometer-level physical encapsulation as a flexible cathode host for high-performance lithium-sulfur batteries. Chem. Eng. J. 2023, 451, 139017. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Liu, C.; Hu, C.; Ma, Y.; Li, X.; Wang, Q.; An, Z.; Liu, S.; Sun, H.; Sun, X. GeO2 Nanoparticles Decorated in Amorphous Carbon Nanofiber Framework as Highly Reversible Lithium Storage Anode. Molecules 2023, 28, 6730. https://doi.org/10.3390/molecules28186730
Xie W, Liu C, Hu C, Ma Y, Li X, Wang Q, An Z, Liu S, Sun H, Sun X. GeO2 Nanoparticles Decorated in Amorphous Carbon Nanofiber Framework as Highly Reversible Lithium Storage Anode. Molecules. 2023; 28(18):6730. https://doi.org/10.3390/molecules28186730
Chicago/Turabian StyleXie, Wenhe, Congcong Liu, Chen Hu, Yuanxiao Ma, Xuefeng Li, Qian Wang, Zhe An, Shenghong Liu, Haibin Sun, and Xiaolei Sun. 2023. "GeO2 Nanoparticles Decorated in Amorphous Carbon Nanofiber Framework as Highly Reversible Lithium Storage Anode" Molecules 28, no. 18: 6730. https://doi.org/10.3390/molecules28186730
APA StyleXie, W., Liu, C., Hu, C., Ma, Y., Li, X., Wang, Q., An, Z., Liu, S., Sun, H., & Sun, X. (2023). GeO2 Nanoparticles Decorated in Amorphous Carbon Nanofiber Framework as Highly Reversible Lithium Storage Anode. Molecules, 28(18), 6730. https://doi.org/10.3390/molecules28186730