HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Rhizoma gastrodiae (Tian Ma) in Yunnan Province
Abstract
:1. Introduction
2. Results
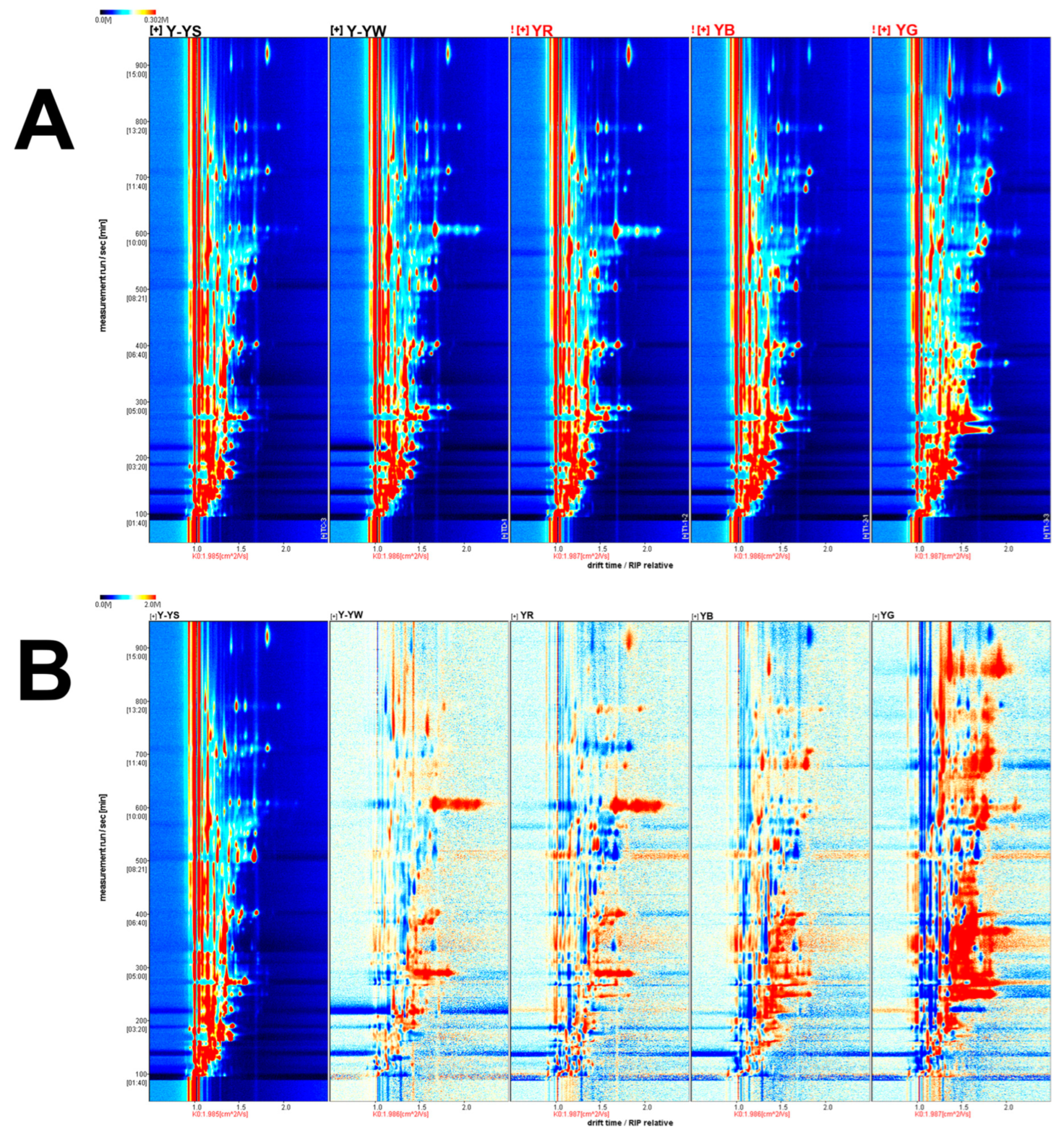
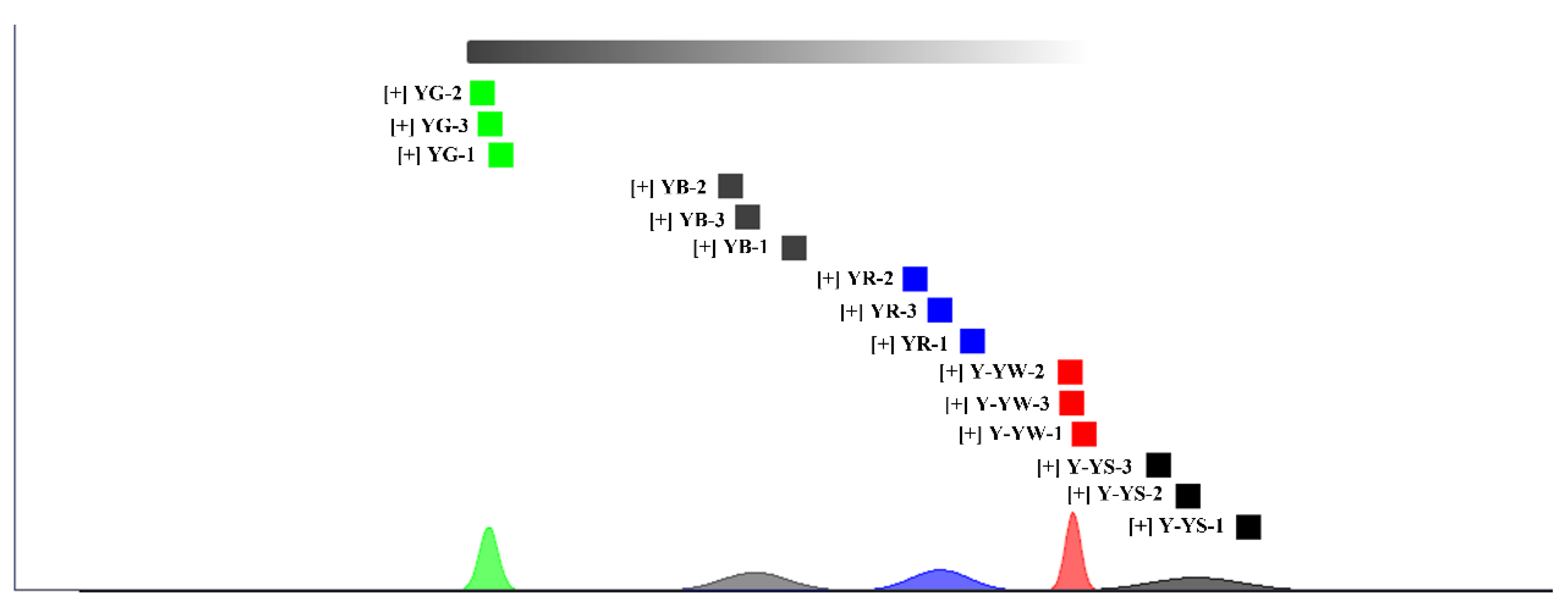
2.1. Analysis of Volatile Components in Different Rhizoma gastrodiae Samples by HS-GC-IMS
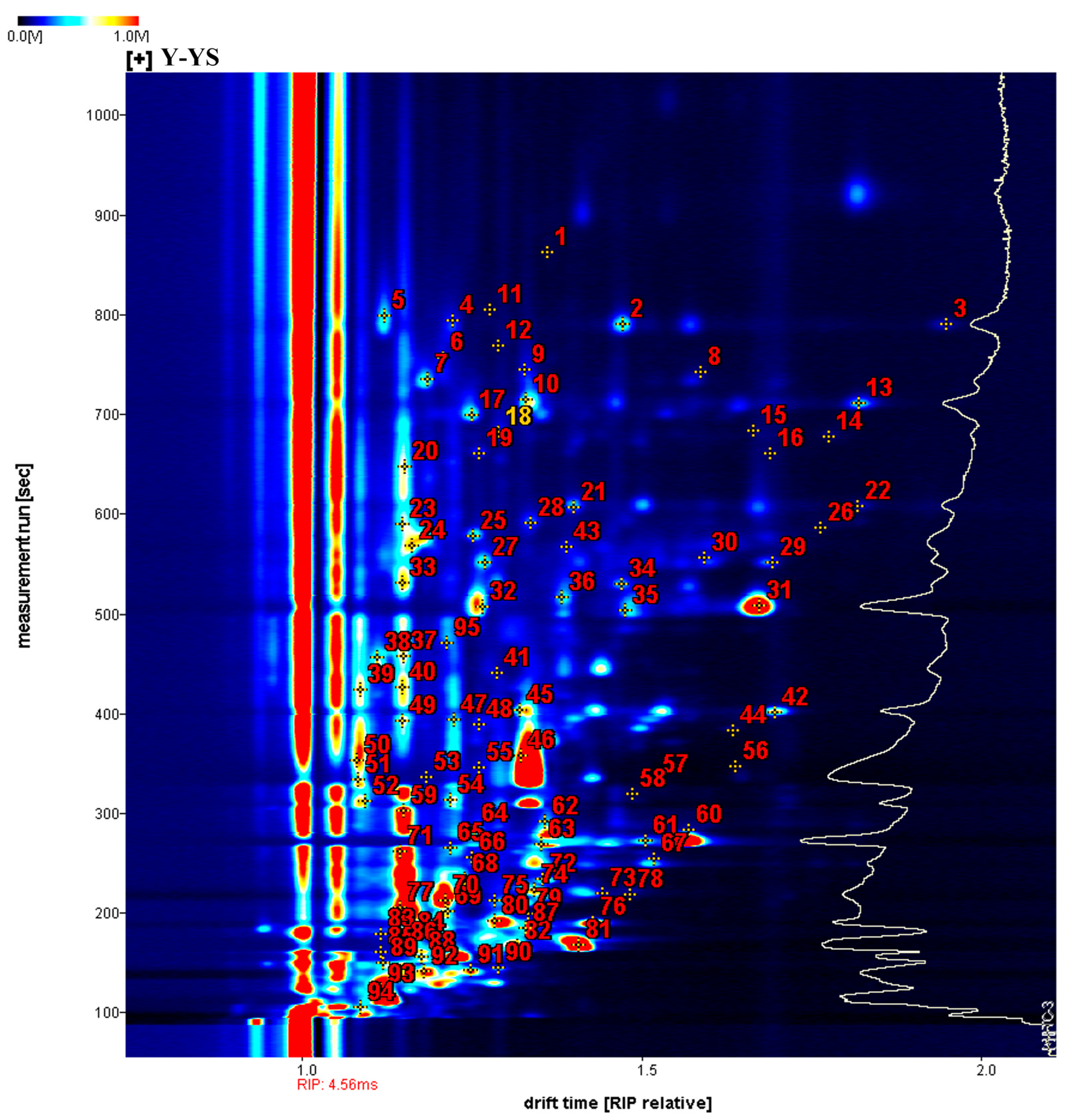
2.2. Identification of Volatile Components in Different Rhizoma gastrodiae Samples
2.3. Topographic Results and Analysis of Five Rhizoma gastrodiae Samples
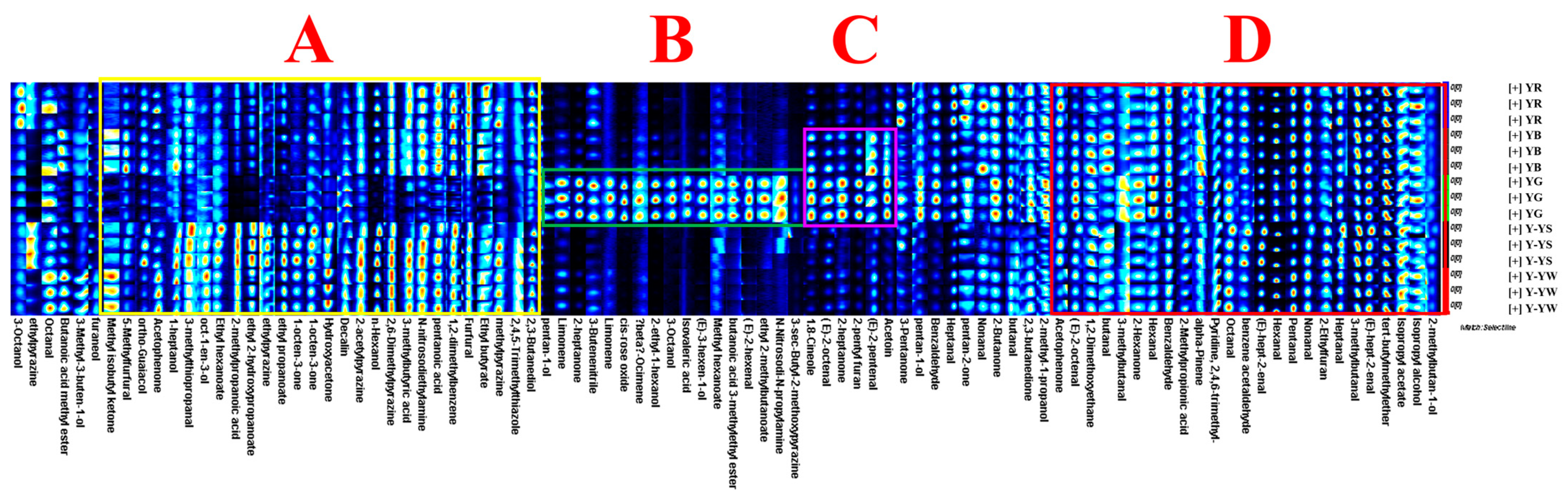
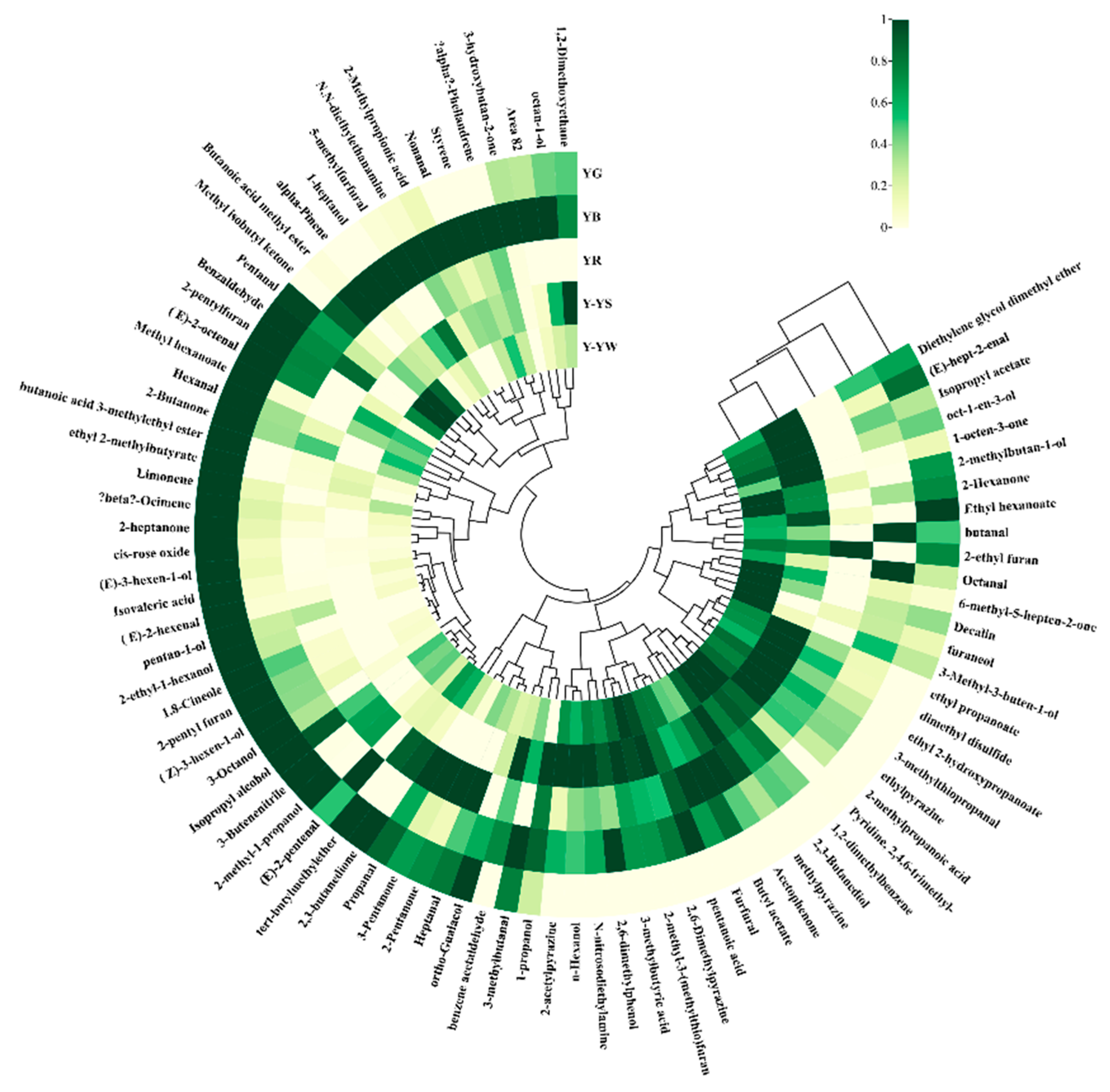
2.4. Cluster Analysis of the Volatile Components of Five Rhizoma gastrodiae Samples
2.4.1. Dynamics of the Sample
2.4.2. Fingerprint Similarity Analysis Based on Euclidean Distance
2.4.3. Hierarchical Cluster Analysis Heatmap
3. Materials and Methods
3.1. Sample Preparation
3.2. HS-GC-IMS System
3.3. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, X.; Chen, X.Q. The collation of the genus Amanita in China. Yunnan Plant Res. 1983, 4, 361–368. [Google Scholar]
- Duan, H.; Zhou, Y.; Zhou, S.; Yan, W. Application of asparagus in health food in China. Food Ind. Sci. Technol. 2023, 44, 403–412. [Google Scholar]
- Fan, Q.; Chen, C.; Huang, Z.; Zhang, C.; Liang, P.; Zhao, S. Discrimination of Rhizoma gastrodiae (Tianma) using 3D synchronous fluorescence spectroscopy coupled with principal component analysis. Spectrochim. Acta Part A Mol. Bio-Mol. Spectrosc. 2015, 136, 1621–1625. [Google Scholar] [CrossRef]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume—An ethnopharma-cological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Liu, Q.; Zhao, Z.; Su, D.; Xiao, C.; Jin, T.; Chen, L.; Xu, C.; You, Z.; et al. Gastrodin programs an Arg-1+ microglial phenotype in hippocampus to ameliorate depression- and anxiety-like behaviors via the Nrf2 pathway in mice. Phytomedicine 2023, 113, 154725. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, H.; Ma, K.; Deng, Y.; Li, M.; Ma, J. A novel alcohol steamed preparation from Gastrodia elata Blume: Pharmaco-logical assessment of a functional food. Front. Pharmacol. 2023, 14, 1092693. [Google Scholar] [CrossRef]
- Sun, H.; Hao, D.; Li, X.; Jin, W. Analysis of the variability of volatile substances in fresh asparagus of different varieties and origins. Food Mach. 2022, 38, 58–64. [Google Scholar]
- Gonçalves, W.B.; Teixeira, W.S.; Cervantes, E.P.; Mioni, M.D.; Sampaio, A.N.; Martins, O.A.; Gruber, J.; Pereira, J.G. Application of an Electronic Nose as a New Technology for Rapid Detection of Adulteration in Honey. Appl. Sci. 2023, 13, 4881. [Google Scholar] [CrossRef]
- Capitain, C.; Weller, P. Non-Targeted Screening Approaches for Profiling of Volatile Organic Compounds Based on Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) and Machine Learning. Molecules 2021, 26, 5457. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Integration of computer vision and electronic nose as non-destructive systems for saffron adulteration detection. Comput. Electron. Agric. 2017, 141, 46–53. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Slimani, S.; Bultel, E.; Cubizolle, T.; Herrier, C.; Rousselle, T.; Livache, T. Opto-Electronic Nose Coupled to a Silicon Micro Pre-Concentrator Device for Selective Sensing of Flavored Waters. Chemosensors 2020, 8, 60. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H.; Wilson, A.D.; Gancarz, M. Performance Analysis of MAU-9 Electronic-Nose MOS Sensor Array Com-ponents and ANN Classification Methods for Discrimination of Herb and Fruit Essential Oils. Chemosensors 2021, 9, 243. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Lin, R.; Li, X.; Ding, H.; Han, L.; Yang, W.; Song, X.; Li, W.; Qu, H.; et al. Application and development trends of gas chromatography–ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J. 2021, 168, 106527. [Google Scholar] [CrossRef]
- Chen, D.; Qin, L.; Geng, Y.; Kong, Q.; Wang, S.; Lin, S. The Aroma Fingerprints and Discrimination Analysis of Shiitake Mushrooms from Three Different Drying Conditions by GC-IMS, GC-MS and DSA. Foods 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Wu, Y.; Qu, F.; Liu, L.; Wang, B.; Wang, P.; Zhang, X. HS−SPME/GC−MS Reveals the Season Effects on Volatile Compounds of Green Tea in High−Latitude Region. Foods 2022, 11, 3016. [Google Scholar] [CrossRef]
- Ning, M.; Rui, G.; Rentang, Z.; Yue, G. GC-IMS-Based Preliminary Analysis of Volatile Flavor Compounds in Ejiao at Different Processing Stages. J. Food Qual. 2022, 2022, 3961593. [Google Scholar]
- Di, W.; Jian, Z.; Zongshuai, Z.; Yang, L.; Suhong, H.; Ming, H. Effect of ageing time on the flavour compounds in Nanjing wa-ter-boiled salted duck detected by HS-GC-IMS. LWT—Food Sci. Technol. 2021, 155, 112870. [Google Scholar]
- Zhang, J.; Zhang, W.; Zhou, L.; Zhang, R. Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS-GC-IMS. Ultrason. Sonochem. 2021, 80, 105807. [Google Scholar] [CrossRef]
- Qiu, H.; Zhou, X.; Wu, L.; Yao, M.; Shen, X.; Xiao, T.; Xu, Q.; Tao, L. Headspace gas chromatography analysis of volatile components of asparagus. Zizhen Guoji Guomao 2019, 30, 2368–2369. [Google Scholar]
- Cao, S.; Zhao, C.F.; Ma, F.W.; Ma, C.; Li, Y.; Wang, R. Evaluation of aromatic quality of asparagus at different harvesting stages based on e-nose and GC-MS. North. Hort. 2019, 19, 87–94. [Google Scholar]
- Lu, Y. Analysis of Volatile Components of Asparagus and Walnut and Development of Their Products. Master’s Thesis, Guizhou University, Guiyang, China, 2016. [Google Scholar]
- Huang, M.Z.; Li, X. Analysis of the species and content of volatile components of asparagus by SDE-GC-MS. J. Guizhou Agric. Sci. 2018, 46, 110–113. [Google Scholar]
- Chen, L.; Li, X.; Li, Z.; Deng, L. Analysis of 17 elements in cow, goat, buffalo, yak, and camel milk by inductively coupled plasma mass spectrometry (ICP-MS). RSC Adv. 2020, 10, 6736–6742. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, R.; Shi, L.; Zhang, F.; Chang, J.; Chu, X. Accurate determination of volatile-flavor components in bos grunniens milk by high-throughput dynamic headspace gas chromatographic-mass spectrometry. J. Chromatogr. A 2019, 1603, 67–82. [Google Scholar] [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. gc-ims-tools—A new Python package for chemometric analysis of GC–IMS data. Food Chem. 2022, 394, 133476. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Feng, D.; Zhou, Y.X.; Zhao, J.; Zhao, J.Y.; Guo, Y.; Yan, W.J. HS-GC-IMS detection of volatile organic compounds in cistanche powders under different treatment methods. LWT—Food Sci. Technol. 2022, 165, 113730. [Google Scholar] [CrossRef]
- Duan, Z.; Dong, S.; Dong, Y.; Gao, Q. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Res. Int. 2021, 145, 110385. [Google Scholar] [CrossRef]
- Lu, W.; Chen, J.; Li, X.; Qi, Y.; Jiang, R. Flavor components detection and discrimination of isomers in Huaguo tea using head-space-gas chromatography-ion mobility spectrometry and multivariate statistical analysis. Anal. Chim. Acta 2023, 1243, 340842. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Liu, T.; Gong, P.; Wang, Y.; Wang, H.; Tian, X.; Liu, Q.; Cui, Q.; Xie, X.; et al. Aroma classification and flavor characterization of Streptococcus thermophilus fermented milk by HS-GC-IMS and HS-SPME-GC-TOF/MS. Food Biosci. 2022, 49, 101832. [Google Scholar] [CrossRef]



| No. | Compound | CAS# | Formula | MW | RI | Rt [sec] | Dt [a.u.] | Category | Odor | Identification Approach |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | cis-rose oxide | C3033236 | C10H18O | 154.3 | 1116.1 | 862.35 | 1.36395 | Ether | sweet, rose | RI, DT |
| 2 | Nonanal a | C124196 | C9H18O | 142.2 | 1085.5 | 790.725 | 1.47499 | Aldehydes | fat, citrus, green | RI, DT |
| 3 | Nonanal b | C124196 | C9H18O | 142.2 | 1085.3 | 790.299 | 1.95001 | Aldehydes | RI, DT | |
| 4 | ortho-Guaiacol b | C90051 | C7H8O2 | 124.1 | 1087 | 794.136 | 1.22412 | Phenols | burnt, phenol, wood | RI, DT |
| 5 | ortho-Guaiacol a | C90051 | C7H8O2 | 124.1 | 1088.8 | 798.400 | 1.12336 | Phenols | RI, DT | |
| 6 | furaneol | C3658773 | C6H8O3 | 128.1 | 1070.8 | 756.192 | 1.20972 | Heterocyclic | caramel | RI, DT |
| 7 | Acetophenone a | C98862 | C8H8O | 120.2 | 1061.5 | 734.449 | 1.18711 | Ketones | must, flower, almond | RI, DT |
| 8 | Acetophenone b | C98862 | C8H8O | 120.2 | 1064.7 | 742.123 | 1.58809 | Ketones | RI, DT | |
| 9 | Decalin | C91178 | C10H18 | 138.3 | 1065.8 | 744.681 | 1.32899 | Hydrocarbons | green, nut, fat | RI, DT |
| 10 | (E)-2-octenal a | C2548870 | C8H14O | 126.2 | 1053.1 | 714.838 | 1.33105 | Aldehydes | / | RI, DT |
| 11 | 3-sec-Butyl-2- methoxypyrazine | C24168705 | C9H14N2O | 166.2 | 1091.5 | 804.689 | 1.27823 | Heterocyclic | / | RI, DT |
| 12 | N-Nitrosodi- N-propylamine | C621647 | C6H14N2O | 130.2 | 1076.2 | 768.858 | 1.29053 | Amines | / | RI, DT |
| 13 | (E)-2-octenal b | C2548870 | C8H14O | 126.2 | 1051.5 | 711.172 | 1.82123 | Aldehydes | green, nut, fat | RI, DT |
| 14 | 2-ethyl-1-hexanol | C104767 | C8H18O | 130.2 | 1037.3 | 677.882 | 1.7773 | Alcohols | green, rose | RI, DT |
| 15 | beta-Ocimene | C13877913 | C10H16 | 136.2 | 1039.7 | 683.473 | 1.66659 | Alkenes | floral | RI, DT |
| 16 | Limonene b | C138863 | C10H16 | 136.2 | 1030.1 | 660.856 | 1.69119 | Alkenes | lemon, orange | RI, DT |
| 17 | benzene acetaldehyde | C122781 | C8H8O | 120.2 | 1046.1 | 698.445 | 1.25225 | Aldehydes | berry, geranium, honey, nut, pungent | RI, DT |
| 18 | 1.8-Cineole | C470826 | C10H18O | 154.3 | 1039.4 | 682.639 | 1.29027 | Alcohols | mint, sweet | RI, DT |
| 19 | Limonene a | C138863 | C10H16 | 136.2 | 1029.8 | 660.325 | 1.26249 | Alkenes | lemon, orange | RI, DT |
| 20 | 2-acetylpyrazine | C22047252 | C6H6N2O | 122.1 | 1024.3 | 647.309 | 1.15281 | Heterocyclic | roast | RI, DT |
| 21 | Octanal a | C124130 | C8H16O | 128.2 | 1006.8 | 606.4 | 1.40141 | Aldehydes | citrus, fat, green, oil, pungent | RI, DT |
| 22 | Octanal b | C124130 | C8H16O | 128.2 | 1007.5 | 607.95 | 1.81965 | Aldehydes | RI, DT | |
| 23 | 2,4,5-Trimethylthiazole | C13623115 | C6H9NS | 127.2 | 999.5 | 589.355 | 1.14912 | Heterocyclic | cocoa, earthy, nut | RI, DT |
| 24 | Pyridine, 2,4,6- trimethyl- | C108758 | C8H11N | 121.2 | 988.9 | 567.97 | 1.16408 | Heterocyclic | / | RI, DT |
| 25 | 2-pentyl furan | C3777693 | C9H14O | 138.2 | 994.2 | 577.962 | 1.25391 | Heterocyclic | butter, floral, fruit, green bean | RI, DT |
| 26 | 3-Octanolb | C589980 | C8H18O | 130.2 | 998.1 | 586.088 | 1.76519 | Alcohols | moss, nut, mushroom | RI, DT |
| 27 | 1-octen-3-one a | C4312996 | C8H14O | 126.2 | 980.3 | 551.638 | 1.27132 | Ketones | earth, mushroom | RI, DT |
| 28 | Ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 1000.2 | 590.895 | 1.3385 | Esters | apple peel, fruit | RI, DT |
| 29 | 1-octen-3-one b | C4312996 | C8H14O | 126.2 | 980.2 | 551.409 | 1.69428 | Ketones | earth, mushroom | RI, DT |
| 30 | oct-1-en-3-ol | C3391864 | C8H16O | 128.2 | 982.5 | 555.758 | 1.59352 | Alcohols | cucumber, earth, fat, floral, mushroom | RI, DT |
| 31 | (E)-hept-2-enal b | C18829555 | C7H12O | 112.2 | 956.9 | 507.516 | 1.67599 | Aldehydes | almond, fat, fruit | RI, DT |
| 32 | (E)-hept-2-enal a | C18829555 | C7H12O | 112.2 | 956.2 | 506.066 | 1.26737 | Aldehydes | RI, DT | |
| 33 | Benzaldehyde a | C100527 | C7H6O | 106.1 | 969.3 | 530.943 | 1.14967 | Aldehydes | almond, burnt sugar | RI, DT |
| 34 | Benzaldehyde b | C100527 | C7H6O | 106.1 | 968.6 | 529.525 | 1.47168 | Aldehydes | RI, DT | |
| 35 | 5-Methylfurfural | C620020 | C6H6O2 | 110.1 | 954.9 | 503.755 | 1.47723 | Heterocyclic | almond, caramel, burnt sugar | RI, DT |
| 36 | 1-heptanol | C111706 | C7H16O | 116.2 | 961.9 | 516.995 | 1.38507 | Alcohols | chemical, green | RI, DT |
| 37 | Ethylpyrazine b | C13925003 | C6H8N2 | 108.1 | 930.5 | 457.601 | 1.15078 | Heterocyclic | burnt, green, iron scorch, must, peanut butter, roasted, rum, wood | RI, DT |
| 38 | Ethylpyrazine a | C13925003 | C6H8N2 | 108.1 | 929.5 | 455.774 | 1.11192 | Heterocyclic | RI, DT | |
| 39 | 3-methylthiopropanal | C3268493 | C4H8OS | 104.2 | 912.8 | 424.181 | 1.0886 | Aldehydes | cooked potato, soy | RI, DT |
| 40 | 2,6-Dimethylpyrazine | C108509 | C6H8N2 | 108.1 | 913.9 | 426.27 | 1.14967 | Heterocyclic | cocoa, coffee, green, roast beef, roasted nut | RI, DT |
| 41 | Methyl hexanoate | C106707 | C7H14O2 | 130.2 | 921.2 | 440.108 | 1.28846 | Esters | fruit, fresh, sweet | RI, DT |
| 42 | Heptanal b | C111717 | C7H14O | 114.2 | 900.3 | 400.683 | 1.69819 | Aldehydes | fat, citrus, rancid | RI, DT |
| 43 | 3-Octanol a | C589980 | C8H18O | 130.2 | 988.3 | 566.739 | 1.39173 | Alcohols | moss, nut, mushroom | RI, DT |
| 44 | 2-heptanone b | C110430 | C7H14O | 114.2 | 890.5 | 383.379 | 1.63571 | Ketones | soap | RI, DT |
| 45 | Heptanal a | C111717 | C7H14O | 114.2 | 901.7 | 403.294 | 1.32286 | Aldehydes | fat, citrus, rancid | RI, DT |
| 46 | n-Hexanol | C111273 | C6H14O | 102.2 | 867.5 | 357.353 | 1.32385 | Alcohols | banana, flower, grass, herb | RI, DT |
| 47 | pentanoic acid | C109524 | C5H10O2 | 102.1 | 896.5 | 393.484 | 1.22546 | Acids | sweat | RI, DT |
| 48 | 2-heptanone a | C110430 | C7H14O | 114.2 | 894 | 388.698 | 1.26285 | Ketones | soap | RI, DT |
| 49 | N-nitrosodiethylamine | C55185 | C4H10N2O | 102.1 | 896.2 | 392.766 | 1.1497 | Amines | / | RI, DT |
| 50 | 1,2-dimethylbenzene | C95476 | C8H10 | 106.2 | 863.3 | 352.567 | 1.0828 | Hydrocarbons | / | RI, DT |
| 51 | Furfural | C98011 | C5H4O2 | 96.1 | 846.8 | 333.904 | 1.08378 | Aldehydes | bread, almond, sweet | RI, DT |
| 52 | methylpyrazine | C109080 | C5H6N2 | 94.1 | 827.3 | 311.89 | 1.09461 | Heterocyclic | cocoa, green, hazelnut, popcorn, roasted | RI, DT |
| 53 | (E)-2-hexenal | C6728263 | C6H10O | 98.1 | 848.3 | 335.578 | 1.18414 | Aldehydes | apple, green | RI, DT |
| 54 | 3-methylbutyric acid | C503742 | C5H10O2 | 102.1 | 829 | 313.804 | 1.21956 | Acids | cheese, pungent | RI, DT |
| 55 | butanoic acid 3- methylethyl ester | C108645 | C7H14O2 | 130.2 | 857.6 | 346.107 | 1.26186 | Esters | apple, fruit, pineapple, sour | RI, DT |
| 56 | ethyl 2-methylbutanoate | C7452791 | C7H14O2 | 130.2 | 858.2 | 346.824 | 1.63966 | Esters | apple, ester, green apple, kiwi, strawberry | RI, DT |
| 57 | (E)-3-hexen-1-ol | C928972 | C6H12O | 100.2 | 847 | 334.143 | 1.5216 | Alcohols | green | RI, DT |
| 58 | Isovaleric acid | C503742 | C5H10O2 | 102.1 | 834.3 | 319.786 | 1.48913 | Acids | sweat, acid, rancid | RI, DT |
| 59 | ethyl 2-hydroxypropanoate | C97643 | C5H10O3 | 118.1 | 819.3 | 302.785 | 1.15121 | Esters | cheese, floral, fruit, pungent, rubber | RI, DT |
| 60 | Hexanal b | C66251 | C6H12O | 100.2 | 802.2 | 283.496 | 1.57059 | Aldehydes | grass, tallow, fat | RI, DT |
| 61 | 2-Hexanone | C591786 | C6H12O | 100.2 | 792.1 | 272.015 | 1.50704 | Ketones | / | RI, DT |
| 62 | 2,3-Butanediol | C513859 | C4H10O2 | 90.1 | 809.1 | 291.304 | 1.35878 | Alcohols | fruit, onion | RI, DT |
| 63 | 2-Methylpropionic acid | C79312 | C4H8O2 | 88.1 | 789.4 | 268.916 | 1.35454 | Acids | burnt, butter, cheese, sweat | RI, DT |
| 64 | Hexanal a | C66251 | C6H12O | 100.2 | 803.5 | 284.874 | 1.2557 | Aldehydes | grass, tallow, fat | RI, DT |
| 65 | Ethyl butyrate | C105544 | C6H12O2 | 116.2 | 786.6 | 265.76 | 1.21954 | Esters | apple | RI, DT |
| 66 | pentan-1-ol a | C71410 | C5H12O | 88.1 | 775 | 255.54 | 1.25267 | Alcohols | balsamic, fruit, green, pungent, yeast | RI, DT |
| 67 | pentan-1-ol b | C71410 | C5H12O | 88.1 | 772.9 | 253.856 | 1.52068 | Alcohols | RI, DT | |
| 68 | 2-methylbutan-1-ol | C137326 | C5H12O | 88.1 | 748.8 | 234.202 | 1.24213 | Alcohols | fish oil, green, malt, onion, wine | RI, DT |
| 69 | N,N-diethylethanamine | C121448 | C6H15N | 101.2 | 709.1 | 201.858 | 1.21653 | Amines | / | RI, DT |
| 70 | Hydroxyacetone | C116096 | C3H6O2 | 74.1 | 721.8 | 212.19 | 1.21352 | Ketones | butter, herb, malt, pungent | RI, DT |
| 71 | 2-methylpropanoic acid | C79312 | C4H8O2 | 88.1 | 782.8 | 261.942 | 1.14576 | Acids | burnt, butter, cheese, sweat | RI, DT |
| 72 | (E)-2-pentenal | C1576870 | C5H8O | 84.1 | 748.8 | 234.202 | 1.35656 | Aldehydes | / | RI, DT |
| 73 | Butanoic acid methyl ester | C623427 | C5H10O2 | 102.1 | 730.3 | 219.153 | 1.44389 | Esters | apple, banana, cheese, ester, floral | RI, DT |
| 74 | Acetoin | C513860 | C4H8O2 | 88.1 | 735 | 222.971 | 1.34451 | Ketones | butter, cream | RI, DT |
| 75 | 3-Methyl-3-buten-1-ol | C763326 | C5H10O | 86.1 | 721.5 | 211.965 | 1.28579 | Alcohols | / | RI, DT |
| 76 | Pentanal | C110623 | C5H10O | 86.1 | 695 | 190.403 | 1.43034 | Aldehydes | almond, malt, pungent | RI, DT |
| 77 | ethyl propanoate | C105373 | C5H10O2 | 102.1 | 712.7 | 204.778 | 1.14576 | Esters | apple, pineapple, rum, strawberry | RI, DT |
| 78 | Methyl isobutyl ketone | C108101 | C6H12O | 100.2 | 729.8 | 218.704 | 1.48454 | Ketones | / | RI, DT |
| 79 | 2,5-Dimethylfuran | C625865 | C6H8O | 96.1 | 705.4 | 198.826 | 1.33548 | Heterocyclic | savory | RI, DT |
| 80 | 2-Ethylfuran | C3208160 | C6H8O | 96.1 | 697.5 | 192.409 | 1.28502 | Heterocyclic | butter, caramel | RI, DT |
| 81 | 3-methylbutanal b | C590863 | C5H10O | 86.1 | 650.1 | 168.205 | 1.40884 | Aldehydes | malt | RI, DT |
| 82 | 1,2-Dimethoxyethane | C110714 | C4H10O2 | 90.1 | 646.3 | 166.547 | 1.31833 | Hydrocarbons | / | RI, DT |
| 83 | pentan-2-one | C107879 | C5H10O | 86.1 | 675 | 179.146 | 1.11851 | Ketones | fruit, pungent | RI, DT |
| 84 | Isopropyl acetate | C108214 | C5H10O2 | 102.1 | 667 | 175.665 | 1.1612 | Esters | banana | RI, DT |
| 85 | 3-Butenenitrile | C109751 | C4H5N | 67.1 | 633.5 | 160.911 | 1.11851 | Nitrile | / | RI, DT |
| 86 | 3-methylbutanal a | C590863 | C5H10O | 86.1 | 643.3 | 165.221 | 1.15181 | Aldehydes | malt | RI, DT |
| 87 | 3-Pentanone | C96220 | C5H10O | 86.1 | 688.8 | 185.28 | 1.33114 | Ketones | / | RI, DT |
| 88 | 2-methyl-1- propanol | C78831 | C4H10O | 74.1 | 622.9 | 156.228 | 1.17831 | Alcohols | apple, bitter, cocoa, wine | RI, DT |
| 89 | Butanal a | C123728 | C4H8O | 72.1 | 608.2 | 149.753 | 1.12118 | Aldehydes | pungent, green | RI, DT |
| 90 | Butanal b | C123728 | C4H8O | 72.1 | 598.2 | 145.366 | 1.29119 | Aldehydes | RI, DT | |
| 91 | 2-Butanone | C78933 | C4H8O | 72.1 | 590.9 | 142.129 | 1.24938 | Ketones | fragrant, fruit, pleasant | RI, DT |
| 92 | 2,3-butanedione | C431038 | C4H6O2 | 86.1 | 589.7 | 141.606 | 1.1818 | Ketones | butter, pastry, yeast | RI, DT |
| 93 | tert-butyl methyl ether | C1634044 | C5H12O | 88.1 | 548.4 | 123.434 | 1.11769 | Ether | / | RI, DT |
| 94 | Isopropyl alcohol | C67630 | C3H8O | 60.1 | 507.1 | 105.262 | 1.08754 | Alcohols | floral | RI, DT |
| 95 | alpha-Pinene | C80568 | C10H16 | 136.2 | 937.2 | 470.198 | 1.21549 | Alkenes | cedarwood, pine, sharp | RI, DT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.; Zhou, S.; Guo, J.; Yan, W. HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Rhizoma gastrodiae (Tian Ma) in Yunnan Province. Molecules 2023, 28, 6705. https://doi.org/10.3390/molecules28186705
Duan H, Zhou S, Guo J, Yan W. HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Rhizoma gastrodiae (Tian Ma) in Yunnan Province. Molecules. 2023; 28(18):6705. https://doi.org/10.3390/molecules28186705
Chicago/Turabian StyleDuan, Hao, Shiqi Zhou, Jinhong Guo, and Wenjie Yan. 2023. "HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Rhizoma gastrodiae (Tian Ma) in Yunnan Province" Molecules 28, no. 18: 6705. https://doi.org/10.3390/molecules28186705
APA StyleDuan, H., Zhou, S., Guo, J., & Yan, W. (2023). HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Rhizoma gastrodiae (Tian Ma) in Yunnan Province. Molecules, 28(18), 6705. https://doi.org/10.3390/molecules28186705





