Abstract
The consumption of food-derived products, including the regular intake of pepper, is increasingly evaluated for its potential benefits in protecting against diverse metabolic complications. The current study made use of prominent electronic databases including PubMed, Google Scholar, and Scopus to retrieve clinical evidence linking the intake of black and red pepper with the amelioration of metabolic complications. The findings summarize evidence supporting the beneficial effects of black pepper (Piper nigrum L.), including its active ingredient, piperine, in improving blood lipid profiles, including reducing circulating levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides in overweight and obese individuals. The intake of piperine was also linked with enhanced antioxidant and anti-inflammatory properties by increasing serum levels of superoxide dismutase while reducing those of malonaldehyde and C-reactive protein in individuals with metabolic syndrome. Evidence summarized in the current review also indicates that red pepper (Capsicum annum), together with its active ingredient, capsaicin, could promote energy expenditure, including limiting energy intake, which is likely to contribute to reduced fat mass in overweight and obese individuals. Emerging clinical evidence also indicates that pepper may be beneficial in alleviating complications linked with other chronic conditions, including osteoarthritis, oropharyngeal dysphagia, digestion, hemodialysis, and neuromuscular fatigue. Notably, the beneficial effects of pepper or its active ingredients appear to be more pronounced when used in combination with other bioactive compounds. The current review also covers essential information on the metabolism and bioavailability profiles of both pepper species and their main active ingredients, which are all necessary to understand their potential beneficial effects against metabolic diseases.
1. Introduction
Metabolic syndrome describes a cluster of metabolic complications, including insulin resistance, hypertension, and hyperlipidemia, that increase the risk for the development of cardiovascular diseases [1,2]. Cardiovascular diseases remain the leading cause of death worldwide [3], especially in people with metabolic disorders [4]. Recent numbers indicate that a growing number of individuals present with a cluster of metabolic disorders such as hyperglycemia and dyslipidemia that are also linked with the development and progression of type 2 diabetes [5]. This emphasizes an urgent need for multisectoral interventions to decrease the global burden of metabolic syndrome and associated complications, especially those involving overweight and obesity (Figure 1). Indeed, the consumption of a high-calorie diet, in combination with reduced physical activity or a sedentary lifestyle, is known to be the major cause of obesity that accelerates the development of metabolic syndrome [6]. An obese state is accompanied by excessive adiposity and enhanced ectopic accumulation, which is associated with increased levels of oxidative stress and inflammation [7]. Both oxidative stress and inflammation are considered prominent pathological mechanisms that alter biochemical processes and cause cellular damage within many metabolic diseases [8,9].
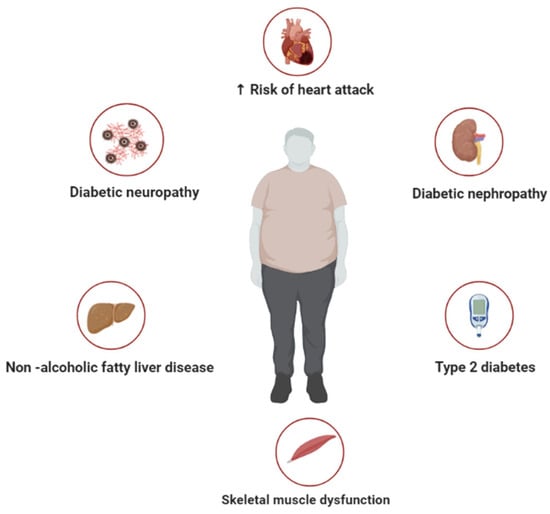
Figure 1.
A general overview of metabolic syndrome, representing some of the diverse pathological conditions associated with this abnormal metabolic state, including diabetic neuropathy, type 2 diabetes, non-alcoholic fatty liver disease, skeletal muscle dysfunction, and increased risk of heart failure.
There has been an increasing interest in evaluating the therapeutic potential of dietary sources, including foods rich in antioxidants, for their ameliorative effects against oxidative stress and inflammation in diverse metabolic conditions. In fact, our group and others have progressively reported on the potential benefits of plant- and/or food-derived bioactive compounds for their capacity to improve metabolic status by blocking the toxic effects of oxidative stress and inflammation [10,11,12,13,14]. A growing body of literature has also progressively reported on the potential benefits of pepper against diverse metabolic complications [15,16,17,18]. Piper, the genus of pepper plants or pepper vines, is contemplated to be part of the most ancient pan-tropical flowering plant groups [19]. With an estimated 1000 species of herbs, encompassing small trees, shrubs, and hanging vines, the genus Piper is considered to have a rich ethnobotanical and ethnopharmaceutical history [20]. The reviewed literature already indicates the potential therapeutic effects of pepper; however, it predominantly focuses on preclinical findings [15,16,17,18]. In particular, reviewed information shows that black and red pepper, including their respective main bioactive compounds piperine and capsaicin, display a variety of biological effects, including antimicrobial, anti-inflammatory, gastro-protective, antidepressant, and antioxidant properties, in preclinical models [16,17]. Azlan and colleagues also recently reviewed evidence of the antioxidant and anti-obesity effects of different chili peppers [18]. However, a gap remains in the evidence on the clinical benefits of pepper against metabolic diseases. Importantly, there are no reviews that have compared the therapeutic effects of both black pepper (Piper nigrum) and red pepper (Capsicum annum) against metabolic diseases. This highlights the importance of the current review, which critically discusses clinical evidence of the potential benefits of both black and red peppers against diverse metabolic complications. The current review also covers essential information on the biological properties, metabolism, and bioavailability profiles, as well as the toxic effects, of pepper types and their main active ingredients, which are all necessary to underscore its potential pharmacological relevance.
2. General Overview of Black Pepper (Piper nigrum), including Its Metabolism and Bioavailability Profile
The genus Piperaceae, of the pepper family, contains flowering plants including small trees, shrubs, or herbs. This class consists of about 3600 species and five genera, including Piper, Peperomia, Zippelia, Manekia, and Verhuellia. Most of the species are found in the Piper genera, with about 2171 species, and Peperomia, with over 1000 species [21]. The most popular species of the Piperaceae family is Piper nigrum, which produces peppercorns that are generally used as spices, including black pepper, which is considered the king of spices [22]. Another well-known species of the Piperaceae is Piper longum, which yields black, white, and green peppercorns [23]. It is believed that the Piper genus is endogenous to India [24], being broadly cultivated within the Karala region [25]. Black pepper contains a major bioactive pungent alkaloid commonly known as piperine, which is found in the fruits of Piper longum and Piper nigrum [23]. Piperine content within black pepper is estimated to range from 2–10% [26,27,28,29,30,31]. Piperine was first extracted around the 1800s, while its chemical composition was elucidated much later, around 1882–1894 [32]. Since then, research has extensively studied black and its constituent piperine, with the description of different isomers of the bioactive compound currently acknowledged, including the trans–trans isomer (piperine), cis–trans isomer (isopiperine), cis–cis isomer (chavicine), and trans–cis isomer (isochevicine) (Figure 2). Other alkaloids that have been identified in black pepper include piperanine, piperettine, piperolein, piperylin, and pipericine [22]. Reviewed information already indicates that other bioactive compounds can be found in the African Piper species [33]. Like other natural compounds that are widely ingested orally, piperine gets broken down within the body into small components or metabolites.
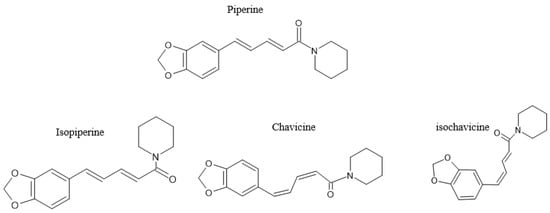
Figure 2.
The chemical structure of piperine, including its isomers isopiperine, chavivine, and isochavicine; adapted from published literature [34].
Evidence from animal studies shows that oral administration of 170 mg of piperine yields approximately 3–4% of the original ingested bioactive compounds, which is detected mainly in feces after 4 or 5 days in rats, while about 96–97% is projected to be absorbed [35,36]. Ingested piperine is normally absorbed within the small intestines of rats [35]. When 170 mg of the bioactive compound is given to rats, about 38.8 µmol of piperine is detected in the serum, while some of the trace elements of the compound are found in the liver and kidneys [36]. Although some studies could not detect the presence of piperine in urine or serum [35,36,37,38], others have reported piperine metabolites including piperonylic acid, piperonyl alcohol, piperonal, and their conjugates in the urine of rats [39]. Piperine, when conjugated with iron, can inhibit the activity of CYP450 3A4 [40], an essential enzyme involved in drug metabolism and detoxication processes within the liver [41]. Additional evidence indicates that twelve metabolites of piperine can be detected in rat plasma, bile, feces, and urine [42]. Apparently, piperine can undergo a series of chemical modifications through the enzymes responsible for the liver first-pass metabolic effect [43]. Studies support effective absorption of piperine in rats, especially after an initial oral dose of 20 mg is given [44]. Others have also affirmed effective absorption of piperine through enhanced levels in the brain and plasma of rats after the ingestion of a dose of 35 mg [45]. The metabolism of 50 mg of piperine in humans translates to about 0.71–0.83 mg of the bioactive compound being detected within the plasma [46]. Importantly, piperine metabolites, including 5-(3-4-dihydroxphenyl) valeric acid piperidide and its derivative 5-(3-4-dihydroxphenyl)valeric acid-4-hydroxypiperidide, have been detected in the urine of humans [47].
Like with other natural compounds, the delivery of piperine is supposedly compromised by its low water solubility, which could lead to poor clinical applications [48]. However, research has evaluated the potential use of delivery systems like nanoparticles, nanoliposomes, and micelles among advances to improve the bioavailability of natural bioactive and food compounds [49]. For example, the encapsulation of piperine in a nanoparticle of sodium chitosan triphosphate could improve its absorption, leading to enhanced biological activity [50]. A combination of piperine and curcumin shows improved efficacy compared to that of each bioactive compound alone [51]. Others have indicated that the use of mixed micelles of D-alpha tocopherol polyethylene glycol succinate and soluplus could improve the efficacy of encapsulated piperine over that of free piperine [52]. Cubic-nanoparticle-encapsulated piperine and protopanaxadiol also show improved bioavailability [53]. Such studies indicate that piperine is released at a much faster rate within in vivo systems, while also promoting increased absorption. Another interesting formulation is a nanoliposome with piperine and gentamicin, as investigated in bacterial growth [54]. It has been found that this liposomal combination was effective in inducing death and bacterial inhibition. Lastly, other researchers have used solid lipid nanoparticles encapsulating piperine to test the ameliorative effect of piperine against the complications of Alzheimer’s disease [55]. Interestingly, it was found that this nano-formulation could reduce the levels of superoxide dismutase (SOD) as well as oxidative stress at a dose of 2 mg/kg [55]. This further indicates that the bioavailability profile or bioactivity of piperine can be enhanced through recent developments in drug discovery, especially when used in combination with other bioactive compounds or food products [56,57].
3. Red Pepper (Capsicum annum), including Its Metabolism and Bioavailability Profile
Red pepper belongs to the Solanaceae family of the genus Capsicum, consisting of five domesticated species such as C. annuum L., C. chinense Jacq., C. frutescens L., C. baccatum L., and C. pubescens Ruiz et Pav [58,59]. It appears the capsicum species were first discovered in Bolivia, with their cultivation expanding to Mexico prior to the Columbian times (7000 B.C.) [60]. The capsicum genus is known by different names including hot pepper, chili pepper, bell pepper, sweet pepper, and sometimes just pepper across the world. Red pepper consists of different secondary metabolites collectively called capsaicinoids [61]. Capsaicinoids include capsaicin (8-methyl-N-vanillyl-6-nonenamide) and homologs of capsaicin with acid amides of vanillyl amine, as well as 8–to–18 carbon fatty acids. Other capsaicinoids that exist in pungent red pepper apart from capsaicin include the 6,7-dihydro analog of capsaicin, called dihydrocapsaicin, and nordihydrocapsaicin, which contains the mono-nor homolog of the acyl residue of dihydrocapsaicin [62].
Figure 3 shows some homo-capsaicinoids, including homocapsaicin, homodihydrocapsaicin and N-vanillyl nanoamide [63]. However, capsaicin, a major bioactive capsaicinoid that occurs as a colorless and odorless hydrophobic compound and is crystalline to waxy [64], is mainly responsible for the burning sensation of the fruit when orally ingested [65]. It has been reported that capsaicin and dihydrocapsaicin make up about 70–90% of the capsaicinoids within the Capsicum genus [66,67]. Studies have also discovered other capsaicinoid-like residues that are structurally related to capsaicin but of the non-pungent cultivar of Capsicum annuum L. [68]. The other structures of these capsaicinoid-like residues have been identified and denoted as capsiate, dihydrocapsiate, and nordihydrocapsiate, with the chemical nomenclature 4-hydroxy-3-methoxybenzyl [E]-8-methyl-6-nonenoate, 4-hydroxy-3-methoxybenzyl 8-methyloctanoate, and 4-hydroxy-3-methoxybenzyl, respectively [68,69]. Structurally, these capsinoids differ from capsaicin by their two moieties that are linked by an ester bond rather than an amine bond [70], and they are called capsinoids [69]. Studies on the pungent components of capsicum with different names such as capsicol, capsaicin, and capsaicin began as early as the 1800s [71]. This is almost the same time when capsaicin was extracted from Capsicum [72], and its chemical composition was then characterized by the 1900s [73].
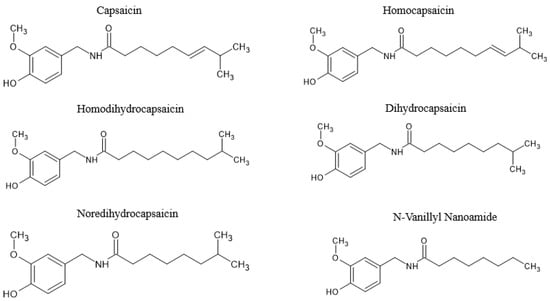
Figure 3.
The chemical structure of capsaicinoids, including capsaicin, homocapsaicin, homodihydrocapsaicin and N-vanillyl nanoamide. Information adapted from previous literature [63,74].
Just like piperine, capsaicin also undergoes liver first-pass metabolism upon oral administration [75]. Evidence indicates that capsaicin can undergo hepatic metabolism, leading to the generation of metabolites like hydroxycapsaicin and dihydrocapsaicin [76]. Apparently, five metabolites of capsaicin have been detected in the human liver, with hydroxycapsaicin, hydroxycapsaicin, and dehydrocapsaicin being the most abundant [77]. Accordingly, the most abundant metabolites in the liver fractions of rats included vanillylamine, hydroxycapsaicin, and dehydrocapsaicin [77]. This finding indicates that capsaicin is metabolized at a higher rate within the liver of rodents. Others have indicated the distribution of capsaicin in different organs of rats, showing that this bioactive compound is abundantly found in the spinal cord and the brain when compared to levels in the liver after intravenous administration [75]. It has also been indicated that reduced levels of capsaicin within the liver may be due to the detoxification process, which produces tolerable conjugates with glucuronic acid or sulfuric acid [75]. This hypothesis has been confirmed by others showing that hydrocapsaicin and other metabolites are found in the urine and feces of rats [71]. It appears that hydrocapsaicin is mainly hydrolyzed by the liver to yield fatty acids and vanillylamine, which are later reduced to vanillin and lastly vanillic acid and/or vanillyl alcohol. Dicapsaicin is another identified metabolite of capsaicin within the liver of rats [78]. Apparently, the P450 enzymes can metabolize capsaicin to produce free-radical intermediates. Other studies have also reported the involvement of a monooxidase system in livers treated with capsaicinoids. For instance, it was previously reported that capsaicinoids were converted to N-(4,5,dihydroxy-3-methoxybenzy1) acylamides in rat livers through a mixed-function monooxidase system promoted by hexobarbital injection [71]. In vitro, it was shown that N-(4,5,dihydroxy-3-methoxybenzy1) acylamide was the only metabolite detected in the incubation medium containing rat liver homogenate and capsaicin [71].
In terms of bioavailability, intestinal absorption of capsaicin in rats and hamsters in vitro has been reported [79]. From these results, it was evident that hamsters had better capsaicin intestinal absorption than rats [71]. Capsaicinoids are absorbed better in the stomach than in the small intestine in vivo, while in vitro evidence through intestinal sacs also supports enhanced intestinal absorption of capsaicin [79]. Capsaicin can also be better absorbed by the jejunum and ileum than by the stomach in rats [80]. The absorption of capsaicin in the lungs appears to be 20–40-fold slower than that in the liver microsomes of both rats and humans [76]. However, the capsaicin metabolites observed in human lung microsomes were similar to those in liver microsomes. Notably, the metabolic profile of red pepper and capsaicin has been poorly investigated in clinical subjects.
With a rapid advancement in science, nanotechnology has been employed as a potential delivery system to enhance the bioavailability of capsaicin. In this context, polymeric nanocapsules have been used to improve the efficacy of capsaicin [81]. Such data has verified that capsaicin is insoluble in water; thus, the introduction of a high-water emulsion reduces its loading efficacy. Solid-lipidic nanoparticles and nanostructured lipid carriers have also been investigated as transdermal transporters of capsaicin. It has been reported that this delivery emulsion exhibits enhanced transdermal permeability and retention in mice skin [82]. Like most of the formulations, the nano-vascular ethosomal formulation was found to improve permeability in ex vivo human skin and improved the anti-arthritic form of capsaicin [83,84]. Also, there has been interest in exploring the use of nanofibers as a capsaicinoid transdermal delivery strategy. A nanofiber was loaded 0.5–2% of capsaicin extract, and it was demonstrated that the release of this bioactive compound and its permeability in snakeskin were high [85]. Lastly, researchers attempted to encapsulate capsaicin in nanoparticles and incorporate chitosan hydrogel to improve its permeability through the skin [86]. This study compiled as many of the formulation strategies for capsaicin delivery systems as possible. Ongoing research continues to cover the different formulations that can be used to enhance the absorption of capsaicin [63].
4. Traditional Uses and Proposed Pharmacological Properties of Pepper and Its Bioactive Compounds
Black pepper and piperine have been acknowledged to have beneficial effects on human health. Starting with preclinical evidence, it has been shown that the administration of piperine at a dose of 50 mg/kg could improve the digestive system while reducing oxidative stress and inflammation in mice [87]. In diverse experimental models of chronic diseases, it was shown that piperine can reduce complications of arthritis [88], hepatic steatosis [89,90], and type 2 diabetes or obesity [91]. It was disclosed that piperine can also reduce depression in mice when given at doses of 2.5, 5, or 10 mg/kg for 14 days [92,93]. Reviewed information has also covered the beneficial effects of black pepper and piperine in various experimental models of disease [94,95]. The molecular mechanisms and signaling pathways that are associated with the ameliorative effects of piperine against the toxic effects of oxidative stress have been discussed, and these include the activation of nuclear factor erythroid 2-related factor 2, peroxisome proliferator-activated receptor-gamma, cyclooxygenase-2, and nitric oxide synthases-2, which is essential to promote intracellular antioxidant responses [96,97]. This bioactive compound can also block inflammation and improve cellular function by effectively modulating or inhibiting multiple signaling pathways, such as those of protein-kinase-activated NLR family pyrin domain containing-3 inflammasome, nuclear factor-κB, Jun N-terminal kinase/p38 mitogen-activated protein kinase, and pro-inflammatory molecules [96,98].
On the other hand, red pepper has been used traditionally to relieve toothache. Other traditional uses of red pepper include its application as a home remedy to heal lung conditions like bronchitis, lower glucose levels in diabetes, stabilize blood pressure, and relieve burning feet [99,100]. Scientific evidence indicates that red pepper can improve blood circulation and gastric abnormalities [101] while ameliorating neuralgia and rheumatism [102,103]. Capsaicin and its derivatives are effective against abdominal pain, bloating [104], and pain [105,106,107], as well as alleviating other complications that underlie diabetes and overweight [108]. In addition, it has been shown that capsaicin alone has anti-inflammatory [109] and antioxidant [110,111,112] properties. Other reviews have also given a general perspective into the diverse biological activities of capsaicin, especially in relation to the alleviation of metabolic-disease-related complications [16,63]. It appears that the secondary metabolites of red pepper are equally important in improving human health [113]. In terms of molecular insights, Caterina and colleagues [114] were fundamental in discovering the role of capsaicin as an analgesic agent. Their findings affirmed that capsaicin receptor is a non-selective cation channel that is structurally linked to members of the TRP family of ion channels. The latter encodes integral membrane proteins that function as ion channels and are broadly expressed in diverse tissues and cell types, where they are involved in different physiological processes, including sensation of different stimuli or ion homeostasis [115]. As a result, accumulative research has explored the potential role of capsaicin in stimulating painful sensations, particularly its chemical modulation of sensory neurons through the vanilloid receptor subtype 1 [116,117,118]. Other studies show that mice lacking TRPV1 exhibit no vanilloid-induced pain behavior, which is related to a reduced capacity to feel pain [117].
5. Potential Toxic Effects of Pepper
An increasing body of evidence shows that pepper has toxicological effects when used at very high doses. In fact, although considered beneficial to human health, even black pepper is a culprit for such toxic effects. For instance, the administration of its active ingredient, piperine, at doses as high as 60 mg/kg could be lethal in female rats, while a dose of 35.5 mg/kg could be toxic in weaning male rats, although this could also be dependent on prolonged exposure to the compound [119]. It appears that doses of piperine ranging from 35.7 mg/kg–140 mg/kg administered orally could cause liver damage, with 140 mg/kg also affecting kidneys and lungs in mice [120]. Also, high doses of piperine could affect sperm quality in rats [121]. Several authors have also reported that piperine can negatively influence maternal reproduction in association with embryonic toxicity in various preclinical models [122,123,124]. However, more work is required to determine the role of dose dependence, as well as intervention period, in driving the toxic effects of piperine or black pepper. Although piperine has also been studied in humans, it is noteworthy that there is lack of information on its toxicity profile. On the other hand, it has been shown that red pepper constituents (capsaicinoids) could induce skin irritation and inflammation in the mucus and eyes [125]. It has been previously reported that high quantity of capsaicinoids have severe effects on the gastrointestinal tract [126,127]. Since the identification of capsaicinoid toxicity, studies have elucidated the lethal dose of capsaicin in mice, which could be about 122–294 mg/kg, whereas a lethal intravenously injected dose was predicted to be 0.36–0.87 mg/kg [128]. In rats, capsaicin was found to damage the liver mitochondria [129,130]. Apart from being fatal, capsaicin was also reported to suppress stimulus response and induce neurotoxicity, mainly when administered in neonates [130,131,132]. In humans, capsaicin at 0.006% is routinely used to induce a burning stimulus [133]. The intolerable effects of capsaicin could include coughing, diarrhea, and vomiting [134]. Also, capsaicin plasters containing 345.8 mg and 34.58 mg tinctures were shown to induce pain and nausea [135]. There is a large body of knowledge on capsaicin toxicity that has been reported elsewhere [71,74,136].
6. Available Clinical Evidence of the Potential Benefits of Pepper
6.1. Characteristic Features of Clinical Studies
To identify relevant clinical studies, a systematic search was conducted using major electronic databases including PubMed, Scopus, and Google Scholar. The search strategy was compiled using the following keywords or Medical Subject Headings (MeSH): “pepper” and “metabolic diseases”, including most relevant synonyms as well as keywords related to the search topic. The literature search was performed from inception until June 2023, while a manual search was performed to identify additional relevant studies. The final search results yielded 14 relevant studies reporting on black pepper or its main active ingredient, piperine, and its potential therapeutic effects against diverse metabolic complications (Table 1), whereas 16 records were identified for clinical studies on red pepper, including its active ingredients, capsinoids, against diverse metabolic complications. Besides those from Argentina, Australia, Brazil, China, India, and Japan, which were outliers, most included studies were from Iran, Europe, and the United States, predominantly focusing on adults over the age of 18 years (Table 1). Summarized literature mainly included overweight and obese subjects and those with metabolic syndrome (Table 1 and Table 2). However, evidence involving healthy subjects was also included, provided it was reporting on the therapeutic effects of pepper or its active ingredients on metabolic parameters in individuals with chronic or metabolic conditions.

Table 1.
An overview of human studies on the effects of black pepper (Piper nigrum) and its active ingredient, piperine, against diverse metabolic complications.

Table 2.
Clinical evidence of the effects of red pepper (Capsicum annum) and its active ingredient, capsaicin, against diverse metabolic complications.
6.2. Evidence of the Effects of Pepper on Overweight and Obese Individuals
Overweight and obesity remain the major contributors to the development of diverse metabolic complications [7,165]. Overnutrition consistent with reduced physical activity is considered the underlying factor driving the development and progression of obesity [166]. In fact, there is an increasing need to investigate the therapeutic effects of pepper against obesity and its associated complications in human subjects. Evidence summarized in this review indicates that several clinical studies have been completed to test the beneficial effects of pepper, including its active ingredients, piperine and capsaicin, on obesity and its related metabolic complications (Table 1). Starting with evidence on black pepper, 3 months of administration of a formulation containing its main ingredient, pure piperine (at 15 mg), together with Camellia sinensis soy distearoylphosphatidylcholine was shown to reduce body weight and fat mass in obese subjects [143]. Captivatingly, reviewed evidence already supports the notion that epigallocatechin, which is one of the major active ingredients of Camellia sinensis, could potentially neutralize oxidative stress and inflammation to amend complications of metabolic syndrome [165]. The 8-week administration of two capsules containing a combination of piper nigrum dry extract (3 mg), capsicum annum (7.5 mg), and decaffeinated dried Camellia sinensis extract (150 mg) could ameliorate obesity-related complications, including insulin resistance, leptin/adiponectin ratio, and low-density lipoprotein (LDL) cholesterol levels, while blocking inflammation in overweight individuals [91]. Similarly, a four-hour administration of a capsule containing a combination of spices (at 2 or 14.5 g) consisting of black pepper, cinnamon, cloves, garlic, ginger, oregano, paprika, rosemary, and turmeric could reduce the levels of triglycerides while alleviating high-fat-meal-induced postprandial interleukin (IL)-1β secretion in overweight and obese subjects [141,147]. Interestingly, an additional study also showed that the beneficial effects of pepper-containing formulations were associated with improved lipid profiles, as seen with reductions in total cholesterol, low-density lipopolysaccharide, and triglyceride levels when individuals with hypercholesterolemia were given capsules comprising different active ingredients such as bioflavonoids, vitamins, omega-3 fatty acids, and black pepper for 30 days [139]. Summarized evidence on the potential therapeutic effects of black pepper against obesity and its associated complications appeared to be more pronounced when used in combination with other active ingredients, with enhanced effects on improving lipid profiles through the reduction of total cholesterol and triglyceride levels, while also lowering pro-inflammatory markers in overweight and obese subjects (Table 1; Figure 4).
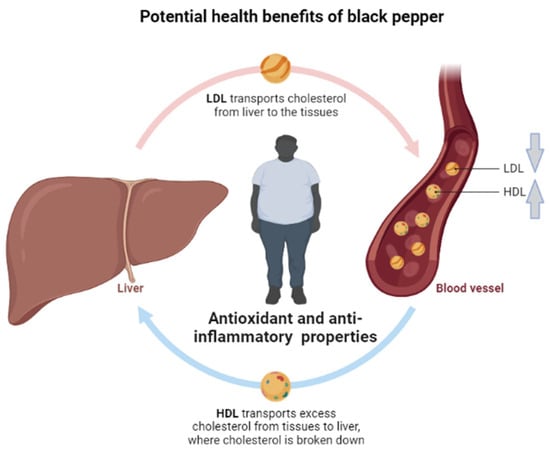
Figure 4.
A general overview of the potential beneficial effects of black pepper against obesity and its associated complications, with evidence indicating that black pepper, including its active ingredient piperine, shows enhanced potential to improve blood lipid profiles, including reducing circulating levels of low-density lipoprotein cholesterol (LDL) while increasing those of high-density lipoprotein (HDL). The strong antioxidant properties of black pepper are attributed to its potential beneficial effects on overweight and obese individuals.
Summarized evidence also reported the therapeutic potential of red pepper against obesity and its related complications (Table 2). Here, it was shown that the 7-day administration of a tablet containing an active ingredient of red pepper, capsaicin (at 0.2 mg), together with green tea extract (at 250 mg), tyrosine (at 203 mg), and anhydrous caffeine (at 25.4 mg) could promote a thermogenic effect and enhance energy expenditure in overweight and obese individuals [152]. This is of great importance since it has been estimated that both common therapies like metformin and prominent bioactive compounds can improve metabolic function by promoting thermogenesis and increasing energy expenditure [167,168]. Interestingly, a 4-week administration of capsinoids (at 3 or 10 mg/kg) could also promote fat oxidation, which was positively correlated with enhanced energy expenditure in overweight subjects [154]. Other studies supported the beneficial effects of capsinoids against obesity-related complications, indicating that consuming these bioactive compounds (at doses between 4–6 mg) for 1 to 3 months could promote fat oxidation while reducing body weight, body mass index, and appetite in overweight subjects [70,162]. Moreover, overweight individuals receiving a 4-week chili blend (at 30 g/d; 55%) intervention displayed reduced postprandial hyperinsulinemia [153]. However, administration of a combination of red pepper spices (at 1 g daily for 4 weeks) did not have a significant effect in improving markers of oxidative stress and inflammation in overweight and obese subjects [157]. Individuals subjected to high-fat and high-carbohydrate meals rich in red pepper (at 10 g) exhibited reduced appetite and subsequently reduced protein and fat intakes, while also limiting energy intake [150]. This may indicate that the therapeutic properties of red pepper and its active ingredients, capsinoids, mainly include promoting energy expenditure, including limiting energy intake in overweight and obese individuals.
This was also verified independently in healthy individuals, where the short-term (at least 30 min) administration of capsinoids (at 10 mg) could enhance adrenergic activity and energy expenditure, leading to a shift in substrate utilization toward lipids at rest, but with little effect during exercise or recovery [156]. Also, individuals receiving capsaicin at a dose of 2.56 mg (1.03 g of red chili pepper) with every meal for 36 h displayed a negative energy balance that was concomitant with a reduction in energy expenditure [158]. Similar results were seen for individuals taking red chili pepper containing capsaicin at 2484 µ/g, nordihydrocapsaicin at 278 µ/g, and dihydrocapsaicin at 1440 µ/g (2.56 mg) in terms of enhancing satiety and fullness while preventing a negative energy balance and desire to eat [159]. Interestingly, the positive effects of black pepper on energy expenditure were very minimal [137,138,144]. In fact, very limited information [160] showed that pepper or its active ingredients could affect or improve the metabolic status of individuals with diverse metabolic complications. The potential beneficial effects of red pepper are summarized in Figure 5.
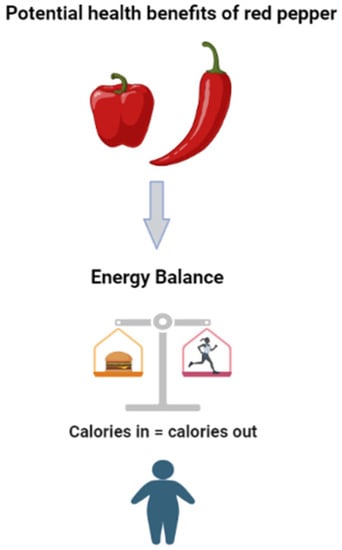
Figure 5.
The potential beneficial effects of red pepper, including its active ingredient capsaicin, could promote energy expenditure and limit energy intake, which is likely to contribute to reduced fat mass in overweight and obese individuals.
6.3. Evidence of the Effects of Pepper on Individuals with Metabolic Syndrome
Overweight and obesity are currently acknowledged to be the leading factors that favor the development of metabolic syndrome. This is a chronic medical condition that describes a cluster of metabolic abnormalities that drive the development of type 2 diabetes and cardiovascular complications. Well beyond assessing the therapeutic effects of pepper against overweight or obesity, it is also important to uncover whether administration of this dietary compound can alleviate the pathological features associated with metabolic syndrome. In fact, some of the evidence included within this review did report the potential benefits of pepper or its active ingredients in complications of metabolic syndrome. (Table 1 and Table 2). For instance, an 8-week co-administration of piperine (at 10 mg) with curcuminoids (at 1 g) could improve oxidative and inflammatory status by enhancing serum levels of SOD while reducing those of malonaldehyde (MDA) and C-reactive protein in individuals with metabolic syndrome [142]. Of course, lipid peroxidation through enhanced levels of MDA is one of the predominant features driving the complications of metabolic disease [169]. It was also shown that a 3-month administration of a formulation containing piperine (at 5 mg) together with resveratrol (at 50 mg) and alpha tocopherol (at 25 mg) could alleviate inflammation by reducing levels of ferritin and C-reactive protein in individuals with metabolic syndrome [148]. The short-term (2 h) administration of capsaicin (at 5 mg) could also reduce plasma glucose levels and maintain insulin levels in individuals subjected to oral glucose tolerance tests [155]. A 4-week administration of a similar dose of capsaicin was also shown to improve plasma postprandial hyperglycemia and hyperinsulinemia in women with gestational diabetes [161]. Notably, limited evidence has directly evaluated the beneficial effects of pepper against the complications linked with metabolic syndrome, while active ingredients such as piperine and capsaicin show the therapeutic potential to improve oxidative stress and inflammation within such pathological conditions.
6.4. Evidence of the Effects of Pepper on Individuals with Metabolic Syndrome
Many other chronic conditions contribute to the development and progression of noncommunicable diseases. The current study also evaluated the therapeutic effects of pepper against diverse chronic conditions, including those involving osteoarthritis, oropharyngeal dysphagia, digestion, hemodialysis, and neuromuscular fatigue (Table 1 and Table 2). For instance, a 4-week administration of a formulation containing piperine (at 3.75 mg) together with curcumin (at 300 mg) and gingerols (at 7.5 mg) could potentially protect against chronic knee osteoporosis by reducing levels of prostaglandin E2 [146]. The administration of piperine (at 1 mM or 150 μM) could improve swallowing in individuals with oropharyngeal dysphagia [140]. Those receiving a 2-week intervention with a formulation containing Trachyspermum ammi (L.) Sprague seed, Zingiber officinale Roscoe. rhizome and Piper nigrum L. berry (at 500 mg) presented with improved bloating status, including eructation, defecation, and borborygmus, compared to that seen in individuals treated with dimethicone [145]. Patients undergoing hemodialysis could benefit from receiving a combination of piperine (at 2 mg) and turmeric (at 3 g) for 12 weeks through an effective reduction in biomarkers of oxidative stress and inflammation, including malonaldehyde and ferritin [164]. On the other hand, a reduction in false alarm errors and mental fatigue at different time periods was also reported in young adults with low energy after receiving black pepper capsules twice a day at 0.504 g for 2 days [149]. Individuals receiving two capsules of capsaicin at 390 mg, with 72 h between sessions, displayed improvements in neuromuscular fatigue through alterations in afferent signaling or neuromuscular relaxation kinetics [163]. The blockage of systematic inflammation, leading to amendments in norepinephrine, heart rate, and systolic blood pressure during the experimental task, is the likely mechanism associated with improvements in stressful conditions in individuals receiving capsaicin at 510 mg for 10 days [151].
7. Summary and Future Perspectives
For centuries now, spices have been an important part of the human diet. This explains the significant interest directed at understanding the therapeutic benefits of species against many diseases, including the use of pepper and its active ingredients [170]. Clinical evidence covered within the current review supports the beneficial effects of pepper against obesity and its associated complications (Table 1 and Table 2). In fact, summarized literature supports the beneficial effects of black pepper (Piper Nigrum L.), including its active ingredient piperine, on lipid profiles, including reducing circulating levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides in overweight and obese individuals (Table 1). Moreover, the potential therapeutic effects of black pepper and piperine also supposedly include enhanced reduction effects on markers of oxidative stress and inflammation. Interestingly, the literature that has already been reviewed indicates that beyond the strong antioxidant effects, black pepper, with its active ingredient, piperine, contains a rich phytochemistry that includes volatile oil, oleoresins, and alkaloids, giving it the biological properties to protect against the toxic effects of oxidative stress and inflammation [15].
Evidence summarized in the current review also indicates that red pepper, together with its active ingredients, capsinoids, displays enhanced benefits in promoting energy expenditure, including limiting energy intake, which is likely to reduce fat mass in overweight and obese individuals. Although red pepper can potentially improve the oxidative and inflammatory status of individuals with metabolic syndrome, very limited information currently affirms the beneficial effects of both black and red pepper on these individuals. However, preclinical evidence supports the beneficial effects of dietary capsaicin in improving glucose homeostasis and lipid metabolism through the modulation of bile acid/gut microbiota in conditions of metabolic disease [171,172,173,174,175,176]. This is in line with the emerging literature highlighting the potential benefits of pepper in improving chronic conditions, including those involving osteoarthritis, oropharyngeal dysphagia, digestion, hemodialysis, and neuromuscular fatigue (Table 1 and Table 2). However, the current literature supports the common use of pepper (including its active ingredients) in combination with other bioactive compounds, which could explain the synergistic effects in protecting against obesity-associated complications. Anyhow, current literature provides an important background for the clinical trials required to better investigate the therapeutic effects of black and red pepper, especially those involving individuals with metabolic syndrome.
Author Contributions
All authors, including P.V.D., I.C., F.M., S.S., P.O., N.M., M.T.M., B.B.N., S.E.M.-M., N.H., S.H., D.N., J.L.M., A.K.B. and L.T., wrote the manuscript, edited the revised draft, and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research is financially backed by the South African Medical Research Council under project code 43500, providing support for covering the expenses related to processing the article. Funding from the National Research Foundation (Grant numbers: 132534 and 117829) for the author (P.V.D.) is also acknowledged. The content herein is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data related to search strategy, study selection, and extraction items will be made available upon request after the manuscript is published.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Not applicable.
References
- Malindisa, E.; Balandya, E.; Njelekela, M.; Kidenya, B.R.; Francis, F.; Mmbaga, B.T.; Dika, H.; Lyamuya, E.; Sunguya, B.; Bartlett, J.; et al. Metabolic syndrome among people living with HIV on antiretroviral therapy in Mwanza, Tanzania. BMC Endocr. Disord. 2023, 23, 88. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Leading Causes of Death Globally; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Nkambule, B.B. Obesity-induced inflammation and insulin resistance: A mini-review on T-cells. Metabol. Open 2019, 3, 100015. [Google Scholar] [CrossRef]
- Matacchione, G.; Gurău, F.; Baldoni, S.; Prattichizzo, F.; Silvestrini, A.; Giuliani, A.; Pugnaloni, A.; Espinosa, E.; Amenta, F.; Bonafè, M.; et al. Pleiotropic effects of polyphenols on glucose and lipid metabolism: Focus on clinical trials. Ageing Res. Rev. 2020, 61, 101074. [Google Scholar] [CrossRef]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 2022, 178, 106163. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Nyambuya, T.M.; Ziqubu, K.; Mabhida, S.E.; Mxinwa, V.; Mokgalaboni, K.; Ndevahoma, F.; Hanser, S.; Mazibuko-Mbeje, S.E.; et al. Vitamin C intake potentially lowers total cholesterol to improve endothelial function in diabetic patients at increased risk of cardiovascular disease: A systematic review of randomized controlled trials. Front. Nutr. 2022, 9, 1011002. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: Updating the status of clinical evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Ziqubu, K.; Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Tiano, L.; Nkambule, B.B.; Kappo, A.P.; Mazibuko-Mbeje, S.E. Isoorientin: A dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol. Res. 2020, 158, 104867. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Pasha, I.; Sultan, M.T.; Randhawa, M.A.; Saeed, F.; Ahmed, W. Black pepper and health claims: A comprehensive treatise. Crit. Rev. Food Sci. Nutr. 2013, 53, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Burger, W.C. Flora Costaricensis; Field Museum of Natural History: Chicago, IL, USA, 1971. [Google Scholar]
- Sen, S.; Dayanandan, S.; Davis, T.; Ganesan, R.; Jagadish, M.R.; Mathew, P.J.; Ravikanth, G. Origin and evolution of the genus Piper in Peninsular India. Mol. Phylogenet Evol. 2019, 138, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P. Angiosperm Phylogeny Website, Version 9; Missouri Botanical Garden: St. Louis, MO, USA, 2001.
- Ravindran, P. Black Pepper: Piper nigrum; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Vasavirama, K.; Upender, M. Piperine: A valuable alkaloid from Piper species. Int. J. Pharm. Pharm. Sci. 2014, 6, 34–38. [Google Scholar]
- Srivastava, S.; Gupta, M.M.; Tripathi, A.K.; Kumar, S. 1,3-Benzodioxole-5-(2,4,8-triene-methyl nonaoate) and 1,3-benzodioxole-5-(2,4,8-triene-isobutyl nonaoate) from Piper mullesua. ChemInform 2000, 32. [Google Scholar] [CrossRef]
- Parthasarathy, U.; Saji, K.; Jayarajan, K.; Parthasarathy, V. Biodiversity of Piper in South India—Application of GIS and cluster analysis. Curr. Sci. 2006, 91, 652–658. [Google Scholar]
- Rohloff, J.; Husøy, T.; Bruzell, E.; Granum, B.; Hetland, R.B.; Wicklund, T.; Steffensen, I.-L. Risk Assessment of “Other Substances”—Piperine. 2018. Available online: https://vkm.no/download/18.645b840415d03a2fe8f25ff2/1502802968337/Risk%2520assessment%2520of%2520%2522other%2520substances%2522%2520%25E2%2580%2593%2520Piperine.pdf (accessed on 2 June 2023).
- Zachariah, T.J.; Parthasarathy, V. Black pepper. In Chemistry of Spices; CABI: Wallingford, UK, 2008; pp. 21–40. [Google Scholar]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Luo, R.; Chen, X.-Q.; Ba, Y.-Y.; Zheng, L.; Guo, W.-W.; Wu, X. Identification and simultaneous quantification of five alkaloids in Piper longum L. by HPLC–ESI-MSn and UFLC–ESI-MS/MS and their application to Piper nigrum L. Food Chem. 2015, 177, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Ochir, S.; Sun, Z.; Borjihan, G.; Yamagishi, T. Occurrence of piperidine alkaloids in Piper species collected in different areas. J. Nat. Med. 2014, 68, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, M.; Khandhar, A.; Patel, S.; Patel, A. Chemistry and pharmacology of Piper longum L. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 67–76. [Google Scholar]
- Ørsted, H.C. HC Ørsted’s Theory of Force: An Unpublished Textbook in Dynamical Chemistry; Kgl. Danske Videnskabernes Selskab: Copenhagen, Denmark, 2003; Volume 86. [Google Scholar]
- Oyemitan, I. African medicinal spices of genus Piper. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 581–597. [Google Scholar]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Bhat, B.G.; Chandrasekhara, N. Studies on the metabolism of piperine: Absorption, tissue distribution and excretion of urinary conjugates in rats. Toxicology 1986, 40, 83–92. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Li, C.; Wang, Q.; Ren, T.; Zhang, Y.; Lam, C.W.K.; Chow, M.S.; Zuo, Z. Non-linear pharmacokinetics of piperine and its herb-drug interactions with docetaxel in Sprague-Dawley rats. J. Pharm. Biomed. Anal. 2016, 128, 286–293. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sharma, A.; Rayees, S.; Kour, G.; Singh, A.; Khullar, M.; Magotra, A.; Paswan, S.K.; Gupta, M.; Ahmad, I. Pharmacokinetic study of piperine in Wistar rats after oral and intravenous administration. Int. J. Drug Deliv. 2014, 6, 82. [Google Scholar]
- Bhat, B.G.; Chandrasekhara, N. Metabolic disposition of piperine in the rat. Toxicology 1987, 44, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Alugolu, V.; Rentala, S.; Komarraju, A.L.; Parimi, U.D. Docking studies of piperine-iron conjugate with human CYP450 3A4. Bioinformation 2013, 9, 334–338. [Google Scholar] [CrossRef]
- N., H.; C., M.; T. R., M.; S., S.; S., N.; K. E., M.; S. C., S.; Y., N.; P. V., D.; R. N., M. In Vitro Hepatic Models to Assess Herb-Drug Interactions: Approaches and Challenges. Pharmaceuticals 2023, 16, 409. [Google Scholar] [CrossRef]
- Gao, T.; Xue, H.; Lu, L.; Zhang, T.; Han, H. Characterization of piperine metabolites in rats by ultra-high-performance liquid chromatography with electrospray ionization quadruple time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 901–910. [Google Scholar] [CrossRef]
- Shang, Z.; Cai, W.; Cao, Y.; Wang, F.; Wang, Z.; Lu, J.; Zhang, J. An integrated strategy for rapid discovery and identification of the sequential piperine metabolites in rats using ultra high-performance liquid chromatography/high resolution mass spectrometery. J. Pharm. Biomed. Anal. 2017, 146, 387–401. [Google Scholar] [CrossRef]
- Bajad, S.; Singla, A.; Bedi, K. Liquid chromatographic method for determination of piperine in rat plasma: Application to pharmacokinetics. J. Chromatogr. B 2002, 776, 245–249. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Q.; Li, C.; Yang, M.; Zuo, Z. Efficient brain uptake of piperine and its pharmacokinetics characterization after oral administration. Xenobiotica 2018, 48, 1249–1257. [Google Scholar] [CrossRef]
- Kakarala, M.; Dubey, S.K.; Tarnowski, M.; Cheng, C.; Liyanage, S.; Strawder, T.; Tazi, K.; Sen, A.; Djuric, Z.; Brenner, D.E. Ultra-low flow liquid chromatography assay with ultraviolet (UV) detection for piperine quantitation in human plasma. J. Agric. Food Chem. 2010, 58, 6594–6599. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, C.; Spiteller, G. Piperin—Ein Beispiel für individuell unterschiedliche (polymorphe) Metabolisierung einer allgegenwärtigen Nahrungskomponente. Liebigs Ann. Chem. 1984, 1984, 1319–1331. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R.; Mukerjee, A. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Anissian, D.; Ghasemi-Kasman, M.; Khalili-Fomeshi, M.; Akbari, A.; Hashemian, M.; Kazemi, S.; Moghadamnia, A.A. Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling model of epilepsy. Int. J. Biol. Macromol. 2018, 107, 973–983. [Google Scholar] [CrossRef]
- Baspinar, Y.; Üstündas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, C.; Wang, Y.; Xu, Y.; Zhao, J.; Gao, M.; Ding, Y.; Peng, J.; Li, L. Development and evaluation of a novel drug delivery: Soluplus®/TPGS mixed micelles loaded with piperine in vitro and in vivo. Drug Dev. Ind. Pharm. 2018, 44, 1409–1416. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Z.H.; Sun, E.; Tan, X.B.; Li, S.L.; Cheng, X.D.; You, M.; Jia, X.B. Enhanced oral absorption of 20(S)-protopanaxadiol by self-assembled liquid crystalline nanoparticles containing piperine: In vitro and in vivo studies. Int. J. Nanomed. 2013, 8, 641–652. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Ghandadi, M.; Ghoochi Atashbeyk, D.; Fazly Bazzaz, B.S.; Iranshahi, M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev. Ind. Pharm. 2015, 41, 989–994. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Khan, R.A.; Ahmed, B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J. Drug Target. 2013, 21, 300–311. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Straight, C.R.; Wang, Q.; Zhou, J.; Sun, Y.; Lo, C.Y.; Yi, L.; Wu, Y.; Huang, J.; et al. Black pepper and vegetable oil-based emulsion synergistically enhance carotenoid bioavailability of raw vegetables in humans. Food Chem. 2022, 373, 131277. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barboza, G.E.; Carrizo García, C.; Leiva González, S.; Scaldaferro, M.; Reyes, X. Four new species of Capsicum (Solanaceae) from the tropical Andes and an update on the phylogeny of the genus. PLoS ONE 2019, 14, e0209792. [Google Scholar] [CrossRef]
- Bosland, P.W.; Votava, E.J.; Votava, E.M. Peppers: Vegetable and Spice Capsicums; Cabi: Wallingford, UK, 2012; Volume 22. [Google Scholar]
- Smith, P.G.; Heiser, C.B., Jr. Taxonomy of Capsicum sinense Jacq. and the geographic distribution of the cultivated Capsicum species. Bull. Torrey Bot. Club 1957, 84, 413–420. [Google Scholar] [CrossRef]
- Zarringhalam, M.; Zaringhalam, J.; Shadnoush, M.; Safaeyan, F.; Tekieh, E. Inhibitory Effect of Black and Red Pepper and Thyme Extracts and Essential Oils on Enterohemorrhagic Escherichia coli and DNase Activity of Staphylococcus aureus. Iran. J. Pharm. Res. 2013, 12, 363–369. [Google Scholar]
- Kirschbaum-Titze, P.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicum annuum). 2. Heterogeneity of capsaicinoid content in individual fruits from one plant. J. Agric. Food Chem. 2002, 50, 1264–1266. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Awang, R.M.; Omar, D.; Rahmani, M.; Rezazadeh, S. Tropical medicinal plant extracts against rice weevil, Sitophilus oryzae L. J. Med. Plants Res. 2011, 5, 259–265. [Google Scholar]
- Turini, D.; Beneforti, P.; Spinelli, M.; Malagutti, S.; Lazzeri, M. Heat/burning sensation induced by topical application of capsaicin on perineal cutaneous area: New approach in diagnosis and treatment of chronic prostatitis/chronic pelvic pain syndrome? Urology 2006, 67, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Naseem, Z.; Imran, K.; Sajila, H.; Ali, J.; Inam, S.M.; Malik, S.M.; Farah, A. Estimation of capsaicin in different chilli varieties using different extraction techniques and HPLC method: A review. Pak. J. Food Sci. 2016, 26, 54–60. [Google Scholar]
- Peña-Alvarez, A.; Ramírez-Maya, E.; Alvarado-Suárez, L.Á. Analysis of capsaicin and dihydrocapsaicin in peppers and pepper sauces by solid phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 2843–2847. [Google Scholar] [CrossRef]
- Yazawa, S.; Yoneda, H.; Hosokawa, M.; Fushiki, T.; Watanabe, T. Novel capsaicinoid like substances in the fruits of new non-pungent cultivar ‘CH-19 Sweet’ of pepper (Capsicum annuum L.). J. Agric. Food Chem. 1998, 46, 1695–1697. [Google Scholar]
- Kobata, K.; Sutoh, K.; Todo, T.; Yazawa, S.; Iwai, K.; Watanabe, T. Nordihydrocapsiate, a new capsinoid from the fruits of a nonpungent pepper, Capsicum annuum. J. Nat. Prod. 1999, 62, 335–336. [Google Scholar] [CrossRef]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2009, 89, 45–50. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwai, K. Constituents of red pepper species: Chemistry, biochemistry, pharmacology, and food science of the pungent principle of Capsicum species. Alkaloids Chem. Pharmacol. 1984, 23, 227–299. [Google Scholar]
- Bucholz, C. Chemical investigation of dry, ripe Spanish peppers. Alm. Oder Taschenb. Scheidekünstler Apoth. 1816, 37, 1–30. [Google Scholar]
- Jones, P.G.; Fenwick, G.R. The glycoalkaloid content of some edible solanaceous fruits and potato products. J. Sci. Food Agric. 1981, 32, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Ueno, S.; Nakayama, A.; Ikeuchi, K.; Ubukata, K.; Mihara, R.; Bernard, B.K. Studies of the toxicological potential of capsinoids, XII: Pharmacokinetic study of capsinoid-containing CH-19 Sweet extract in rats. Int. J. Toxicol. 2010, 29, 15S–21S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saria, A.; Skofitsch, G.; Lembeck, F. Distribution of capsaicin in rat tissues after systemic administration. J. Pharm. Pharmacol. 1982, 34, 273–275. [Google Scholar] [CrossRef]
- Reilly, C.A.; Ehlhardt, W.J.; Jackson, D.A.; Kulanthaivel, P.; Mutlib, A.E.; Espina, R.J.; Moody, D.E.; Crouch, D.J.; Yost, G.S. Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chem. Res. Toxicol. 2003, 16, 336–349. [Google Scholar] [CrossRef]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Henion, F.; Bugni, T.S.; Ethirajan, M.; Stockmann, C.; Pramanik, K.C.; Srivastava, S.K.; Yost, G.S. Reactive intermediates produced from the metabolism of the vanilloid ring of capsaicinoids by p450 enzymes. Chem. Res. Toxicol. 2013, 26, 55–66. [Google Scholar] [CrossRef]
- Monsereenusorn, Y. In vitro intestinal absorption of capsaicin. Toxicol. Appl. Pharmacol. 1980, 53, 134–139. [Google Scholar] [CrossRef]
- Kawada, T.; Suzuki, T.; Takahashi, M.; Iwai, K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984, 72, 449–456. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.C.; Sul, D.; Hwang, S.W.; Lee, S.H.; Kim, Y.H.; Tae, G. Nanoparticle formulation for controlled release of capsaicin. J. Nanosci. Nanotechnol. 2011, 11, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Katare, O.P. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J. Liposome Res. 2014, 24, 290–296. [Google Scholar] [CrossRef]
- Sarwa, K.K.; Das, P.J.; Mazumder, B. A nanovesicle topical formulation of Bhut Jolokia (hottest capsicum): A potential anti-arthritic medicine. Expert. Opin. Drug Deliv. 2014, 11, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Sarwa, K.K.; Mazumder, B.; Rudrapal, M.; Verma, V.K. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015, 22, 638–646. [Google Scholar] [CrossRef]
- Opanasopit, P.; Sila-On, W.; Rojanarata, T.; Ngawhirunpat, T. Fabrication and properties of capsicum extract-loaded PVA and CA nanofiber patches. Pharm. Dev. Technol. 2013, 18, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Contri, R.V.; Katzer, T.; Ourique, A.F.; da Silva, A.L.M.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Combined effect of polymeric nanocapsules and chitosan hydrogel on the increase of capsaicinoids adhesion to the skin surface. J. Biomed. Nanotechnol. 2014, 10, 820–830. [Google Scholar] [CrossRef]
- Selvendiran, K.; Sakthisekaran, D. Chemopreventive effect of piperine on modulating lipid peroxidation and membrane bound enzymes in benzo(a)pyrene induced lung carcinogenesis. Biomed. Pharmacother. 2004, 58, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Golam Sarwar, A.H.; Umar, K.; Ahmad, N.; Sajad, M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen induced arthritis. Cell Immunol. 2013, 284, 51–59. [Google Scholar] [CrossRef]
- Hwahng, S.H.; Ki, S.H.; Bae, E.J.; Kim, H.E.; Kim, S.G. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-alpha-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology 2009, 49, 1913–1925. [Google Scholar] [CrossRef]
- Choi, S.; Choi, Y.; Choi, Y.; Kim, S.; Jang, J.; Park, T. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013, 141, 3627–3635. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Perna, S.; Faliva, M.; Solerte, S.B.; Fioravanti, M.; Klersy, C.; Cava, E.; Paolini, M.; Scavone, L.; et al. Improvement in insulin resistance and favourable changes in plasma inflammatory adipokines after weight loss associated with two months’ consumption of a combination of bioactive food ingredients in overweight subjects. Endocrine 2013, 44, 391–401. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Wang, M.; Li, W.; Matsumoto, K.; Tang, Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007, 80, 1373–1381. [Google Scholar] [CrossRef]
- Kirschenbaum, B.; Goldman, S.A. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc. Natl. Acad. Sci. USA 1995, 92, 210–214. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Quijia, C.R.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Piperine: A Review. Crit. Rev. Anal. Chem. 2020, 50, 62–77. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni Suef Univ. J. Basic. Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Rashid, S.; Arafah, A.; Qamar, W.; Alsaffar, R.M.; Ahmad, A.; Almatroudi, N.M.; Alqahtani, S.M.A.; Rashid, S.M.; Ahmad, S.B. Piperine Regulates Nrf-2/Keap-1 Signalling and Exhibits Anticancer Effect in Experimental Colon Carcinogenesis in Wistar Rats. Biology 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.I.; Yoo, M.C.; Hahm, D.H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Sunil, P.; Sanjay, Y.; Vinod, S. Pharmacognostical investigation and standardization of Capsicum annum L. Roots. Chem. Pharm. Bull. 1990, 38, 1299. [Google Scholar]
- Magdy Beshbishy, A.; Alghamdi, S.; Onyiche, T.E.; Zahoor, M.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Ghorab, M.A.; Meshaal, A.K.; El-Esawi, M.A.; Hetta, H.F. Biogenesis, biologic function and clinical potential of exosomes in different diseases. Appl. Sci. 2020, 10, 4428. [Google Scholar] [CrossRef]
- Gonzalez, R.; Dunkel, R.; Koletzko, B.; Schusdziarra, V.; Allescher, H.D. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig. Dis. Sci. 1998, 43, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J. The Natural History of Medicinal Plants; Timber Press: Portland, OR, USA, 2000. [Google Scholar]
- Hui, Y.H.; Meunier-Goddik, L.; Josephsen, J.; Nip, W.-K.; Stanfield, P.S. Handbook of Food and Beverage Fermentation Technology; CRC Press: Boca Raton, FL, USA, 2004; Volume 134. [Google Scholar]
- Bortolotti, M.; Porta, S. Effect of red pepper on symptoms of irritable bowel syndrome: Preliminary study. Dig. Dis. Sci. 2011, 56, 3288–3295. [Google Scholar] [CrossRef]
- Winter, J.; Bevan, S.; Campbell, E. Capsaicin and pain mechanisms. Br. J. Anaesth. 1995, 75, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Guedes, V.; Castro, J.P.; Brito, I. Topical capsaicin for pain in osteoarthritis: A literature review. Reumatol. Clín. Engl. Ed. 2018, 14, 40–45. [Google Scholar] [CrossRef]
- Evangelista, S. Novel therapeutics in the field of capsaicin and pain. Expert Rev. Clin. Pharmacol. 2015, 8, 373–375. [Google Scholar] [CrossRef]
- Varghese, S.; Kubatka, P.; Rodrigo, L.; Gazdikova, K.; Caprnda, M.; Fedotova, J.; Zulli, A.; Kruzliak, P.; Büsselberg, D. Chili pepper as a body weight-loss food. Int. J. Food Sci. Nutr. 2017, 68, 392–401. [Google Scholar] [CrossRef]
- Fusco, B.M.; Giacovazzo, M. Peppers and pain: The promise of capsaicin. Drugs 1997, 53, 909–914. [Google Scholar] [CrossRef]
- Lee, C.Y.J.; Kim, M.; Yoon, S.W.; Lee, C.H. Short-term control of capsaicin on blood and oxidative stress of rats in vivo. Phytother. Res. 2003, 17, 454–458. [Google Scholar] [CrossRef]
- Srivastava, S.K. Role of Capsaicin in Oxidative Stress and Cancer; Springer: Berlin/Heidelberg, Germany, 2013; Volume 3. [Google Scholar]
- Abdel-Salam, O.M.; Abdel-Rahman, R.F.; Sleem, A.A.; Farrag, A.R. Modulation of lipopolysaccharide-induced oxidative stress by capsaicin. Inflammopharmacology 2012, 20, 207–217. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Tominaga, M.; Julius, D. Capsaicin receptor in the pain pathway. Jpn. J. Pharmacol. 2000, 83, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Piyachaturawat, P.; Glinsukon, T.; Toskulkao, C. Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol. Lett. 1983, 16, 351–359. [Google Scholar] [CrossRef]
- Makiyah, S.N.N.; Tasminatun, S.; Arsito, P.N.; Fauziah, K.N.; Nugrahanti, D.R.; Putriani, A. Subchronic toxicity of piperine in piper nigrum on the histology of the kidney, liver, and lungs of mice (Mus musculus L.). Bali Med. J. 2021, 10, 1161–1167. [Google Scholar] [CrossRef]
- Malini, T.; Manimaran, R.; Arunakaran, J.; Aruldhas, M.; Govindarajulu, P. Effects of piperine on testis of albino rats. J. Ethnopharmacol. 1999, 64, 219–225. [Google Scholar] [CrossRef]
- Piyachaturawat, P.; Glinsukon, T.; Peugvicha, P. Postcoital antifertility effect of piperine. Contraception 1982, 26, 625–633. [Google Scholar] [CrossRef]
- Daware, M.B.; Mujumdar, A.M.; Ghaskadbi, S. Reproductive toxicity of piperine in Swiss albino mice. Planta Med. 2000, 66, 231–236. [Google Scholar] [CrossRef]
- Chandhoke, N.; Gupta, S.; Dhar, S. Interceptive activity of various species of Piper, their natural amides and semi-synthetic analogs. Indian J. Pharm. Sci. 1978, 40, 113–116. [Google Scholar]
- Glas, B.; Claeson, A.S. Skin sensitivity to capsaicin, perceived stress and burn out among patients with building-related symptoms. Int. Arch. Occup. Env. Health 2021, 94, 791–797. [Google Scholar] [CrossRef]
- Winek, C.L.; Markie, D.C.; Shanor, S.P. Pepper sauce toxicity. Drug Chem. Toxicol. 1982, 5, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Nopanitaya, W. Effects of capsaicin in combination with diets of varying protein content on the duodenal absorptive cells of the rat. Am. J. Dig. Dis. 1974, 19, 439–448. [Google Scholar] [CrossRef]
- Glinsukon, T.; Stitmunnaithum, V.; Toskulkao, C.; Buranawuti, T.; Tangkrisanavinont, V. Acute toxicity of capsaicin in several animal species. Toxicon 1980, 18, 215–220. [Google Scholar] [CrossRef]
- Chudapongse, P.; Janthasoot, W. Studies on the effect of capsaicin on metabolic reactions of isolated rat liver mitochondria. Toxicol. Appl. Pharmacol. 1976, 37, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Joó, F.; Jancsó-Gábor, A. Mitochondrial changes in preoptic neurons after capsaicin desensitization of the hypothalamic thermodetectors in rats. Nature 1971, 229, 116–117. [Google Scholar] [CrossRef]
- Makara, G.B. Superficial and deep chemonociception: Differential inhibition by pretreatment with capsaicin. Acta Physiol. Acad. Sci. Hung. 1970, 38, 393–399. [Google Scholar]
- Szolcsányi, J.; Jancśo-Gábor, A.; Joo, F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch. Pharmacol. 1975, 287, 157–169. [Google Scholar] [CrossRef]
- Lysy, J.; Sistiery-Ittah, M.; Israelit, Y.; Shmueli, A.; Strauss-Liviatan, N.; Mindrul, V.; Keret, D.; Goldin, E. Topical capsaicin—A novel and effective treatment for idiopathic intractable pruritus ani: A randomised, placebo controlled, crossover study. Gut 2003, 52, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Moser, J.; Phadke, H.; Basha, A.A.; Spencer, S.L. Endogenous replication stress in mother cells leads to quiescence of daughter cells. Cell Rep. 2017, 19, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, K.N.; Hwang, K.G.; Park, C.J. Capsicum plaster at the Hegu point reduces postoperative analgesic requirement after orthognathic surgery. Anesth. Analg. 2009, 108, 992–996. [Google Scholar] [CrossRef]
- Johnson, W., Jr. Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int. J. Toxicol. 2007, 26 (Suppl. S1), 3–106. [Google Scholar]
- Gregersen, N.T.; Belza, A.; Jensen, M.G.; Ritz, C.; Bitz, C.; Hels, O.; Frandsen, E.; Mela, D.J.; Astrup, A. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br. J. Nutr. 2013, 109, 556–563. [Google Scholar] [CrossRef]
- O’Connor, A.; Corbin, K.D.; Nieman, D.C.; Swick, A.G. A randomized, controlled trial to assess short-term black pepper consumption on 24-hour energy expenditure and substrate utilization. Funct. Foods Health Dis. 2013, 3, 377–388. [Google Scholar] [CrossRef][Green Version]
- Hobbs, T.; Caso, R.; McMahon, D.; Nymark, M. A novel, multi-ingredient supplement to manage elevated blood lipids in patients with no evidence of cardiovascular disease: A pilot study. Altern. Ther. Health Med. 2014, 20, 18–23. [Google Scholar]
- Rofes, L.; Arreola, V.; Martin, A.; Clavé, P. Effect of oral piperine on the swallow response of patients with oropharyngeal dysphagia. J. Gastroenterol. 2014, 49, 1517–1523. [Google Scholar] [CrossRef]
- McCrea, C.E.; West, S.G.; Kris-Etherton, P.M.; Lambert, J.D.; Gaugler, T.L.; Teeter, D.L.; Sauder, K.A.; Gu, Y.; Glisan, S.L.; Skulas-Ray, A.C. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: Results of a randomized crossover study and in vitro experiments. J. Transl. Med. 2015, 13, 7. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gilardini, L.; Pasqualinotto, L.; Di Pierro, F.; Risso, P.; Invitti, C. Effects of Greenselect Phytosome® on weight maintenance after weight loss in obese women: A randomized placebo-controlled study. BMC Complement. Altern. Med. 2016, 16, 233. [Google Scholar] [CrossRef]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Ostman, E. Black pepper-based beverage induced appetite-suppressing effects without altering postprandial glycaemia, gut and thyroid hormones or gastrointestinal well-being: A randomized crossover study in healthy subjects. Food Funct. 2018, 9, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, Z.; Shokri, J.; Kamalinejad, M.; Meftah, N.; Khafri, S.; Mozaffarpur, S.A.; Shirafkan, H. The efficacy of a Persian herbal formulation on functional bloating: A double-blind randomized controlled trial. J. Integr. Med. 2019, 17, 344–350. [Google Scholar] [CrossRef]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Oh, E.S.; Petersen, K.S.; Kris-Etherton, P.M.; Rogers, C.J. Spices in a High-Saturated-Fat, High-Carbohydrate Meal Reduce Postprandial Proinflammatory Cytokine Secretion in Men with Overweight or Obesity: A 3-Period, Crossover, Randomized Controlled Trial. J. Nutr. 2020, 150, 1600–1609. [Google Scholar] [CrossRef]
- Pastor, R.F.; Repetto, M.G.; Lairion, F.; Lazarowski, A.; Merelli, A.; Manfredi Carabetti, Z.; Pastor, I.; Pastor, E.; Iermoli, L.V.; Bavasso, C.A.; et al. Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome. Nutrients 2020, 12, 3149. [Google Scholar] [CrossRef]
- Lindheimer, J.B.; Loy, B.D.; O’Connor, P.J. Short-term effects of black pepper (Piper nigrum) and rosemary (Rosmarinus officinalis and Rosmarinus eriocalyx) on sustained attention and on energy and fatigue mood states in young adults with low energy. J. Med. Food 2013, 16, 765–771. [Google Scholar] [CrossRef]
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.; Logan, H.; Kirchner, H.L.; Rothrock, N.; Svengalis, S.; Iverson, K.; Lubaroff, D. Effects of relaxation and stress on the capsaicin-induced local inflammatory response. Psychosom. Med. 2000, 62, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Belza, A.; Jessen, A.B. Bioactive food stimulants of sympathetic activity: Effect on 24-h energy expenditure and fat oxidation. Eur. J. Clin. Nutr. 2005, 59, 733–741. [Google Scholar] [CrossRef][Green Version]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am. J. Clin. Nutr. 2006, 84, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Matsunaga, Y.; Satoh, H.; Takahashi, M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci. Biotechnol. Biochem. 2007, 71, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai 2009, 92, 108–113. [Google Scholar]
- Josse, A.R.; Sherriffs, S.S.; Holwerda, A.M.; Andrews, R.; Staples, A.W.; Phillips, S.M. Effects of capsinoid ingestion on energy expenditure and lipid oxidation at rest and during exercise. Nutr. Metab. 2010, 7, 65. [Google Scholar] [CrossRef]
- Nieman, D.C.; Cialdella-Kam, L.; Knab, A.M.; Shanely, R.A. Influence of red pepper spice and turmeric on inflammation and oxidative stress biomarkers in overweight females: A metabolomics approach. Plant Foods Hum. Nutr. 2012, 67, 415–421. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Martens, E.A.; Westerterp-Plantenga, M.S. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS ONE 2013, 8, e67786. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite 2014, 77, 44–49. [Google Scholar] [CrossRef]
- Galgani, J.E.; Ryan, D.H.; Ravussin, E. Effect of capsinoids on energy metabolism in human subjects. Br. J. Nutr. 2010, 103, 38–42. [Google Scholar] [CrossRef]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; Zhang, Q.Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; John, F.; Thomas, J.V.; Das, S.; Maliakel, B.; Mohan, R.; Krishnakumar, I.M. Influence of a Novel Food-Grade Formulation of Red Chili Extract (Capsicum annum) on Overweight Subjects: Randomized, Double-Blinded, Placebo-Controlled Study. J. Diet. Suppl. 2021, 18, 387–405. [Google Scholar] [CrossRef]
- Giuriato, G.; Venturelli, M.; Matias, A.; Soares, E.; Gaetgens, J.; Frederick, K.A.; Ives, S.J. Capsaicin and Its Effect on Exercise Performance, Fatigue and Inflammation after Exercise. Nutrients 2022, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santana, N.C.F.E.; Rodrigues, H.C.N.; Pereira Martins, T.F.; Braga, C.C.; Silva, M.A.C.; Carlos da Cunha, L.; de Souza Freitas, A.T.V.; Costa, N.A.; Peixoto, M. Turmeric supplementation with piperine is more effective than turmeric alone in attenuating oxidative stress and inflammation in hemodialysis patients: A randomized, double-blind clinical trial. Free Radic. Biol. Med. 2022, 193, 648–655. [Google Scholar] [CrossRef]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L.; et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Mathur, P.; Pillai, R. Overnutrition: Current scenario & combat strategies. Indian J. Med. Res. 2019, 149, 695–705. [Google Scholar] [CrossRef]
- Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Mabhida, S.E.; Jack, B.U.; Nyambuya, T.M.; Nkambule, B.B.; Basson, A.K.; Tiano, L.; Dludla, P.V. Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target. Int. J. Mol. Sci. 2023, 24, 2227. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Tiano, L.; Louw, J.; Jastroch, M.; Mazibuko-Mbeje, S.E. Uncoupling proteins as a therapeutic target to protect the diabetic heart. Pharmacol. Res. 2018, 137, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- National Library of Medicine, Pepper. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=pepper (accessed on 22 June 2022).
- Gong, T.; Wang, H.; Liu, S.; Zhang, M.; Xie, Y.; Liu, X. Capsaicin regulates lipid metabolism through modulation of bile acid/gut microbiota metabolism in high-fat-fed SD rats. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Zeng, H.; Shi, N.; Peng, W.; Yang, Q.; Ren, J.; Yang, H.; Chen, L.; Chen, Y.; Guo, J. Effects of Capsaicin on Glucose Uptake and Consumption in Hepatocytes. Molecules 2023, 28, 5258. [Google Scholar] [CrossRef]
- Song, J.X.; Ren, H.; Gao, Y.F.; Lee, C.Y.; Li, S.F.; Zhang, F.; Li, L.; Chen, H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Kawada, T.; Hagihara, K.; Iwai, K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J. Nutr. 1986, 116, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Zhou, Y.; Zhang, L.; Wang, H.; Zhang, M.; Liu, X. Capsaicin combined with dietary fiber prevents high-fat diet associated aberrant lipid metabolism by improving the structure of intestinal flora. Food Sci. Nutr. 2023, 11, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, X.; Zhang, L.; Sun, H.; Liu, X. Capsaicin Reduces Blood Glucose by Increasing Insulin Levels and Glycogen Content Better than Capsiate in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2017, 65, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).