Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives
Abstract
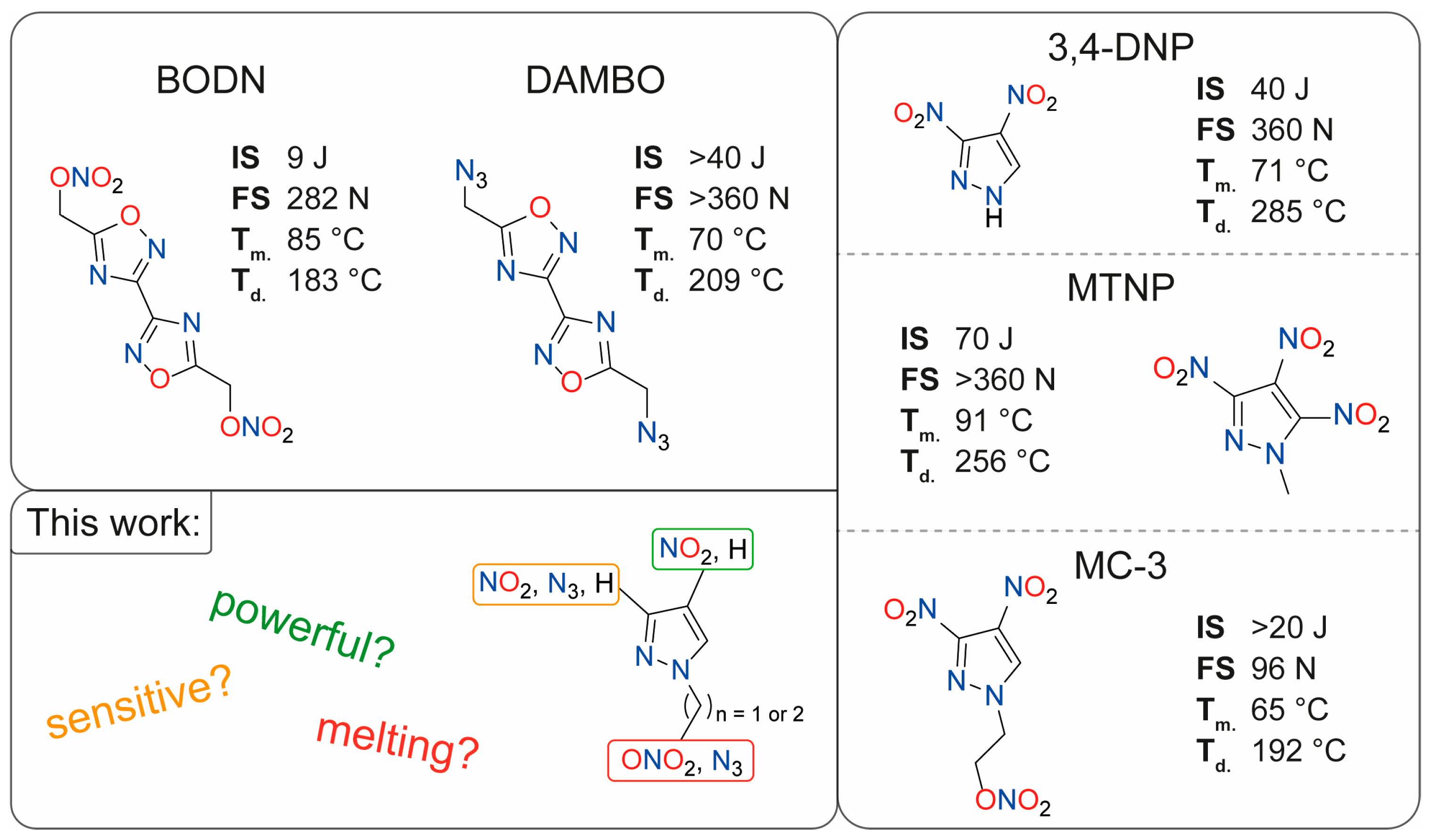
:1. Introduction
2. Results and Discussion
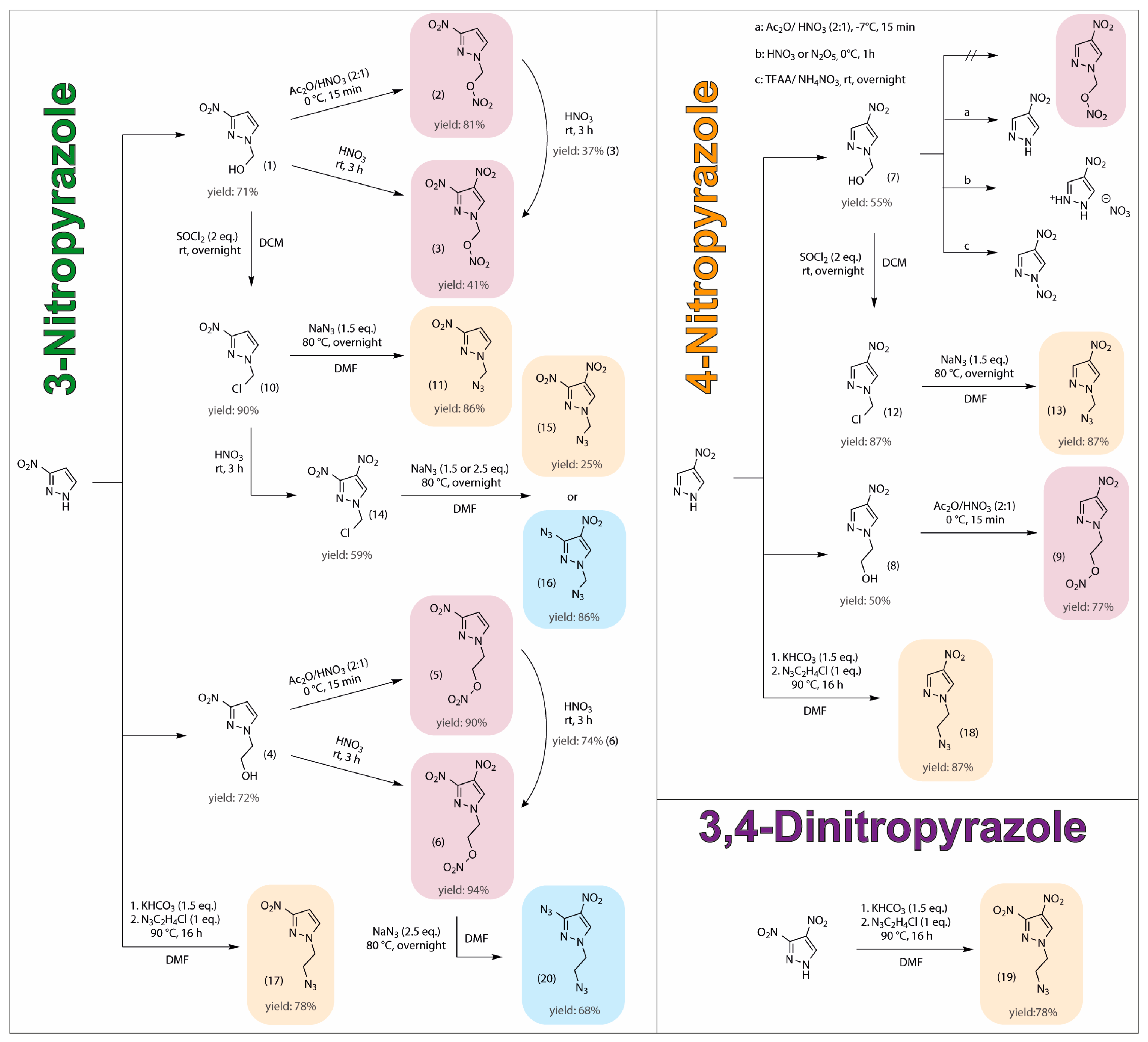
2.1. Synthesis
2.2. Crystal Structures
2.3. Physicochemical Properties
2.4. NMR Spectroscopy
2.5. SSRT (Small-Scale Shock Reactivity Test)
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brinck, T. Green Energetic Materials; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Klapoetke, T.M. Chemistry of High-Energy Materials, 6th ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022. [Google Scholar]
- Agrawal, J.P. High Energy Materials; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Koehler, J.; Meyer, R.; Homburg, A. Explosivstoffe, 10th ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Spencer, A.F. Melt-Castable Explosive Composition. U.S. Patent US4747892A, 31 May 1988. [Google Scholar]
- Wang, S.-W.; Zhang, Y.-L.; Wu, C.; Xiao, L.; Lin, G.-M.; Hu, Y.-B.; Hao, G.-Z.; Guo, H.; Zhang, G.-P.; Jiang, W. Equal-Material Manufacturing of a Thermoplastic Melt-Cast Explosive Using Thermal-Pressure Coupling Solidification Treatment Technology. ACS Omega 2023, 8, 16251–16262. [Google Scholar] [CrossRef] [PubMed]
- Chavez, D.E.; Hiskey, M.A.; Naud, D.L.; Parrish, D. Synthesis of an Energetic Nitrate Ester. Angew. Chem. Int. Ed. 2008, 47, 8307–8309. [Google Scholar] [CrossRef]
- Technical Fact Sheet2–,4,6-Trinitrotoluene (TNT); U.S. Environmental Protection Agency: Washington, DC, USA, 2021.
- Maloney, S.W.; Boddu, V.M.; Phull, K.K.; Hao, O.J. TNT Redwater Treatment by Wet Air Oxidation; US Army Corps of Engeneering: Champaige, IL, USA, 1994.
- Crouse, L.C.B.; Michie, M.W.; Major, M.; Johnson, M.S.; Lee, R.B.; Paulus, H.I. Subchronic Oral Toxicity of RDX in Rats; U.S. Army Center for Health Promotion and Preventive Medicine, Aberdeen Proving Ground: Aberdeen, MD, USA, 2006. [Google Scholar]
- Blessinger, T.; D’Amico, L.; Subramaniam, R.; Brinkerhoff, C. Toxicological Review of RDX; U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- Nair, U.R.; Sivabalan, R.; Gore, G.M.; Geetha, M.; Asthana, S.N.; Singh, H. Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-Based Formulations (Review). Combust. Explos. Shock. Waves 2005, 41, 121–132. [Google Scholar] [CrossRef]
- Fischer, N.; Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the Limits of Energetic Materials–the Synthesis and Characterization of Dihydroxylammonium 5,5′-Bistetrazole-1,1′-Diolate. J. Mater. Chem. 2012, 22, 20418. [Google Scholar] [CrossRef]
- Sabatini, J.J.; Johnson, E.C. A Short Review of Nitric Esters and Their Role in Energetic Materials. ACS Omega 2021, 6, 11813–11821. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, F.; Wang, Y.; Wang, K.; Yan, M.; Zhang, Q. Accelerating the Discovery of Energetic Melt-Castable Materials by a High-Throughput Virtual Screening and Experimental Approach. J. Mater. Chem. A 2021, 9, 21723–21731. [Google Scholar] [CrossRef]
- Johnson, E.C.; Sabatini, J.J.; Chavez, D.E.; Sausa, R.C.; Byrd, E.F.C.; Wingard, L.A.; Guzmàn, P.E. Bis(1,2,4-Oxadiazole)Bis(Methylene) Dinitrate: A High-Energy Melt-Castable Explosive and Energetic Propellant Plasticizing Ingredient. Org. Process Res. Dev. 2018, 22, 736–740. [Google Scholar] [CrossRef]
- Bauer, L.; Benz, M.; Klapötke, T.M.; Lenz, T.; Stierstorfer, J. Polyazido-Methyl Derivatives of Prominent Oxadiazole and Isoxazole Scaffolds: Synthesis, Explosive Properties, and Evaluation. J. Org. Chem. 2021, 86, 6371–6380. [Google Scholar] [CrossRef]
- Ek, S.; Wahlström, L.Y.; Latypov, N. Derivatives of 3(5),4-Dinitropyrazole as Potential Energetic Plasticisers. J. Chem. Chem. Eng. 2011, 5, 929–935. [Google Scholar]
- Bölter, M.F.; Harter, A.; Klapötke, T.M.; Stierstorfer, J. Isomers of Dinitropyrazoles: Synthesis, Comparison and Tuning of Their Physicochemical Properties. ChemPlusChem 2018, 83, 804–811. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Z.; Yang, W.; Li, W.; Ju, J.; Fan, G. Strategies for Constructing Melt-Castable Energetic Materials: A Critical Review. Energetic Mater. Front. 2021, 2, 69–85. [Google Scholar] [CrossRef]
- Benz, M.; Gruhne, M.S.; Klapötke, T.M.; Krüger, N.; Lenz, T.; Lommel, M.; Stierstorfer, J. Evolving the Scope of 5,5’-Azobistetrazoles in the Search for High Performing Green Energetic Materials. Eur. J. Org. Chem. 2021, 2021, 4388–4392. [Google Scholar] [CrossRef]
- Lenz, T.; Klapötke, T.M.; Mühlemann, M.; Stierstorfer, J. About the Azido Derivatives of Pentaerythritol Tetranitrate. Prop. Explos. Pyrotech. 2021, 46, 723–731. [Google Scholar] [CrossRef]
- Lease, N.; Kay, L.M.; Brown, G.W.; Chavez, D.E.; Robbins, D.; Byrd, E.F.C.; Imler, G.H.; Parrish, D.A.; Manner, V.W. Synthesis of Erythritol Tetranitrate Derivatives: Functional Group Tuning of Explosive Sensitivity. J. Org. Chem. 2020, 85, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Tselinskii, I.V.; Tolstyakov, V.V.; Putis, S.M.; Mel’nikova, S.F. Synthesis and Study of Thermal Stability of 3-Nitro-1,2,4-Triazole N-Nitroxy-and N-Azidomethyl Derivatives. Russ. Chem. Bull. 2009, 58, 2356–2361. [Google Scholar] [CrossRef]
- Yuan, L.; Yuji, L.; Wei, H.; Zhiwei, Z.; Hongwei, Y.; Yongxing, T. Synthesis and Characterization of Pyrazole-and Imidazole- Derived Energetic Compounds Featuring Ortho Azido/Nitro Groups. FirePhysChem 2022, 2, 140–146. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Vatsadze, I.A.; Shkineva, T.K.; Kormanov, A.V.; Struchkova, M.I.; Suponitsky, K.Y.; Bragin, A.A.; Monogarov, K.A.; Sinditskii, V.P.; Sheremetev, A.B. Novel Highly Energetic Pyrazoles: N-Trinitromethyl-Substituted Nitropyrazoles. Chem. Asian J. 2015, 10, 1987–1996. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Z.; Lan, D.; Jia, Q.; Liu, N.; Zhang, J.; Kou, K. Recent Advances in Synthesis and Properties of Nitrated-Pyrazoles Based Energetic Compounds. Molecules 2020, 25, 3475. [Google Scholar] [CrossRef]
- Ding, L.; Ge, R.; Wang, P.; Li, D.; Lin, Q.; Lu, M.; Xu, Y. A Series of N -Trinitromethyl-Substituted Polynitro-Pyrazoles: High-Energy-Density Materials with Positive Oxygen Balances. RSC Adv. 2022, 12, 33304–33312. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.; Parrish, D.A.; Shreeve, J.M. Energetic N,N ′-Ethylene-Bridged Bis(Nitropyrazoles): Diversified Functionalities and Properties. Chem. Eur. J. 2014, 20, 16529–16536. [Google Scholar] [CrossRef]
- Reinhardt, E.; Thamm, S.; Stierstorfer, J.; Klapötke, T.M. Alkyl-Bridged Nitropyrazoles–Adjustment of Performance and Sensitivity Parameters. Eur. J. Org. Chem. 2023, 26, e202300304. [Google Scholar] [CrossRef]
- Chinnam, A.K.; Yu, Q.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Azo- and Methylene-Bridged Mixed Azoles for Stable and Insensitive Energetic Applications. Dalton. Trans. 2020, 49, 11498–11503. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.W.; Chavez, D.E.; Hanson, S.K.; Scharff, R.J.; Scott, B.L.; Veauthier, J.M.; Wu, R. Independent Control of Optical and Explosive Properties: Pyrazole–Tetrazine Complexes of First Row Transition Metals. Inorg. Chem. 2015, 54, 8077–8086. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xiong, H.; Yang, H.; Tang, J.; Cheng, G. Nitropyrazole Based Tricyclic Nitrogen-Rich Cation Salts: A New Class of Promising Insensitive Energetic Materials. FirePhysChem 2021, 1, 71–75. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Parrish, D.A.; Shreeve, J.M. 4-Chloro-3,5-Dinitropyrazole: A Precursor for Promising Insensitive Energetic Compounds. J. Mater. Chem. A 2013, 1, 2863. [Google Scholar] [CrossRef]
- Lei, C.; Yang, H.; Cheng, G. New Pyrazole Energetic Materials and Their Energetic Salts: Combining the Dinitromethyl Group with Nitropyrazole. Dalton Trans. 2020, 49, 1660–1667. [Google Scholar] [CrossRef]
- Gross, R.S.; Guo, Z.; Dyck, B.; Coon, T.; Huang, C.Q.; Lowe, R.F.; Marinkovic, D.; Moorjani, M.; Nelson, J.; Zamani-Kord, S.; et al. Design and Synthesis of Tricyclic Corticotropin-Releasing Factor-1 Antagonists. J. Med. Chem. 2005, 48, 5780–5793. [Google Scholar] [CrossRef]
- Meng, W.; Cheng, P.T.W. Noval Glucokinase Activators and Methods of Using Same. WO2011/130459A1, 20 October 2011. [Google Scholar]
- Treu, M.; Zahn, S. Karl New 5,8-Dimethyl-9-Phenyl-5,8-Dihydro-6H-Pyrazolo[3,4-H]Quinazolin-2-Yl)-(1H-Pyrazol-3-Yl)-Amines and Derivatives as IGF-1 R/IR Inhibitors. WO2016/180843A1, 17 November 2016. [Google Scholar]
- Machin, P.J. Substituierte Phenoxy-Aminopropanol Derivate. EP0034831A2, 2 September 1981. [Google Scholar]
- Dalinger, I.; Vatsadze, I.; Shkineva, T.; Popova, G.; Shevelev, S. Efficient Procedure for High-Yield Synthesis of 4-Substituted 3,5-Dinitropyrazoles Using 4-Chloro-3,5-Dinitropyrazole. Synthesis 2012, 44, 2058–2064. [Google Scholar] [CrossRef]
- Bauer, L.; Kirchhoff, L.; Stierstorfer, J.; Klapötke, T.M. Development of an Azidoethyl-Transfer Reaction Protocol for Azoles. In Proceedings of the 25th NTREM Seminar, Pardubice, Czech Repuplic, 19–21 April 2023. [Google Scholar]
- CCDC Database. Available online: https://www.ccdc.cam.ac.uk/structures (accessed on 27 July 2023).
- Cottrell, T.L. The Strengths of Chemical Bonds, 2nd ed.; Butterworths: London, UK, 1958. [Google Scholar]
- Klapötke, T.M. Energetic Materials Encyclopedia; 2. Auflage-; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; ISBN 978-3-11-067465-1. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; 4. rev.enl. ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93809-5. [Google Scholar]
- Cabildo, P.; Claramunt, R.M.; Elguero, J. 13C NMR Chemical Shifts OfN-Unsubstituted- AndN-Methyl-Pyrazole Derivatives. Org. Magn. Reson. 1984, 22, 603–607. [Google Scholar] [CrossRef]
- Viesser, R.V.; Ducati, L.C.; Tormena, C.F.; Autschbach, J. The Unexpected Roles of σ and π Orbitals in Electron Donor and Acceptor Group Effects on the 13 C NMR Chemical Shifts in Substituted Benzenes. Chem. Sci. 2017, 8, 6570–6576. [Google Scholar] [CrossRef]
- Sandusky, H.W.; Granholm, R.H.; Bohl, D.G. Small-Scale Shock Reactivity Test (SSRT); Naval Surfave Warfare Center, Indian Head Division: Charles County, MD, USA, 2005.
- Bauer, L.; Benz, M.; Klapötke, T.M.; Selmeier, A. Evaluation of SSRT-Test by Classical Gravimetric Analysis and Optical Topographic Measurement: A Comparative Study. Propellants Explos. Pyrotech. 2022, 47, e202200113. [Google Scholar] [CrossRef]
- Bauer, L.; Benz, M.; Klapötke, T.M. Linear Correlation Between Confined Explosive Quantity and Dent Volume of an Underlying Aluminium Block Using the SSRT Setup. Propellants Explos. Pyrotech. 2022, 47, e202100332. [Google Scholar] [CrossRef]
- CrysAlisPro Software; Version 171.33.41; Rigaku Corporation: Wroclaw, Poland, 2009; Available online: http://www.rigaku.com (accessed on 4 July 2023).
- Sheldrick, G.M. SHELXT Integrated space-group and structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A Complete Structure Solution, Refinement and Analysis Programm. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SCALE3 ABSPACK—An Oxford Diffraction Program; Version 1.0.4; Rigaku Corporation: Wroclaw, Poland, 2009; Available online: http://www.rigaku.com (accessed on 4 July 2023).
- APEX3; Version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2018; Available online: http://www.bruker.com (accessed on 4 July 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.04; Gaussian, Inc: Wallingford, CT, USA, 2016. [Google Scholar]
- Ochterski, J.W.; Petersson, G.A.; Montgomery, J.A. A complete basis set model chemistry. V. Extensions to sex or more heavy atoms. J. Chem. Phys. 1996, 104, 2598–2619. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 12, 6532–6542. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J. Chem. Phys. 1997, 106, 1063–1079. [Google Scholar] [CrossRef]
- Rice, B.M.; Pai, S.V.; Hare, J. Predicting heats of formation of energetic materials using quantum mechanical calculations. Combust. Flame 1999, 118, 445–458. [Google Scholar] [CrossRef]
- Byrd, E.F.C.; Rice, B.M. Improved Prediction of Heats of Formation of Energetic Materials Using Quantum Mechanical Calculations. J. Phys. Chem. A 2006, 110, 1005–1013. [Google Scholar] [CrossRef]
- Linstrom, J.; Mallard, W.G. National Institute of Standards and Technology Standard Reference Database Number 69. Available online: http://www.webbook.nist.gov/chemistry (accessed on 4 July 2022).
- Wahler, S.; Chung, P.; Klapoetke, T.M. Training machine learning models based on the structural formula for the enthalpy of vaporization and sublimation and a thorough analysis of Trouton’s rules. J. Energ. Mater. 2023. [Google Scholar] [CrossRef]
- STANAG 4147; Chemical Compatibility of Ammunition Components with Explosives (Non-Nuclear Application). NATO: Brussels, Belgium, 2001.
- Koehler, J.; Meyer, R.; Homburg, A. Explosivstoffe, 6th ed.; Wiley: Weinheim, Germany, 2007. [Google Scholar]
- Bundesanstalt für Materialforschung und -Prüfung. Available online: http://www.bam.de (accessed on 4 July 2023).
- STANAG 4489; Explosives, Impact Sensitivity Tests. NATO: Brussels, Belgium, 1999.
- Standardarbeitsanweisung 4-5.1.02; Ermittlung der Explosionsgefährlichkeit, hier der Schlagempfindlichkeit mit dem Fallhammer. WIWEB: Erding, Germany, 2002.
- STANAG 4489; Explosives, Friction Sensitivity Tests. NATO: Brussels, Belgium, 2002.
- Standardarbeitsanweisung 4-5.1.03; Ermittlung der Explosionsgefährlichkeit oder der Reibeempfindlichkeit mit dem Reibeapparat. WIWEB: Erding, Germany, 2002.
- OZM Research. Available online: http://www.ozm.cz (accessed on 4 July 2023).
- Impact: Insensitive > 40 J, Less Sensitive ≤ 35 J, Sensitive ≤ 4 J, Very Sensitive ≤ 3 J; Friction: Insensitive > 360 N, Less Sensitive = 360 N, Sensitive 80 N, Very Sensitive ≤ 80 N, Extreme Sensitive ≤ 10 N; According to the UN Recommendations on the Transport of Dangerous Goods (+) Indicates: Not Safe for Transport. Available online: https://www.un-ilibrary.org/content/books/9789210019057 (accessed on 4 July 2023).



| IS a (J) | FS b (N) | Tmelt c (°C) | Texo. d (°C) | ρ e (g/cm3) | ΔfH° f (kJ/mol) | DC-J g (m/s) | pC-J h (GPa) | |
|---|---|---|---|---|---|---|---|---|
| (2) | >40 | >360 | 70 | 161 | 1.696 | −20.2 | 7675 | 24.3 |
| (3) | 4 | 288 | 93 | 159 | 1.848 | 8.7 | 8668 | 33.6 |
| (5) | >40 | >360 | 78 | 198 | 1.629 | −67.1 | 7163 | 19.8 |
| (6) | 30 | >360 | 61 | 198 | 1.734 | −43.1 | 7932 | 26.7 |
| (9) | >40 | >360 | 52 | 191 | 1.613 | −65.0 | 7110 | 19.5 |
| (11) | >40 | >360 | 40 | 179 | 1.513 | 401.8 | 6972 | 19.9 |
| (13) | >40 | >360 | 42 | 174 | 1.547 | 398.7 | 7100 | 19.8 |
| (15) i | >40 | >360 | / | 154 | 1.58 j | 433.9 | 7679 | 23.1 |
| (17) i | >40 | >360 | / | 214 | 1.28 j | 369.6 | 6235 | 11.9 |
| (18) i | >40 | >360 | / | 214 | 1.34 j | 372.1 | 6483 | 13.3 |
| (19) | 25 | >360 | 50 | 216 | 1.664 | 382.5 | 7639 | 22.9 |
| (16) | <1 | 10 | 57 | 157 | 1.670 | 755.8 | 7945 | 24.4 |
| (20) | 2 | 80 | 41 | 172 | 1.554 | 707.4 | 7274 | 20.1 |
| TNT | 15 | >360 | 81 | 289 | 1.65 | −185 | 6950 | 20.5 |
| (3) | (6) | PETN | HMX | |
|---|---|---|---|---|
| Dent volume | 1216.36 | 1135.83 | 1107.81 | 1212.39 |
| m (g) a | 496 | 447 | 478 | 511 |
| ρ (g/cm3) b | 1.848 | 1.664 | 1.778 | 1.904 |
| PCJ (kbar) c | 34.2 | 27.3 | 30.8 | 37.8 |
| Vdet (m/s) d | 8734 | 8004 | 8429 | 9193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhardt, E.; Lenz, T.; Bauer, L.; Stierstorfer, J.; Klapötke, T.M. Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives. Molecules 2023, 28, 6489. https://doi.org/10.3390/molecules28186489
Reinhardt E, Lenz T, Bauer L, Stierstorfer J, Klapötke TM. Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives. Molecules. 2023; 28(18):6489. https://doi.org/10.3390/molecules28186489
Chicago/Turabian StyleReinhardt, Elena, Tobias Lenz, Lukas Bauer, Jörg Stierstorfer, and Thomas M. Klapötke. 2023. "Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives" Molecules 28, no. 18: 6489. https://doi.org/10.3390/molecules28186489
APA StyleReinhardt, E., Lenz, T., Bauer, L., Stierstorfer, J., & Klapötke, T. M. (2023). Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives. Molecules, 28(18), 6489. https://doi.org/10.3390/molecules28186489








