Variation of Sequential Ligandrol (LGD-4033) Metabolite Levels in Routine Anti-Doping Urine Samples Detected with or without Other Xenobiotics
Abstract
:1. Introduction
2. Results and Discussion
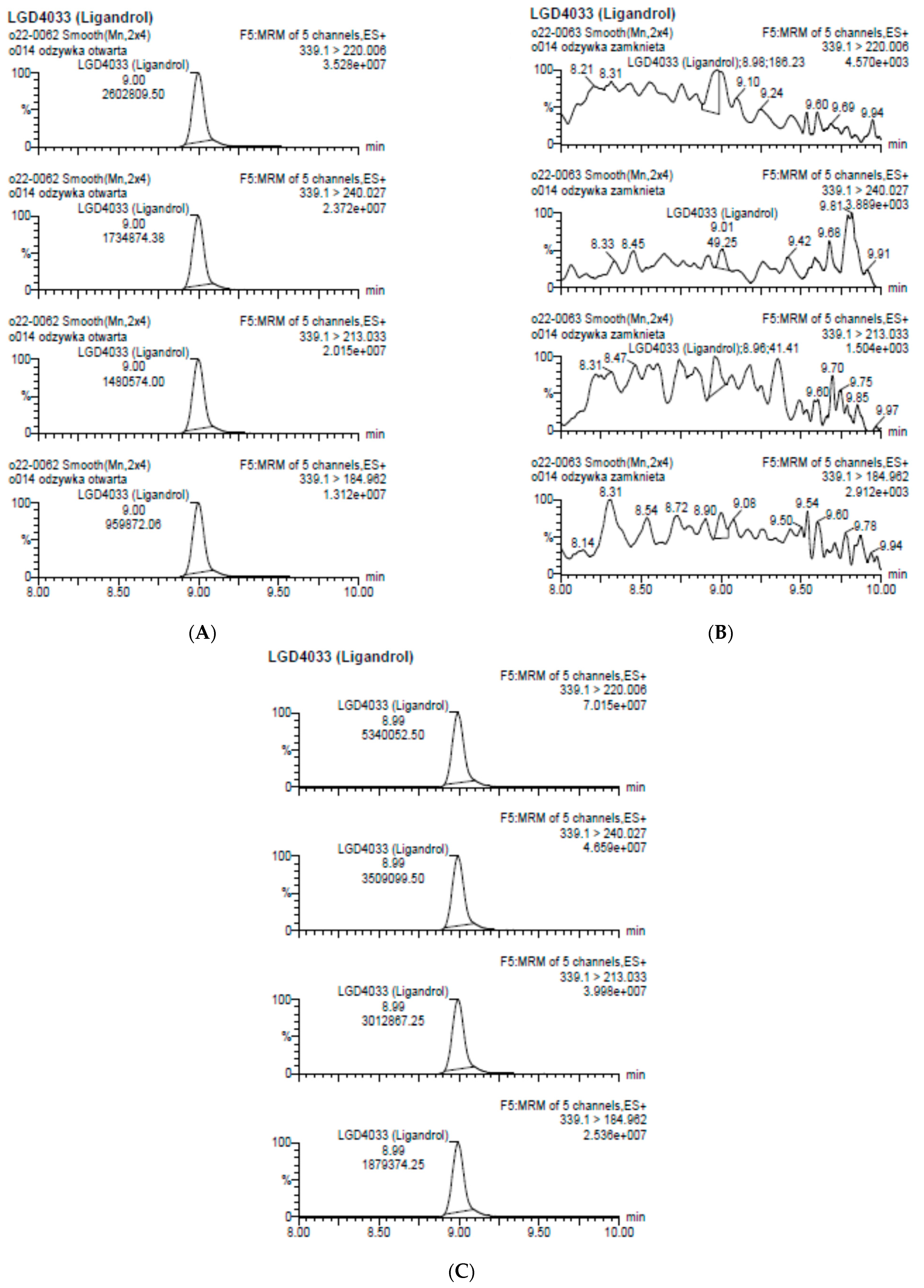
2.1. LGD-4033 Metabolites
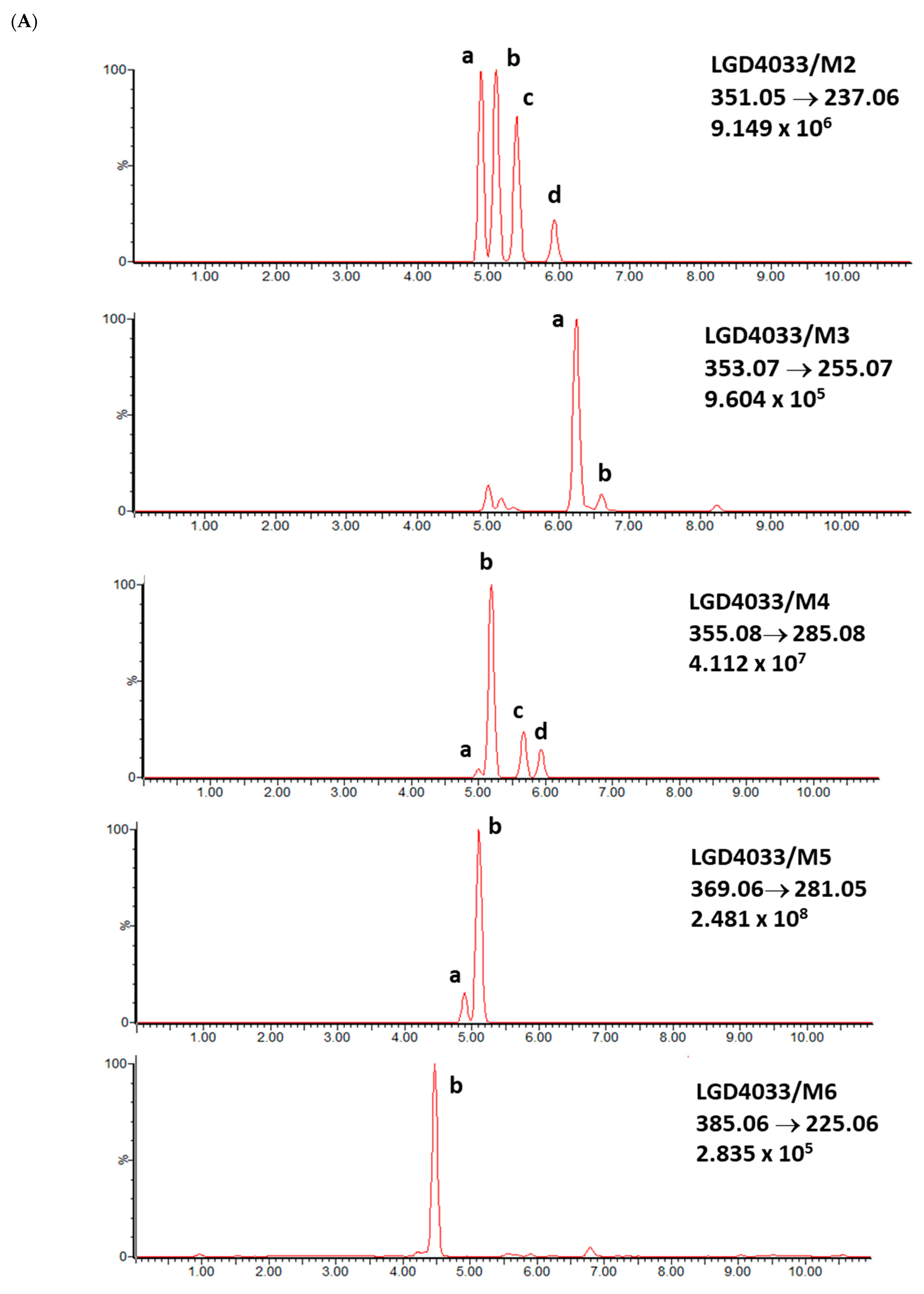

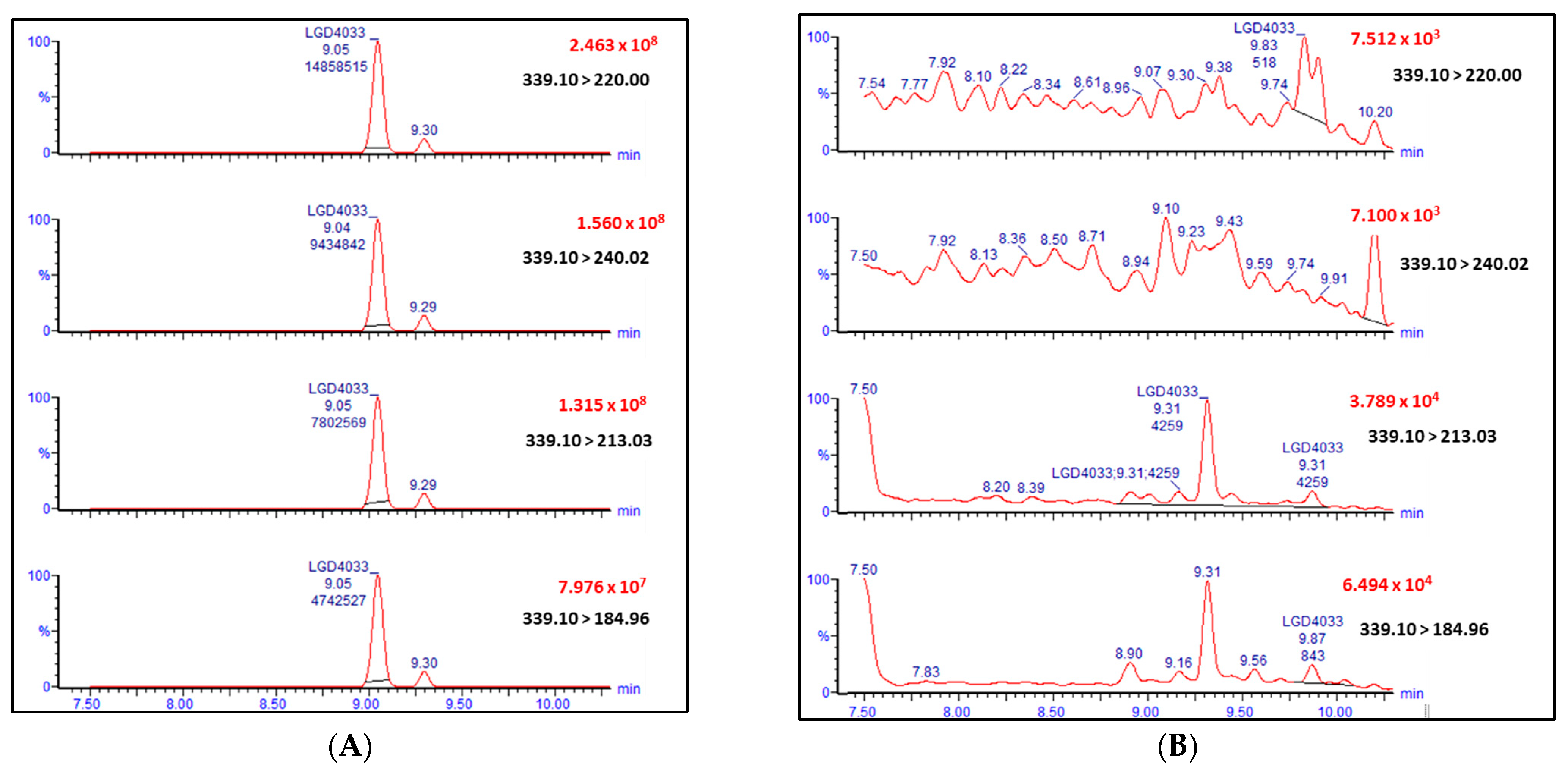
2.2. Real Samples and Other Prohibited Substances in Sport
2.3. Analysis of Dietary Supplements for the Purposes of Disciplinary Proceedings
3. Materials and Methods
3.1. Chemical and Materials
3.2. Sample Preparation
3.2.1. Urine Samples
3.2.2. Dietary Supplements
3.3. Instrumental Analysis
3.3.1. Liquid Chromatography
3.3.2. Mass Spectrometry
3.3.3. Assay Characterization
3.3.4. Linearity, LOD, LOQ
3.3.5. Relative Extraction Efficiency
3.3.6. Selectivity, Sample Carry-Over, and Method Robustness Control
3.3.7. Intra-Laboratory Repeatability and Reproducibility of the Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Esposito, M.; Salerno, M.; Calvano, G.; Agliozzo, R.; Ficarra, V.; Sessa, F.; Favilla, V.; Cimino, S.; Pomara, C. Impact of anabolic androgenic steroids on male sexual and reproductive function: A systematic review. Panminerva Med. 2023, 65, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Linhares, B.L.; Miranda, E.P.; Cintra, A.R.; Reges, R.; Torres, L.O. Use, Misuse and Abuse of Testosterone and Other Androgens. Sex Med. Rev. 2022, 10, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Krishnan, V.; Storer, T.W.; Steiner, M.; Dobs, A.S. Androgens and Selective Androgen Receptor Modulators to Treat Functional Limitations Associated with Aging and Chronic Disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78 (Suppl. 1), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Vignali, J.D.; Pak, K.C.; Beverley, H.R.; DeLuca, J.P.; Downs, J.W.; Kress, A.T.; Sadowski, B.W.; Selig, D.J. Systematic Review of Safety of Selective Androgen Receptor Modulators in Healthy Adults: Implications for Recreational Users. J. Xenobiot. 2023, 13, 218–236. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency. The 2018, Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/prohibited_list_2018_en.pdf (accessed on 26 July 2023).
- Thevis, M.; Lagojda, A.; Kuehne, D.; Thomas, A.; Dib, J.; Hansson, A.; Hedeland, M.; Bondesson, U.; Wigger, T.; Karst, U.; et al. Characterization of a non-approved selective androgen receptor modulator drug candidate sold via the Internet and identification ofinvitrogenerated phase-I metabolites for human sports drug testing. Rapid Commun. Mass Spectrom. 2015, 29, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.; Knych, H.; Stanley, S.; Berndtson, E.; Jackson, L.; Bondesson, U.; Thevis, M.; Hedeland, M. Equine in vivo-derived metabolites of the SARM LGD-4033, and comparison with human and fungal metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1074, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Geldof, L.; Pozo, O.J.; Lootens, L.; Morthier, W.; Van Eenoo, P.; Deventer, K. In vitro metabolism study of a black market product containing SARM LGD-4033. Drug Test. Anal. 2017, 9, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.D.; Eichner, D. Detection of LGD-4033, and its metabolites in athlete urine samples. Drug Test. Anal. 2017, 9, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Pitsinos, E.N.; Angelis, Y.S.; Petrou, M. Structure revision and chemical synthesis of ligandrol’s main bishydroxylated long-term metabolic marker. Org. Biomol. Chem. 2022, 20, 9112–9116. [Google Scholar] [CrossRef] [PubMed]
- Fragkaki, A.G.; Sakellariou, P.; Kiousi, P.; Kioukia-Fougia, N.; Tsivou, M.; Petrou, M.; Angelis, Y. Human in vivo metabolism study of LGD-4033. Drug Test. Anal. 2018, 10, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency. The 2023, Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/2023-05/2023list_en_final_9_september_2022.pdf (accessed on 26 July 2023).
- World Anti-Doping Agency. WADA Technical Document—TD2023IDCR—Minimum Criteria for Chromatographic-Mass Spectrometric Confirmation of the Identity of Analytes for Doping Control Purposes. Available online: https://www.wada-ama.org/sites/default/files/2023-02/td2023idcrv1.1_eng_final.pdf (accessed on 26 July 2023).
- Wagener, F.; Guddat, S.; Görgens, C.; Angelis, Y.S.; Petrou, M.; Lagojda, A.; Kühne, D.; Thevis, M. Investigations into the elimination profiles and metabolite ratios of micro-dosed selective androgen receptor modulator LGD-4033, for doping control purposes. Anal. Bioanal. Chem. 2022, 414, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Martinez Brito, D.; de la Torre, X.; Botrè, F. Detection of urinary metabolites of arimistane in humans by gas chromatography coupled to high-accuracy mass spectrometry for antidoping analyses. Rapid Commun. Mass Spectrom. 2019, 33, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Martinez Brito, D.; Leogrande, P.; Botrè, F.; de la Torre, X. Detection of urinary arimistane metabolites in humans using liquid chromatography-mass spectrometry: Complementary results to gas chromatography mass spectrometric data and its application to antidoping analyses. Rapid Commun. Mass Spectrom. 2021, 35, e9080. [Google Scholar] [CrossRef] [PubMed]
- Sobolevsky, T.; Prasolov, I.; Rodchenkov, G. Urinary metabolism of ibutamoren, a small molecule growth hormone secretagogue. Recent Adv. Doping Anal. 2013, 21, 182–186. [Google Scholar]
- Walpurgis, K.; Rubio, A.; Wagener, F.; Krug, O.; Knoop, A.; Görgens, C.; Guddat, S.; Thevis, M. Elimination profiles of micro dosed ostarine mimicking contaminated products ingestion. Drug Test. Anal. 2020, 12, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Grucza, K.; Kowalczyk, K.; Wicka, M.; Szutowski, M.; Bulska, E.; Kwiatkowska, D. The use of valid and straightforward method for the identification of higenamine in dietary supplements in view of the anti-doping rule violation cases. Drug Test. Anal. 2019, 11, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Grucza, K.; Wicka, M.; Drapała, A.; Kwiatkowska, D. Determination of Ecdysterone in Dietary Supplements and Spinach by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations 2022, 9, 8. [Google Scholar] [CrossRef]
- Kwiatkowska, D.; Kowalczyk, K.; Grucza, K.; Szutowski, M.; Bulska, E.; Wicka, M. Detection of bemitil and its metabolite in urine by means of LC-MS/MS in view of doping control analysis. Drug Test. Anal. 2018, 10, 1682–1688. [Google Scholar] [CrossRef] [PubMed]

| Compound | RE (%) (Mean) | Precision (%RSD) |
|---|---|---|
| LGD-4033 | 89.91 | 7.40 |
| Compound | Urine | ||||||
|---|---|---|---|---|---|---|---|
| 1C | 2C | 3C | 4C | 5C | 6C | %RSD | |
| R2 | |||||||
| LD-4033 | 0.9993 | 0.9978 | 0.9996 | 0.9943 | 0.9982 | 0.9969 | 0.19 |
| Substance | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|
| LGD-4033 | 0.5 | 0.80 |
| Measurements over One Day | |||
|---|---|---|---|
| Compound | Concentration (ng/mL) | Precision (%RSD) | Accuracy (%) |
| LGD-4033 | 0.500 | 10.73 | 8.33 |
| 0.750 | 5.96 | 0.00 | |
| 1 | 4.29 | 2.50 | |
| 2 | 2.12 | 1.25 | |
| 5 | 5.72 | 2.50 | |
| 10 | 1.21 | 0.33 | |
| Analyte | Proposed Chemical Structure | Metabolic Transformation | Precursor Ion (m/z) [M]+ Parent Compound and [M]− Metabolites | Product Ion (m/z) | Retention Times (min) Determined Experimentally |
|---|---|---|---|---|---|
| LGD-4033 | 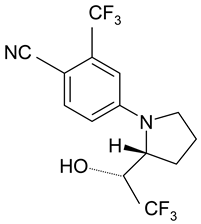 | 339.10 | 220.00; 240.02; 213.03; 184.96 | 9.05 | |
| M2-a |  | Hydroxylation, Dehydrogenation | 351.05 | 237.06; 253.02; 281.05 | 4.90 |
| M2-b | 237.06; 253.02; 281.05 | 5.11 | |||
| M2-c | 237.06; 253.02; 281.05 | 5.40 | |||
| M2-d | 237.06; 253.02; 281.05 | 5.93 | |||
| M3-a |  | Hydroxylation | 353.07 | 255.07; 199.04; 185.03 | 6.25 |
| M3-b | 255.07; 185.03 | 6.40 | |||
| M4-a | 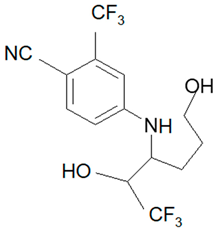 | Hydroxylation, Ring cleavage | 355.08 | 285.08; 257.09; 185.03 | 5.00 |
| M4-b M4-c | 285.08; 257.09; 185.03 | 5.19 | |||
| 285.08; 185.03 | 5.67 | ||||
| M4-d | 285.08; 185.03 | 5.93 | |||
| M5-a | 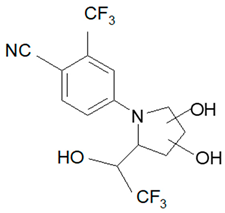 | bis-Hydroxylation | 369.06 | 281.05; 237.06; 253.05 | 4.89 |
| M5-b | 281.05; 237.06; 255.07 | 5.10 | |||
| M6-b | 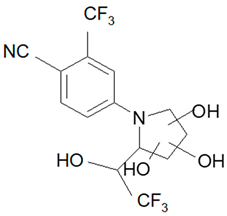 | tri-Hydroxylation | 385.06 | 225.06; 227.04 | 4.48 |
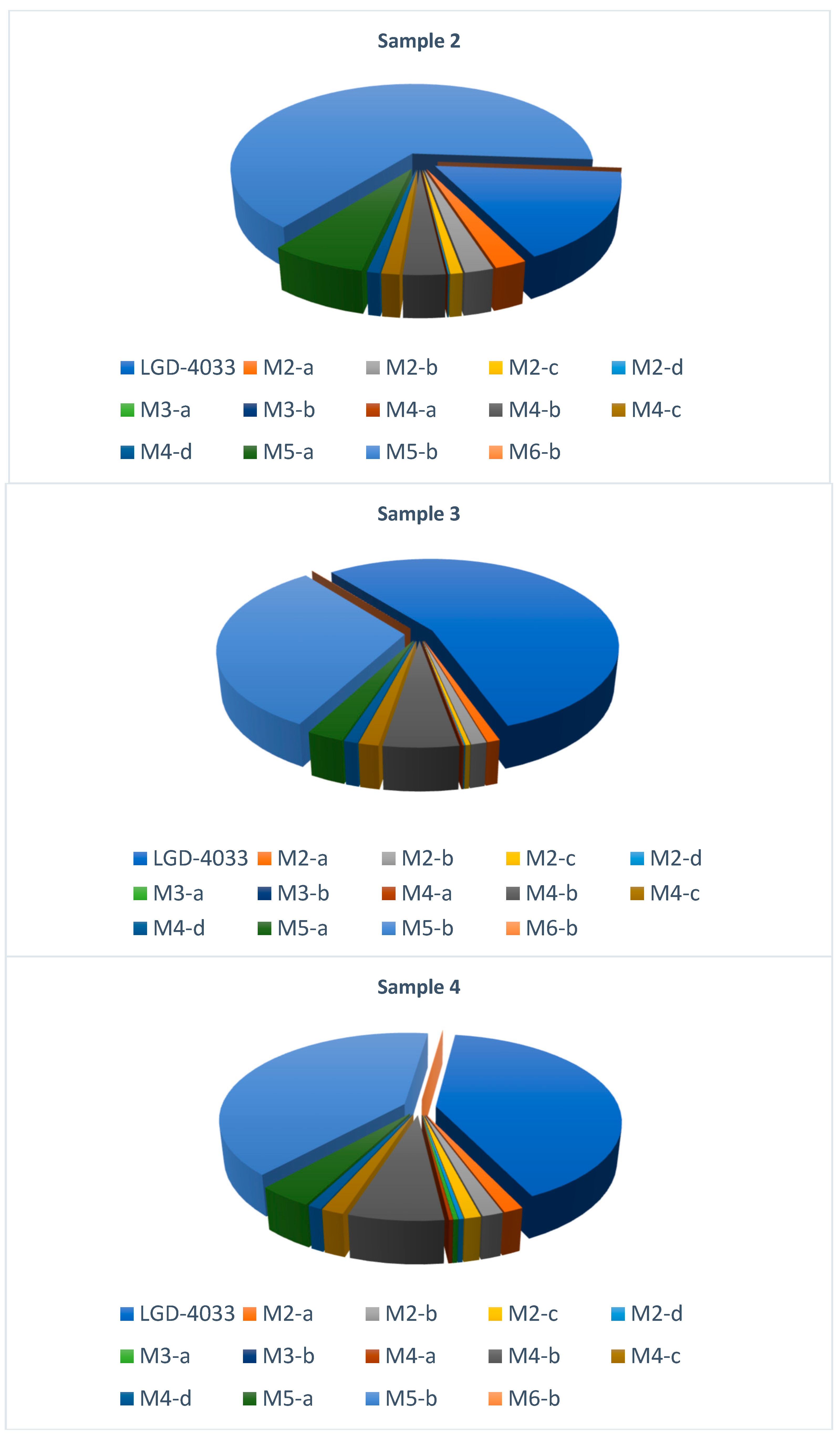
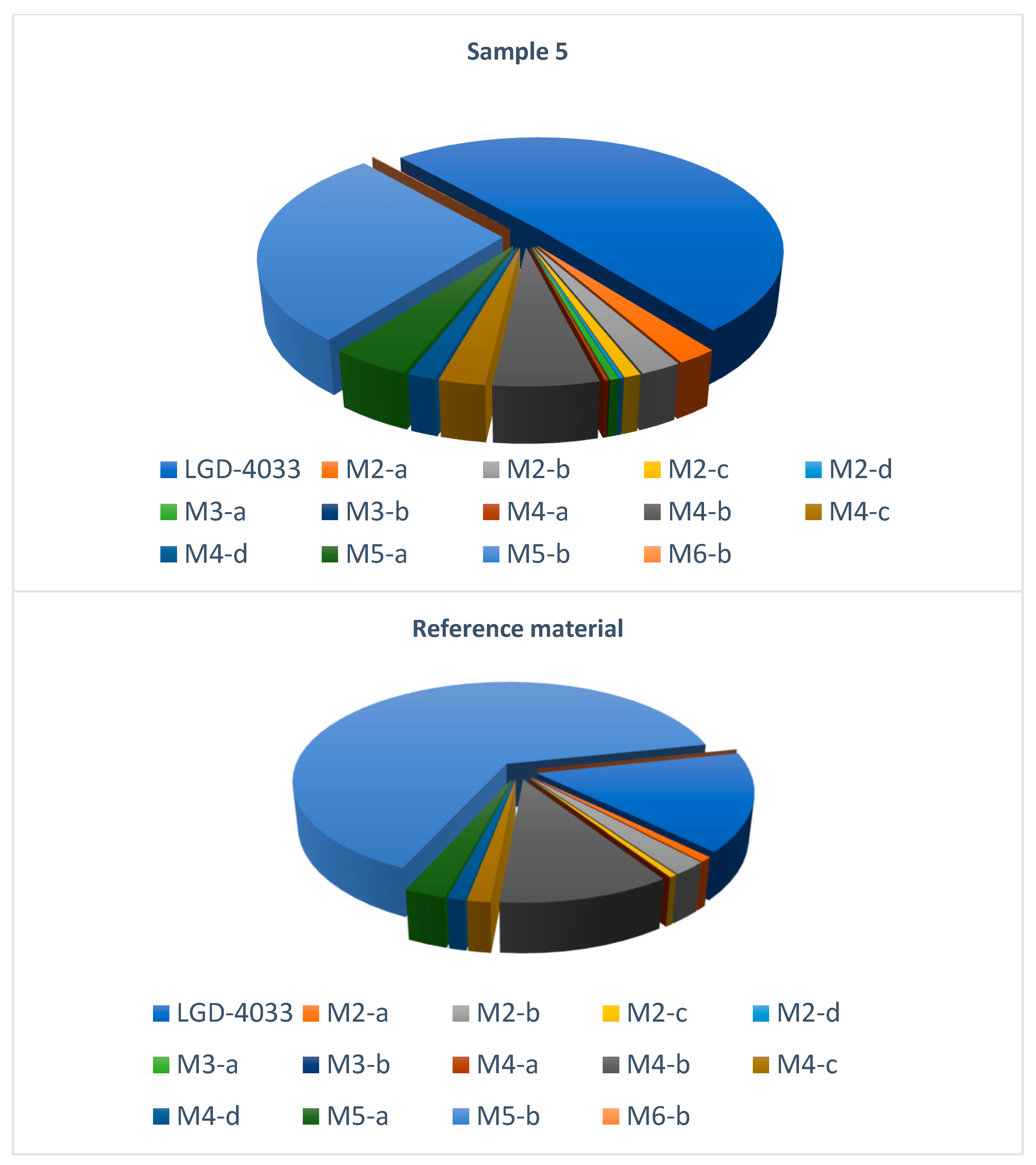
| Analyte | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|
| LGD-4033 | 45.5 | 1.45 | 324 | 125 | 545 |
| M2-a | + | + | + | + | + |
| M2-b | + | + | + | + | + |
| M2-c | + | + | + | + | + |
| M2-d | + | + | + | + | + |
| M3-a | - | - | - | + | + |
| M3-b | - | - | - | - | + |
| M4-a | - | - | + | + | + |
| M4-b | + | + | + | + | + |
| M4-c | + | + | + | + | + |
| M4-d | + | + | + | + | + |
| M5-a | + | + | + | + | + |
| M5-b | + | + | + | + | + |
| M6-b | + | - | + | + | + |
| Analyte | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Reference Material | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC/PC | TC/ISTD | TC/PC | TC/ISTD | TC/PC | TC/ISTD | TC/PC | TC/ISTD | TC/PC | TC/ISTD | TC/PC | TC/ISTD | |
| LGD-4033 | 1 | 1.7625 | 1 | 0.1498 | 1 | 28.0490 | 1 | 6.8591 | 1 | 101.9872 | 1 | 0.0831 |
| M2-a | 0.0594 | 0.0886 | 0.1624 | 0.0213 | 0.0199 | 0.4629 | 0.0434 | 0.2437 | 0.0756 | 4.5054 | 0.0514 | 0.0042 |
| M2-b | 0.0534 | 0.0796 | 0.1464 | 0.0192 | 0.0246 | 0.5727 | 0.0462 | 0.2594 | 0.0764 | 4.5539 | 0.1406 | 0.0116 |
| M2-c | 0.0338 | 0.0504 | 0.0628 | 0.0082 | 0.0075 | 0.1734 | 0.0358 | 0.2012 | 0.0313 | 1.8633 | 0.0330 | 0.0027 |
| M2-d | 0.0069 | 0.0103 | 0.0073 | 0.0010 | 0.0023 | 0.0539 | 0.0106 | 0.0595 | 0.0086 | 0.5103 | - | - |
| M3-a | - | - | - | - | - | - | 0.0109 | 0.0610 | 0.0168 | 1.0008 | - | - |
| M3-b | - | - | - | - | - | - | - | - | 0.0010 | 0.0621 | - | - |
| M4-a | - | - | - | - | 0.0020 | 0.0458 | 0.0082 | 0.0462 | 0.0079 | 0.4706 | - | - |
| M4-b | 0.2818 | 0.4205 | 0.2131 | 0.0279 | 0.1189 | 2.7653 | 0.2082 | 1.1704 | 0.1968 | 11.7233 | 0.6973 | 0.0574 |
| M4-c | 0.0467 | 0.0697 | 0.0918 | 0.0120 | 0.0317 | 0.7381 | 0.0492 | 0.2766 | 0.0865 | 5.1555 | 0.0943 | 0.0078 |
| M4-d | 0.0245 | 0.0365 | 0.0643 | 0.0084 | 0.0216 | 0.5023 | 0.0303 | 0.1704 | 0.0536 | 3.1921 | 0.0718 | 0.0059 |
| M5-a | 0.1902 | 0.2838 | 0.4915 | 0.0644 | 0.0588 | 1.3668 | 0.1207 | 0.6784 | 0.1479 | 8.8091 | 0.1684 | 0.0139 |
| M5-b | 1.5928 | 2.3765 | 4.5244 | 0.5928 | 0.6918 | 16.0886 | 1.2134 | 6.8203 | 0.9451 | 56.3030 | 4.2050 | 0.3463 |
| M6-b | 0.0031 | 0.0047 | - | - | 0.0011 | 0.0256 | 0.0014 | 0.0081 | 0.0035 | 0.2115 | - | - |
| Compounds Detected | Estimated Concentration (ng/mL) |
|---|---|
| Sample 1 | |
| Arimistane (Androst-3,5-diene-7,17-dione) [15] | 110.65 |
| Arimistane M (Androst-3,5-diene-7β-ol-17-one) [16] | 454.38 |
| Ibutamoren (MK-677) | 44.46 |
| Hydroxyibutamoren M1 [17] | not determined |
| Hydroxyibutamoren M2 [17] | not determined |
| Dihydroxyibutamoren M3 [17] | not determined |
| Desbenzylibutamoren M4 [17] | not determined |
| Ostarine | 924.42 |
| o-Dephenylostarine | 11.14 |
| Hydroxyostarine M1a [18] | not determined |
| Hydroxyostarine M1b [18] | not determined |
| Sample 3 | |
| Ibutamoren (MK-677) | 0.13 |
| Sample 5 | |
| Ibutamoren (MK-677) | 203.25 |
| Dehydrochlormethyltestosterone M3 (4-chloro-18-nor-17β-hydroxymethyl,17α-methyl-5α-androst-13-en-3α-ol) | 1.1 |
| LGD-4033 | 544.86 |
| LGD-4033 M5a (bis-hydroxylation) | not determined |
| Ostarine | 1.03 |
| RAD140 | 844.97 |
| Nandrolone M (19-norandrosterone) | 3.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, D.; Wicka, M.; Grucza, K.; Konarski, P.; Drapała, A.; Kaliszewski, P. Variation of Sequential Ligandrol (LGD-4033) Metabolite Levels in Routine Anti-Doping Urine Samples Detected with or without Other Xenobiotics. Molecules 2023, 28, 6486. https://doi.org/10.3390/molecules28186486
Kwiatkowska D, Wicka M, Grucza K, Konarski P, Drapała A, Kaliszewski P. Variation of Sequential Ligandrol (LGD-4033) Metabolite Levels in Routine Anti-Doping Urine Samples Detected with or without Other Xenobiotics. Molecules. 2023; 28(18):6486. https://doi.org/10.3390/molecules28186486
Chicago/Turabian StyleKwiatkowska, Dorota, Mariola Wicka, Krzysztof Grucza, Patryk Konarski, Aleksandra Drapała, and Paweł Kaliszewski. 2023. "Variation of Sequential Ligandrol (LGD-4033) Metabolite Levels in Routine Anti-Doping Urine Samples Detected with or without Other Xenobiotics" Molecules 28, no. 18: 6486. https://doi.org/10.3390/molecules28186486
APA StyleKwiatkowska, D., Wicka, M., Grucza, K., Konarski, P., Drapała, A., & Kaliszewski, P. (2023). Variation of Sequential Ligandrol (LGD-4033) Metabolite Levels in Routine Anti-Doping Urine Samples Detected with or without Other Xenobiotics. Molecules, 28(18), 6486. https://doi.org/10.3390/molecules28186486







