Surface Modification of ZnO with Sn(IV)-Porphyrin for Enhanced Visible Light Photocatalytic Degradation of Amaranth Dye
Abstract
:1. Introduction
2. Results and Discussion
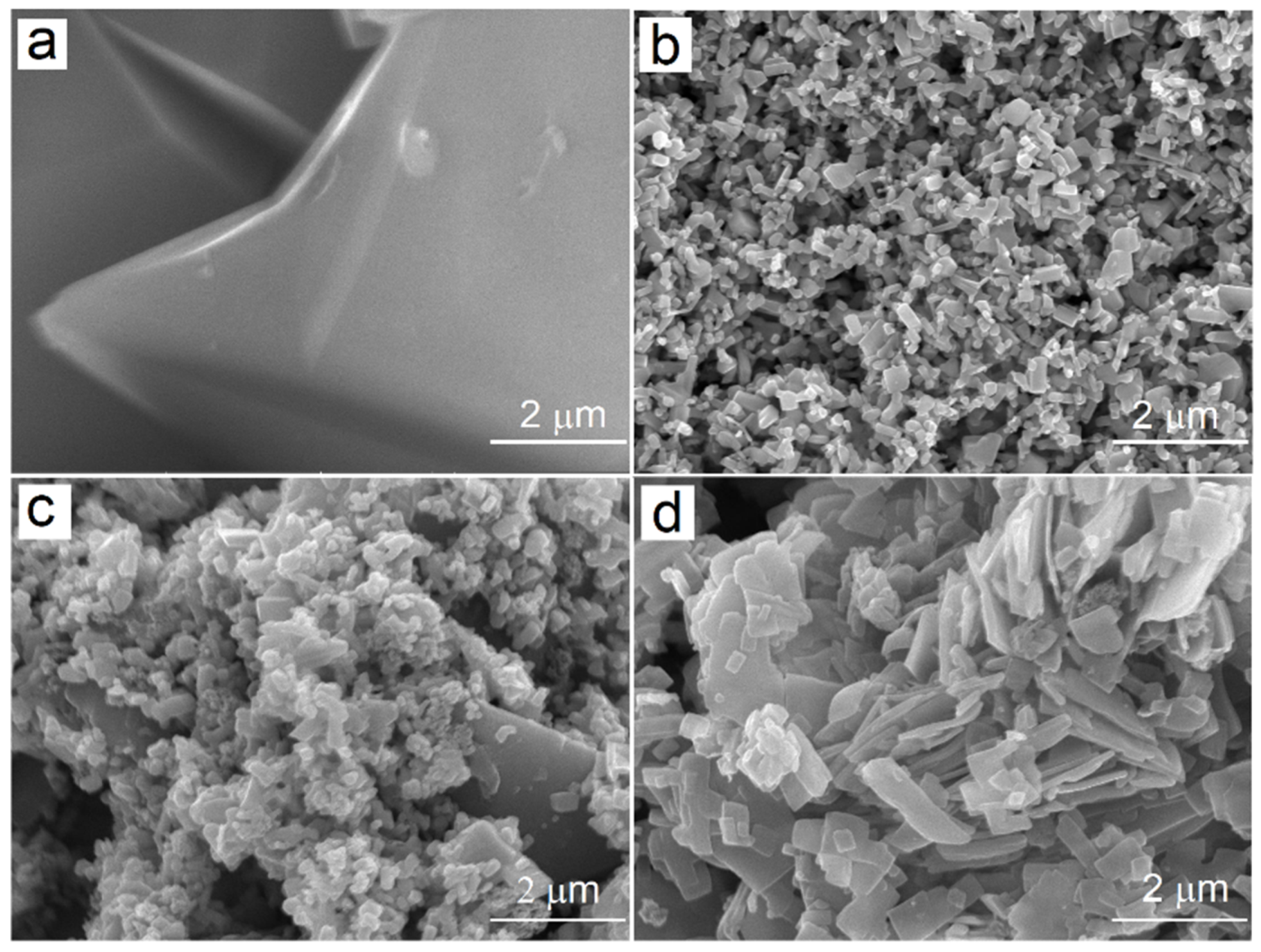
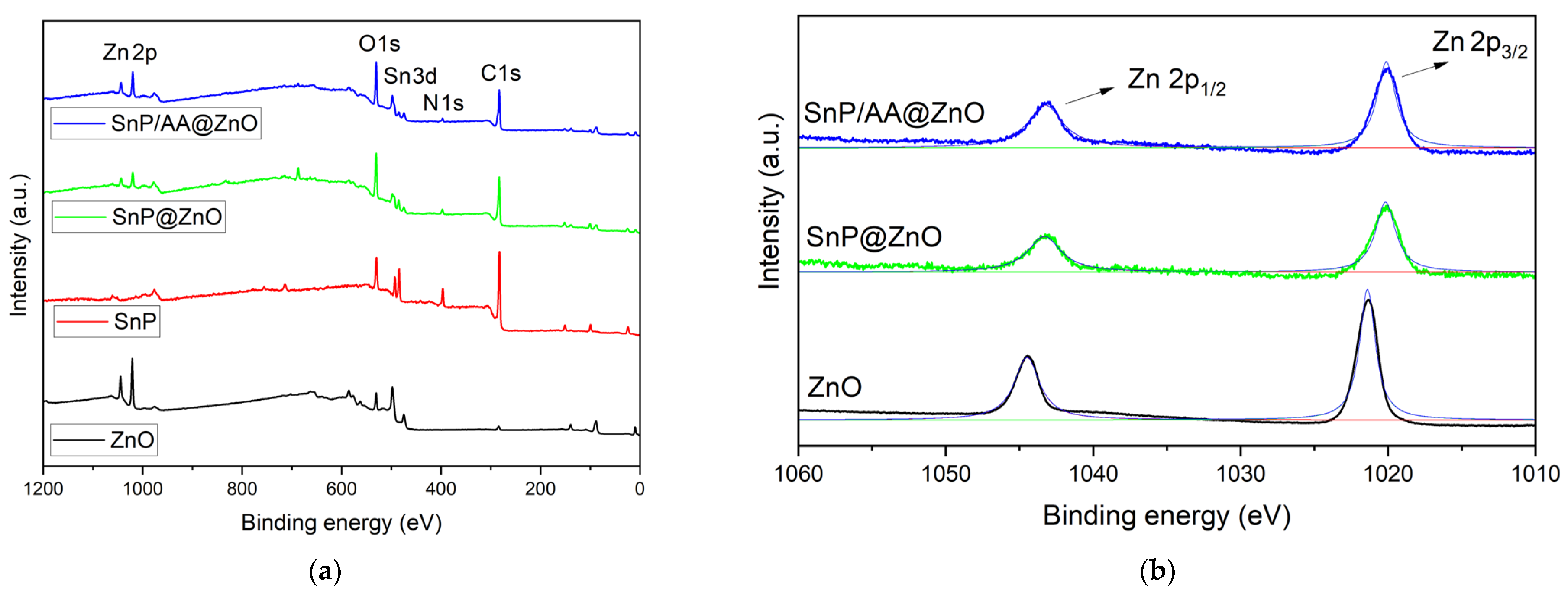
2.1. Fabrication and Characterization of Hybrid Photocatalysts
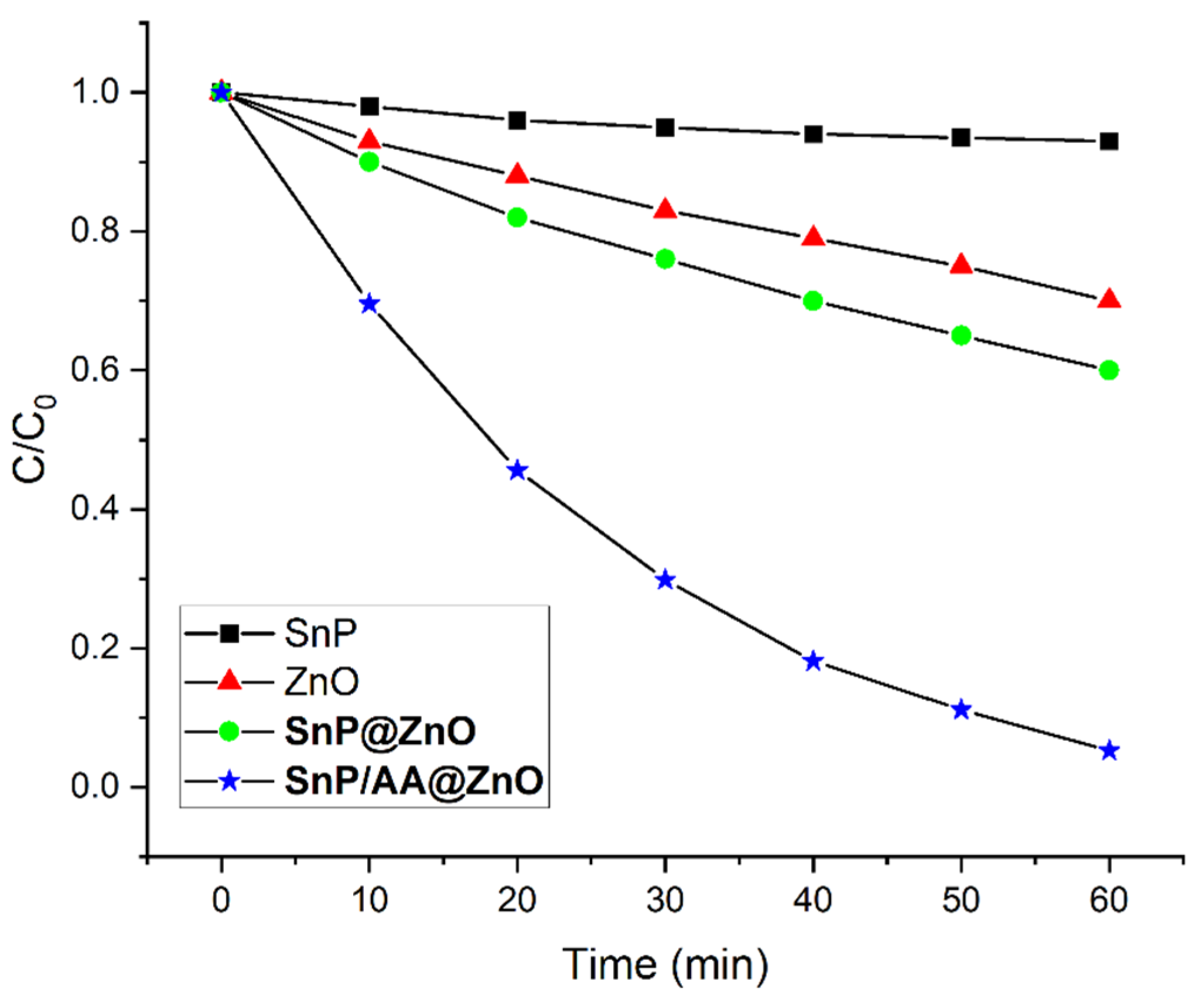
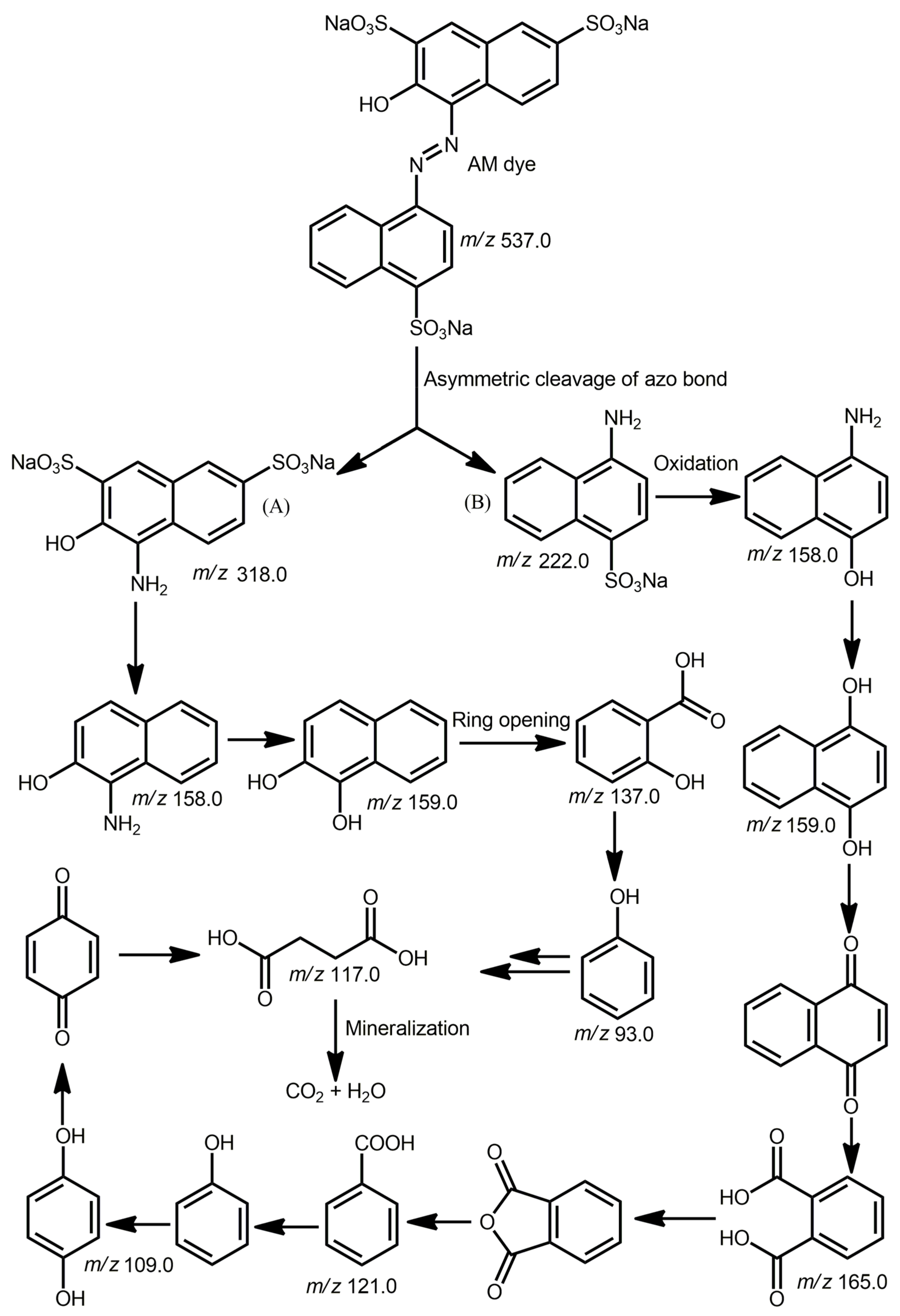
2.2. Photocatalytic Degradation of an Organic Dye
3. Materials and Methods
3.1. Synthesis of SnP/AA@ZnO
3.2. Synthesis of SnP@ZnO
3.3. Photocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health C 2016, 34, 233–261. [Google Scholar]
- Alsantali, R.I.; Alam Raja, Q.; Alzahrani, A.Y.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Al-Rooqi, M.M.; El Guesmi, N.; Moussa, Z.; Ahmed, S.A. Miscellaneous azo dyes: A comprehensive review on recent advancements in biological and industrial applications. Dye. Pigment. 2022, 199, 110050. [Google Scholar]
- Hossain, M.R.; Rashid, T.U.; Lata, N.P.; Dey, S.C.; Sarker, M.; Shamsuddin, S.M. Fabrication of Novel Nanohybrid Material for the Removal of Azo Dyes from Wastewater. J. Compos. Sci. 2022, 6, 304. [Google Scholar]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent Progress and Prospects in Catalytic Water Treatment. Chem. Rev. 2022, 122, 2981–3121. [Google Scholar]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterials 2021, 11, 2045. [Google Scholar]
- O’ Shea, K.E.; Dionysiou, D.D. Advanced oxidation processes for water treatment. J. Phys. Chem. Lett. 2012, 3, 2112–2113. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar]
- Shee, N.K.; Park, B.-H.; Kim, H.-J. Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules 2023, 28, 1886. [Google Scholar]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Kamran, M.A.; Al-Habardi, M. Green Synthesis of Spherical TiO2 Nanoparticles Using Citrus Limetta Extract: Excellent Photocatalytic Water Decontamination Agent for Rhb Dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar]
- Spoială, A.; Ilie, C.-I.; Trușcă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [PubMed]
- Al-Hamdi, A.M.; Rinner, U.; Sillanpää, M. Tin Dioxide as a Photocatalyst for Water Treatment: A Review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar]
- Ighalo, J.O.; Sagboye, P.A.; Umenweke, G.; Ajala, O.J.; Omoarukhe, F.O.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G. CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100443. [Google Scholar]
- Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Ismail, A.F.; Matsuura, T.; Othman, M.H.D.; Rahman, M.A.; Yusof, N. Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination. Membranes 2023, 13, 475. [Google Scholar] [PubMed]
- Shen, Y.; Tang, J.; Nie, Z.; Wang, Y.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar]
- Abdullah, T.A.; Juzsakova, T.; Rasheed, R.T.; Salman, A.D.; Adelikhah, M.; Cuong, L.P.; Cretescu, I. V2O5 Nanoparticles for Dyes Removal from Water. Chem. J. Mold. 2021, 16, 102–111. [Google Scholar]
- Li, H.; Wang, G.; Zhang, F.; Cai, Y.; Wang, Y.; Djerdj, I. Surfactant-assisted synthesis of CeO2 nanoparticles and their application in wastewater treatment. RSC Adv. 2012, 2, 12413–12423. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar]
- Chai, L.; Zhang, L.; Wang, X.; Xu, L.; Han, C.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Bottom-up synthesis of MOF-derived hollow N-doped carbon materials for enhanced ORR performance. Carbon 2019, 146, 248–256. [Google Scholar]
- Diaz-Angulo, J.; Gomez-Bonilla, I.; Jimenez-Tohapanta, C.; Mueses, M.; Pinzon, M.; Machuca-Martinez, F. Visible-light activation of TiO2 by dye-sensitization for degradation of pharmaceutical compounds. Photochem. Photobiol. Sci. 2019, 18, 897–904. [Google Scholar] [PubMed]
- Wang, C.; Li, J.; Mele, G.; Yang, G.-M.; Zhang, F.-X.; Palmisano, L.; Vasapollo, G. Efficient degradation of 4-nitrophenol by using functionalized porphyrin-TiO2 photocatalysts under visible irradiation. Appl. Catal. B Environ. 2007, 76, 218–226. [Google Scholar]
- Sun, W.-J.; Li, J.; Mele, G.; Zhang, Z.-Q.; Zhang, F.-X. Enhanced photocatalytic degradation of rhodamine B by surface modification of ZnO with copper (II) porphyrin under both UV–vis and visible light irradiation. J. Mol. Catal. A Chem. 2013, 366, 84–91. [Google Scholar]
- Yaghoubi-berijani, M.; Bahramian, B.; Zargari, S. Synthesis, characterization, and design of a photocatalyst based on BiOBr nanoplates and tin porphyrin with enhanced visible light photocatalytic activity. Res. Chem. Intermed. 2020, 46, 197–213. [Google Scholar]
- Zhang, J.; Wang, A.; Zhao, W.; Li, C.; Chen, X.; Wang, Y.; Zhu, W.; Zhong, Q. Influence of Metal-Porphyrins on the Photocatalysis of Graphitic Carbon Nitride. Dye. Pigment. 2018, 153, 241–247. [Google Scholar]
- Liu, S.; Xia, S.; Wang, J.; Ren, X.; Chen, S.; Zhong, Y.; Bai, F. Synthesis of the ZnTPyP/WO3 nanorod-on-nanorod heterojunction direct Z-scheme with spatial charge separation ability for enhanced photocatalytic hydrogen generation. Nanoscale 2023, 15, 2871–2881. [Google Scholar]
- Mele, G.; Del Sole, R.; Vasapollo, G.; García-López, E.; Palmisano, M.; Schiavello, M. Photocatalytic degradation of 4-nitrophenol in aqueous suspension by using polycrystalline TiO2 impregnated with functionalized Cu(II)–porphyrin or Cu(II)–phthalocyanine. J. Catal. 2003, 217, 334–342. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Ji, H.-B.; Huang, X.-J. Photocatalytic Degradation of Methyl Orange over Metalloporphyrins Supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Marcelo, A.M.P.; Da Silva, K.T.; Da Silva, F.L.F.; Mota, J.P.F.; Do Nascimento, J.P.C.; Sombra, A.S.B.; Clemente, C.D.S.; Mele, G.; Carbone, L.; et al. New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation. Materials 2017, 10, 1114. [Google Scholar]
- Vaz, B.; Pérez-Lorenzo, M. Unraveling Structure–Performance Relationships in Porphyrin-Sensitized TiO2 Photocatalysts. Nanomaterials 2023, 13, 1097. [Google Scholar]
- Rochford, J.; Chu, D.; Hagfeldt, A.; Galoppini, E. Tetrachelate porphyrin chromophores for metal oxide semiconductor sensitization: Effect of the spacer length and anchoring group position. J. Am. Chem. Soc. 2007, 129, 4655–4665. [Google Scholar] [PubMed]
- Yao, B.; Peng, C.; Zhang, W.; Zhang, Q.; Niu, J.; Zhao, J. A Novel Fe(III) Porphyrin-Conjugated TiO2 Visible-Light Photocatalyst. Appl. Catal. B Environ. 2015, 174, 77–84. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Balakrishna, A.; Arranja, C.T.; Dias, C.M.F.; Sobral, A.J. Chemically modified amino porphyrin/TiO2 for the degradation of Acid Black 1 under day light illumination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 176, 134–141. [Google Scholar]
- Krishnakumar, B.; Balakrishna, A.; Nawabjan, S.A.; Pandiyan, V.; Aguiar, A.; Sobral, A.J.F.N. Solar and Visible Active Amino Porphyrin/SiO2ZnO for the Degradation of Naphthol Blue Black. J. Phys. Chem. Solids 2017, 111, 364–371. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Łabuz, P.; Dubin, G.; Dąbrowski, J.M. Surface Modification of Nanocrystalline TiO2 Materials with Sulfonated Porphyrins for Visible Light Antimicrobial Therapy. Catalysts 2019, 9, 821. [Google Scholar] [CrossRef]
- Jiang, J.; Luo, R.; Zhou, X.; Chen, Y.; Ji, H. Photocatalytic Properties and Mechanistic Insights into Visible Light-Promoted Aerobic Oxidation of Sulfides to Sulfoxides via Tin Porphyrin-Based Porous Aromatic Frameworks. Adv. Synth. Catal. 2018, 360, 4402–4411. [Google Scholar]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular porphyrin nanostructures based on coordination driven self-assembly and their visible light catalytic degradation of methylene blue dye. Nanomaterials 2020, 10, 2314. [Google Scholar]
- Shee, N.K.; Kim, H.-J. Sn(IV) Porphyrin-Based Ionic Self-Assembled Nanostructures and Their Application in Visible Light Photo-Degradation of Malachite Green. Catalysts 2022, 12, 799. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Morphology-controlled self-assembled nanostructures of complementary metalloporphyrin triads obtained through tuning their intermolecular coordination and their photocatalytic degradation of Orange II dye. Inorg. Chem. Front. 2022, 9, 5538–5548. [Google Scholar]
- Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(IV)porphyrins with Re(I)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Inorg. Chem. Front. 2023, 10, 174–183. [Google Scholar]
- Duan, M.Y.; Li, J.; Mele, G.; Wang, C.; Lu, X.F.; Vasapollo, G.; Zhang, F.X. Photocatalytic Activity of Novel Tin Porphyrin/TiO2 Based Composites. J. Phys. Chem. C 2010, 114, 7857–7862. [Google Scholar] [CrossRef]
- Zargari, S.; Rahimi, R.; Yousefi, A. An efficient visible light photocatalyst based on tin porphyrin intercalated between TiO2–graphene nanosheets for inactivation of E. coli and investigation of charge transfer mechanism. RSC Adv. 2016, 6, 24218–24228. [Google Scholar]
- Shee, N.K.; Kim, H.-J. Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation. Inorganics 2023, 11, 336. [Google Scholar]
- Daphnomili, D.; Landrou, G.; Prakash Singh, S.; Thomas, A.; Yesudas, K.; Bhanuprakash, K.; Sharma, G.D.; Coutsolelos, A.G. Photophysical, electrochemical and photovoltaic properties of dye sensitized solar cells using a series of pyridyl functionalized porphyrin dyes. RSC Adv. 2012, 2, 12899–12908. [Google Scholar]
- Shahmoradi, B.; Maleki, A.; Byrappa, K. Photocatalytic degradation of Amaranth and Brilliant Blue FCF dyes using in situ modified tungsten doped TiO2 hybrid nanoparticles. Catal. Sci. Technol. 2011, 1, 1216–1223. [Google Scholar]
- Gupta, V.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. 2012, 32, 12–17. [Google Scholar]
- Kumar, J.; Bansal, A. Photodegradation of amaranth in aqueous solution catalyzed by immobilized nanoparticles of titanium dioxide. Int. J. Environ. Sci. Technol. 2012, 9, 479–484. [Google Scholar]
- Barros, W.R.; Steter, J.R.; Lanza, M.R.; Motheo, A.J. Degradation of amaranth dye in alkaline medium by ultrasonic cavitation coupled with electrochemical oxidation using a boron-doped diamond anode. Electrochim. Acta 2014, 143, 180–187. [Google Scholar]
- Roşu, M.C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; PogǍcean, F.; Coroş, M.; MǍgeruşan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar]
- Naik, A.P.; Salkar, A.V.; Majik, M.S.; Morajkar, P.P. Enhanced photocatalytic degradation of Amaranth dye on mesoporous anatase TiO2: Evidence of C-N, N=N bond cleavage and identification of new intermediates. Photochem. Photobiol. Sci. 2017, 16, 1126–1138. [Google Scholar]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Enhanced visible light driven photocatalytic application of Ag2O decorated ZnO nanorods heterostructures. Sep. Purif. Technol. 2017, 183, 341–349. [Google Scholar]
- Thor, S.-H.; Ho, L.-N.; Ong, S.-A.; Abidin, C.Z.A.; Heah, C.-Y.; Nordin, N.; Ong, Y.-P.; Yap, K.-L. Advanced oxidation treatment of amaranth dye synchronized with electricity generation using carbon-based cathodes in a sustainable photocatalytic fuel cell integrated electro-fenton system. J. Environ. Chem. Eng. 2021, 9, 106439. [Google Scholar]
- Makula, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R.; Attri, P. Transition Metal Oxides and Their Composites for Photocatalytic Dye Degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Feng, H.P.; Niu, C.G.; Huang, D.W.; Guo, H.; Liang, C.; Liu, H.Y.; Chen, S.; Tang, N.; Li, L. Constructing a plasma-based Schottky heterojunction for near-infrared-driven photothermal synergistic water disinfection: Synergetic effects and antibacterial mechanisms. Chem. Eng. J. 2021, 426, 131902. [Google Scholar]
- Feng, H.; Yu, J.; Tang, J.; Tang, L.; Liu, Y.; Lu, Y.; Wang, J.; Ni, T.; Yang, Y.; Yi, Y. Enhanced electro-oxidation performance of FeCoLDH to organic pollutants using hydrophilic structure. J. Hazard. Mater. 2022, 430, 128464. [Google Scholar]
- Steter, J.R.; Barros, W.R.P.; Lanza, M.R.V.; Motheo, A.J. Electrochemical and sonoelectrochemical processes applied to amaranth dye degradation. Chemosphere 2014, 117, 200–207. [Google Scholar]
- Zhang, M.; Lin, K.A.; Huang, C.; Tong, S. Enhanced Degradation of Toxic Azo Dye, Amaranth, in Water Using Oxone Catalyzed by MIL-101-NH2 under Visible Light Irradiation. Sep. Purif. Technol. 2019, 227, 115632. [Google Scholar]
- Valencia-Lopez, C.D.; Zafra-Calvo, M.; Martín de Vidales, M.J.; Blanco-Gutierrez, V.; Atanes-Sanchez, E.; Merayo, N.; Fernandez-Martinez, F.; Nieto-Marquez, A.; Dos santos-Garcia, A.J. Synthesis of NiFe2O4-LDH Composites with High Adsorption and Photocatalytic Activity for Methyl Orange Degradation. Inorganics 2018, 6, 98. [Google Scholar] [CrossRef]
- Jo, H.J.; Jung, S.H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of trans-Dihydroxotin(IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]

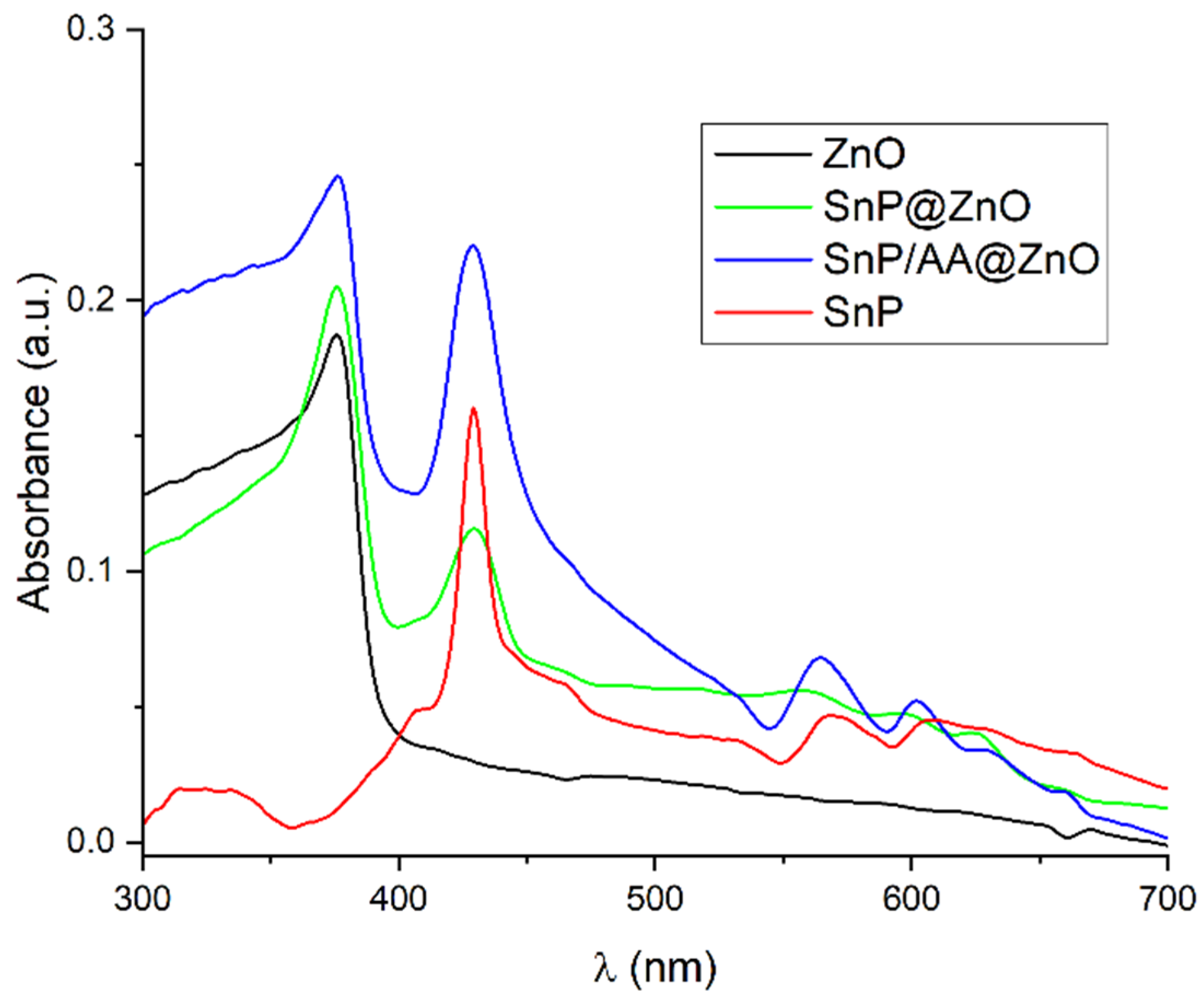
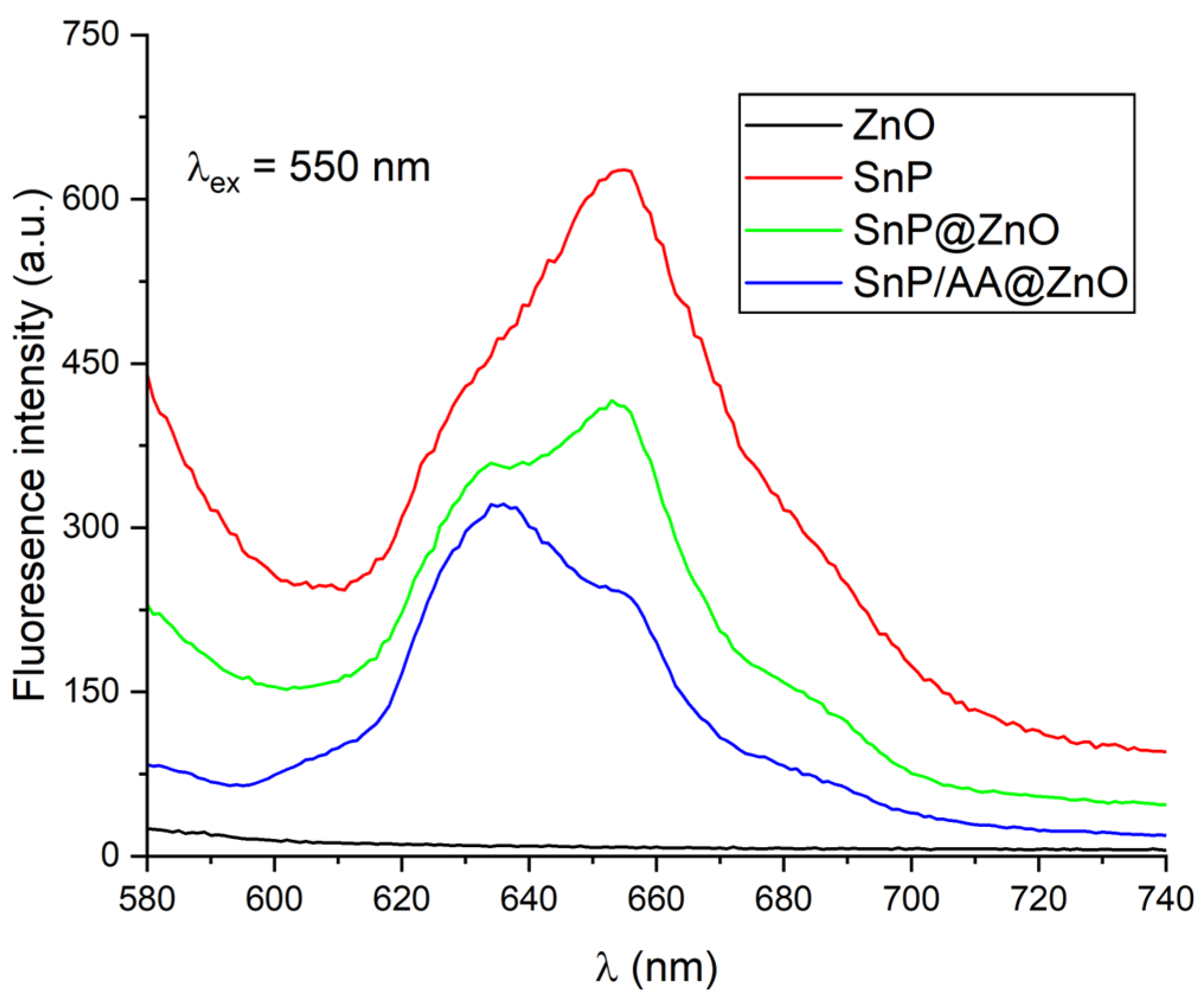
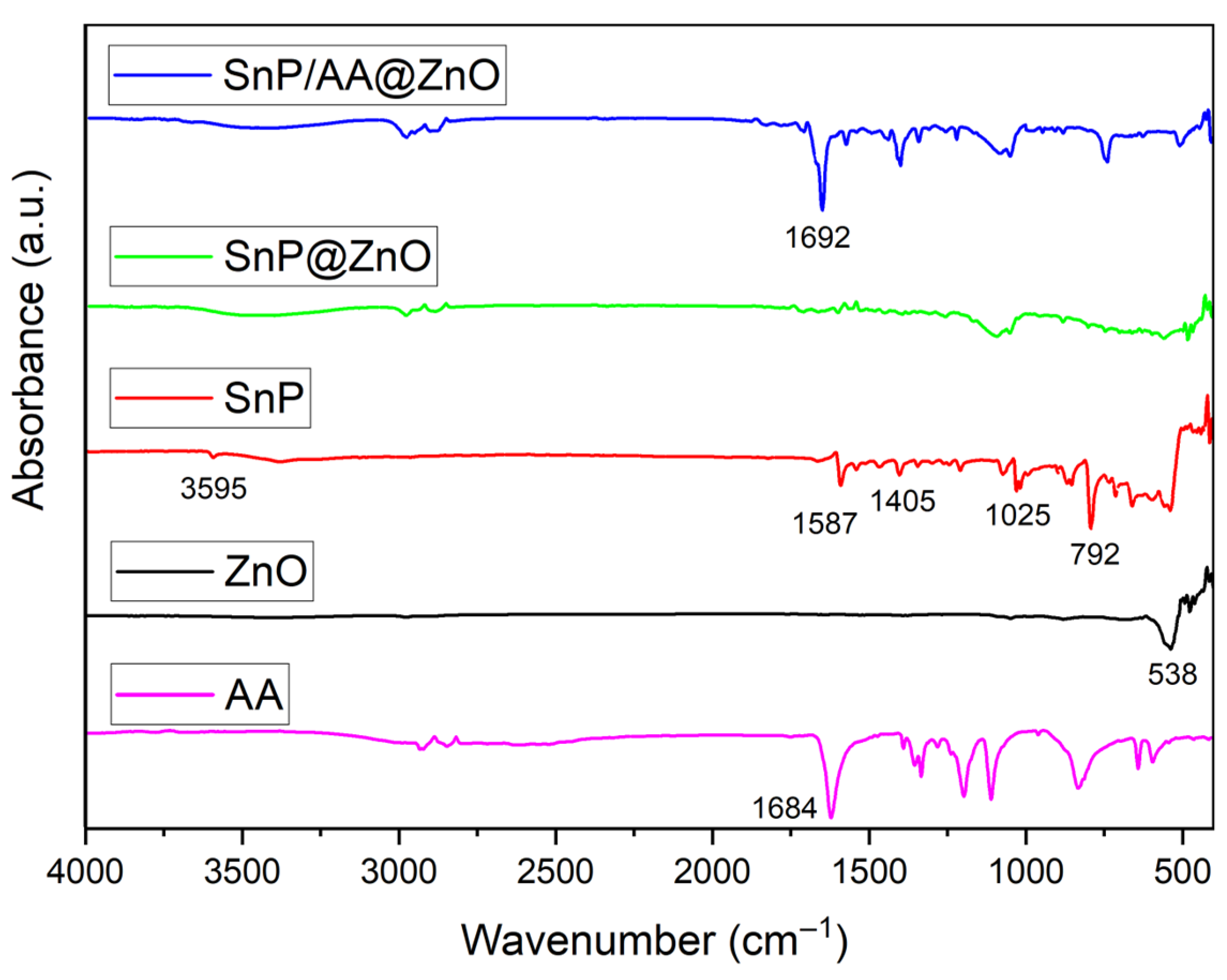
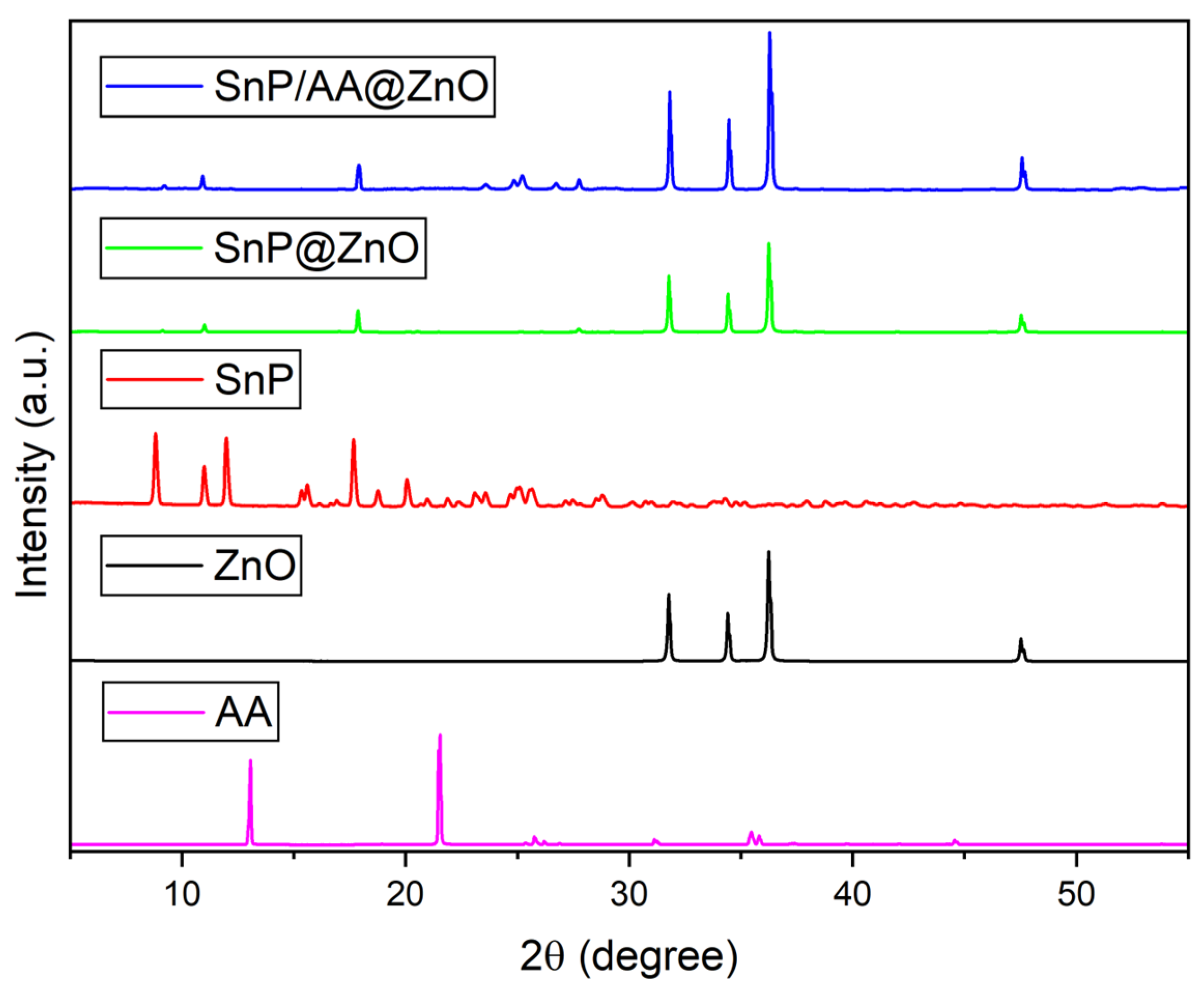
| Photocatalyst | Rate Constant (min−1) | Reference |
|---|---|---|
| W/TiO2 | 0.063 | [46] |
| H2O2-TiO2 | 0.016 | [47] |
| Immobilized TiO2 | 0.0098 | [48] |
| Boron-doped diamond anode | 0.046 | [49] |
| Graphene/TiO2-Ag | 0.0583 | [50] |
| Anatase TiO2 | 0.042 | [51] |
| Ag2O-ZnO | 0.088 | [52] |
| Photocatalytic fuel cell-electro fenton (PEC-EF) | 0.007 | [53] |
| Porphyrin nanostructure | 0.031 | [55] |
| SnP | 0.001 | This study |
| ZnO | 0.006 | This study |
| SnP@ZnO | 0.008 | This study |
| SnP/AA@ZnO | 0.048 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Surface Modification of ZnO with Sn(IV)-Porphyrin for Enhanced Visible Light Photocatalytic Degradation of Amaranth Dye. Molecules 2023, 28, 6481. https://doi.org/10.3390/molecules28186481
Shee NK, Kim H-J. Surface Modification of ZnO with Sn(IV)-Porphyrin for Enhanced Visible Light Photocatalytic Degradation of Amaranth Dye. Molecules. 2023; 28(18):6481. https://doi.org/10.3390/molecules28186481
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2023. "Surface Modification of ZnO with Sn(IV)-Porphyrin for Enhanced Visible Light Photocatalytic Degradation of Amaranth Dye" Molecules 28, no. 18: 6481. https://doi.org/10.3390/molecules28186481
APA StyleShee, N. K., & Kim, H.-J. (2023). Surface Modification of ZnO with Sn(IV)-Porphyrin for Enhanced Visible Light Photocatalytic Degradation of Amaranth Dye. Molecules, 28(18), 6481. https://doi.org/10.3390/molecules28186481





