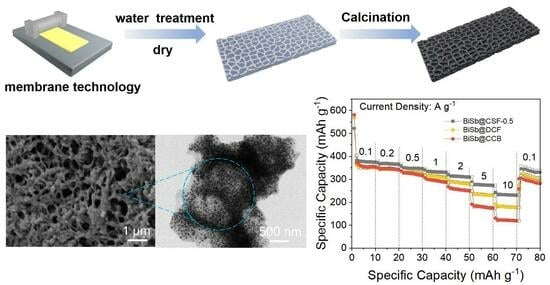
Bismuth-Antimony Alloy Nanoparticles Embedded in 3D Hierarchical Porous Carbon Skeleton Film for Superior Sodium Storage
Abstract
:1. Introduction
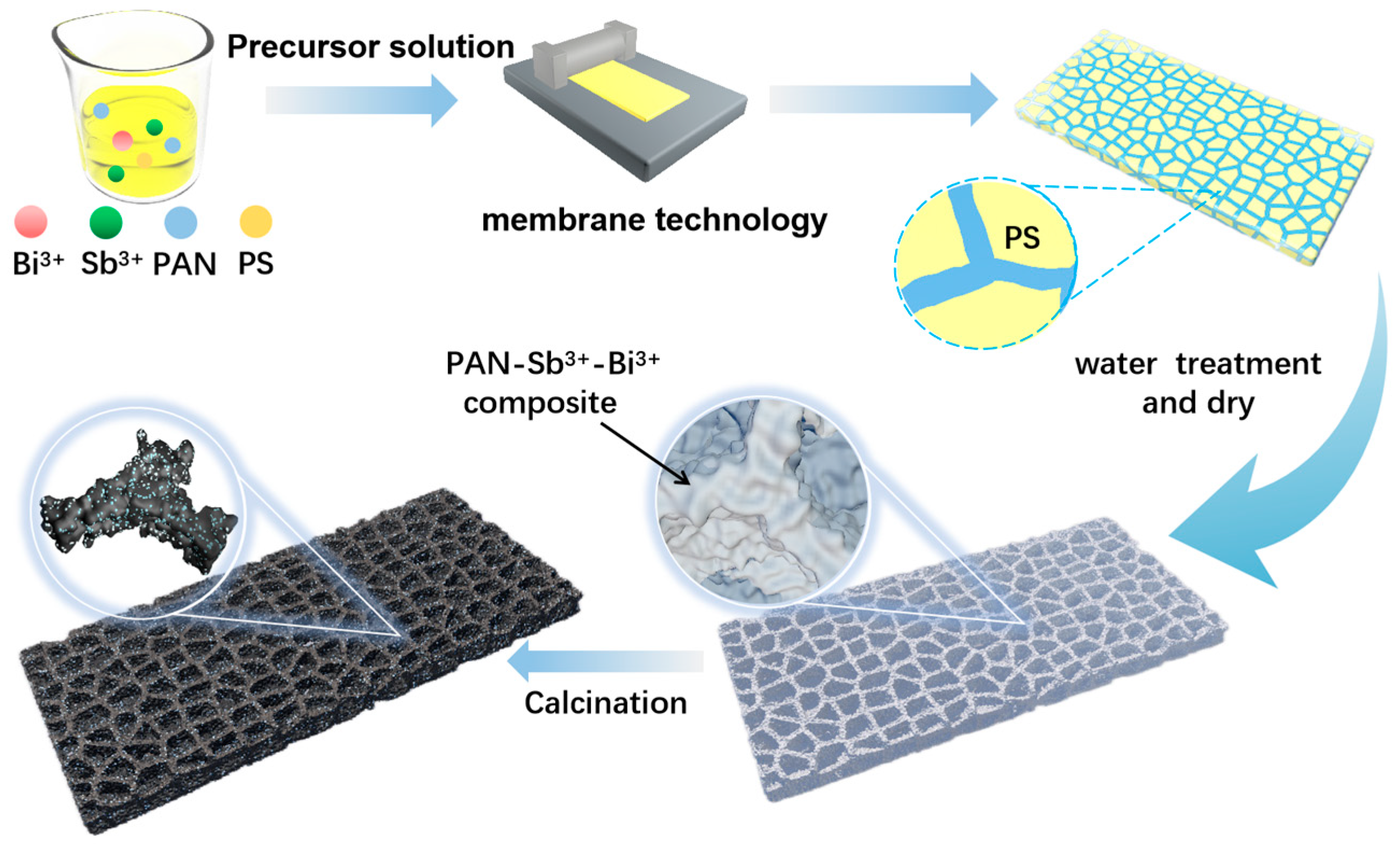
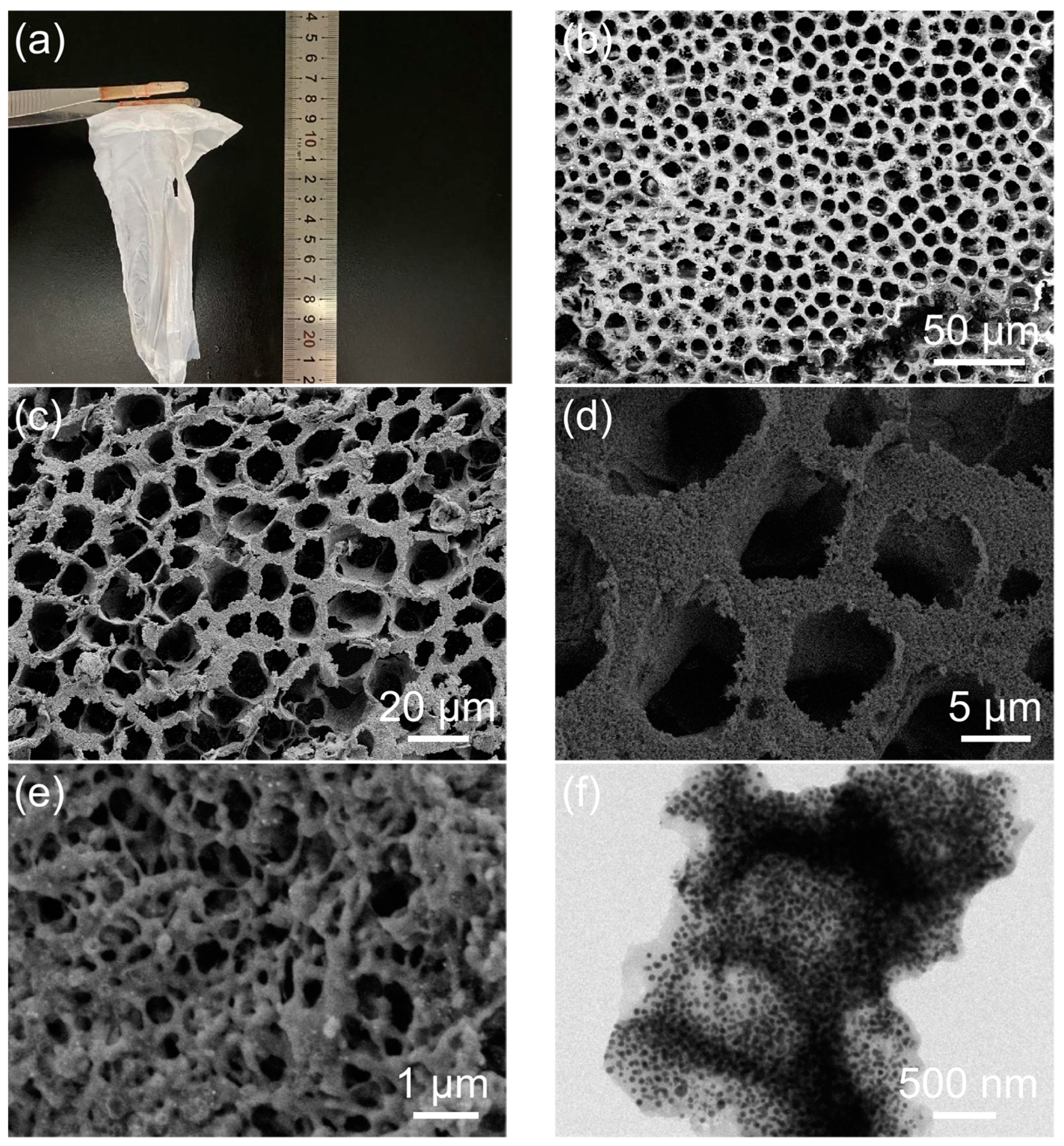
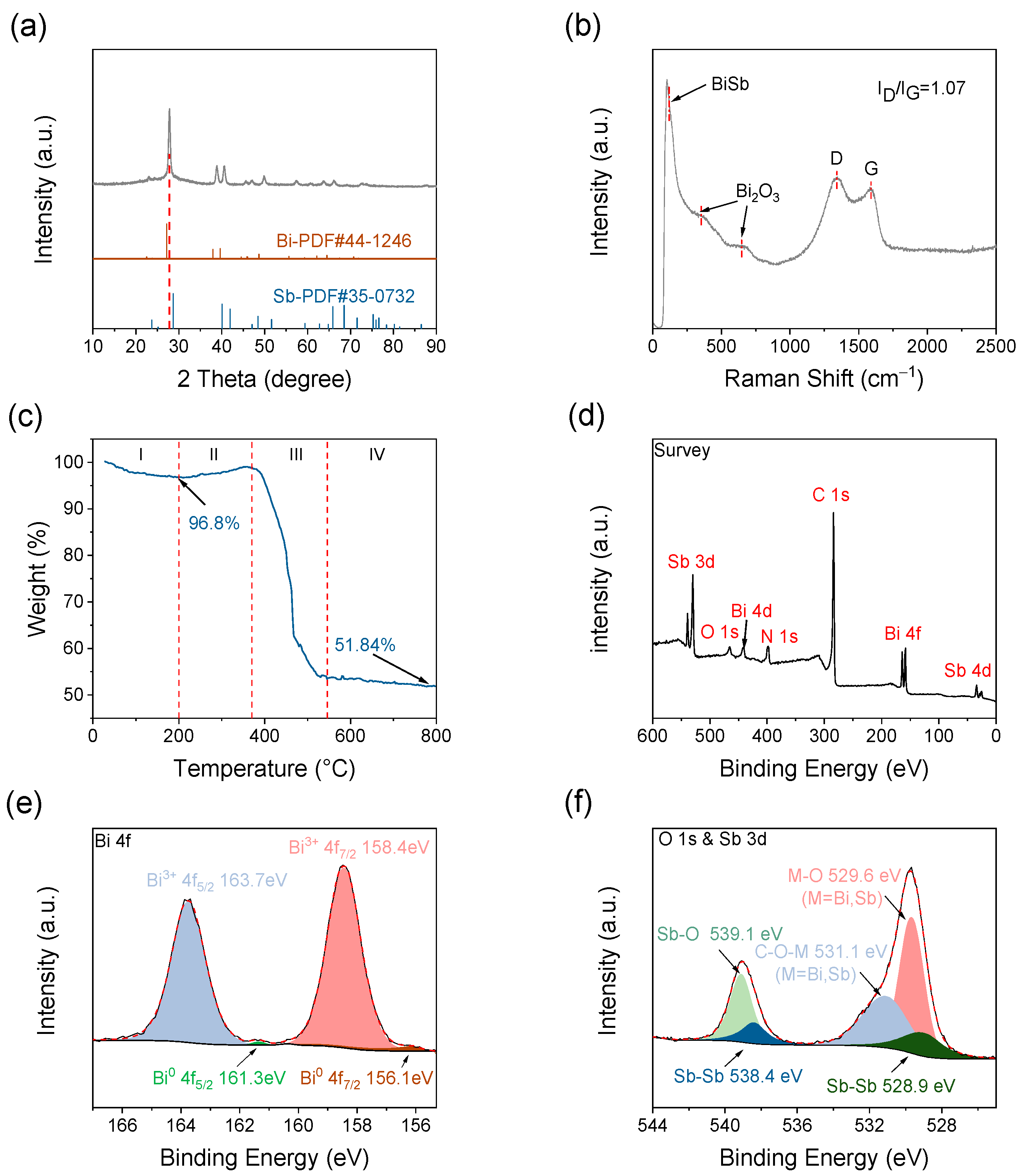
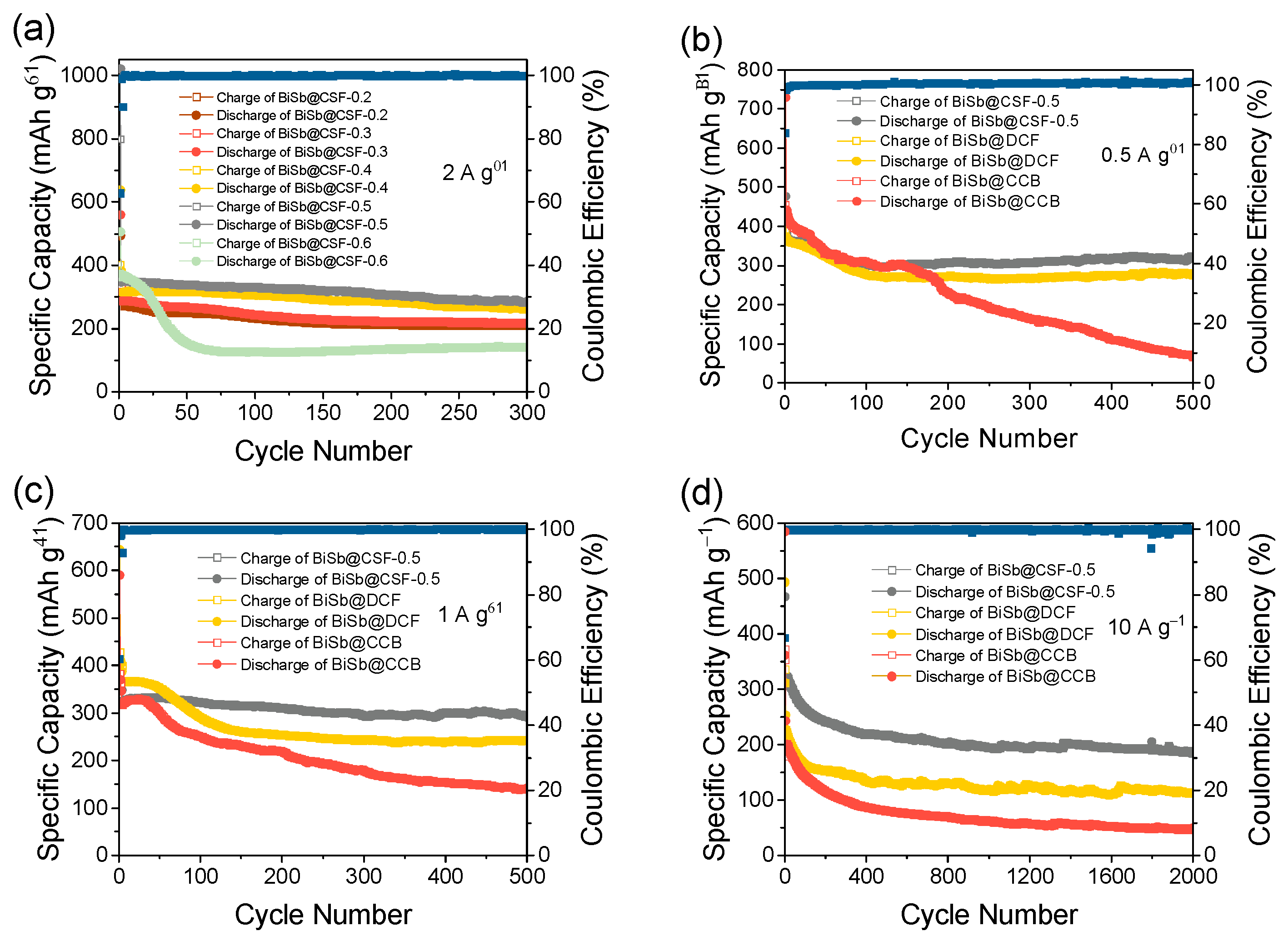
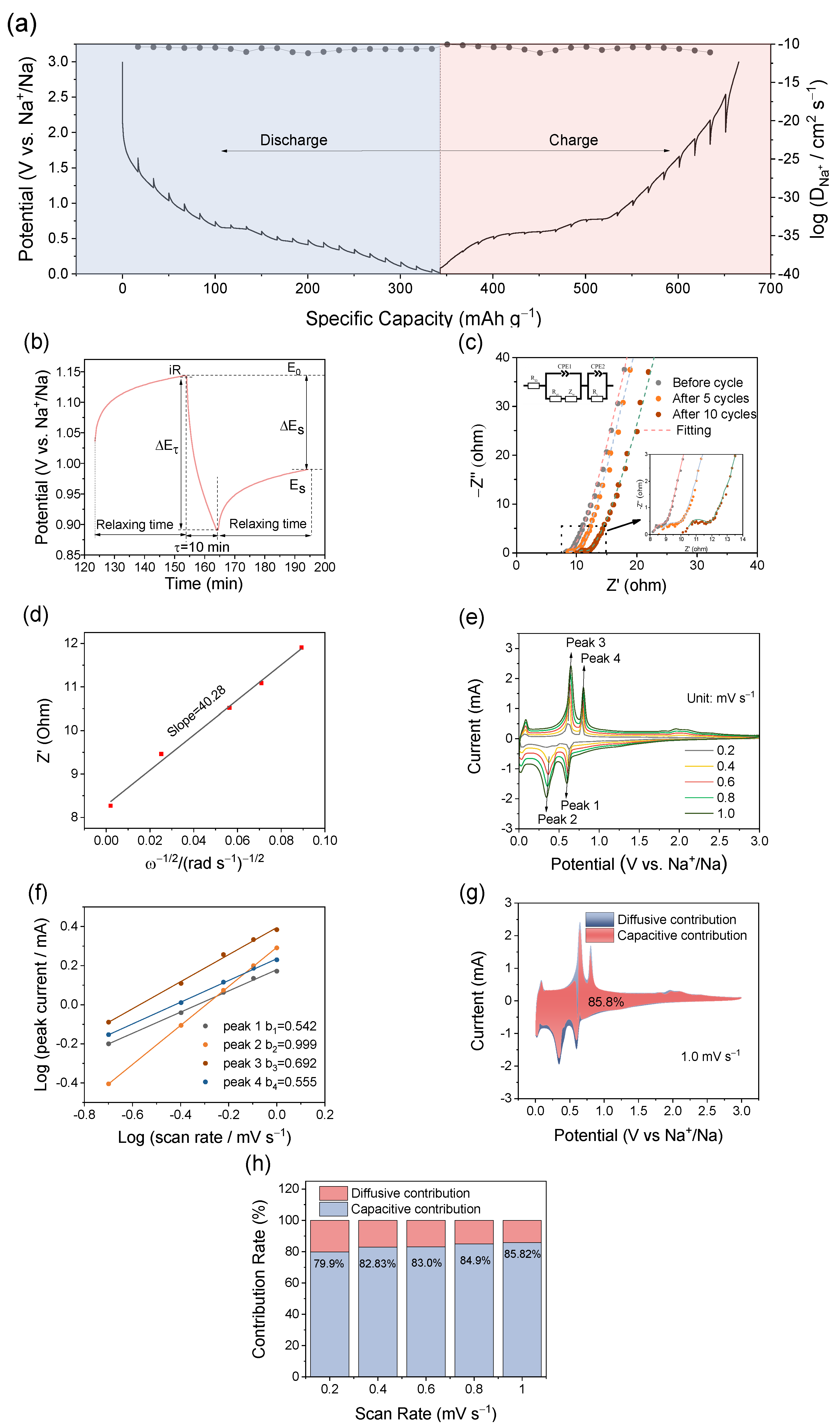
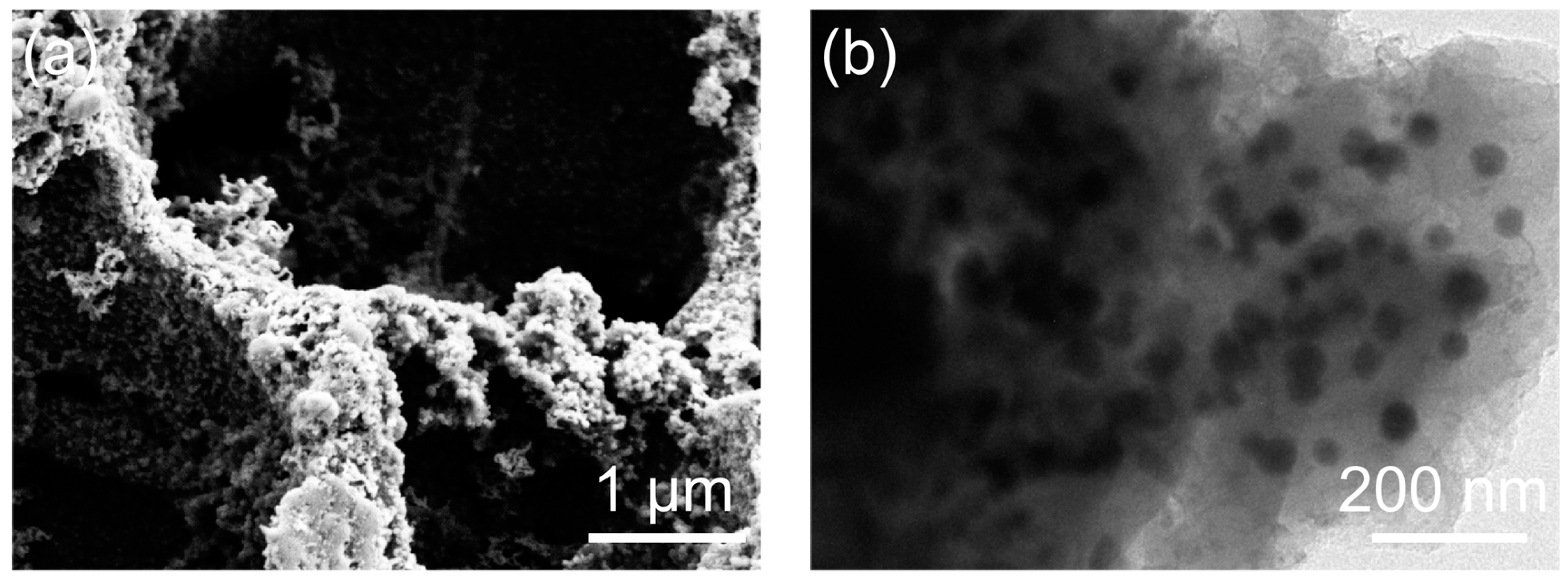
2. Results and Discussion
3. Experimental
3.1. Material Synthesis
3.2. Material Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, W.C.; Yuan, Y.F.; Du, P.F.; Yin, S.M.; Guo, S.Y. Intimately coupled MoP nanocrystalline@carbon nanosheets-assembled hierarchical mesoporous nanospheres for high-performance sodium-ion storage. Electrochim. Acta 2021, 389, 138712. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.J.; Liu, X.; Chen, Z.S.; Li, H.Y. Nitrogen-coordinated antimony atom anchored on carbon matrix as efficient active sites to enhance sodium/potassium ion storage. J. Colloid Interface Sci. 2023, 648, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.X.; Liu, C.; Sun, Q.Q.; Ren, G.Y.; Li, Y.X.; Wang, Y.T.; Gao, J.Y.; Yao, Z.J.; Yang, Y.F. Ion-exchange route induced heterostructured CoS2/FeS nanoparticles confined in hollow N-doped carbon frameworks for enhanced sodium storage performance, ACS Appl. Nano Mater. 2023, 6, 11944–11954. [Google Scholar] [CrossRef]
- Cheng, J.P.; Wang, W.D.; Wang, X.C.; Liu, F. Recent research of core-shell structured composites with NiCo2O4 as scaffolds for electrochemical capacitors. Chem. Eng. J. 2020, 393, 124747. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Zhang, T.; Yang, Z.Y.; Yao, Z.J.; Wang, B.X.; Yang, Q.H.; Guo, S.Y. Nano VS4 in-situ grown in three-dimensional honeycomb macroporous carbon enabling high-rate and long-life lithium storage. J. Alloys Compd. 2023, 942, 169021. [Google Scholar] [CrossRef]
- Bai, B.; Qiu, L.L.; Yuan, Y.F.; Song, L.X.; Xiong, J.; Du, P.F. Nitrogen doped siloxene and composite with graphene for high performance fiber-based supercapacitors. J. Energy Storage 2023, 63, 106984. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.J.; Xu, J.H.; He, P.F.; Ding, X.L. Hexagonal Sb nanocrystals as high-capacity and long-cycle anode materials for sodium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 26728–26736. [Google Scholar] [CrossRef]
- Fang, J.; Yuan, Y.F.; Wang, L.K.; Ni, H.L.; Zhu, H.L.; Gui, J.S.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Hierarchical ZnO@NiO core-shell nanorod array as high performance anode material for lithium-ion batteries. Mater. Lett. 2013, 111, 1–4. [Google Scholar] [CrossRef]
- Wang, B.Q.; Gong, S.H.; Wang, X.C.; Wu, J.F.; Liu, F.; Cheng, J.P. Controllable reduction of NiCoO2@NiCo core-shell nanospheres on CNTs for high-performance electrochemical energy storage. J. Colloid Interface Sci. 2023, 645, 154–164. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Cao, F.; Zhang, Y.Q.; Xia, X.H. The development trend of graphene derivatives. J. Electron. Mater. 2022, 51, 4107. [Google Scholar] [CrossRef]
- Tang, T.T.; Ren, R.Y.; Wen, Y.; Lu, M.X.; Yao, Z.J.; Liu, T.C.; Shen, S.H.; Xie, H.J.; Xia, X.H.; Yang, Y.F. Spatially confined Fe7S8 nanoparticles anchored on a porous nitrogen-doped carbon nanosheet skeleton for high-rate and durable sodium storage. ACS Appl. Mater. Interfaces 2023, 15, 30249–30261. [Google Scholar] [CrossRef]
- Zhang, T.F.; Li, C.; Wang, F.; Noori, A.H.; Mousavi, M.F.; Xia, X.H.; Zhang, Y.Q. Recent advances in carbon anodes for sodium-ion batteries. Chem. Rec. 2022, 22, e202200083. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.Q.; Wang, Y.; Shui, J.X.; Jin, Z.F.; Bai, B.; Qiu, L.L.; Du, P.F. Fabrication of high-performance silicon anode materials for lithium-ion batteries by the impurity compensation doping method. J. Solid State Electrochem. 2023, 27, 969–976. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Y.; Chen, H.X.; Feng, K.J.; Jia, K.X.; Pan, K.M.; Wang, G.X.; Huang, T.; Pang, X.C.; Zhang, Q.B. Emerging bismuth-based materials: From fundamentals to electrochemical energy storage applications. Energy Storage Mater. 2023, 58, 232–270. [Google Scholar] [CrossRef]
- You, J.; Sun, H.R.; Wang, X.J.; Li, M.; Sun, J.R.; Wang, P.; He, Y.; Liu, Z.M. Sb ultra-small nanoparticles embedded within N, S co-doped flexible carbon nanofiber films with longitudinal tunnels as high performance anode materials for sodium-ion batteries. Batter. Supercaps 2023, 6, e202300022. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Choi, M.; Ku, M.K.Y.; Kam, D.; Kim, S.O.; Choi, W. Sb/C composite embedded in SiOC buffer matrix via dispersion property control for novel anode material in sodium-ion batteries. J. Power Sources 2023, 568, 232908. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ding, Y.F.; Gao, J.W.; Zhang, X.; Sun, H.T.; Wang, G.K. Microwave-regulated Bi nanoparticles on carbon nanotube networks as a freestanding electrode for flexible sodium-ion capacitors. J. Colloid Interface Sci. 2023, 643, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qing, Y.; Zhou, B.; Wang, L.D.; Pu, B.; Zhou, X.F.; Wang, Y.B.; Zhang, M.Z.; Bai, J.; Tang, Q.; et al. Yolk-shell Sb@void@graphdiyne nanoboxes for high-rate and long cycle life sodium-ion batteries. ACS Nano 2023, 17, 2431–2439. [Google Scholar] [CrossRef]
- Qian, K.; Mu, Z.Y.; Wang, X.; Zhang, Y.Q.; Zhu, M.Z.; Zhang, C.Z.; Li, J.F. Sb particles embedded in N-doped carbon spheres wrapped by graphene for superior K+-Storing performances. Ceram. Int. 2023, 49, 4273–4280. [Google Scholar] [CrossRef]
- Zhang, X.S.; Qiu, X.Q.; Lin, J.X.; Lin, Z.H.; Sun, S.R.; Yin, J.; Alshareef, H.N.; Zhang, W.L. Structure and interface engineering of ultrahigh-rate 3D bismuth anodes for sodium-ion batteries. Small 2023, 19, 2302071. [Google Scholar] [CrossRef]
- Gao, H.; Lee, J.; Lu, Q.X.; Kim, Y.; Shin, K.H.; Park, H.S.; Zhang, Z.H.; Lee, L.Y.S. Highly stable Sb/C anode for K+ and Na+ energy storage enabled by pulsed laser ablation and polydopamine coating. Small 2023, 19, 2205681. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.P.; Xue, F.F.; Zhang, Z.G.; Li, Q.H. Sb nanoparticles encapsulated in N-doped carbon nanotubes as freestanding anodes for high-performance lithium and potassium ion batteries. Rare Met. 2022, 42, 449–458. [Google Scholar] [CrossRef]
- Feng, B.; Long, T.; Yang, C.L.; Wang, K.R.; Wang, Z.Y.; Ding, Y.L. Porous Sb nanocubes embedded in three-dimensional interconnected nitrogen-doped carbon frameworks for enhanced sodium storage. ACS Appl. Energy Mater. 2022, 5, 14107–14118. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Shao, J.; Jiang, Y.; Zhang, K.J.; Shi, Q.; Qu, Q.T.; Zheng, H.H. Sb nanocrystallites derived from industrial antimony white as promising alloying-type anodes for Na-ion batteries. J. Alloys Compd. 2022, 926, 166808. [Google Scholar] [CrossRef]
- Gu, Y.; Cui, R.C.; Wang, G.Y.; Yang, C.C.; Jiang, Q. Sb/N-doped carbon nanofiber as a sodium-ion battery anode. Energy Technol. 2022, 10, 2200746. [Google Scholar] [CrossRef]
- Chen, N.N.; Shen, N.L.; Yi, X.P.; Pang, Y.S.; Zheng, J.; Lai, Q.X.; Liang, Y.Y. Achieving stable K-storage performance of carbon sphere-confined Sb via electrolyte regulation. J. Energy Chem. 2023, 76, 51–58. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, D.; Ma, C.S.; Feng, L.; Zhang, Y.H.; Zhang, L.F.; Liu, Y.; Guo, S.W. Vapor-phase derived ultra-fine bismuth nanoparticles embedded in carbon nanotube networks as anodes for sodium and potassium ion batteries. J. Colloid Interface Sci. 2023, 643, 409–419. [Google Scholar] [CrossRef]
- Han, J.; Liu, D.; Liu, S.; Shao, W.; Yang, E.; Zhang, T.; Jin, X.; Jian, X.; Hu, F. A collaborative strategy for encapsulating Sb nanoparticles into porous carbon toward high and stable sodium storage. J. Alloys Compd. 2022, 921, 166054. [Google Scholar] [CrossRef]
- Yang, L.; Liu, M.L.; Xiang, Y.E.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; Ji, X.B. Carbon skeleton confined Sb chalcogenides nanodots for stable sodium storage. Carbon 2022, 197, 341–349. [Google Scholar] [CrossRef]
- Xie, M.G.; Li, C.G.; Ren, S.Y.; Ma, Y.; Chen, X.B.; Fan, X.F.; Han, Y.; Shi, Z.; Feng, S.H. Ultrafine Sb nanoparticles in situ confined in covalent organic frameworks for high-performance sodium-ion battery anodes. J. Mater. Chem. A 2022, 10, 15089–15100. [Google Scholar] [CrossRef]
- Guo, S.T.; Li, H.; Lu, Y.; Liu, Z.F.; Hu, X.L. Lattice softening enables highly reversible sodium storage in anti-pulverization Bi–Sb alloy/carbon nanofibers. Energy Storage Mater. 2020, 27, 270–278. [Google Scholar] [CrossRef]
- Wang, X.X.; Feng, B.; Huang, L.M.; Fu, Q.F.; Li, W.Z.; Zhu, C.; Chen, P.; Yang, C.L.; Ding, Y.L. Superior electrochemical performance of Sb–Bi alloy for sodium storage: Understanding from alloying element effects and new cause of capacity attenuation. J. Power Sources 2022, 520, 230826. [Google Scholar] [CrossRef]
- Ni, J.F.; Li, X.Y.; Sun, M.L.; Yuan, Y.F.; Liu, T.C.; Li, L.; Lu, J. Durian-inspired design of bismuth−antimony alloy arrays for robust sodium storage. ACS Nano 2020, 14, 9117–9124. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Xu, A.D.; Li, G.L.; Sun, H.; Wu, S.P.; Xu, Z.G.; Yan, Y.R. Alloyed BiSb nanoparticles confined in tremella-like carbon microspheres for ultralong-life potassium ion batteries. Small 2021, 17, 2100685. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Z.; Chen, B.C.; Xie, H.N.; Bai, X.R.; Liang, M.; Wu, Z.Y.; Jin, X.Y.; He, C.N.; Zhao, N.Q. Bismuth-antimony alloy nanoparticles encapsulated in 3D carbon framework: Synergistic effect for enhancing interfacial potassium storage. Chem. Eng. J. 2022, 430, 132906. [Google Scholar] [CrossRef]
- Chen, K.T.; Yang, Y.C.; Lyu, L.M.; Lu, M.Y.; Tuan, H.Y. In situ formed robust submicron-sized nanocrystalline aggregates enable highly-reversible potassium ion storage. Nano Energy 2021, 88, 106233. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, W.; Jiang, H.Q.; Wang, C.; Zhou, Y.; Ke, F.S.; Cong, H.J.; Deng, H.X. Uniform Bi−Sb alloy nanoparticles synthesized from MOFs by laser metallurgy for sodium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 335–342. [Google Scholar] [CrossRef]
- Miao, W.F.; Zhang, Y.; Li, H.T.; Zhang, Z.H.; Li, L.; Yu, Z.; Zhang, W.M. ZIF-8/ZIF-67-derived 3D amorphous carbon-encapsulated CoS/N-CNTs supported on CoS-coated carbon nanofibers as an advanced potassium-ion battery anode. J. Mater. Chem. A 2019, 7, 5504–5512. [Google Scholar] [CrossRef]
- Gu, H.C.; Yang, L.P.; Zhang, Y.; Wang, C.Y.; Zhang, X.; Xie, Z.J.; Wei, J.P.; Zhou, Z. Highly reversible alloying/dealloying behavior of SnSb nanoparticles incorporated into N-rich porous carbon nanowires for ultra-stable Na storage. Energy Storage Mater. 2019, 21, 203–209. [Google Scholar] [CrossRef]
- Gao, H.; Niu, J.Z.; Zhang, C.; Peng, Z.Q.; Zhang, Z.H. A dealloying synthetic strategy for nanoporous bismuth-antimony anodes for sodium ion batteries. ACS Nano 2018, 12, 3568–3577. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.S.; Chen, X.H.; Song, H.H. Graphene-loaded Bi2Se3: A conversion-alloying-type anode material for ultrafast gravimetric and volumetric Na storage. ACS Appl. Mater. Interfaces 2018, 10, 30379–30387. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.L.; Xu, C.F.; Li, Y.L.; Zhang, C.L.; Zhao, H.P.; Kaiser, U.; Lei, Y. Ultrahigh-rate and ultralong-duration sodium storage enabled by sodiation-driven reconfiguration. Adv. Energy Mater. 2023, 13, 2204324. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, A.D.; Chen, H.M.; Liu, C.X.; Yan, Y.R.; Wu, S.P. CuSe2 nanocubes enabling efficient sodium storage. ACS Appl. Mater. Interfaces 2023, 15, 12976–12985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Wang, J.; Ni, J.F.; Li, L. Structurally durable bimetallic alloy anodes enabled by compositional gradients. Adv. Sci. 2022, 9, 2201209. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Duan, C.Q.; Wang, D.; Dong, K.Z.; Luo, S.H.; Liu, Y.G.; Wang, Q.; Zhang, Y.H.; Hao, A.M. BiSb@Bi2O3/SbOx encapsulated in porous carbon as anode materials for sodium/potassium-ion batteries with a high pseudocapacitive contribution. J. Colloid Interface Sci. 2020, 580, 429–438. [Google Scholar] [CrossRef]
- Ababaikeri, R.; Sun, Y.; Wang, X.C.; Li, X.F.; Li, M.H.; Zhang, F.; Li, Y.; Wang, P.Y.; Guo, J.X.; Cao, Y.L. Scalable fabrication of Bi@N-doped carbon as anodes for sodium/potassium-ion batteries with enhanced electrochemical performances. J. Alloys Compd. 2023, 935, 168207. [Google Scholar] [CrossRef]
- Cheng, X.L.; Li, D.J.; Wu, Y.; Xu, R.; Yu, Y. Bismuth nanospheres embedded in three-dimensional (3D) porous graphene frameworks as high performance anodes for sodium- and potassium-ion batteries. J. Mater. Chem. A 2019, 7, 4913–4921. [Google Scholar] [CrossRef]
- Chong, S.K.; Ma, M.; Yuan, L.L.; Qiao, S.Y.; Dong, S.H.; Liu, H.K.; Dou, S.X. Hierarchical encapsulation and rich sp2 N assist Sb2Se3-based conversion-alloying anode for long-life sodium-and potassium-ion storage. Energy Environ. Mater. 2022, e12458. [Google Scholar] [CrossRef]
- Qin, J.; Wang, T.S.; Liu, D.Y.; Liu, E.Z.; Zhao, N.Q.; Shi, C.S.; He, F.; Ma, L.Y.; He, C.N. A top-down strategy toward SnSb in-plane nanoconfined 3D N-doped porous graphene composite microspheres for high performance Na-ion battery anode. Adv. Mater. 2018, 30, 1704670. [Google Scholar] [CrossRef]
- Song, Z.Y.; Wang, G.; Chen, Y.; Lu, Y.; Wen, Z.Y. In situ three-dimensional cross-linked carbon nanotube-interspersed SnSb@CNF as freestanding anode for long-term cycling sodium-ion batteries. Chem. Eng. J. 2023, 463, 142289. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Dong, K.Z.; Wang, D.; Chen, F.; Luo, S.H.; Liu, Y.G.; He, C.N.; Shi, C.S.; Zhao, N.Q. Monodisperse multicore-shell SnSb@SnOx/SbOx@C nanoparticles space-confined in 3D porous carbon networks as high-performance anode for Li-ion and Na-ion batteries. Chem. Eng. J. 2019, 371, 356–365. [Google Scholar] [CrossRef]



| Composites | Current Density (mA·g−1) | Capacity (mA·g−1) | Cycling Number | Ref. |
|---|---|---|---|---|
| G-Bi0.33Sb0.67 np-Bi2Sb6 | 125 | 328 | 1000 | [44] |
| 1000 | 150 | 10,000 | [40] | |
| Bi0.70Sb0.30alloys-MOF | 200 | 259.8 | 500 | [36] |
| 1000 | 180.9 | 1000 | ||
| BiSb@Bi2O3/SbOx | 100 | 293 | 100 | [45] |
| 1000 | 248.4 | 500 | ||
| Bi-Sb/C nanofibers | 2000 | 233.2 | 2000 | [31] |
| Bi@N-C | 1000 | 307 | 400 | [46] |
| Bi2Se3/GNS | 2000 | 222 | 1000 | [41] |
| 10,000 | 183 | 1000 | ||
| Bi@3DGFs | 100 | 208 | 95 | [47] |
| Sb@P-N/C | 500 | 295.6 | 400 | [7] |
| Sb2Se3@rGO@NC | 200 | 180 | 1000 | [48] |
| 3D SnSb@N–PG | 100 | 400 | 100 | [49] |
| 10,000 | 190 | 4000 | ||
| SnSb@CNF/CNT | 500 | 210 | 700 | [50] |
| 1000 | 161 | 1000 | ||
| SnSb@SnOx/SbOx@C | 100 | 360 | 200 | [51] |
| 2000 | 195 | 500 | ||
| BiSb@CSF-0.5 | 500 | 323 | 500 | This work |
| 1000 | 291 | 500 | ||
| 10,000 | 186.5 | 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lin, Y.; Lv, W.; Yuan, Y.; Guo, S.; Yan, W. Bismuth-Antimony Alloy Nanoparticles Embedded in 3D Hierarchical Porous Carbon Skeleton Film for Superior Sodium Storage. Molecules 2023, 28, 6464. https://doi.org/10.3390/molecules28186464
Wang J, Lin Y, Lv W, Yuan Y, Guo S, Yan W. Bismuth-Antimony Alloy Nanoparticles Embedded in 3D Hierarchical Porous Carbon Skeleton Film for Superior Sodium Storage. Molecules. 2023; 28(18):6464. https://doi.org/10.3390/molecules28186464
Chicago/Turabian StyleWang, Jiafan, Yonghui Lin, Wei Lv, Yongfeng Yuan, Shaoyi Guo, and Weiwei Yan. 2023. "Bismuth-Antimony Alloy Nanoparticles Embedded in 3D Hierarchical Porous Carbon Skeleton Film for Superior Sodium Storage" Molecules 28, no. 18: 6464. https://doi.org/10.3390/molecules28186464
APA StyleWang, J., Lin, Y., Lv, W., Yuan, Y., Guo, S., & Yan, W. (2023). Bismuth-Antimony Alloy Nanoparticles Embedded in 3D Hierarchical Porous Carbon Skeleton Film for Superior Sodium Storage. Molecules, 28(18), 6464. https://doi.org/10.3390/molecules28186464