Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease
Abstract
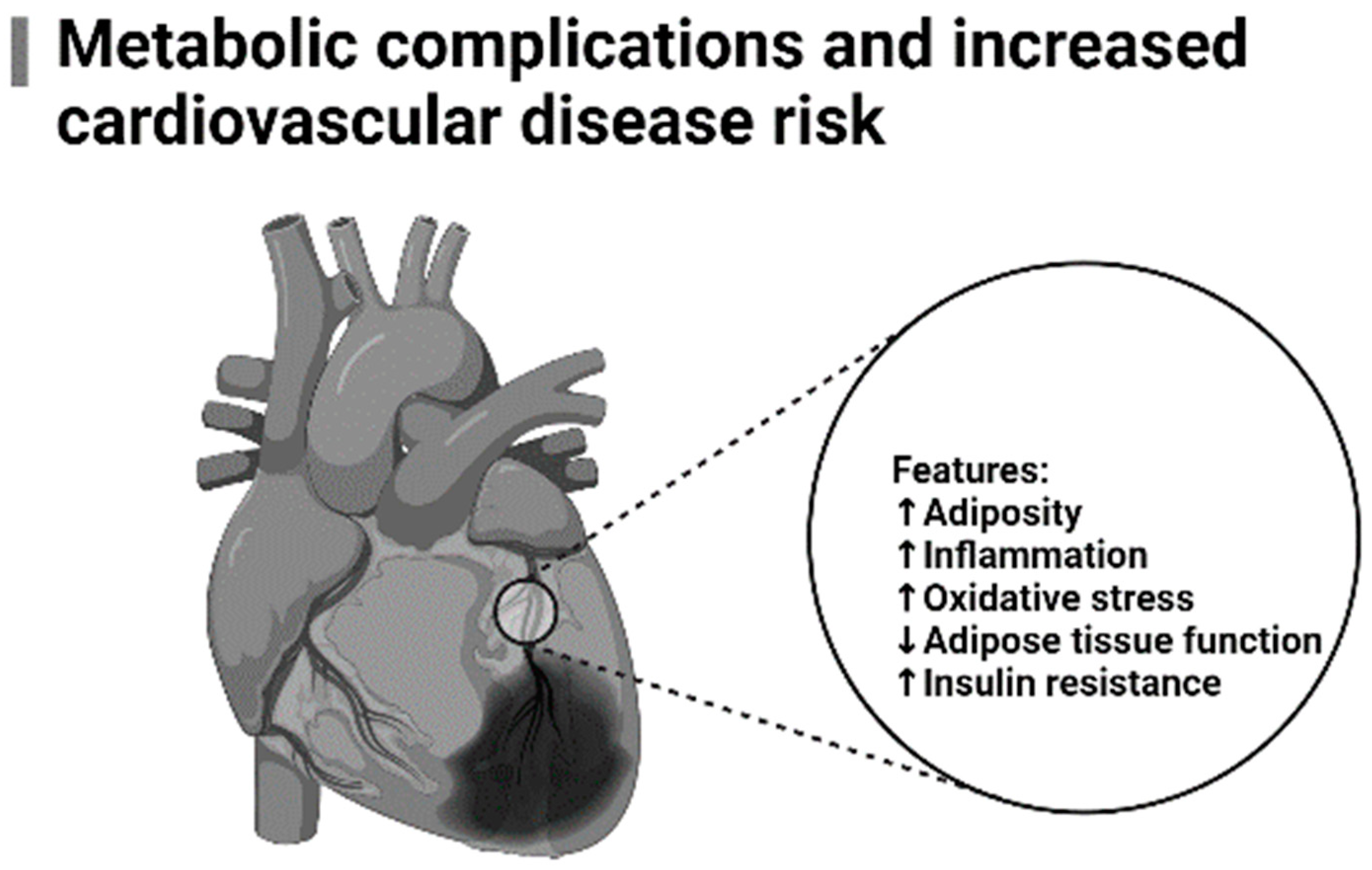
:1. Introduction
2. Methodology for Study Inclusion
3. Metabolism and Bioavailability Profile of Coffee, Especially Caffeine as One of Its Major Bioactive Compounds
4. Characteristic Features of Included Clinical Studies
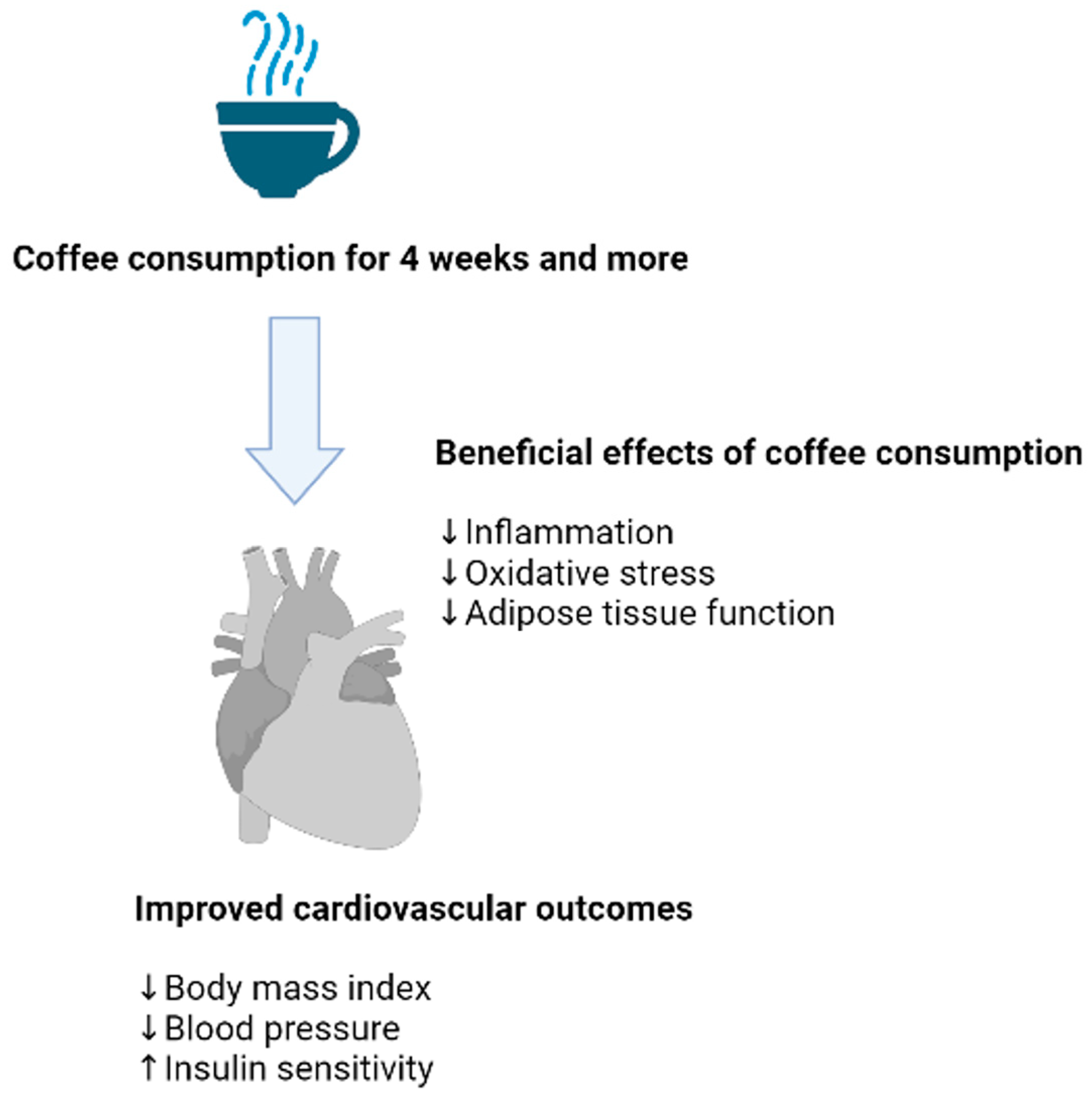
4.1. Evidence on the Effects of Coffee Consumption on Biomarkers of Oxidative Stress
4.2. Evidence on the Effects of Coffee Consumption on Biomarkers of Inflammation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef]
- Samoggia, A.; Rezzaghi, T. The Consumption of Caffeine-Containing Products to Enhance Sports Performance: An Application of an Extended Model of the Theory of Planned Behavior. Nutrients 2021, 13, 344. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Dippong, T.; Dan, M.; Kovacs, M.H.; Kovacs, E.D.; Levei, E.A.; Cadar, O. Analysis of Volatile Compounds, Composition, and Thermal Behavior of Coffee Beans According to Variety and Roasting Intensity. Foods 2022, 11, 3146. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- George, S.E.; Ramalakshmi, K.; Mohan Rao, L.J. A perception on health benefits of coffee. Crit. Rev. Food Sci. Nutr. 2008, 48, 464–486. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Jang, J.Y.; Cho, Y.O. Coffee intake can promote activity of antioxidant enzymes with increasing MDA level and decreasing HDL-cholesterol in physically trained rats. Nutr. Res. Pract. 2010, 4, 283–289. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J. Oxidative Stress and Inflammation in Heart Disease: Do Antioxidants Have a Role in Treatment and/or Prevention? Int. J. Inflam. 2011, 2011, 514623. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Ndevahoma, F.; Nkambule, B.B.; Dludla, P.V.; Mukesi, M.; Natanael, K.N.; Nyambuya, T.M. The effect of underlying inflammation on iron metabolism, cardiovascular risk and renal function in patients with type 2 diabetes. EJHaem 2021, 2, 357–365. [Google Scholar] [CrossRef]
- Tangvarasittichai, S.; Pongthaisong, S.; Tangvarasittichai, O. Tumor Necrosis Factor-A, Interleukin-6, C-Reactive Protein Levels and Insulin Resistance Associated with Type 2 Diabetes in Abdominal Obesity Women. Indian J. Clin. Biochem. 2016, 31, 68–74. [Google Scholar] [CrossRef]
- Ngcobo, S.R.; Nkambule, B.B.; Nyambuya, T.M.; Mokgalaboni, K.; Ntsethe, A.; Mxinwa, V.; Ziqubu, K.; Ntamo, Y.; Nyawo, T.A.; Dludla, P.V. Activated monocytes as a therapeutic target to attenuate vascular inflammation and lower cardiovascular disease-risk in patients with type 2 diabetes: A systematic review of preclinical and clinical studies. Biomed. Pharmacother. 2022, 146, 112579. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Giuliani, A.; Mensà, E.; Sabbatinelli, J.; De Nigris, V.; Rippo, M.R.; La Sala, L.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Pleiotropic effects of metformin: Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res. Rev. 2018, 48, 87–98. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Arif, Z.; Kabir, A.; Mehmood, I.; Munir, D.; Razzaq, A.; Ali, A.; Goksen, G.; Coşier, V.; Ahmad, N.; et al. Oxidative stress and metabolic diseases: Relevance and therapeutic strategies. Front. Nutr. 2022, 9, 994309. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Ho, C.K. Antioxidant properties of drugs used in Type 2 diabetes management: Could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. 2018, 23, 1–24. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Ceriello, A. Is time ready for combination therapy at diagnosis of type 2 diabetes? Diabetes Metab. Res. Rev. 2021, 37, e3460. [Google Scholar] [CrossRef] [PubMed]
- Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Mxinwa, V.; Mokgalaboni, K.; Orlando, P.; Silvestri, S.; Louw, J.; Tiano, L.; Dludla, P.V. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules 2020, 25, 5645. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Silvestri, S.; Orlando, P.; Gabuza, K.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Johnson, R.; Muller, C.J.F.; et al. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-associated Complications: A Systematic Review of Preclinical Studies. Nutrients 2020, 12, 739. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; DiNicolantonio, J.J.; Lavie, C.J. Coffee for Cardioprotection and Longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar] [CrossRef]
- Teramoto, M.; Yamagishi, K.; Muraki, I.; Tamakoshi, A.; Iso, H. Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension. J. Am. Heart Assoc. 2023, 12, e026477. [Google Scholar] [CrossRef]
- Lim, D.; Chang, J.; Ahn, J.; Kim, J. Conflicting Effects of Coffee Consumption on Cardiovascular Diseases: Does Coffee Consumption Aggravate Pre-existing Risk Factors? Processes 2020, 8, 438. [Google Scholar] [CrossRef]
- Shahinfar, H.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Coffee consumption and cardiovascular diseases and mortality in patients with type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2526–2538. [Google Scholar] [CrossRef]
- Campos, H.; Baylin, A. Coffee consumption and risk of type 2 diabetes and heart disease. Nutr. Rev. 2007, 65, 173–179. [Google Scholar] [CrossRef]
- Kwok, M.K.; Leung, G.M.; Schooling, C.M. Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer’s disease: A Mendelian randomization study. Sci. Rep. 2016, 6, 36500. [Google Scholar] [CrossRef] [PubMed]
- Ranheim, T.; Halvorsen, B. Coffee consumption and human health-beneficial or detrimental?—Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol. Nutr. Food Res. 2005, 49, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. The impact of coffee consumption on blood pressure, cardiovascular disease and diabetes mellitus. Expert. Rev. Cardiovasc. Ther. 2017, 15, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Barton, S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. Bmj 2000, 321, 255–256. [Google Scholar] [CrossRef]
- Hemraj, S. A Detail Chemistry of Coffee and Its Analysis. In Coffee; Dalyse Toledo, C., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 5. [Google Scholar]
- Moreira, A.S.P.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Data on coffee composition and mass spectrometry analysis of mixtures of coffee related carbohydrates, phenolic compounds and peptides. Data Brief 2017, 13, 145–161. [Google Scholar] [CrossRef]
- Saud, S.; Salamatullah, A.M. Relationship between the Chemical Composition and the Biological Functions of Coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef]
- Wei, F.; Tanokura, M. Chapter 10—Chemical Changes in the Components of Coffee Beans during Roasting. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 83–91. [Google Scholar]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb. Exp. Pharmacol. 2011, 200, 33–91. [Google Scholar] [CrossRef]
- Teekachunhatean, S.; Tosri, N.; Rojanasthien, N.; Srichairatanakool, S.; Sangdee, C. Pharmacokinetics of Caffeine following a Single Administration of Coffee Enema versus Oral Coffee Consumption in Healthy Male Subjects. ISRN Pharmacol. 2013, 2013, 147238. [Google Scholar] [CrossRef]
- Newton, R.; Broughton, L.J.; Lind, M.J.; Morrison, P.J.; Rogers, H.J.; Bradbrook, I.D. Plasma and salivary pharmacokinetics of caffeine in man. Eur. J. Clin. Pharmacol. 1981, 21, 45–52. [Google Scholar] [CrossRef]
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef]
- Nuhu, A.A. Bioactive micronutrients in coffee: Recent analytical approaches for characterization and quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef] [PubMed]
- Mumford, G.K.; Benowitz, N.L.; Evans, S.M.; Kaminski, B.J.; Preston, K.L.; Sannerud, C.A.; Silverman, K.; Griffiths, R.R. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur. J. Clin. Pharmacol. 1996, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Rodopoulos, N.; Wisén, O.; Norman, A. Caffeine metabolism in patients with chronic liver disease. Scand. J. Clin. Lab. Investig. 1995, 55, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G. The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiol. Behav. 2010, 100, 33–41. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Tabrez, S.; Jabir, N.R.; Ali, M.; Kamal, M.A.; da Silva Araujo, L.; De Oliveira Santos, J.V.; Da Mata, A.; De Aguiar, R.P.S.; de Carvalho Melo Cavalcante, A.A. An Insight into the Therapeutic Potential of Major Coffee Components. Curr. Drug Metab. 2018, 19, 544–556. [Google Scholar] [CrossRef]
- Thorn, C.F.; Aklillu, E.; Klein, T.E.; Altman, R.B. PharmGKB summary: Very important pharmacogene information for CYP1A2. Pharmacogenet. Genom. 2012, 22, 73–77. [Google Scholar] [CrossRef]
- Banks, N.F.; Tomko, P.M.; Colquhoun, R.J.; Muddle, T.W.D.; Emerson, S.R.; Jenkins, N.D.M. Genetic Polymorphisms in ADORA2A and CYP1A2 Influence Caffeine’s Effect on Postprandial Glycaemia. Sci. Rep. 2019, 9, 10532. [Google Scholar] [CrossRef]
- Djordjevic, N.; Ghotbi, R.; Bertilsson, L.; Jankovic, S.; Aklillu, E. Induction of CYP1A2 by heavy coffee consumption in Serbs and Swedes. Eur. J. Clin. Pharmacol. 2008, 64, 381–385. [Google Scholar] [CrossRef]
- Acheson, K.J.; Zahorska-Markiewicz, B.; Pittet, P.; Anantharaman, K.; Jéquier, E. Caffeine and coffee: Their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 1980, 33, 989–997. [Google Scholar] [CrossRef]
- Martínez-López, S.; Sarriá, B.; Baeza, G.; Mateos, R.; Bravo-Clemente, L. Pharmacokinetics of caffeine and its metabolites in plasma and urine after consuming a soluble green/roasted coffee blend by healthy subjects. Food Res. Int. 2014, 64, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a Factor Influencing the Functioning of the Human Body-Friend or Foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.; Sawers, S.J. The absolute bioavailability of caffeine in man. Eur. J. Clin. Pharmacol. 1983, 24, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kamimori, G.H.; Karyekar, C.S.; Otterstetter, R.; Cox, D.S.; Balkin, T.J.; Belenky, G.L.; Eddington, N.D. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int. J. Pharm. 2002, 234, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Booth, G.H., Jr. Gastric acid secretion and lower-esophageal-sphincter pressure in response to coffee and caffeine. N. Engl. J. Med. 1975, 293, 897–899. [Google Scholar] [CrossRef]
- Marks, V.; Kelly, J.F. Absorption of caffeine from tea, coffee, and coca cola. Lancet 1973, 1, 827. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Greenblatt, D.J.; Ehrenberg, B.L.; Goddard, J.E.; Cotreau, M.M.; Harmatz, J.S.; Shader, R.I. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J. Clin. Pharmacol. 1997, 37, 693–703. [Google Scholar] [CrossRef]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bhatti, S.K.; Patil, H.R.; DiNicolantonio, J.J.; Lucan, S.C.; Lavie, C.J. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 1043–1051. [Google Scholar] [CrossRef]
- Clark, I.; Landolt, H.P. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep. Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef]
- Scherer, P.E.; Hill, J.A. Obesity, Diabetes, and Cardiovascular Diseases: A Compendium. Circ. Res. 2016, 118, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Alfthan, G.; Virtanen, J.K.; Rissanen, T.H.; Happonen, P.; Nyyssönen, K.; Kaikkonen, J.; Salonen, R.; et al. The effects of coffee consumption on lipid peroxidation and plasma total homocysteine concentrations: A clinical trial. Free Radic. Biol. Med. 2005, 38, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Moura-Nunes, N.; Perrone, D.; Farah, A.; Donangelo, C.M. The increase in human plasma antioxidant capacity after acute coffee intake is not associated with endogenous non-enzymatic antioxidant components. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S6), 173–181. [Google Scholar] [CrossRef]
- Mišík, M.; Hoelzl, C.; Wagner, K.H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat. Res. 2010, 692, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kotyczka, C.; Boettler, U.; Lang, R.; Stiebitz, H.; Bytof, G.; Lantz, I.; Hofmann, T.; Marko, D.; Somoza, V. Dark roast coffee is more effective than light roast coffee in reducing body weight, and in restoring red blood cell vitamin E and glutathione concentrations in healthy volunteers. Mol. Nutr. Food Res. 2011, 55, 1582–1586. [Google Scholar] [CrossRef]
- Corrêa, T.A.; Monteiro, M.P.; Mendes, T.M.; Oliveira, D.M.; Rogero, M.M.; Benites, C.I.; Vinagre, C.G.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; et al. Medium light and medium roast paper-filtered coffee increased antioxidant capacity in healthy volunteers: Results of a randomized trial. Plant Foods Hum. Nutr. 2012, 67, 277–282. [Google Scholar] [CrossRef]
- Teekachunhatean, S.; Tosri, N.; Sangdee, C.; Wongpoomchai, R.; Ruangyuttikarn, W.; Puaninta, C.; Srichairatanakool, S. Antioxidant effects after coffee enema or oral coffee consumption in healthy Thai male volunteers. Hum. Exp. Toxicol. 2012, 31, 643–651. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee Consumption Increases the Antioxidant Capacity of Plasma and Has No Effect on the Lipid Profile or Vascular Function in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef]
- Katada, S.; Watanabe, T.; Mizuno, T.; Kobayashi, S.; Takeshita, M.; Osaki, N.; Kobayashi, S.; Katsuragi, Y. Effects of Chlorogenic Acid-Enriched and Hydroxyhydroquinone-Reduced Coffee on Postprandial Fat Oxidation and Antioxidative Capacity in Healthy Men: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2018, 10, 525. [Google Scholar] [CrossRef]
- Shaposhnikov, S.; Hatzold, T.; Yamani, N.E.; Stavro, P.M.; Lorenzo, Y.; Dusinska, M.; Reus, A.; Pasman, W.; Collins, A. Coffee and oxidative stress: A human intervention study. Eur. J. Nutr. 2018, 57, 533–544. [Google Scholar] [CrossRef]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzmán, O.J.; Medina, S.; Álvarez, R.; Oger, C.; Durand, T.; Galano, J.M.; Zuluaga, N.; Gil-Izquierdo, Á.; Muñoz-Durango, K. Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells. Free Radic. Biol. Med. 2020, 160, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Domínguez-Perles, R.; Rosi, A.; Tassotti, M.; Angelino, D.; Medina, S.; Ricci, C.; Guy, A.; Oger, C.; Gigliotti, L.; et al. Effect of Coffee and Cocoa-Based Confectionery Containing Coffee on Markers of DNA Damage and Lipid Peroxidation Products: Results from a Human Intervention Study. Nutrients 2021, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.P.; Liu, C.; Chan, L.P.; Liang, C.H. Coffee pulp supplement affects antioxidant status and favors anti-aging of skin in healthy subjects. J. Cosmet. Dermatol. 2022, 21, 2189–2199. [Google Scholar] [CrossRef]
- Vuckovic, B.A.; Cabarkapa, V.S.; Ilic, T.A.; Salatic, I.R.; Lozanov-Crvenkovic, Z.S.; Mitic, G.P. Clinical significance of determining plasma homocysteine: Case-control study on arterial and venous thrombotic patients. Croat. Med. J. 2013, 54, 480–488. [Google Scholar] [CrossRef]
- Fakhrzadeh, H.; Ghotbi, S.; Pourebrahim, R.; Nouri, M.; Heshmat, R.; Bandarian, F.; Shafaee, A.; Larijani, B. Total plasma homocysteine, folate, and vitamin B12 status in healthy Iranian adults: The Tehran homocysteine survey (2003–2004)/a cross-sectional population based study. BMC Public Health 2006, 6, 29. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef]
- Gavrieli, A.; Yannakoulia, M.; Fragopoulou, E.; Margaritopoulos, D.; Chamberland, J.P.; Kaisari, P.; Kavouras, S.A.; Mantzoros, C.S. Caffeinated coffee does not acutely affect energy intake, appetite, or inflammation but prevents serum cortisol concentrations from falling in healthy men. J. Nutr. 2011, 141, 703–707. [Google Scholar] [CrossRef]
- Corrêa, T.A.; Rogero, M.M.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; César, L.A.; Torres, E.A. Paper-filtered coffee increases cholesterol and inflammation biomarkers independent of roasting degree: A clinical trial. Nutrition 2013, 29, 977–981. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Strom, A.; Nowotny, B.; Zahiragic, L.; Nowotny, P.J.; Carstensen-Kirberg, M.; Herder, C.; Roden, M. Effect of Low-Energy Diets Differing in Fiber, Red Meat, and Coffee Intake on Cardiac Autonomic Function in Obese Individuals with Type 2 Diabetes. Diabetes Care 2015, 38, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Goodman, C.L.; Capps, C.R.; Shue, Z.L.; Arnot, R. Influence of 2-Weeks Ingestion of High Chlorogenic Acid Coffee on Mood State, Performance, and Postexercise Inflammation and Oxidative Stress: A Randomized, Placebo-Controlled Trial. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 55–65. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death Leading Causes of Death. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 2 July 2023).
- Pickering, R.J. Oxidative Stress and Inflammation in Cardiovascular Diseases. Antioxidants 2021, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; VAnil Kumar, N.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Parihar, A.; Parihar, M.S. Bioactive Food Components in the Prevention of Cardiovascular Diseases. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 137–157. [Google Scholar]
- Pan, S.Y.; Nie, Q.; Tai, H.C.; Song, X.L.; Tong, Y.F.; Zhang, L.J.; Wu, X.W.; Lin, Z.H.; Zhang, Y.Y.; Ye, D.Y.; et al. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef]
- Zhang, D.; Ferguson, K.; Troester, M.A.; Bensen, J.T.; Cai, J.; Milne, G.L.; Sandler, D.P.; Nichols, H.B. Tea consumption and oxidative stress: A cross-sectional analysis of 889 premenopausal women from the Sister Study. Br. J. Nutr. 2019, 121, 582–590. [Google Scholar] [CrossRef]
- Basu, A.; Lucas, E.A. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 2007, 65, 361–375. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Ndevahoma, F.; et al. Tea consumption and its effects on primary and secondary prevention of coronary artery disease: Qualitative synthesis of evidence from randomized controlled trials. Clin. Nutr. ESPEN 2021, 41, 77–87. [Google Scholar] [CrossRef]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L.; et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Martin, S. Health Effects of Coffee: Mechanism Unraveled? Nutrients 2020, 12, 1842. [Google Scholar] [CrossRef]
- Lonati, E.; Carrozzini, T.; Bruni, I.; Mena, P.; Botto, L.; Cazzaniga, E.; Del Rio, D.; Labra, M.; Palestini, P.; Bulbarelli, A. Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules 2022, 27, 1049. [Google Scholar] [CrossRef]
- Boettler, U.; Sommerfeld, K.; Volz, N.; Pahlke, G.; Teller, N.; Somoza, V.; Lang, R.; Hofmann, T.; Marko, D. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J. Nutr. Biochem. 2011, 22, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo-Clemente, L.; Goya, L.; Sarriá, B. Colonic Coffee Phenols Metabolites, Dihydrocaffeic, Dihydroferulic, and Hydroxyhippuric Acids Protect Hepatic Cells from TNF-α-Induced Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 1440. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-d-glucoside. Nutr. Metab. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]

| References | Country | Study Population | Intervention | Main Findings |
| Mursu et al., 2005 [64] | Finland | Healthy nonsmoking men (n = 45), with age range of 20–26 years | Received two cups of filtered coffee (300 mL/day) for 3 weeks | Improved plasma homocysteine levels, although did not affect markers of lipid peroxidation |
| Moura-Nunes et al., 2009 [65] | Brazil | Healthy individuals (n = 10), with age range of 22–57 years | Received instant coffee (200 mL) for 90 min | Improved antioxidant capacity, correlating with uric acid and α-tocopherol |
| Mišík et al., 2010 [66] | Austria | Individuals (n = 38), with age range of 19–36 years | Received filtered coffee (800 mL/day) for 5 days | Protected against formation of endogenous oxidative DNA-damage. But did not affect levels of malondialdehyde (MDA), glutathione (GSH), intracellular reactive oxygen species (ROS) levels and the activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in lymphocytes |
| Kotyczka et al., 2011 [67] | Germany | Obese individuals (n = 30), age not disclosed | Received roast coffee (500 mL/day) for 4 weeks | Reduced body weights, consistent with improving the antioxidant status of erythrocytes, including enhancing levels of SOD, GPx. Whereas tocopherol and GSH concentrations were also increased |
| Corrêa et al., 2012 [68] | Brazil | Healthy individuals (n = 20), with age range of 20–65 years | Received medium light roast and medium roast coffee (482 mL/day) for 4 weeks | Both coffees increased antioxidant status, including plasma levels of catalase (CAT), SOD, and GPx. But did not affect lipid peroxidation |
| Teekachunhatean et al., 2012 [69] | Thailand | Healthy individuals (n = 11), with age range of 13–30 years | Received ready to drink coffee (approximately 500 mL) daily for 11 days | Serum antioxidant status was improved, but did not directly affect GSH or MDA levels |
| Agudelo-Ochoa et al., 2016 [70] | Colombia | Healthy individuals (n = 75), with age range of 20–60 years | Received filtered coffee (400 mL/day), with either high or low chlorogenic acids for 8 weeks | Acute effect on the plasma antioxidant capacity, although did not have an effect on blood lipids or vascular function |
| Katada et al., 2018 [71] | Japan | Healthy individuals (n = 15), with age range of 20–60 years | Received rich in chlorogenic acids (185 mL) for 4 weeks | Effective in reducing chlorogenic acids-induced fat oxidation, while enhancing the antioxidant status |
| Shaposhnikov et al., 2018 [72] | Norway | Healthy individuals (n = 160), with age range of 35–65 years | Received five cups of filtered coffee for 8 weeks | Increased serum creatinine and the liver enzyme γ-glutamyl transaminase, while not affecting markers of oxidation of DNA and lipid |
| Martínez-López et al., 2019 [73] | Spain | Hypercholesterolemic individuals (n = 52), with age range of 18–45 years | Received 6 g/day of soluble green/roasted (35:65) coffee for 8 weeks | Improved plasma antioxidant capacity, while decreasing markers of lipid peroxidation (MDA), while reducing systolic and diastolic blood pressure, including heart rate and body weight |
| Lara-Guzmán et al., 2020 [74] | Colombia | Healthy individuals (n = 74), with age range of 20–60 years | Received two types of coffee that provided 787 mg chlorogenic acids/day (Coffee A) and 407 mg chlorogenic acids/day (Coffee B) for 8 weeks | Both coffees decreased urine oxylipins, while coffee A showed a stronger effect in reducing prostaglandins and prostaglandin metabolites. However, neither of the two coffees reduced the levels of oxidized low-density lipoprotein (oxLDL) |
| Martini et al., 2021 [75] | Italy | Healthy individuals (n = 21), with age range of 22–24 years | Received one/three cup of espresso coffee/day, and one cup of espresso coffee plus two cocoa-based products containing coffee for 4 weeks | No significant modulation of DNA and lipid damage markers was recorded, although DNA strand breaks and some markers of lipid peroxidation were modulated |
| Tseng et al., 2022 [76] | Taiwan | Healthy individuals (n = 40), with age range of 35–55 years | Received a coffee pulp drink (50 mL/day) for 8 weeks | Slowed the skin aging process and improved skin health. The radical scavenging activity was enhanced through inhibition of tyrosinase activity |
| References | Country | Study Population | Intervention | Main Findings |
| Kempf et al., 2010 [80] | Germany | Healthy individuals (n = 47), younger than the age of 65 years | Received 4 cups of filtered coffee/d and in the third month 8 cups of filtered coffee/d (150 mL/cup) for 4 weeks | Showed beneficial effects on subclinical inflammation and high-density lipoprotein (HDL) cholesterol, although not affecting glucose metabolism. Significant changes were also observed for serum concentrations of interleukin-18, 8-isoprostane, and adiponectin |
| Gavrieli et al., 2011 [81] | Greece | Healthy individuals (n = 16), with age range of 21–39 years | Received caffeinated coffee (3 mg caffeine/kg body weight) for 180 min before consuming meal ad libitum | Did not affect appetite-related ratings, the appetite plasma hormonal responses as well as the plasma glucose, serum insulin, and plasma and serum inflammatory marker responses. However, serum cortisol; cortisol concentrations were significantly higher following the caffeinated coffee intervention |
| Corrêa et al., 2013 [82] | Brazil | Healthy individuals (n = 20), with age range of 20–65 years | Received medium light roast and medium roast coffee (482 mL/day) for 4 weeks | Did not affect plasma total cholesterol, low-density lipoprotein-cholesterol, and soluble vascular cell adhesion molecule-1 concentrations. No changes were observed for lipoprotein, total homocysteine, glycemic biomarkers, and blood pressure |
| Ziegler et al., 2015 [84] | Germany | Obese and type 2 diabetic individuals (n = 28), with age range of 18–69 years | Received a diet high in cereal fiber, free of red meat, and high in coffee for 8 weeks | Improved cardiac vagal function and oxidative glucose utilization. Moreover, there was a reduction in body weight and heart rate with the heart rate variability being promoted. However, there was no effect on insulin sensitivity and inflammatory status |
| Nieman et al., 2018 [85] | United states | Cyclists (n = 15), with age range of 19–51 years | Received high chlorogenic acid coffee for 2 weeks | Improved total mood disturbance scores but did not affect blood inflammatory biomarker interleukin (IL)-6 and oxidative stress biomarker hydroxyoctade cadienoic acids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dludla, P.V.; Cirilli, I.; Marcheggiani, F.; Silvestri, S.; Orlando, P.; Muvhulawa, N.; Moetlediwa, M.T.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hlengwa, N.; et al. Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease. Molecules 2023, 28, 6440. https://doi.org/10.3390/molecules28186440
Dludla PV, Cirilli I, Marcheggiani F, Silvestri S, Orlando P, Muvhulawa N, Moetlediwa MT, Nkambule BB, Mazibuko-Mbeje SE, Hlengwa N, et al. Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease. Molecules. 2023; 28(18):6440. https://doi.org/10.3390/molecules28186440
Chicago/Turabian StyleDludla, Phiwayinkosi V., Ilenia Cirilli, Fabio Marcheggiani, Sonia Silvestri, Patrick Orlando, Ndivhuwo Muvhulawa, Marakiya T. Moetlediwa, Bongani B. Nkambule, Sithandiwe E. Mazibuko-Mbeje, Nokulunga Hlengwa, and et al. 2023. "Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease" Molecules 28, no. 18: 6440. https://doi.org/10.3390/molecules28186440
APA StyleDludla, P. V., Cirilli, I., Marcheggiani, F., Silvestri, S., Orlando, P., Muvhulawa, N., Moetlediwa, M. T., Nkambule, B. B., Mazibuko-Mbeje, S. E., Hlengwa, N., Hanser, S., Ndwandwe, D., Marnewick, J. L., Basson, A. K., & Tiano, L. (2023). Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease. Molecules, 28(18), 6440. https://doi.org/10.3390/molecules28186440







