Abstract
Chemical profiling for quality monitoring and evaluation of medicinal plants is gaining attention. This study aims to develop an HPLC method followed by multivariate analysis to obtain HPLC profiles of five specific flavonoids, including rutin (1), hyperin (2), isoquercitrin (3), quercitrin (4), and quercetin (5) from Houttuynia cordata leaves and powder products and assess the quality of H. cordata samples. Eventually, we successfully established HPLC-based flavonoid profiles and quantified the contents of 32 H. cordata fresh leave samples and four powder products. The study also quantified the contents of those five essential flavonoids using an optimized RP-HPLC method. Peak areas of samples were then investigated with principal component analysis (PCA) and hierarchical cluster analysis (HCA) to evaluate the similarity and variance. Principal components in PCA strongly influenced by hyperin and quercetin showed that the samples were clustered into subgroups, demonstrating H. cordata samples’ quality. The results of HCA showed the similarity and divided the samples into seven subgroups. In conclusion, we have successfully developed a practical methodology that combined the HPLC-based flavonoid profiling and multivariate analysis for the quantification and quality control of H. cordata samples from fresh leaves and powder products. For further studies, we will consider various environmental factors, including climate and soil factors, to investigate their effects on the flavonoid contents of H. cordata.
1. Introduction
The chemical profile describes the representation of chemical information of medicinal plants into a chromatogram or spectrogram as meaningful evidence for the rapid identification of herbs. It has been an attractive option in assessing the quality of medicinal herbs with various benefits, such as detecting counterfeit, adulterated, or poor-quality herbal plants through their principal natural compound groups. Currently, the chemical profiles of medicinal plants have been recommended as a technique for quality estimation of medicinal plants by the US Food and Drug Administration (FDA) [1], the European Medicines Agency (EMA) [2], the World Health Organization (WHO) [3], and many monographs of Chinese Pharmacopoeia [4] and Vietnamese Pharmacopoeia [5].
Among numerous analytical techniques for obtaining medicinal herb chemical profiles, including thin-layer chromatography (TLC) [6], high-performance thin-layer chromatography (HPTLC) [7], Fourier-transform infrared spectroscopy (FTIR) [8], high-performance liquid chromatography (HPLC) [9], and gas chromatography (GC) [10], HPLC is widely recognized as a powerful and conventional technique due to its high sensitivity, specificity, low detection limit, high accuracy, and precision [11]. Furthermore, HPLC provides qualitative and quantitative information about the sample more efficiently than other analytical techniques. However, an efficient and intuitive method to integrate and describe the data is required because HPLC generates a large amount of data. Multivariate statistical analysis approaches can be deployed for reducing data and extracting relevant information. In particular, principal component analysis (PCA) and hierarchical clustering analysis (HCA) are most commonly used in HPLC chromatography analysis [12,13,14]. PCA reduces raw data size and turns it into smaller data size without losing information in the original data. Therefore, fewer principal components (PCs) are needed to explain the correlation between abundant variables. Meanwhile, HCA aids in grouping data clusters, thereby visualizing the relationship of the analytic samples. HPLC-based profiles combined with PCA and HCA could effectively identify the classification and quality control of herbal medicines [9,15,16].
Houttuynia cordata (H. cordata, HC) is a popular vegetable grown throughout Vietnam. H. cordata belongs to the Saururaceae family and was originally described as the only species in the Houttuynia genus. In 2001, Zhu et al. discovered and characterized a second species, Houttuynia emeiensis, found in China; however, its validity is still unestablished [17]. Due to their heat-clearing, detoxifying, diuretic, and antiseptic properties, H. cordata has long been used to treat many diseases, such as constipation, hemorrhoids, furuncle, and urinary retention [18,19]. Many pharmacological effects of H. cordata have been demonstrated, including antioxidant [20], anti-inflammatory [21], and antibacterial properties [22]. These potentials are primarily due to flavonoid chemicals found in H. cordata, namely, rutin, quercetin, quercitrin, hyperin, and isoquercitrin [23,24]. Flavonoids are one of the main bioactive compounds and are of particular interest since they define the quality and capacity of H. cordata. Although the uses of H. cordata are undisputed, numerous studies in recent years have revealed many important newly discovered biological properties. Most studies on the flavonoid composition of H. cordata preferred to use the leaves components as the analysis sample because the highest content of total flavonoids was found in leaves, followed by roots and stems, with substantial differences [25]. The presence of quercitrin, quercetin, and hyperoside in H. cordata leaves was demonstrated to have potent ferric-reducing antioxidant power and DPPH radical-scavenging activity in vitro by lowering CCl4-induced oxidative stress in mouse liver [26]. Flavonoids of H. cordata were proposed to improve H1N1-induced acute lung injury in mice through inhibition of influenzal neuraminidase activity and TLR signaling, in which hyperin and quercitrin played the main roles in the therapeutic effect [27]. On the other hand, a flavonoid hyperoside-enriched fraction from H. cordata leaves was proposed to be UVB-induced photoprotective or anti-skin aging agent via the MAPK signaling pathway [28]. This clarified the usages of H. cordata in the pharmaceutical cosmetic industry.
Because of the potential and promising applications of H. cordata, it is necessary to develop an effective classification and quality assessment method to evaluate the flavonoid content in H. cordata across a variety of geographical locations in Vietnam and the commercial products after processing. The present study aims to develop an HPLC method to obtain chromatographic profiles of specific flavonoids of H. cordata leaves collected from different sites in Vietnam and H. cordata leave powder products available in the market. Simultaneously, this research quantified these five main flavonoids in H. cordata leaves and applied hierarchical cluster analysis (HCA) and principal component analysis (PCA) to evaluate the similarity and variance between H. cordata samples. It is essential to highlight the high-quality H. cordata growing regions and the quality of commercial products.
2. Results
2.1. Optimization of RP-HPLC Conditions for Flavonoid Analysis
Table 1 displays the various RP-HPLC conditions used to investigate the optimal method for the H. cordata leaves analysis, represented by each experiment’s retention time, resolution, tailing factor, and the number of theoretical plates. Firstly, we used the Phenomenex column (250 mm × 4.6 mm, 5 µm) to examine the ideal mobile phase. With acetonitrile as solvent B and formic acid in water at 0.1% (A), the chromatograms demonstrated more excellent resolution, tailing factor, and the number of theoretical plates of the analytical peaks than acetic acid at 0.2% (A). As a result, formic acid 0.1% (A) and acetonitrile (B) were utilized as mobile phases for further investigation with a 150 mm column at two column temperatures of 25 °C and 40 °C and a lower flow rate (0.6 mL min−1). At 25 °C, the two closest peaks, hyperin and isoquercitrin, have the highest resolutions (5.221 and 0.909, respectively). The tailing factor of peaks range from 0.8 to 1.2, and the number of theoretical plates greater than 5000 indicate that the peaks have good symmetrical shapes. By increasing the column temperature to 40 °C, the parameters are not better than at 25 °C. Thus, a 150 mm column at 25 °C was selected as the optimal condition for HPLC-based profiling and quantifying five specific flavonoids from H. cordata leaves.

Table 1.
Different RP-HPLC analysis conditions of Houttuynia cordata leave extracts and the results of parameters including retention time, resolution, tailing factor, and the number of theoretical plates.
The chromatogram denoted the target flavonoid peaks with the best separation under the optimal HPLC conditions using Phenomenex C18 reversed-phase column (150 mm × 4.6 mm, 5 μm), and the mobile phase consisted of formic acid 0.1% in water (A) and acetonitrile (B) with a gradient program of 20% B in 0–5 min, 20–21% B in 5–25 min, and 21–50% B in 25–45 min, a flow rate of 0.6 mL min−1, an injection volume of 2 μL, and a column temperature kept at 25 °C. The wavelength of detection was 355 nm for acquiring chromatograms. The total analysis duration was 45 min. The typical optimized HPLC chromatograms are shown in Figure 1.
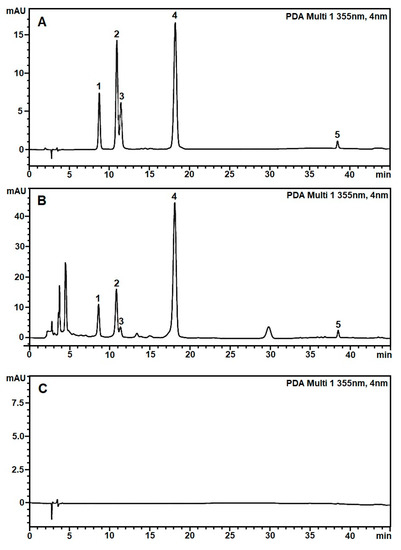
Figure 1.
The typical RP-HPLC profile of five standard flavonoids (A), flavonoids in the leave extract of H. cordata (B), and solvent mobile phase baseline (C) were achieved under the optimized RP-HPLC methods with elution program C, Phenomenex column 150 mm × 4.6 mm × 5 μm at a flow rate 0.6 mL/min, an injection volume of 2 μL, and a column temperature at 25 °C. The peaks marked with 1 to 5 were of rutin (8.74 min), hyperin (10.93 min), isoquercitrin (11.43 min), quercitrin (18.22 min), and quercetin (38.46 min), respectively.
2.2. Validation of Optimized RP-HPLC Method
2.2.1. Specificity
The specificity of the method was validated by comparing the chromatograms of the mobile phase blank, the standard sample, and the H. cordata leaves extract sample using the optimized HPLC conditions in Section 2.1. Figure 2 shows the retention times of five specific flavonoids, including rutin, hyperin, isoquercitrin, quercitrin, and quercetin in the extracts, which were monitored at 8.74, 10.93, 11.43, 18.22, and 38.46 min, respectively. The chromatograms of the blank showed no analytes that interfered with absorption at 355 nm. The specificity ensured that the method was suitable for the qualitative and quantitative analysis of five flavonoids in H. cordata leaves and powder products.
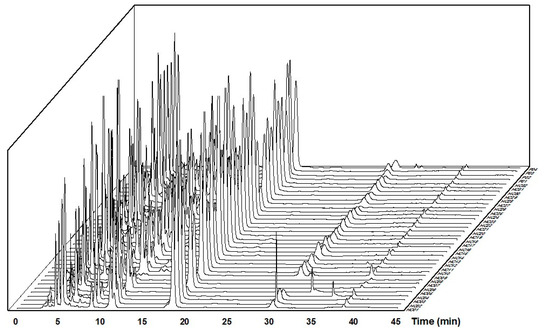
Figure 2.
HPLC-based flavonoid profiles from 36 samples of Houttuynia cordata leaves are designated HC01-HC32 and P01-P04 on the z-axis.
2.2.2. Precision, Repeatability, Intermediate Precision, and Stability
The HC27 leaves sample was analyzed six times to assess the precision. Stability was evaluated by analyzing samples of HC27 at different time points (0, 3, 6, 9, and 12 h). The H. cordata sample was analyzed five times to evaluate the repeatability. %RSD for peak area of five flavonoids, including rutin, hyperin, isoquercitrin, quercitrin, and quercetin, was less than 2% for all, reflecting the method’s precision. The H. cordata leaves test sample was determined within 12 h. The results are shown in Table 2.

Table 2.
Validation results of precision, intermediate precision, repeatability, and stability for determination of five flavonoids.
2.2.3. Accuracy
Three concentrations (80%, 100%, and 120%) of the standards were added to the HC27 sample to evaluate the accuracy of the quantitative method. The results are presented in Table 3. The recoveries of rutin, hyperin, isoquercitrin, quercitrin, and quercetin were all in the 98–102% range, indicating the method’s accuracy.

Table 3.
Validation result of accuracy of the quantitative method RP-HPLC.
2.2.4. Linearity, Range, Limit of Detection, and Limit of Quantification
Five calibration curves all had good linearity with R2 > 0.9999 and the quantification range was relatively wide. The limit of detection and quantification were also calculated based on the parameters’ standard deviation and slope of the calibration curve. The results are shown in Table 4.

Table 4.
Calibration curves, correlation coefficient, linear range, LOD, and LOQ were obtained using the optimized RP-HPLC analysis condition for the five flavonoids. Calibration curves are in the form of regression equation y = ax + b, where a is the slope of the line, b is the y-intercept, x is the peak area, and y is the flavonoid content.
2.3. HPLC-Based Flavonoid Profiles
HPLC-based flavonoid profiles of 36 samples of H. cordata leaves and four samples of powder products processed with Origin 2021 software were presented in Figure 2.
Figure 2 shows the corresponding chromatograms at the standard peaks, including the five specific flavonoids: rutin, hyperin, isoquercitrin, quercitrin, and quercetin. Chromatograms of samples HC04 and HC05 have strange peaks at 36.15 min and 33.24 min, respectively. The chromatogram of sample HC09 has differences in the retention time of rutin, hyperin, and isoquercitrin peaks. In sample HC05, the peak of quercetin was challenging to identify on the chromatogram due to low concentration.
2.4. Quantification Results of 36 H. cordata Leaves Samples
Five flavonoids, including rutin, hyperin, isoquercitrin, quercitrin, and quercetin, were quantitatively determined using substitutive standard substances. Table 5 describes the calibration curves for the quantification of each flavonoid. In the H. cordata samples, the content of quercitrin was the most (0.25–13.13 mg/g), followed by hyperin (0.02–2.22 mg/g), rutin (0.04–1.64 mg/g), isoquercitrin (0.02–0.98 mg/g), and quercetin (0.006–0.12 mg/g). In general, samples of HC10 and HC14 (Tien Giang and Quang Tri province, respectively) have high flavonoid content.

Table 5.
Contents of the five flavonoids in Houttuynia cordata leaves (mg flavonoid/g extract). Values are presented as mean ± SD (n = 3).
It was evident that quercitrin was one of the most abundant components in H. cor-data. It deserves to be a reference substance and index for quality assessment and control of H. cordata. Meanwhile, the results in Table 5 illustrated remarkable differences in the contents of the five marker substances from different regions, which could be attributed to the variations in genetics, plant origins, environmental factors, drying process, and storage conditions.
2.5. Principal Component Analysis (PCA)
The five flavonoid peak areas of 36 samples of H. cordata leaves were used as variables in the PCA. The PCs analysis results are presented in Table 6. PC1 and PC2 had the highest eigenvalues (3.47 and 0.96), explaining most of the total variance of the data, and they described 69.41% and 19.56% of the cumulative variance, respectively. PC3, PC4, and PC5 had little interaction with the variable.

Table 6.
Eigenvalues of the correlation matrix.
A loading plot (Figure 3) is provided to examine the degree of influence of each flavonoid on the principal components. The closer the loadings are to ±1, the stronger the variable affects the component. Hyperin, isoquercitrin, rutin, and quercitrin vectors have significant positive loadings toward PC1. Hyperin, isoquercitrin, rutin, and quercitrin vectors have significant positive loadings toward PC1 (Table S3). Moreover, the vectors of hyperin, isoquercitrin, rutin, and quercitrin were close to one another, forming small angles, showing that they were positively correlated. Meanwhile, quercetin significantly affects PC2 with a strong coefficient (0.94097), and the quercetin vector formed an angle close to 90° with the other four vectors, showing that quercetin was almost uncorrelated with other flavonoids. Among the flavonoids in the PCA, hyperin and quercetin have the most contribution toward PCs with coefficients of 0.5115 (PC1) and 0.94097 (PC2), respectively. Therefore, we used hyperin and quercetin as principal components to analyze the relationship between samples.
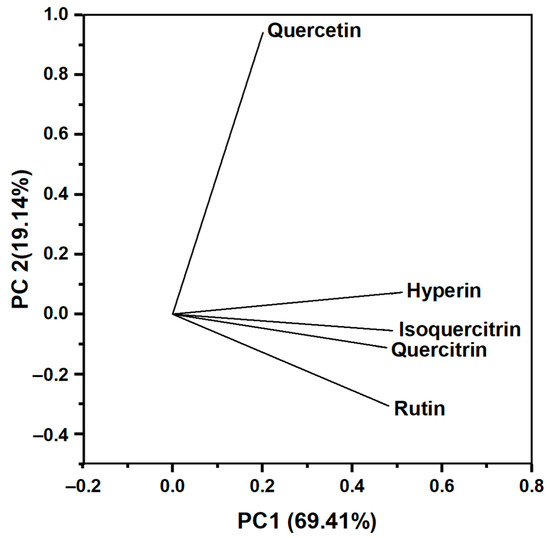
Figure 3.
The loading plot illustrates the influence of flavonoids on the principal components.
The score plot represents the distribution of H. cordata leaves samples in the same plane (Figure 4). Quadrant I indicates the samples having a high content of hyperin, while quadrant II consists of the samples with an average hyperin amount. Quadrant III shows the cluster of samples with low concentrations of hyperin and quercetin. The group of samples with abnormally high quercetin content is in quadrant IV with HC09 and HC20 (0.1126 ± 0.0001 and 0.1265 ± 0.0012 mg/g extract, respectively). HC04 sample located in quadrant IV has a high concentration of quercetin, but it lies closer to the cluster in quadrant III due to the low amount of hyperin in this sample.
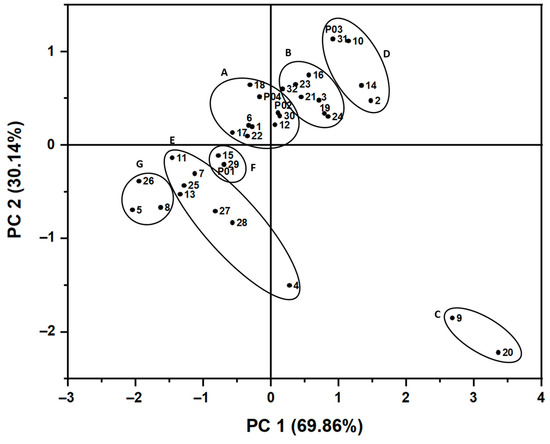
Figure 4.
The score plot presents the distribution of H. cordata leaves samples on the same plane as well as the correlation between them and the first and second principal components. Based on HCA results, 36 samples (1–32 and P01–P04) are divided into seven groups labeled A–G.
Moreover, the clusters of four commercial products labeled P01-P04 also showed that these products are different in flavonoid content from other samples. Three of H. cordata’s commercial products are in quadrant I (P02, P03) and quadrant II (P04), whereas P01 is located in quadrant III, meaning it has low hyperin and quercetin contents. Besides the culturing and harvesting conditions, the score plot may also indicate different drying methods. The freeze-drying method achieved P02, P03, and P04, while P01 was processed using the hot air-drying method.
2.6. Hierarchical Cluster Analysis (HCA)
The similarity analysis of the H. cordata samples was performed based on the content of the five flavonoids of 36 samples, and the results are shown in Table S5. Within the same squared Euclidean distance, the HCA shows that there are seven subgroups from A to G (Figure 5) similar to the clustering results of PCA score plot.
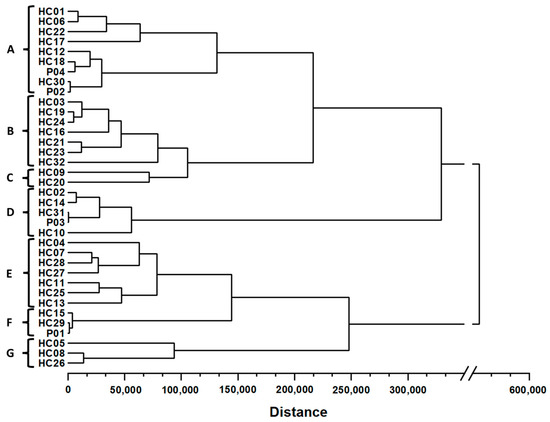
Figure 5.
Hierarchical cluster analysis of 32 H. cordata leaves samples and four powder products. All samples were classified into nine clusters labeled A–G.
In subgroup A (HC01, HC06, HC22, HC17, HC12, HC30, P04, and P02), most samples were collected from the southern provinces, but two were from the central provinces (HC17 and HC12). In contrast, most samples in subgroup B (HC03, HC19, HC24, HC16, HC21, HC23, and HC32) were collected mainly in provinces in the central region, excluding HC24 in Binh Thuan (southeast region) and HC32 in the north of Vietnam. Meanwhile, the samples of subgroup D (HC02, HC19, HC24, P04, and HC10) all had high contents of flavonoids, but they were collected from three different regions. The same pattern can be observed for subgroup E (HC04, HC07, HC11, HC13, HC25, HC27, and HC28). With high concentrations of quercetin, samples HC09 and HC20 formed a separate subgroup C matching the PCA score plot. Subgroups E, F, and G had a greater distance than subgroups A, B, C, and D and were separated due to low concentrations of hyperin and quercetin. Generally, the HCA results strongly support and validate the qualitative analysis of the samples conducted using PCA.
Figure 6 demonstrates the differences between hyperin and quercetin contents divided into seven groups from multivariate analysis results. In terms of hyperin, there was a significant difference in the content of each group, excluding group B and group C, and have similar amounts of hyperin, 1.70697 ± 0.07343 and 1.65627 ± 0.03986, respectively (Table S4). However, the quercetin concentration of group C is 3.5 times higher than group B, leading to the clustering of H. cordata samples.
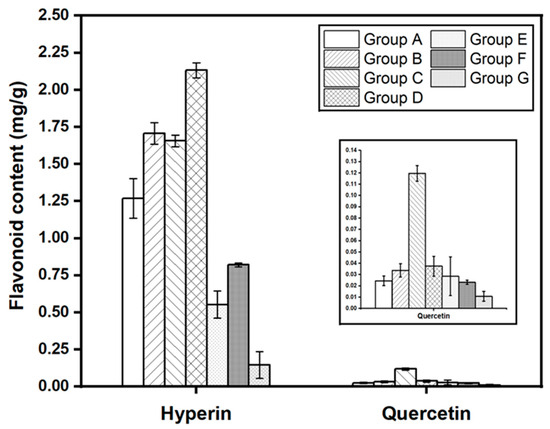
Figure 6.
Comparison of hyperin and quercetin contents in H. cordata samples among seven groups.
3. Discussion
Our studies successfully developed the RP-HPLC method for qualitative and quantitative analysis of five specific flavonoids in H. cordata leaves growing in Vietnam. Yang et al. (2014) studied the HPLC-based flavonoid profiling of 35 H. cordata samples grown in different regions of Guizhou province in China [24]. They determined that the rutin, isoquercitrin, quercitrin, and quercetin contents were 0.03–1.7 mg/mg, 0.01–1.19 mg/g, 0.02–5.50 mg/g, and 0.01–0.18 mg/g, respectively. Notably, the quercitrin content of Vietnamese H. cordata was 2.4 times higher than Chinese H. cordata. The concentrations of rutin, isoquercitrin, and quercetin were approximately the same as those found in H. cordata samples of this study. Wu et al. (2009) evaluated the flavonoid content in 22 H. cordata samples grown in different regions of China, in which the average hyperin, quercitrin, and quercetin content were 4.313 mg/g, 5.738 mg/ g, and 0.096 mg/g, respectively [29]. Quantitative results showed that the HC14 sample had a quercitrin content 2.3 times higher than the average quercitrin content of the Chinese H. cordata samples.
We performed a principal component analysis and hierarchical cluster analysis to evaluate the similarities and distinctions among samples based on the HPLC profiles. The purpose of using PCA is to reduce the dimensionality of the datasets and enhance data interpretability by preserving essential information [30]. When using five flavonoids to analyze, we found that clustering in the score plot did not clearly show the difference in flavonoid components among the samples (Figure S1). Meanwhile, when we selected hyperin and quercetin, the two flavonoids that most affect PCs, the score plot shows the clustering of the samples match with the HCA results. Therefore, the use of PCA based on two flavonoids contents, hyperin and quercetin, can demonstrate quality of H. cordata.
Previously, Ling-Shang et al. (2009) [29] have quantified the content of three flavonoids, including hyperin, quercitrin, and quercetin, in 23 H. cordata samples from different regions in China. Similar to our study, the results reveal that geographic factors did not significantly correlate to the flavonoid contents in H. cordata. Furthermore, Ling-Shang et al. evaluated the correlation between the content of those three flavonoids and plant morphology in these samples. On the other hand, our study used extracts from fresh leaves and powdered samples of H. cordata to quantify five major flavonoids: rutin, hyperin, quercitrin, isoquercitrin, and quercetin using RP-HPLC. Then, PCA was used as a preprocessing step before clustering to reduce the dimension from five flavonoids to two flavonoids (hyperin and quercetin). Eventually, this enabled us to explain almost the variances of the samples in HCA and PCA results. In our research, the herbal quality of H. cordata was determined based on quantitative results accompanied by multivariate analysis, not based on plant morphology, which is suitable for evaluating the quality of H. cordata powder products on the market. In the study of Qi et al. (2022) [25], they utilized the headspace solid-phase micro-extraction technique (HS-SPME-GC-MS), followed by orthogonal partial least squares discriminant analysis and clustering analysis to discriminate the parts of H. cordata by differentiating the volatile metabolites. It is noteworthy that the pharmacological activities of H. cordata are mainly from volatile compounds and flavonoids. Therefore, besides volatile components, the flavonoids are the crucial markers to assess the quality of H. cordata. Our study then focused on quantifying five major flavonoids for further application using fresh leaves and powder products in the cosmetic and pharmaceutical industries.
Moreover, the HCA of four H. cordata commercial samples also showed that the results were highly similar, proving that these products are pure and unadulterated. Figure S2 shows that the four samples were evidently classified into two clusters (P01 and P04 vs. P02 and P03), and there were no significant differences in drying method that affected the flavonoid contents. The samples P02, P03, and P04 were processed by the freeze-drying method, while P01 was processed by the hot air-drying method. However, a previous study reported that freeze-drying method can retain the quality of herbal compounds [31]. This difference in our result may result from quality of raw herbal materials during farming, harvesting, and storage before processing. Therefore, assessing the quality of H. cordata raw materials is essential in research and industrial manufacturing.
4. Materials and Methods
4.1. Materials and Reagents
Ethanol for the extraction and the HPLC solvents (acetonitrile, glacial acetic acid, formic acid, and methanol) were purchased from Merck KGaA (Darmstadt, Germany). Five chemical standards, including rutin hydrate, hyperin, isoquercitrin, quercitrin hydrate, and quercetin, were provided by Sigma-Aldrich (St. Louis, MO, USA).
4.2. Instrumentations
Shimadzu LC-2030C 3D HPLC System (Shimadzu, Tokyo, Japan), Ultrasonic Water Baths (Grant, Cambridge, UK), Memmert Waterbath WNB 14 (Memmert, Bayern, Germany), Hettich EBA20S Portable Centrifuge (Hettich, Tuttlingen, Germany), HPLC Column Phenomenex C18 Prodigy™ ODS-3 100 Å (150 mm × 4.6 mm, 5 µm) (Phenomenex, CA, USA), and HPLC Column Phenomenex C18 Prodigy™ ODS-3 100 Å (250 mm × 4.6 mm, 5 µm) (Phenomenex, CA, USA).
4.3. Sampling H. cordata Leaves
A total of 32 samples of H. cordata leaves were collected from 32 different provinces of Vietnam from January to March 2022 and authenticated by Dr. Hoang Viet, Department of Ecology-Evolutionary Biology, University of Sciences, Vietnam National University, Ho Chi Minh City. Sample collection information is presented in Table S1. Fresh H. cordata leaves were dried at 50 °C (moisture content less than 10%), then ground and sieved through a 0.63 mm sieve to obtain H. cordata leaves powder. The powder was stored at room temperature and protected from sunlight. Our research also used four samples of H. cordata powder commercially available and circulating in the market (Table S2).
4.4. Preparation of Flavonoids Standard Solution
The five standards, including rutin hydrate, hyperin, isoquercitrin, quercitrin hydrate, and quercetin, were dissolved in a 5 mL volumetric flask with methanol to obtain a stock solution of 100.0, 200.0, 100.0, 360.0, and 2.0 µg/mL, respectively. Accurately diluting 1.0 mL of each standard solution into a 5 mL volumetric flask by methanol to obtain 20.0 µg/mL rutin, 40.0 µg/mL hyperin, 20.0 µg/mL isoquercitrin, 72.0 µg/mL quercitrin, and 0.4 µg/mL quercetin. The solutions were then filtered through a 0.45 µm filter before HPLC analysis.
4.5. Preparation of Houttuynia Cordata Leaves Sample Solution
Extraction of H. cordata leaves powder with optimized conditions has been previously published [32]. Briefly, 0.5 g of H. cordata powder was weighed and extracted with 30 mL of 80% ethanol (solid:liquid ratio 1:60, g/L) at 60 °C for 38 min using ultrasound (Ultrasonic bath XUB10), power 75%. The extract was then filtered through Newstar 102 filter paper and dried. The residue was dissolved in 10 mL of HPLC grade methanol, then centrifuged at 3000 rpm for 15 min. The solution after centrifugation was filtered through a filter of 0.45 μm before HPLC analysis.
4.6. RP-HPLC Method Optimization for the Analysis of the Flavonoids
The Shimadzu HPLC system model LC-2030C 3D with a 200–800 nm PDA probe (Shimadzu, Japan) was used to optimize the analysis method for flavonoids of H. cordata leaves. The acetic or formic acid in water (A) and acetonitrile (B) mobile phases, flow rates of 0.6, 0.9, and 1.0 mL min−1, and gradient programs were designed under different conditions and analyzed via Phenomenex C18 columns (4.6 mm, 5 μm) with the length of 250 mm and 150 mm. The sample injection volume was fixed at 2 μL. A resolution value greater than or equal to 1.5, a tailing factor ranging from 1.8 to 2.0, and a number of theoretical plates greater than 5000 were considered ideal for HPLC analysis.
4.7. RP-HPLC Method Validation
The optimized HPLC method was validated according to ICH Quality Guidelines Q2(R1) Validation of Analytical Procedures (2018) with parameters including specificity, stability, accuracy, precision, linearity, range, the limit of detection (LOD), the limit of quantification (LOQ), and robustness [33].
4.7.1. Specificity
The specificity of the RP-HPLC method was evaluated by injecting the blank, the standard, and the H. cordata extract solution three times with a volume of 2.0 μL to observe interference peaks and compare the retention times and shapes of the peaks.
4.7.2. Stability
The stability of the extracts was evaluated at 0, 3, 6, 9, and 12 consecutive hours with the optimized HPLC method.
4.7.3. Accuracy
The accuracy of the RP-HPLC method was verified by preparing five standard solutions mixed in methanol (HPLC grade), accurately adding approximately 80%, 100%, and 120% of the standards to the test sample. Five standards were analyzed with the optimized HPLC analytical method. Each injection sample was repeated three times. A recovery rate (%) between 98–102% is considered appropriate.
4.7.4. Precision
The repeatability of the RP-HPLC method was performed by analyzing H. cordata leaves samples six times. The percentage relative standard deviation (%RSD) was calculated based on the peak areas.
Intermediate precision was validated by analyzing H. cordata leaves samples six times over two consecutive days and calculating %RSD.
4.7.5. Linearity and Range
Five standards were diluted to obtain six different concentrations of rutin (5, 10, 25, 50, 100, and 200 μg/mL), hyperin (16, 24, 32, 40, 80, and 200 μg/mL), isoquercitrin (1, 5, 10, 25, 50, and 100 μg/mL), quercitrin (48, 96, 120, 240, 600, and 1000 μg/mL), and quercetin (0.2, 0.5, 1, 2, 4, and 8 μg/mL). Each concentration was injected in triplicate with the same analytical conditions. A calibration curve was constructed using a linear regression equation between mean peak area (x) and standard concentration (y). The correlation coefficient (R2) greater than or equal to 0.999 proves the linearity of the analytical method.
4.7.6. LOD and LOQ
The analytical procedure’s limit of detection (LOD) and limit of quantification (LOQ) were determined based on linearity. LOD and LOQ were calculated according to the formula:
in which, S is the standard deviation; a is the slope of the calibration curve y = ax + b.
4.8. Quantitative Analysis of Flavonoids Content
The flavonoids of H. cordata were quantified using the calibration curve method. The flavonoid contents in the leaves of H. cordata were determined by the formula:
in which, C: flavonoid content in 1 g H. cordata powder (mg/g extract); y: flavonoid concentration calculated from calibration curve (µg/mL); V: volume of extract (mL); w: weight of H. cordata powder (g); and M: moisture content of H. cordata powder (%).
4.9. Data Analysis
The raw HPLC chromatographic data were examined and exported as *.txt format files using LabSolutions software (Version 5.87 SP1; Shimadzu, Tokyo, Japan). Quantitative data and HPLC method validation were analyzed using Microsoft Excel 2016. HPLC-based flavonoid profiling chromatogram, similarity analysis, principal component analysis (PCA), and hierarchical cluster analysis (HCA) were performed using the software Origin 2021 (Version 9.8; OriginLab Corp., Northampton, MA, USA). All experiments were repeated in triplicate, and results were presented as mean ± SD.
5. Conclusions
This study established an effective RP-HPLC method followed by the multivariate analysis to quantify and assess the quality of 32 H. cordata leaves samples from different provinces throughout Vietnam and four powder products. From the PCA analysis, two flavonoids, hyperin and quercetin, can demonstrate the quality of H. cordata samples from fresh leaves and powder products. For further studies, we will consider various environmental factors, including climate and soil factors, to investigate their effects on the quality of H. cordata in particular and medicinal plants in general.
Supplementary Materials
The following supporting information is available online at https://www.mdpi.com/article/10.3390/molecules28176378/s1, Table S1: Information on collecting H. cordata leaves from different regions in Vietnam from January to March 2022; Table S2: Samples of H. cordata powder available in market; Table S3: Extracted Eigenvectors; Table S4: Average hyperin and quercetin content of each seven groups; Table S5: Similarity of HPLC-based flavonoid profiling chromatograms of H. cordata leaves; Figure S1: The score plot presents the distribution of H. cordata leaves samples on the same plane as well as the correlation between them and the first and second principal components (by analyzing five flavonoids); Figure S2: Hierarchical cluster analysis of four H. cordata commercial products.
Author Contributions
M.T.L., D.L.H. and M.H.N. contributed to conceptualization of the experiments and reviewing the manuscript. M.H.N., B.M.D., N.T.N.C. and T.H.T. performed the experiments, and wrote the manuscript. M.H.N. and N.T.H.L. performed data analysis and visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Vietnam National University HoChiMinh City (VNU-HCM) under grant number DN2022-44-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- FDA. Botanical Drug Development: Guidance for Industry. Available online: https://www.fda.gov/media/93113/download (accessed on 12 May 2022).
- EMA. Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-herbal-medicinal-products-traditional-herbal-medicinal-products-revision-2_en.pdf (accessed on 12 May 2022).
- WHO. Programme on Traditional Medicine. Guidelines for the Assessment of Herbal Medicines. Available online: https://apps.who.int/iris/bitstream/handle/10665/58865/WHO_TRM_91.4.pdf?sequence=1&isAllowed=y (accessed on 12 May 2022).
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2015.
- Ministry of Health. Vietnamese Pharmacopoeia V; Medical Publshing House: Hanoi, Vietnam, 2017.
- Hawrył, A.; Hawrył, M.; Litwińczuk, W.; Bogucka-Kocka, A. Thin-layer chromatographic fingerprint of selected Paulownia species with chemometrics and antioxidant activity. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 367–374. [Google Scholar] [CrossRef]
- Pedan, V.; Weber, C.; Do, T.; Fischer, N.; Reich, E.; Rohn, S. HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chem. 2018, 267, 277–287. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, T.; Wu, L.; Zhang, J.; Zuo, Z.; Wang, Y. Identification of Gentiana rigescens from different geographical origins based on HPLC and FTIR fingerprints. Anal. Methods 2020, 12, 2260–2271. [Google Scholar] [CrossRef]
- Zhuo, Z.; Xu, D.; Li, Y.; Pu, B.; Ye, M. Fingerprint analysis of Zanthoxylum armatum DC. by HPLC. J. Food Compos. Anal. 2021, 96, 103736. [Google Scholar] [CrossRef]
- Yan, X.; Wang, W.; Chen, Z.; Xie, Y.; Li, Q.; Yu, Z.; Hu, H.; Wang, Z. Quality assessment and differentiation of Aucklandiae Radix and Vladimiriae Radix based on GC-MS fingerprint and chemometrics analysis: Basis for clinical application. Anal. Bioanal. Chem. 2020, 412, 1535–1549. [Google Scholar] [CrossRef]
- Xie, P.; Chen, S.; Liang, Y.-z.; Wang, X.; Tian, R.; Upton, R. Chromatographic fingerprint analysis—A rational approach for quality assessment of traditional Chinese herbal medicine. J. Chromatogr. A 2006, 1112, 171–180. [Google Scholar] [CrossRef]
- Xie, B.; Gong, T.; Tang, M.; Mi, D.; Zhang, X.; Liu, J.; Zhang, Z. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers. J. Pharm. Biomed. Anal. 2008, 48, 1261–1266. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Xi, J.; Pan, C.; Xu, Z.; Xia, W.; Zhang, W. Multiple-fingerprint analysis of Poria cocos polysaccharide by HPLC combined with chemometrics methods. J. Pharm. Biomed. Anal. 2021, 198, 114012. [Google Scholar] [CrossRef]
- Patel, P.R.; Patel, N.D.; Patel, S.G.; Kanaki, N.S.; Patel, A.J. Fingerprint Analysis of Vitex Negundo by HPLC Coupled with Multi-components Analysis. Curr. Pharm. Anal. 2020, 16, 743–751. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.-H.; Zhang, Q.; Lai, M.-X.; Wang, Q. Quality assessment of Cortex cinnamomi by HPLC chemical fingerprint, principle component analysis and cluster analysis. J. Sep. Sci. 2007, 30, 1276–1283. [Google Scholar] [CrossRef]
- Yu, B.; Li, Z.; Wu, J.; Ying, J.; Tang, Y.; Wu, B.; Tang, C.; Xu, J. Quality Control of Gastrodia elata by High-Performance Liquid Chromatography with Fluorescence Detection (HPLC–FLD) and Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA). Anal. Lett. 2020, 53, 746–759. [Google Scholar] [CrossRef]
- Wei, W.; Youliang, Z.; Li, C.; Yuming, W.; Zehong, Y.; Ruiwu, Y. PCR-RFLP analysis of cpDNA and mtDNA in the genus Houttuynia in some areas of China. Hereditas 2005, 142, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Loi, D.T. Vietnamese Medicinal Plants and Remedies; Medical Publishing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Bich, D.H.; Chung, D.Q.; Chuong, B.X.; Dong, N.T.; Dam, D.T.; Hien, P.V.; Lo, V.N.; Mai, P.D.; Man, P.K.; Nhu, D.T.; et al. Plants and Animals Used as Medicine in Vietnam; Science and Technics Publishing House: Hanoi, Vietnam, 2004; Volume 1 & 2. [Google Scholar]
- Chen, A.; Xiang, W.; Liu, D.; Liu, C.; Yang, L. Determination of total flavonoids and its antioxidant ability in Houttuynia cordata. MSCE 2016, 4, 131–136. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Mizuguchi, H.; Amoh, T.; Ogino, S.; Matsuo, T.; Miyake, Y.; Fukui, H.; Kashiwada, Y. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem. 2016, 80, 1205–1213. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Yang, L.P. Systematic analysis of components and contents in Houttuynia cordata Thunb. TMR 2017, 2, 176–188. [Google Scholar] [CrossRef]
- Yang, Z.-n.; Sun, Y.-m.; Luo, S.-q.; Chen, J.-w.; Yu, Z.-w.; Sun, M. Quality evaluation of Houttuynia cordata Thunb. by high performance liquid chromatography with photodiode-array detection (HPLC-DAD). Pak. J. Pharm. Sci. 2014, 27, 223–231. [Google Scholar]
- Qi, S.; Zha, L.; Peng, Y.; Luo, W.; Chen, K.; Li, X.; Huang, D.; Yin, D. Quality and Metabolomics Analysis of Houttuynia cordata Based on HS-SPME/GC-MS. Molecules 2022, 27, 3921. [Google Scholar] [CrossRef]
- Tian, L.; Shi, X.; Yu, L.; Zhu, J.; Ma, R.; Yang, X. Chemical Composition and Hepatoprotective Effects of Polyphenol-Rich Extract from Houttuynia cordata Tea. J. Agric. Food Chem. 2012, 60, 4641–4648. [Google Scholar] [CrossRef]
- Ling, L.-j.; Lu, Y.; Zhang, Y.-y.; Zhu, H.-y.; Tu, P.; Li, H.; Chen, D.-f. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef] [PubMed]
- Ling-Shang, W.; Jin-Ping, S.; Xiao-Qing, Y.; Xue-Rong, S. Quantitive variation of flavonoids in Houttuynia cordata from different geographic origins in China. Chin. J. Nat. Med. 2009, 7, 40–46. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Transactions. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Ramirez-Gomez, X.S.; Beltran-Campos, V.; Contreras-Medina, L.M.; Garcia-Trejo, J.F.; Feregrino-Pérez, A.A. Changes in the Content of Phenolic Compounds and Biological Activity in Traditional Mexican Herbal Infusions with Different Drying Methods. Molecules 2020, 25, 1601. [Google Scholar] [CrossRef] [PubMed]
- Hien, N.M.; Huong, T.T.; Minh, D.B.; Loan, H.T.; Tri, L.M. Application of response surface methodology to optimize the ultrasound-assisted flavonoid-rich extraction of fish mint (Houttuynia cordata Thunb.). Sci. Technol. Dev. J. 2021, 24, 1994–2003. [Google Scholar]
- Teasdale, A.; Elder, D.; Nims, R.W. ICH Quality Guidelines: An Implementation Guide; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).