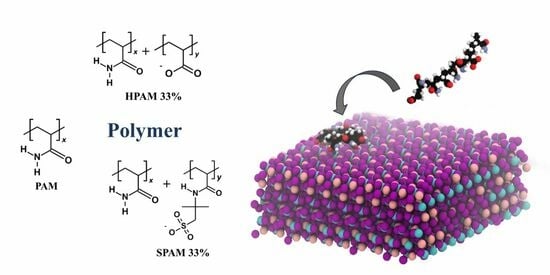
Molecular Dynamics Simulation of Polyacrylamide Adsorption on Calcite
Abstract
:1. Introduction
2. Results and Discussion
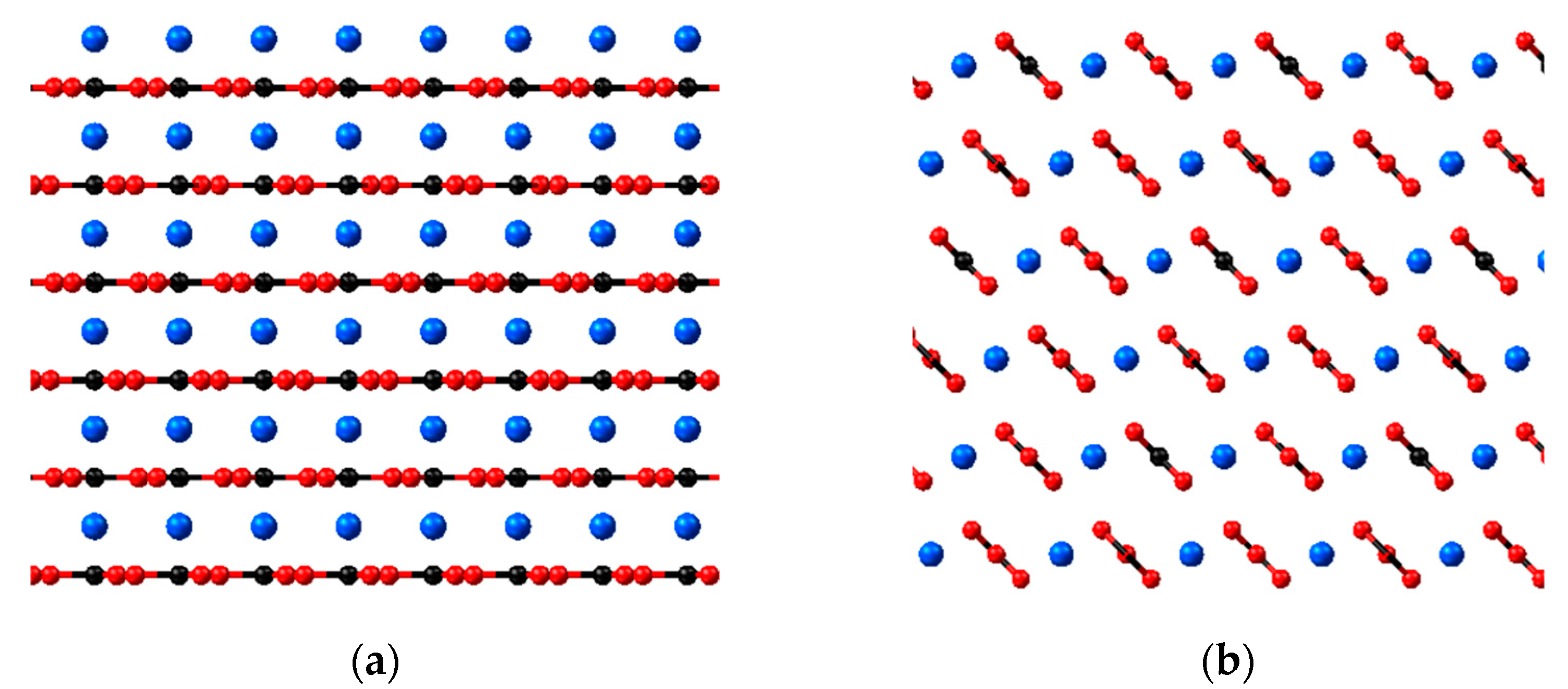
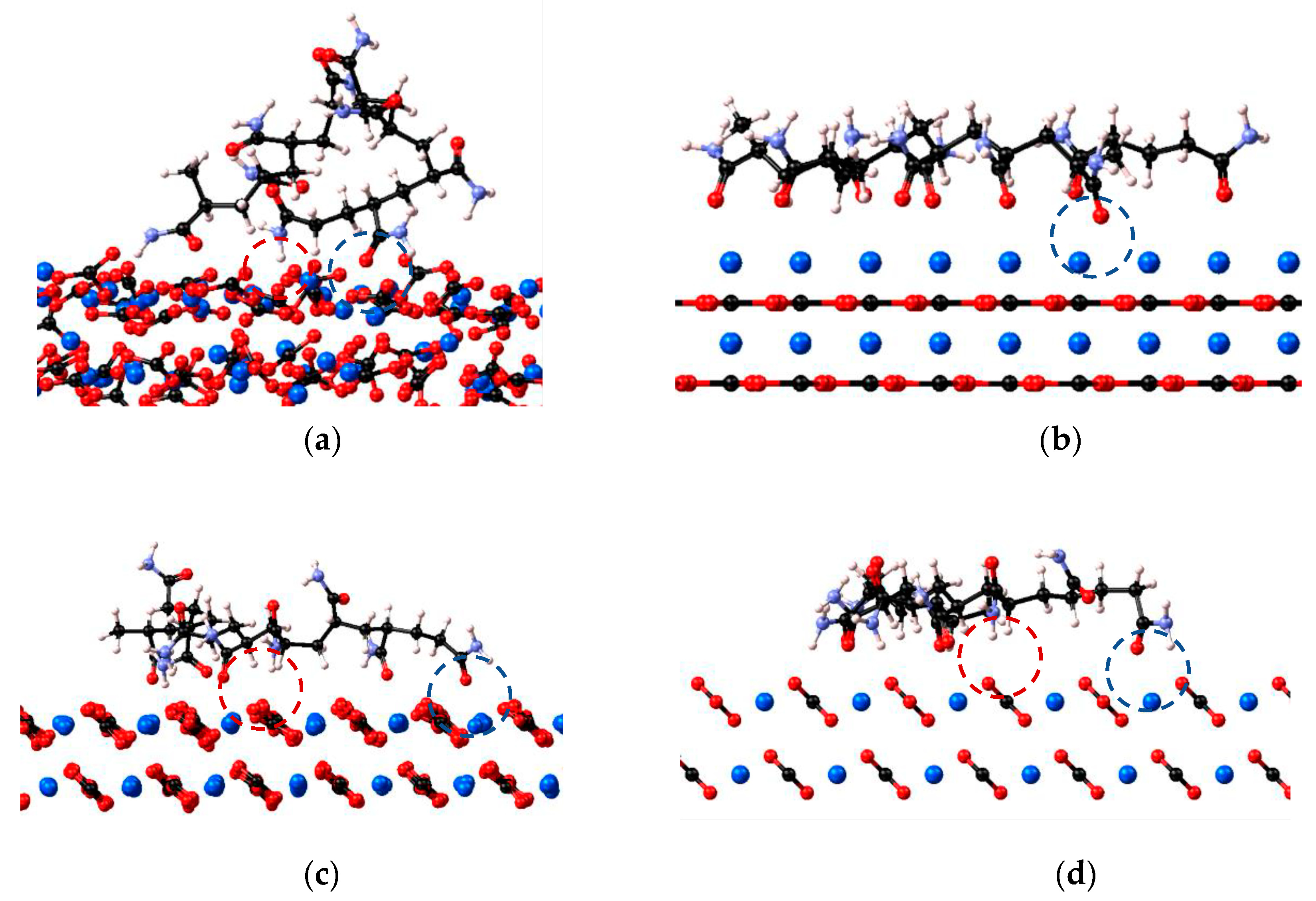
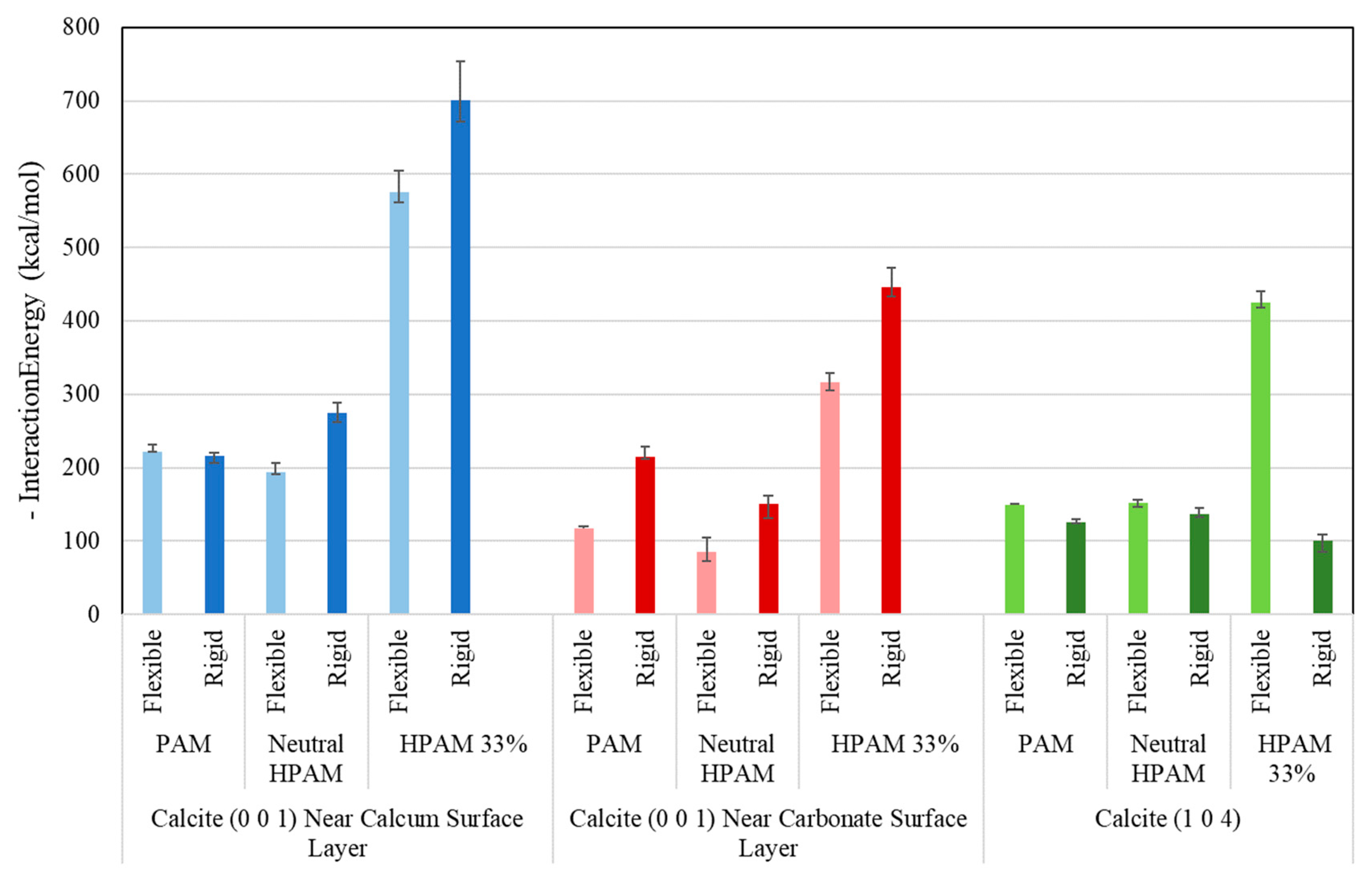
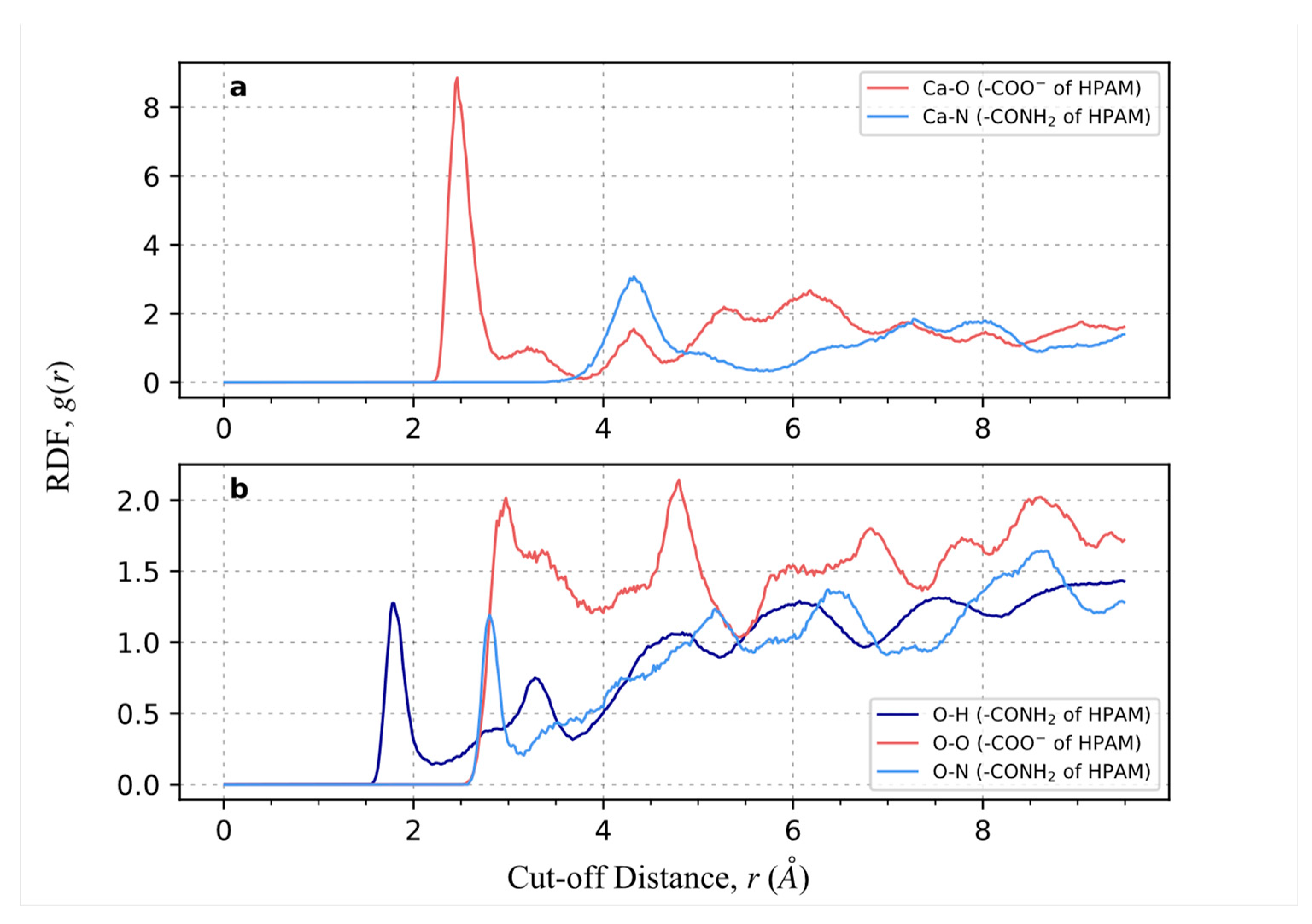
2.1. Crystal Plane Evaluation and Solid-Fluid Interactions
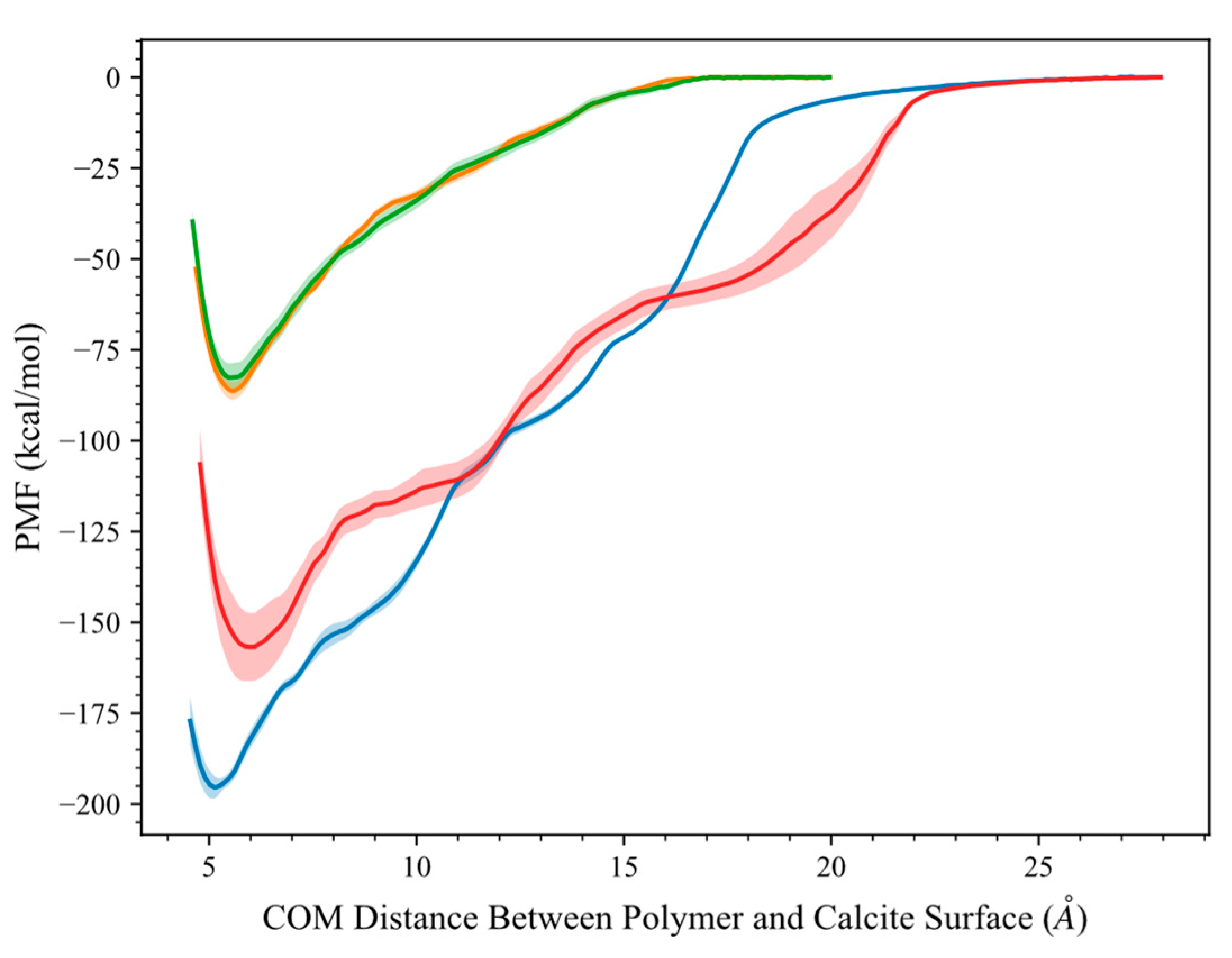
2.2. Umbrella Sampling Adsorption Free Energy Analysis
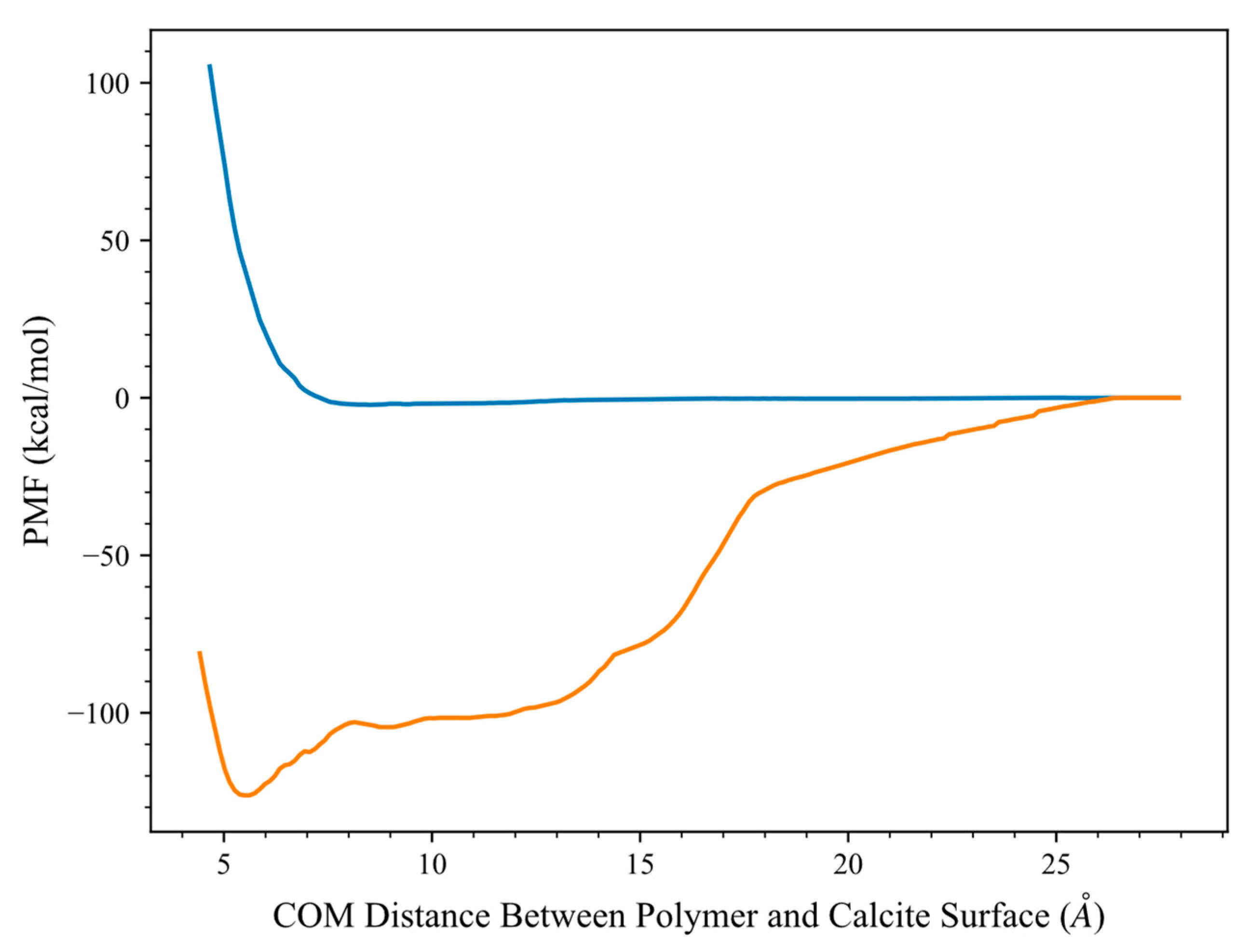
Evaluation of HPAM 33% Adsorption on Calcite in the Presence of Solvents
3. Materials and Methods
3.1. General Simulation Details
3.2. Polymer Models
3.3. Calcite Model
Crystal Plane and Rigidity of the Calcite Model
3.4. Umbrella Sampling Adsorption Free Energy Analysis
Free Energy Analysis in the Presence of Solvents
3.5. Experimental Adsorption Studies and AFM Analysis
3.5.1. Material
3.5.2. Adsorption Study
3.5.3. Atomic Force Microscopy (AFM) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Awad, M.N.J.; El-Sayed, A.-A.H.; Desouky, S.E.-D.M. Factors Affecting Sand Production from Unconsolidated Sandstone Saudi Oil and Gas Reservoir. J. King Saud Univ.-Eng. Sci. 1999, 11, 151–172. [Google Scholar] [CrossRef]
- Bopp, P.A.; Hawlicka, E.; Fritzsche, S. The Hitchhiker’s guide to molecular dynamics. ChemTexts 2018, 4, 2. [Google Scholar] [CrossRef]
- Choi, I.; Kim, I.W. Molecular Dynamics Simulation to Understand the Ability of Anionic Polymers to Alter the Morphology of Calcite. Int. J. Polym. Sci. 2017, 2017, 7594950. [Google Scholar] [CrossRef]
- Ji, Y.-X.; Wang, F.-H.; Duan, L.-C.; Zhang, F.; Gong, X.-D. Effect of temperature on the adsorption of sulfanilamide onto aluminum oxide and its molecular dynamics simulations. Appl. Surf. Sci. 2013, 285, 403–408. [Google Scholar] [CrossRef]
- Krishna, S.; Sreedhar, I.; Patel, C.M. Molecular dynamics simulation of polyamide-based materials—A review. Comput. Mater. Sci. 2021, 200, 110853. [Google Scholar] [CrossRef]
- Chen, H.; Panagiotopoulos, A.Z.; Giannelis, E.P. Atomistic molecular dynamics simulations of carbohydrate-calcite interactions in concentrated brine. Langmuir 2015, 31, 2407–2413. [Google Scholar] [CrossRef]
- Meissner, R.H.; Wei, G.; Ciacchi, L.C. Estimation of the free energy of adsorption of a polypeptide on amorphous SiO2 from molecular dynamics simulations and force spectroscopy experiments. Soft Matter 2015, 11, 6254–6265. [Google Scholar] [CrossRef]
- Willemsen, J.A.R.; Myneni, S.C.B.; Bourg, I.C. Molecular Dynamics Simulations of the Adsorption of Phthalate Esters on Smectite Clay Surfaces. J. Phys. Chem. C 2019, 123, 13624–13636. [Google Scholar] [CrossRef]
- Gaberle, J.; Gao, D.Z.; Shluger, A.L. Calculating free energies of organic molecules on insulating substrates. Beilstein J. Nanotechnol. 2017, 8, 667–674. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, P.; Cardellini, A.; Asinari, P. Exploring the Free Energy Landscape To Predict the Surfactant Adsorption Isotherm at the Nanoparticle-Water Interface. ACS Cent. Sci. 2019, 5, 1804–1812. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wang, J. Energetics of Interfacial Interactions of Hydrocarbon Fluids with Kerogen and Calcite Using Molecular Modeling. Energy Fuels 2020, 34, 4251–4259. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Mahmood, S.M.; Abdulrahman, A.; Abdulelah, H.; Akbari, S.; Saraih, N. An Experimental Study on Hydrodynamic Retention of Low and High Molecular Weight Sulfonated Polyacrylamide Polymer. Polymers 2019, 11, 1453. [Google Scholar] [CrossRef]
- Herth, G.; Schornick, G.L.; Buchholz, F. Polyacrylamides and Poly (Acrylic Acids). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–16. [Google Scholar]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef]
- Beteta, A.; Nurmi, L.; Rosati, L.; Hanski, S.; McIver, K.; Sorbie, K.; Toivonen, S. Polymer Chemical Structure and its Impact on EOR Performance. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 31 August–4 September 2020. [Google Scholar]
- API. Recommended Practices for Evaluation of Polymers Used in Enhanced Oil Recovery Operations; API Recommended Practice 63: Washington, DC, USA, 1990. [Google Scholar]
- Zhang, W.; Feng, Q.; Jin, Z.; Xing, X.; Wang, S. Molecular simulation study of oil-water two-phase fluid transport in shale inorganic nanopores. Chem. Eng. Sci. 2021, 245, 116948. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Koleini, M.M.; Ayatollahi, S. Molecular dynamics simulation investigation of hexanoic acid adsorption onto calcite (101¯4)surface. Fluid Phase Equilibria 2015, 387, 24–31. [Google Scholar] [CrossRef]
- Cooke, D.J.; Gray, R.J.; Sand, K.K.; Stipp, S.L.; Elliott, J.A. Interaction of ethanol and water with the {1014} surface of calcite. Langmuir 2010, 26, 14520–14529. [Google Scholar] [CrossRef]
- Lowry, E.; Sedghi, M.; Goual, L. Molecular simulations of NAPL removal from mineral surfaces using microemulsions and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 485–494. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, S.; Wang, X.; Guo, M.; Wang, Y.; Wang, D. Molecular dynamics simulation of oil detachment from calcite surface in aqueous surfactant solution. Comput. Theor. Chem. 2016, 1092, 82–89. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, S.; Yan, H.; Zhao, X. Mechanism of oil detachment from a silica surface in aqueous surfactant solutions: Molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 2867–2875. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, P.; Zhang, Y.; Yan, Y.; Hu, S.; Zhang, J. Adsorption mechanism of oil components on water-wet mineral surface: A molecular dynamics simulation study. Energy 2013, 59, 295–300. [Google Scholar] [CrossRef]
- Gurina, D.; Surov, O.; Voronova, M.; Zakharov, A. Molecular Dynamics Simulation of Polyacrylamide Adsorption on Cellulose Nanocrystals. Nanomaterials 2020, 10, 1256. [Google Scholar] [CrossRef]
- Ma, X.; Sun, X.; Chang, M.; Liu, Q.; Dong, X.; Fan, Y.; Chen, R. Adsorption of Different Ionic Types of Polyacrylamide on Montmorillonite Surface: Insight from QCM-D and Molecular Dynamic Simulation. Molecules 2023, 28, 4417. [Google Scholar] [CrossRef]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Polyacrylamide adsorption on (1 0 1) quartz surfaces in saltwater for a range of pH values by molecular dynamics simulations. Miner. Eng. 2021, 162, 106741. [Google Scholar] [CrossRef]
- Sparks, D.J.; Romero-Gonzalez, M.E.; El-Taboni, E.; Freeman, C.L.; Hall, S.A.; Kakonyi, G.; Swanson, L.; Banwart, S.A.; Harding, J.H. Adsorption of poly acrylic acid onto the surface of calcite: An experimental and simulation study. Phys. Chem. Chem. Phys. 2015, 17, 27357–27365. [Google Scholar] [CrossRef] [PubMed]
- Ahsani, T.; Tamsilian, Y.; Rezaei, A. Molecular dynamic simulation and experimental study of wettability alteration by hydrolyzed polyacrylamide for enhanced oil recovery: A new finding for polymer flooding process. J. Pet. Sci. Eng. 2021, 196, 108029. [Google Scholar] [CrossRef]
- Abdel-Azeim, S.; Kanj, M.Y. Dynamics, Aggregation, and Interfacial Properties of the Partially Hydrolyzed Polyacrylamide Polymer for Enhanced Oil Recovery Applications: Insights from Molecular Dynamics Simulations. Energy Fuels 2018, 32, 3335–3343. [Google Scholar] [CrossRef]
- Lew, J.H.; Matar, O.K.; Muller, E.A.; Maung, M.T.M.; Luckham, P.F. Adsorption of Hydrolysed Polyacrylamide onto Calcium Carbonate. Polymers 2022, 14, 405. [Google Scholar] [CrossRef]
- Rasteiro, M.G.; Pinheiro, I.; Ahmadloo, H.; Hunkeler, D.; Garcia, F.A.P.; Ferreira, P.; Wandrey, C. Correlation between flocculation and adsorption of cationic polyacrylamides on precipitated calcium carbonate. Chem. Eng. Res. Des. 2015, 95, 298–306. [Google Scholar] [CrossRef]
- Ekanem, E.M.; Rücker, M.; Yesufu-Rufai, S.; Spurin, C.; Ooi, N.; Georgiadis, A.; Berg, S.; Luckham, P.F. Novel adsorption mechanisms identified for polymer retention in carbonate rocks. JCIS Open 2021, 4, 100026. [Google Scholar] [CrossRef]
- Ricci, M.; Spijker, P.; Stellacci, F.; Molinari, J.-F.; Voïtchovsky, K. Direct Visualization of Single Ions in the Stern Layer of Calcite. Langmuir 2013, 29, 2207–2216. [Google Scholar] [CrossRef]
- Rezaei Gomari, K.A.; Hamouda, A.A. Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface. J. Pet. Sci. Eng. 2006, 50, 140–150. [Google Scholar] [CrossRef]
- Legens, C.; Toulhoat, H.; Cuiec, L.; Villiera, F.; Palermol, T. Wettability Change Related to the Adsorption of Organic Acids on Calcite: Experimental and Ab Initio Computational Studies. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Otteans, LA, USA, 27–30 September 1998. [Google Scholar]
- Leeuw, N.H.d.; Parker, S.C. Surface Structure and Morphology of Calcium Carbonate Polymorphs Calcite, Aragonite, and Vaterite: An Atomistic Approach. J. Phys. Chem. B 1998, 102, 2914–2922. [Google Scholar] [CrossRef]
- Hwang, S.; Blanco, M.; Goddard-III, W.A. Atomistic Simulations of Corrosion Inhibitors Adsorbed on Calcite Surfaces I. Force field Parameters for Calcite. J. Phys. Chem. B 2001, 105, 10746–10752. [Google Scholar] [CrossRef]
- Donnini, S.; Mark, A.E.; Juffer, A.H.; Villa, A. Incorporating the effect of ionic strength in free energy calculations using explicit ions. J. Comput. Chem. 2005, 26, 115–122. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids, 2nd ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Gaberle, J.; Gao, D.Z.; Watkins, M.B.; Shluger, A.L. Calculating the Entropy Loss on Adsorption of Organic Molecules at Insulating Surfaces. J. Phys. Chem. C 2016, 120, 3913–3921. [Google Scholar] [CrossRef]
- Jabes, B.S.; Bratko, D.; Luzar, A. Universal Repulsive Contribution to the Solvent-Induced Interaction Between Sizable, Curved Hydrophobes. J. Phys. Chem. Lett. 2016, 7, 3158–3163. [Google Scholar] [CrossRef]
- Mintis, D.G.; Mavrantzas, V.G. Effect of pH and Molecular Length on the Structure and Dynamics of Short Poly(acrylic acid) in Dilute Solution: Detailed Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 4204–4219. [Google Scholar] [CrossRef]
- Materials, D. MedeA 3.5 (Materials Exploration and Design Analysis); Materials Design Inc.: San Diego, CA, USA, 2022. [Google Scholar]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Yiannourakou, M.; Ungerer, P.; Leblanc, B.; Ferrando, N.; Teuler, J.-M. Overview of MedeA®-GIBBS capabilities for thermodynamic property calculation and VLE behaviour description of pure compounds and mixtures: Application to polar compounds generated from ligno-cellulosic biomass. Mol. Simul. 2013, 39, 1165–1211. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; Adam Hilger: New York, NY, UDA, 1989. [Google Scholar]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Tillotson, M.J.; Diamantonis, N.I.; Buda, C.; Bolton, L.W.; Muller, E.A. Molecular modelling of the thermophysical properties of fluids: Expectations, limitations, gaps and opportunities. Phys. Chem. Chem. Phys. 2023, 25, 12607–12628. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Temperature Dependence of the Structural Parameters in the Transformation of Aragonite to Calcite, as Determined from in Situ Synchrotron Powder X-Ray-Diffraction Data. Can. Mineral. 2010, 48, 1225–1236. [Google Scholar] [CrossRef]
- Maslen, E.N.; Streltsov, V.A.; Streltsova, N.R. X-ray Study of the Electron Density in Calcite, CaCO3. Acta Crystallogr. 1993, B49, 636–641. [Google Scholar] [CrossRef]
- Effenberger, H.; Mereiter, K.; Zemann, J. Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithonite, and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates. Z. Für Krist. 1981, 156, 233–243. [Google Scholar]
- Taguta, J.; O’Connor, C.T.; McFadzean, B. The relationship between enthalpy of immersion and flotation response. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 263–270. [Google Scholar] [CrossRef]
- Zoungrana, T.; Berrada, A.; Douillard, J.-M.; Partyka, S. Competitive Interactions between Water and Organic Solvents onto Mineral Solid Surfaces Studied by Calorimetry. Langmuir 1995, 11, 1760–1767. [Google Scholar] [CrossRef]
- Lagerge, S.; Rousset, P.; Zoungrana, T.; Douillard, J.M.; Partyka, S. Adsorption of benzoic acid from organic solvents on calcite and dolomite: Influence of water. Colloids Surf. A Physicochem. Eng. Asp. 1993, 80, 261–272. [Google Scholar] [CrossRef]
- Wade, W.H.; Hackerman, N. Heats of Immersion. II. Calcite and Kaolinite—The Effect of Pretreatment. J. Phys. Chem. 1959, 63, 1639–1641. [Google Scholar] [CrossRef]
- Chun, B.J.; Lee, S.G.; Choi, J.I.; Jang, S.S. Adsorption of carboxylate on calcium carbonate (1 0 14) surface: Molecular simulation approach. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 474, 9–17. [Google Scholar] [CrossRef]
- Ataman, E.; Andersson, M.P.; Ceccato, M.; Bovet, N.; Stipp, S.L.S. Functional Group Adsorption on Calcite: I. Oxygen Containing and Nonpolar Organic Molecules. J. Phys. Chem. C 2016, 120, 16586–16596. [Google Scholar] [CrossRef]
- Kästner, J. Umbrella sampling. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 932–942. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, H.; Zhou, H.X.; Marszalek, P.E.; Yang, W. Equilibrium sampling for biomolecules under mechanical tension. Biophys. J. 2010, 98, 733–740. [Google Scholar] [CrossRef]


| Polymer Type | MD Simulation | Experiment | |
|---|---|---|---|
| Adsorption Free Energy (kcal/mol) | Equilibrium Adsorbed Amount (mg/m2) | AFM Interaction Energy (aJ) | |
| HPAM | 197 | 0.25 | 34.903 |
| SPAM | 160 | 0.14 | 9.413 |
| Vacuum | Water | Dodecane | |
|---|---|---|---|
| Negative adsorption-free energy (kcal/mol) | 197 | 2.25 | 126.2 |
| Polymer | Repeat Unit Composition | Copolymer Ratio/Total Repeat Units for a Polymer Chain | Molecular Weight (Da) |
|---|---|---|---|
| PAM | 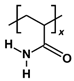 | x = 3, repeat units = 3 | 641.725 |
| HPAM 33% | 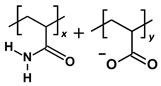 | x:y = 2:1, repeat units = 3 | 641.656 |
| Neutral HPAM | 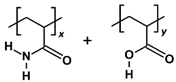 | x:y = 2:1, repeat units = 3 | 644.680 |
| SPAM 33% | 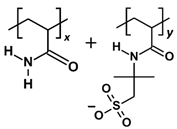 | x:y = 2:1, repeat units = 3 | 1116.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hue, K.Y.; Lew, J.H.; Myo Thant, M.M.; Matar, O.K.; Luckham, P.F.; Müller, E.A. Molecular Dynamics Simulation of Polyacrylamide Adsorption on Calcite. Molecules 2023, 28, 6367. https://doi.org/10.3390/molecules28176367
Hue KY, Lew JH, Myo Thant MM, Matar OK, Luckham PF, Müller EA. Molecular Dynamics Simulation of Polyacrylamide Adsorption on Calcite. Molecules. 2023; 28(17):6367. https://doi.org/10.3390/molecules28176367
Chicago/Turabian StyleHue, Keat Yung, Jin Hau Lew, Maung Maung Myo Thant, Omar K. Matar, Paul F. Luckham, and Erich A. Müller. 2023. "Molecular Dynamics Simulation of Polyacrylamide Adsorption on Calcite" Molecules 28, no. 17: 6367. https://doi.org/10.3390/molecules28176367
APA StyleHue, K. Y., Lew, J. H., Myo Thant, M. M., Matar, O. K., Luckham, P. F., & Müller, E. A. (2023). Molecular Dynamics Simulation of Polyacrylamide Adsorption on Calcite. Molecules, 28(17), 6367. https://doi.org/10.3390/molecules28176367