Synthesis of Bis-Chalcones and Evaluation of Its Effect on Peroxide-Induced Cell Death and Lipopolysaccharide-Induced Cytokine Production
Abstract
:1. Introduction
2. Results and Discussion
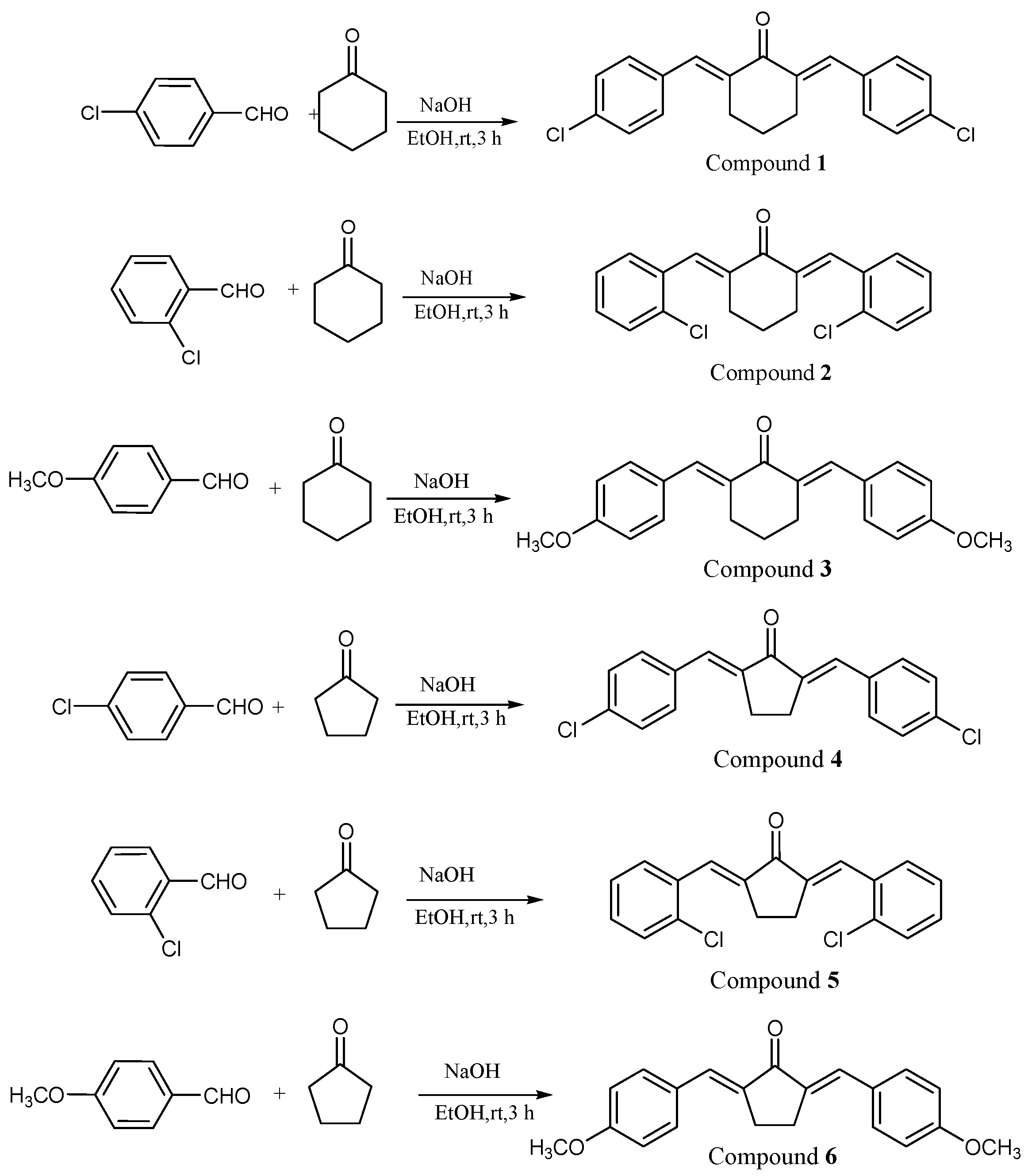
2.1. Characterization of Compounds
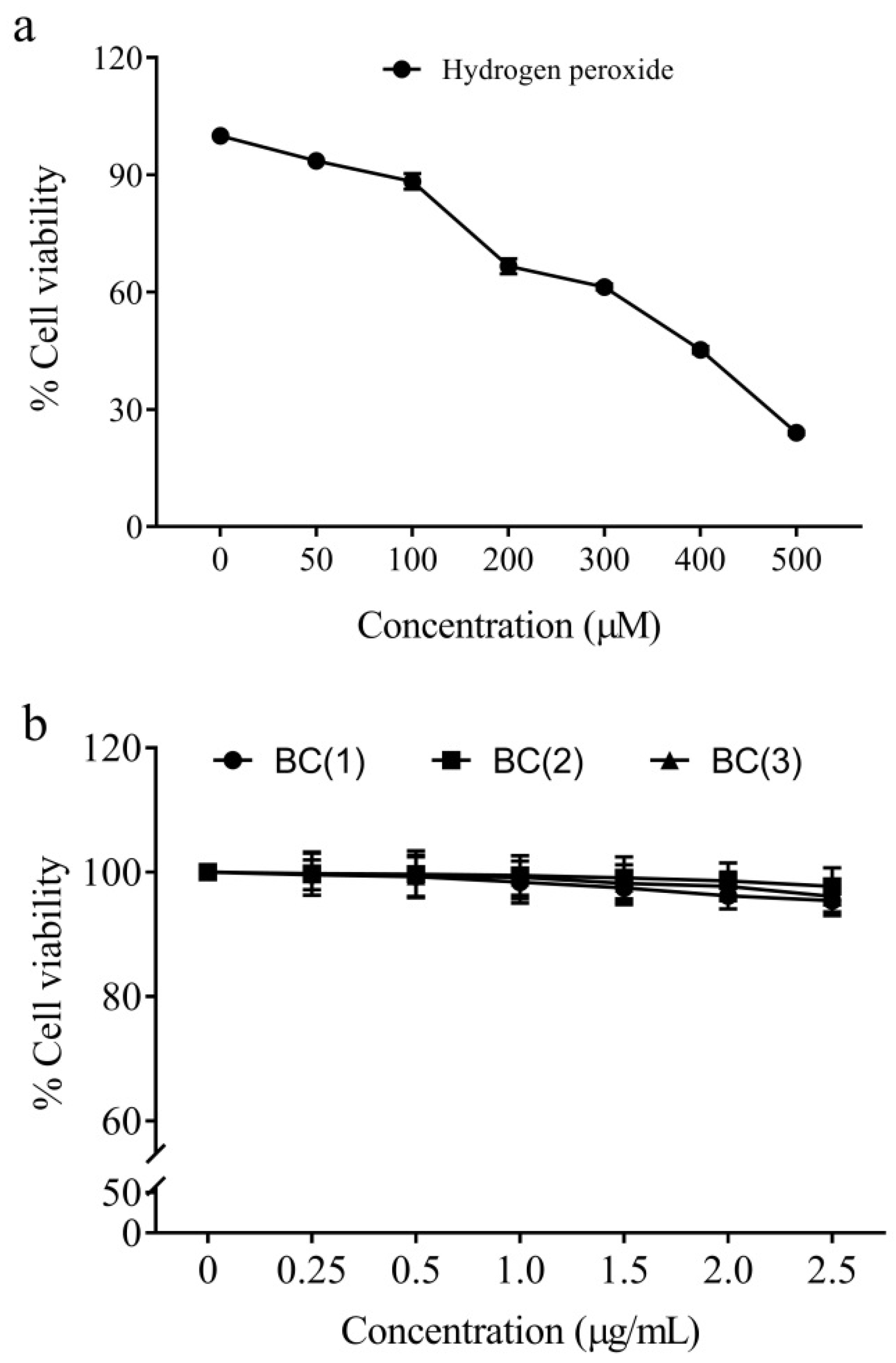
2.2. Cytotoxicity of Peroxide Radicals and Bio-Safe Concentration of Bis-Chalcones
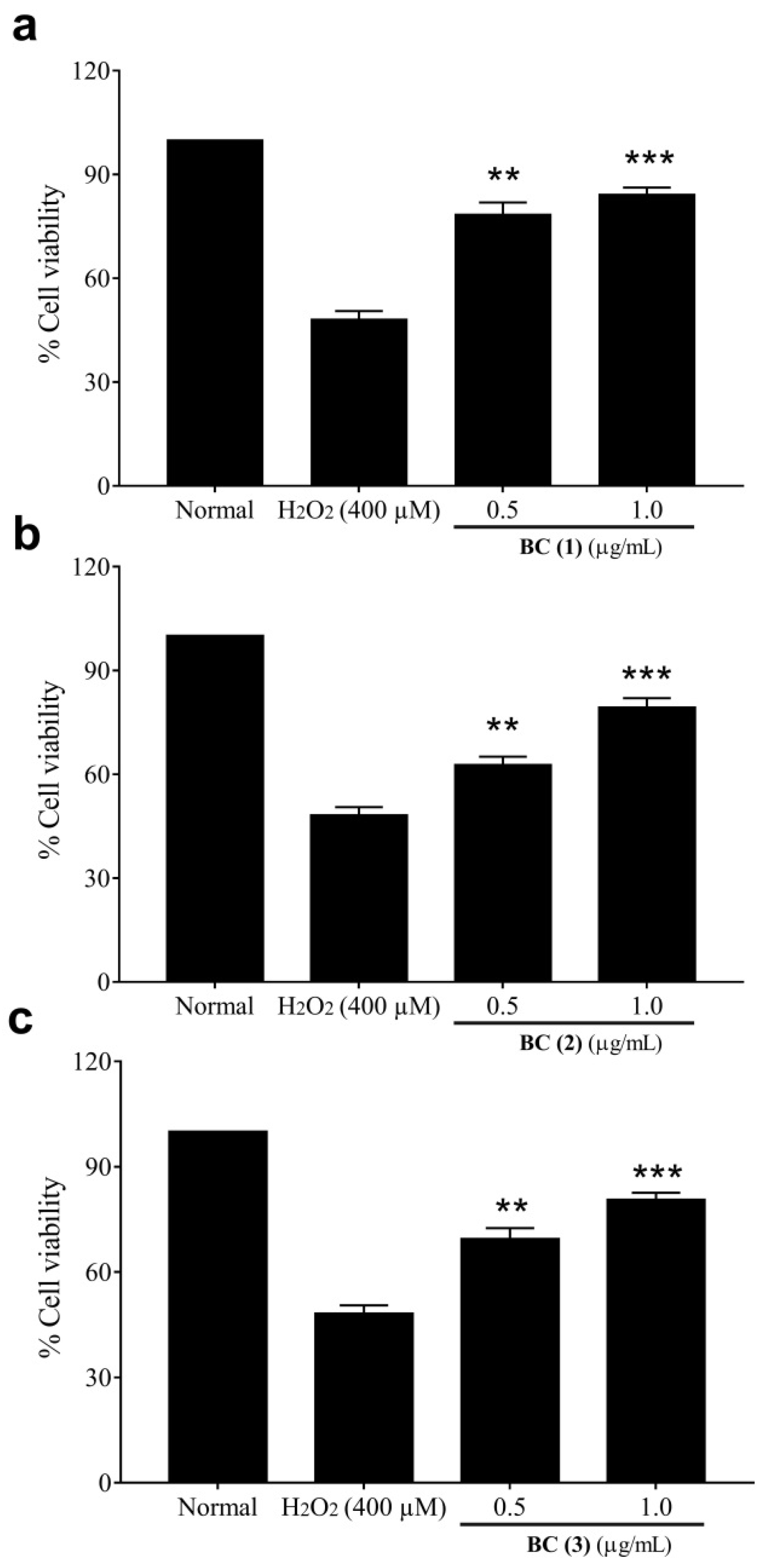
2.3. Cytoprotective Effect of Bis-Chalcones
2.4. Anti-Inflammatory Effects of Bis-Chalcones
3. Materials and Methods
3.1. Chemicals, Cells and Media
3.2. Synthesis and Characterization of Bis-Chalcones
3.3. Cytotoxicity Analysis of Peroxides and Biologically Safe Concentration of Bis-Chalcones
3.4. Analysis of the Effect of Bis-Chalcones against Peroxide-Induced Damage in Cells
3.5. Effect of Bis-Chalcones on LPS-Stimulated Macrophages
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Cheryala, M.; Aloysius, M.; Zheng, B.; Yang, K.; Chen, B.; Fang, Q.; Chowdary, S.B.; Abougergi, M.S.; et al. Global increase of colorectal cancer in young adults over the last 30 years: An analysis of the Global Burden of Disease Study 2019. J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sifaki-Pistolla, D.; Poimenaki, V.; Fotopoulou, I.; Saloustros, E.; Mavroudis, D.; Vamvakas, L.; Lionis, C. Significant Rise of Colorectal Cancer Incidence in Younger Adults and Strong Determinants: 30 Years Longitudinal Differences between under and over 50s. Cancers 2022, 14, 4799. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Patil, P.S.; Saklani, A.; Gambhire, P.; Mehta, S.; Engineer, R.; De’Souza, A.; Chopra, S.; Bal, M. Colorectal Cancer in India: An Audit from a Tertiary Center in a Low Prevalence Area. Indian J. Surg. Oncol. 2017, 8, 484–490. [Google Scholar] [CrossRef]
- Cao, S.; Chen, C.; Gu, D.; Wang, Z.; Xu, G. Establishment and external verification of an oxidative stress-related gene signature to predict clinical outcomes and therapeutic responses of colorectal cancer. Front. Pharmacol. 2023, 13, 991881. [Google Scholar] [CrossRef]
- Basak, D.; Uddin, M.N.; Hancock, J. The Role of Oxidative Stress and Its Counteractive Utility in Colorectal Cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular Mechanisms behind Free Radical Scavengers Function against Oxidative Stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Azzolin, V.F.; Cadoná, F.C.; Machado, A.K.; Berto, M.D.; Barbisan, F.; Dornelles, E.B.; Glanzner, W.G.; Gonçalves, P.B.; Bica, C.G.; da Cruz, I.B. Superoxide-hydrogen peroxide imbalance interferes with colorectal cancer cells viability, proliferation and oxaliplatin response. Toxicol. Vitr. 2016, 32, 8–15. [Google Scholar] [CrossRef]
- Zhu, J.W.; Yu, B.M.; Ji, Y.B.; Zheng, M.H.; Li, D.H. Upregulation of vascular endothelial growth factor by hydrogen peroxide in human colon cancer. World J. Gastroenterol. WJG 2002, 8, 153–157. [Google Scholar] [CrossRef] [PubMed]
- van der Waals, L.M.; Jongen, J.M.J.; Elias, S.G.; Veremiyenko, K.; Trumpi, K.; Trinh, A.; Laoukili, J.; Ubink, I.; Schenning-van Schelven, S.J.; van Diest, P.J.; et al. Increased Levels of Oxidative Damage in Liver Metastases Compared with Corresponding Primary Colorectal Tumors: Association with Molecular Subtype and Prior Treatment. Am. J. Pathol. 2018, 188, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Pravda, J. Evidence-based pathogenesis and treatment of ulcerative colitis: A causal role for colonic epithelial hydrogen peroxide. World J. Gastroenterol. WJG 2022, 28, 4263–4298. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. WJG 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Soleimani, A.; Rahmani, F.; Ferns, G.A.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Role of the NF-κB signaling pathway in the pathogenesis of colorectal cancer. Gene 2020, 726, 144132. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Marotta, L.; Rossi, S.; Ibba, R.; Brogi, S.; Calderone, V.; Butini, S.; Campiani, G.; Gemma, S. The green chemistry of chalcones: Valuable sources of privileged core structures for drug discovery. Front. Chem. 2022, 10, 988376. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Liu, X.; Shang, J.; Zhang, A.; Zhu, Z.; Zha, D. Chalcone synthase (CHS) family members analysis from eggplant (Solanum melongena L.) in the flavonoid biosynthetic pathway and expression patterns in response to heat stress. PLoS ONE 2020, 15, e0226537. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Cabral, I.S.R.; d’Arce, M.A.B.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Escobar-Ramos, A.; Lobato-García, C.E.; Zamilpa, A.; Gómez-Rivera, A.; Tortoriello, J.; González-Cortazar, M. Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L., with Spasmolytic Activity. Molecules 2017, 22, 1405. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Rehman, A.U.; Arshad, H.; Malik, A.; Fatima, M.; Tabassum, T.; Raza, A.R.; Bukhsh, M.; Murtaza, M.A.; Mehmood, M.H.; et al. In Vitro Antioxidant Activities and the Therapeutic Potential of Some Newly Synthesized Chalcones against 4-Acetaminophenol Induced Hepatotoxicity in Rats. Dose-Response 2021, 19, 1559325821996955. [Google Scholar] [CrossRef]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Chalcones as putative hepatoprotective agents: Preclinical evidence and molecular mechanisms. Pharmacol. Res. 2018, 129, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Zhang, Y.; Chen, R.; Cui, Y.; Wang, Q. Anti-inflammatory Chalcone–Isoflavone Dimers and Chalcone Dimers from Caragana jubata. J. Nat. Prod. 2019, 82, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Sha, F.; Zhao, C.; Zheng, Z.; Zhao, S.; Zhu, Z.; Zhu, H.; Chen, J.; Chen, Y. Synthesis and anti-inflammatory activity of novel steroidal chalcones with 3β-pregnenolone ester derivatives in RAW 264.7 cells in vitro. Steroids 2021, 171, 108830. [Google Scholar] [CrossRef]
- ur Rashid, H.; Xu, Y.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg. Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef]
- Pozzetti, L.; Ibba, R.; Rossi, S.; Taglialatela-Scafati, O.; Taramelli, D.; Basilico, N.; D’Alessandro, S.; Parapini, S.; Butini, S.; Campiani, G.; et al. Total Synthesis of the Natural Chalcone Lophirone E, Synthetic Studies toward Benzofuran and Indole-Based Analogues, and Investigation of Anti-Leishmanial Activity. Molecules 2022, 27, 463. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Kuttithodi, A.M.; Nikhitha, D.; Jacob, J.; Narayanankutty, A.; Mathews, M.; Olatunji, O.J.; Rajagopal, R.; Alfarhan, A.; Barcelo, D. Antioxidant, Antimicrobial, Cytotoxicity, and Larvicidal Activities of Selected Synthetic Bis-Chalcones. Molecules 2022, 27, 8209. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Dao, T.T.; Linthorst, H.J.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Vilema-Enríquez, G.; Arroyo, A.; Grijalva, M.; Amador-Zafra, R.I.; Camacho, J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxidative Med. Cell. Longev. 2016, 2016, 1908164. [Google Scholar] [CrossRef]
- Xiang, J.; Wan, C.; Guo, R.; Guo, D. Is Hydrogen Peroxide a Suitable Apoptosis Inducer for All Cell Types? BioMed Res. Int. 2016, 2016, 7343965. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Franco, R.; Panayiotidis, M.I.; Cidlowski, J.A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J. Biol. Chem. 2007, 282, 30452–30465. [Google Scholar] [CrossRef]
- Liu, N.; Ma, X.; Luo, X.; Zhang, Y.; He, Y.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. L-Glutamine Attenuates Apoptosis in Porcine Enterocytes by Regulating Glutathione-Related Redox Homeostasis. J. Nutr. 2018, 148, 526–534. [Google Scholar] [CrossRef]
- Kachadourian, R.; Day, B.J.; Pugazhenti, S.; Franklin, C.C.; Genoux-Bastide, E.; Mahaffey, G.; Gauthier, C.; Di Pietro, A.; Boumendjel, A. A synthetic chalcone as a potent inducer of glutathione biosynthesis. J. Med. Chem. 2012, 55, 1382–1388. [Google Scholar] [CrossRef]
- Andrés, C.M.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Martins, D.; English, A.M. Catalase activity is stimulated by H2O2 in rich culture medium and is required for H2O2 resistance and adaptation in yeast. Redox Biol. 2014, 2, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Greene, A.E.; Li, J.; Wang, H.; Volpe, J.J.; Rosenberg, P.A. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J. Neurosci. 2004, 24, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, J.; Wang, Y.; Huang, X.; Shao, T.; Deng, X.; Cao, Y.; Zhou, M.; Zhao, C. Oxidative Stress and Lipid Peroxidation: Prospective Associations Between Ferroptosis and Delayed Wound Healing in Diabetic Ulcers. Front. Cell Dev. Biol. 2022, 10, 898657. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 12, 61122. [Google Scholar]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef]
- Means, A.L.; Freeman, T.J.; Zhu, J.; Woodbury, L.G.; Marincola-Smith, P.; Wu, C.; Meyer, A.R.; Weaver, C.J.; Padmanabhan, C.; An, H.; et al. Epithelial Smad4 Deletion Up-Regulates Inflammation and Promotes Inflammation-Associated Cancer. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 257–276. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Buccini, D.F.; Roriz, B.C.; Rodrigues, J.M.; Franco, O.L. Antimicrobial peptides could antagonize uncontrolled inflammation via Toll-like 4 receptor. Front. Bioeng. Biotechnol. 2022, 10, 1037147. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, Z.; Schindler, F.; Bahiraii, S.; Brenner, M.; Heiss, E.H.; Weckwerth, W. Norbergenin prevents LPS-induced inflammatory responses in macrophages through inhibiting NFκB, MAPK and STAT3 activation and blocking metabolic reprogramming. Front. Immunol. 2023, 14, 1117638. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Apte, R.N. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015, 8, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Pappan, L.; Galliher-Beckley, A.; Shi, J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer 2012, 11, 1476–4598. [Google Scholar] [CrossRef]
- Lin, Y.; He, Z.; Ye, J.; Liu, Z.; She, X.; Gao, X.; Liang, R. Progress in understanding the IL-6/STAT3 pathway in colorectal cancer. OncoTargets Ther. 2020, 13, 13023–13032. [Google Scholar] [CrossRef]
- Waldner, M.J.; Foersch, S.; Neurath, M.F. Interleukin-6—A key regulator of colorectal cancer development. Int J. Biol. Sci. 2012, 8, 1248–1253. [Google Scholar] [CrossRef]
- Al Obeed, O.A.; Alkhayal, K.A.; Al Sheikh, A.; Zubaidi, A.M.; Vaali-Mohammed, M.A.; Boushey, R.; McKerrow, J.H.; Abdulla, M.H. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J. Gastroenterol. 2014, 20, 18390–18396. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Fattori, V.; Bussmann, A.J.C.; Bottura, C.; Fonseca, M.J.V.; Vignoli, J.A.; Baracat, M.M.; et al. trans-Chalcone, a flavonoid precursor, inhibits UV-induced skin inflammation and oxidative stress in mice by targeting NADPH oxidase and cytokine production. Photochem. Photobiol. Sci. 2017, 16, 1162–1173. [Google Scholar] [CrossRef]
- Lee, J.S.; Bukhari, S.N.; Fauzi, N.M. Effects of chalcone derivatives on players of the immune system. Drug Des. Devel. Ther. 2015, 9, 4761–4778. [Google Scholar]
- Khanapure, S.; Jagadale, M.; Bansode, P.; Choudhari, P.; Rashinkar, G. Anticancer activity of ruthenocenyl chalcones and their molecular docking studies. J. Mol. Struct. 2018, 1173, 142–147. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kunnath, K.; Famurewa, A.C.; Ramesh, V.; Rajagopal, R.; Alfarhan, A. Variations in the composition, cytoprotective and anti-inflammatory effects of natural polyphenols of edible oils extracted from fresh and dried coconut testa. Physiol. Mol. Plant Pathol. 2022, 117, 101742. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, L.; Ma, J.; Wang, Y.; Ding, L.; Zhao, F.; Chen, L.; Qiu, F. Sesquiterpenes from the essential oil of Curcuma wenyujin and their inhibitory effects on nitric oxide production. Fitoterapia 2015, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Catalase (U/mg Protein) | GSH (µmoles/mg Protein) | GPx (U/mg Protein) | TBARS (nmoles/mg Protein) |
|---|---|---|---|---|
| Normal | 47.64 ± 3.7 | 4.68 ± 0.22 | 65.65 ± 3.94 | 1.75 ± 0.34 |
| H2O2 (400 µM) | 88.19 ± 4.3 | 2.31 ± 0.24 | 103.10 ± 4.82 | 6.55 ± 0.45 |
| BC(1) 0.5 µg/mL | 65.62 ± 3.4 ** | 3.07 ± 0.21 * | 85.64 ± 4.12 ** | 4.59 ± 0.21 * |
| BC(1) 1.0 µg/mL | 50.04 ± 4.2 *** | 3.78 ± 0.35 ** | 75.05 ± 3.14 *** | 3.25 ± 0.42 ** |
| BC(2) 0.5 µg/mL | 72.11 ± 2.3 * | 2.94 ± 0.23 ** | 90.04 ± 2.15 * | 4.76 ± 0.40 * |
| BC(2) 1.0 µg/mL | 59.15 ± 6.1 *** | 3.15 ± 0.18 | 84.11 ± 3.45 ** | 4.05 ± 0.51 ** |
| BC(3) 0.5 µg/mL | 70.55 ± 4.8 * | 3.13 ± 0.30 * | 80.17 ± 4.03 * | 5.01 ± 0.17 * |
| BC(3) 1.0 µg/mL | 61.82 ± 6.4 ** | 3.85 ± 0.41 ** | 70.52 ± 4.16 ** | 4.11 ± 0.38 ** |
| IL-1β (pg/mg Protein) | IL-6 (pg/mg Protein) | TNF-α (pg/mg Protein) | NO (µM/mg Protein) | |
|---|---|---|---|---|
| Untreated | 54.5 ± 2.9 | 103.4 ± 10.2 | 259.4 ± 10.9 | 8.5 ± 0.7 |
| LPS | 503.2 ± 12.3 | 1185.2 ± 24.6 | 1635.0 ± 22.5 | 67.8 ± 2.7 |
| BC(1) 0.5 µg/mL | 407.8 ± 15.6 * | 851.1 ± 20.6 * | 1367.0 ± 18.3 ** | 39.5 ± 1.2 * |
| BC(1) 1.0 µg/mL | 298.4 ± 12.4 *** | 756.1 ± 22.4 *** | 1015.1 ± 18.6 *** | 26.5 ± 1.4 *** |
| BC(2) 0.5 µg/mL | 421.8 ± 14.5 * | 927.5 ± 27.3 * | 1475.8 ± 10.4 * | 51.0 ± 0.5 ns |
| BC(2) 1.0 µg/mL | 365.7 ± 15.5 ** | 835.1 ± 17.2 ** | 1300.7 ± 33.4 ** | 39.2 ± 1.1 * |
| BC(3) 0.5 µg/mL | 434.5 ± 10.9 * | 964.7 ± 19.5 * | 1404.1 ± 28.2 * | 46.7 ± 0.8 * |
| BC(3) 1.0 µg/mL | 389.4 ± 16.2 ** | 876.1 ± 27.4 ** | 1288.5 ± 16.3 ** | 31.2 ± 1.8 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tom, A.; Jacob, J.; Mathews, M.; Rajagopal, R.; Alfarhan, A.; Barcelo, D.; Narayanankutty, A. Synthesis of Bis-Chalcones and Evaluation of Its Effect on Peroxide-Induced Cell Death and Lipopolysaccharide-Induced Cytokine Production. Molecules 2023, 28, 6354. https://doi.org/10.3390/molecules28176354
Tom A, Jacob J, Mathews M, Rajagopal R, Alfarhan A, Barcelo D, Narayanankutty A. Synthesis of Bis-Chalcones and Evaluation of Its Effect on Peroxide-Induced Cell Death and Lipopolysaccharide-Induced Cytokine Production. Molecules. 2023; 28(17):6354. https://doi.org/10.3390/molecules28176354
Chicago/Turabian StyleTom, Alby, Jisha Jacob, Manoj Mathews, Rajakrishnan Rajagopal, Ahmed Alfarhan, Damia Barcelo, and Arunaksharan Narayanankutty. 2023. "Synthesis of Bis-Chalcones and Evaluation of Its Effect on Peroxide-Induced Cell Death and Lipopolysaccharide-Induced Cytokine Production" Molecules 28, no. 17: 6354. https://doi.org/10.3390/molecules28176354
APA StyleTom, A., Jacob, J., Mathews, M., Rajagopal, R., Alfarhan, A., Barcelo, D., & Narayanankutty, A. (2023). Synthesis of Bis-Chalcones and Evaluation of Its Effect on Peroxide-Induced Cell Death and Lipopolysaccharide-Induced Cytokine Production. Molecules, 28(17), 6354. https://doi.org/10.3390/molecules28176354






