The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue
Abstract
:1. Introduction
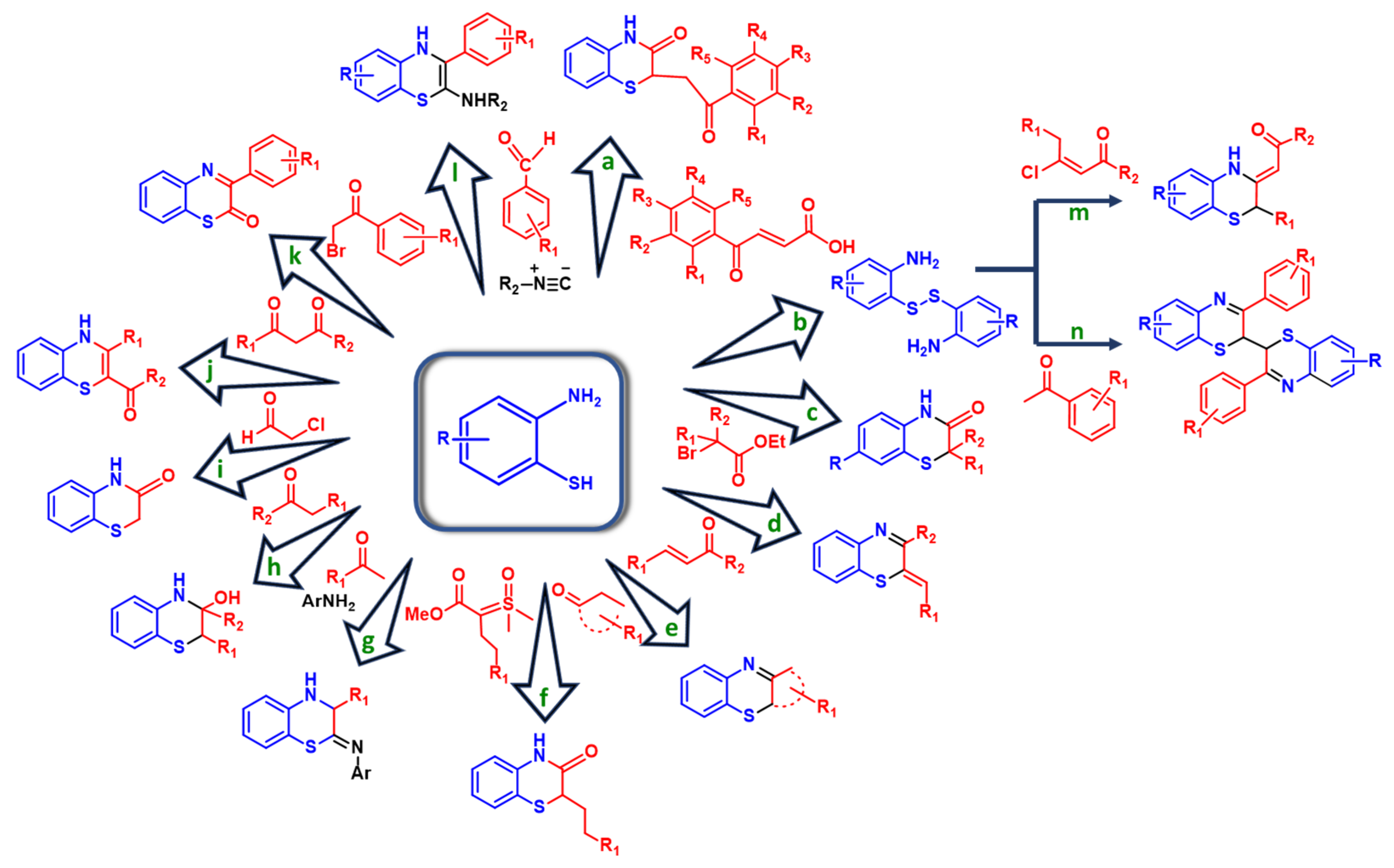
2. Recent Synthetic Approaches to 1,4-Benzothiazines
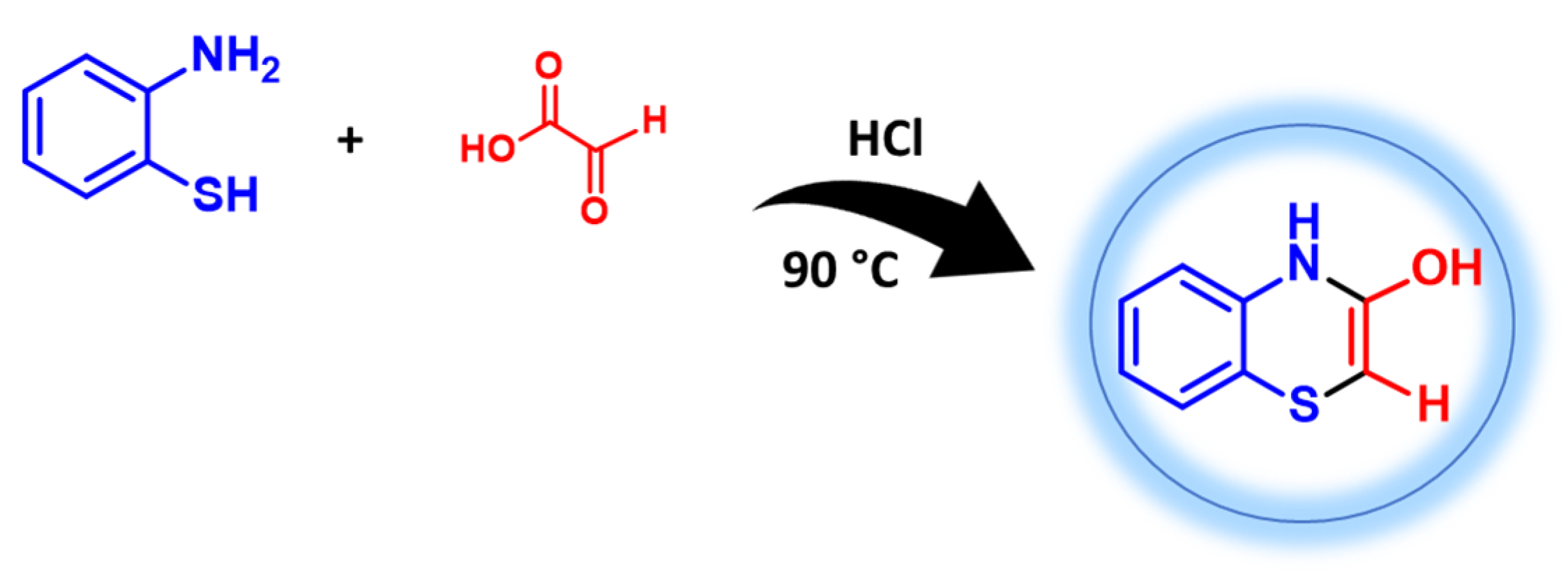
2.1. Coupling of 2-Aminothiophenols with Carbonyl Compounds
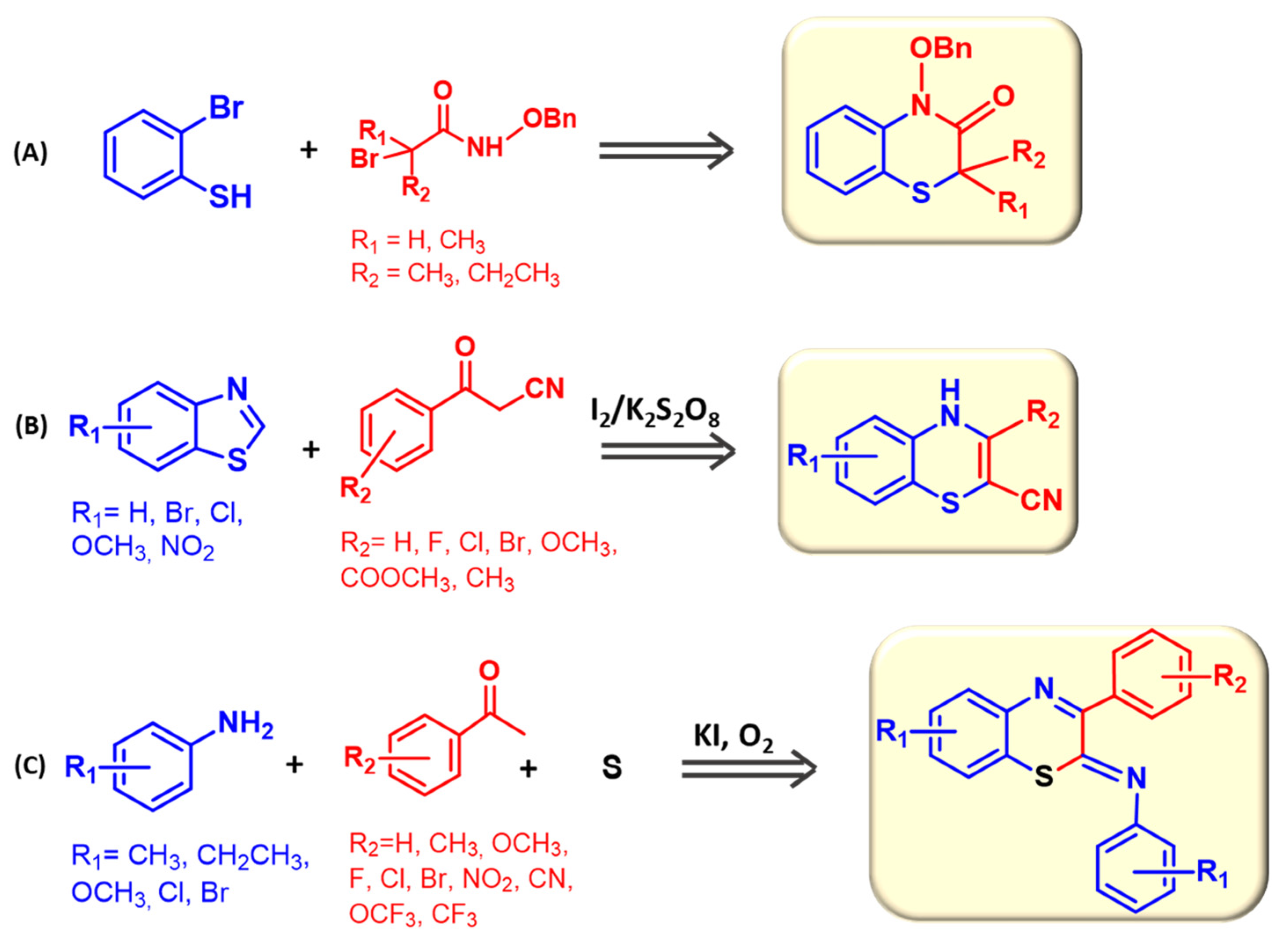
2.2. Other Synthetic Routes
3. Exploitation of the Chromophoric Properties of the 1,4-Benzothiazine System
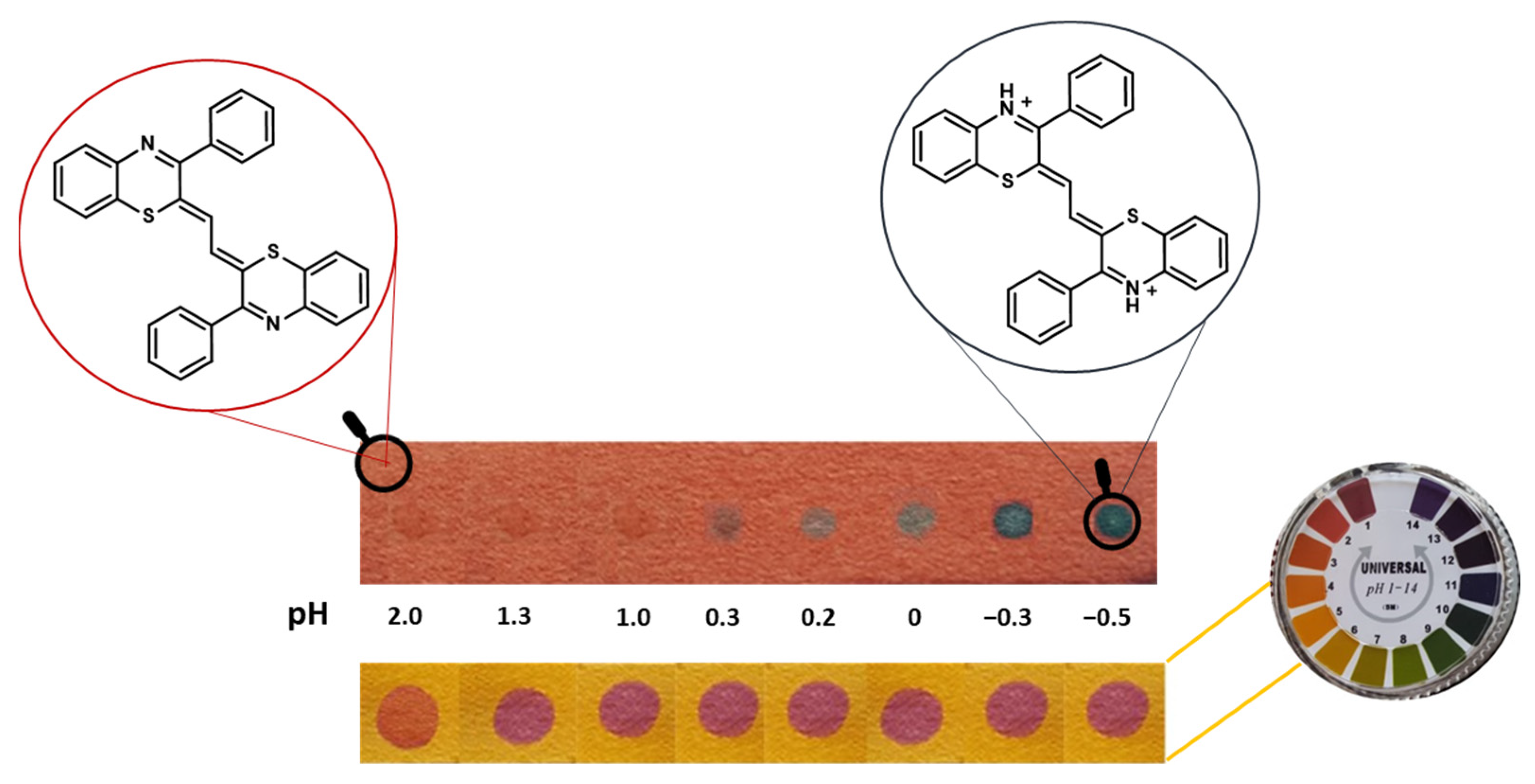
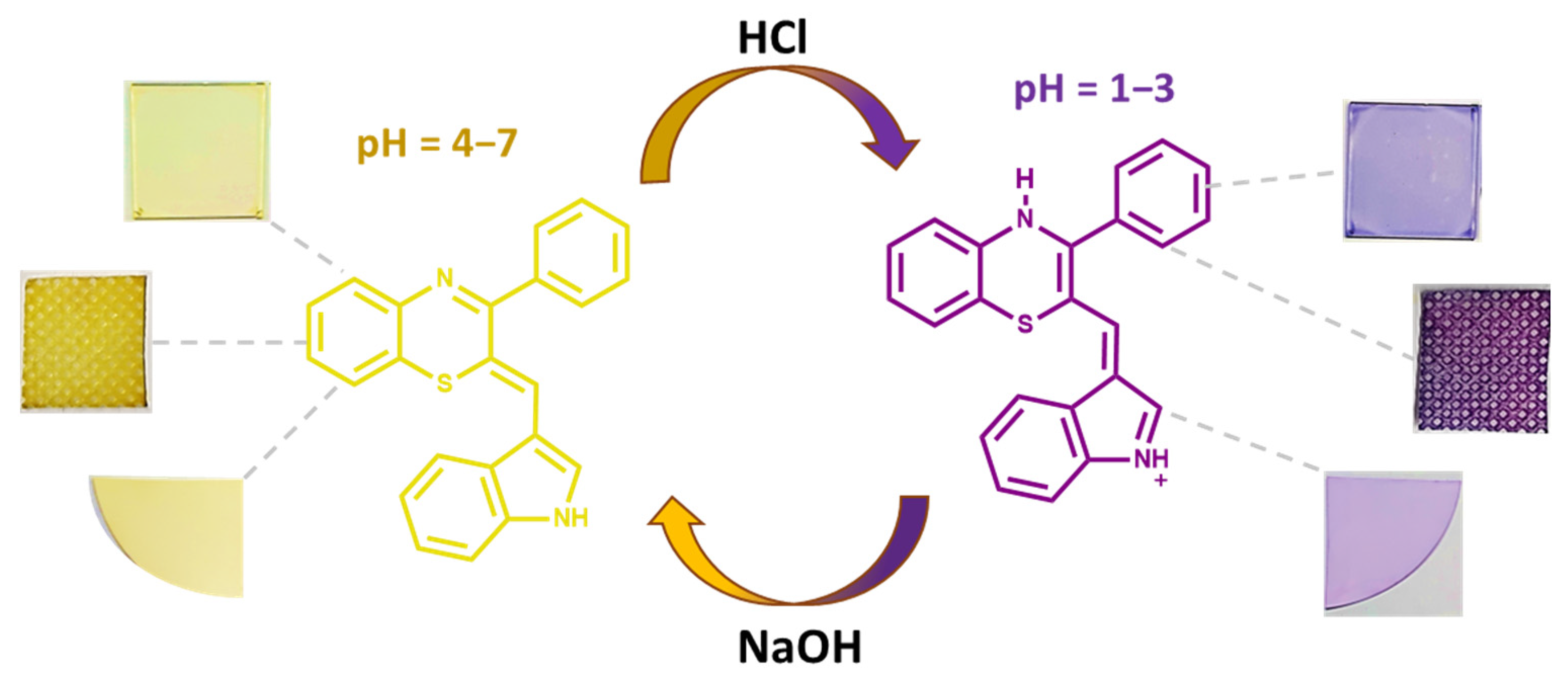
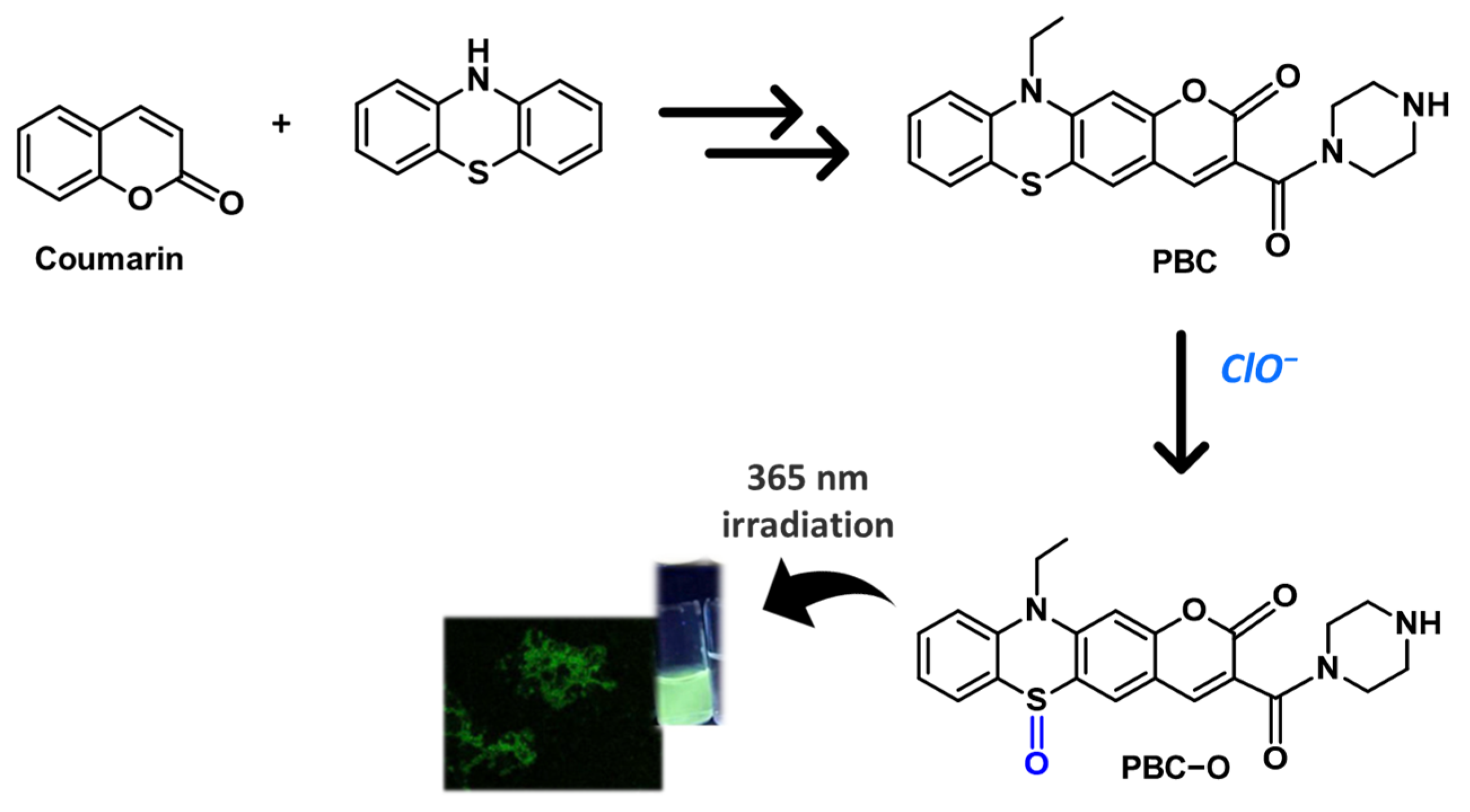
3.1. pH Sensing
3.2. Filter Permeability Control
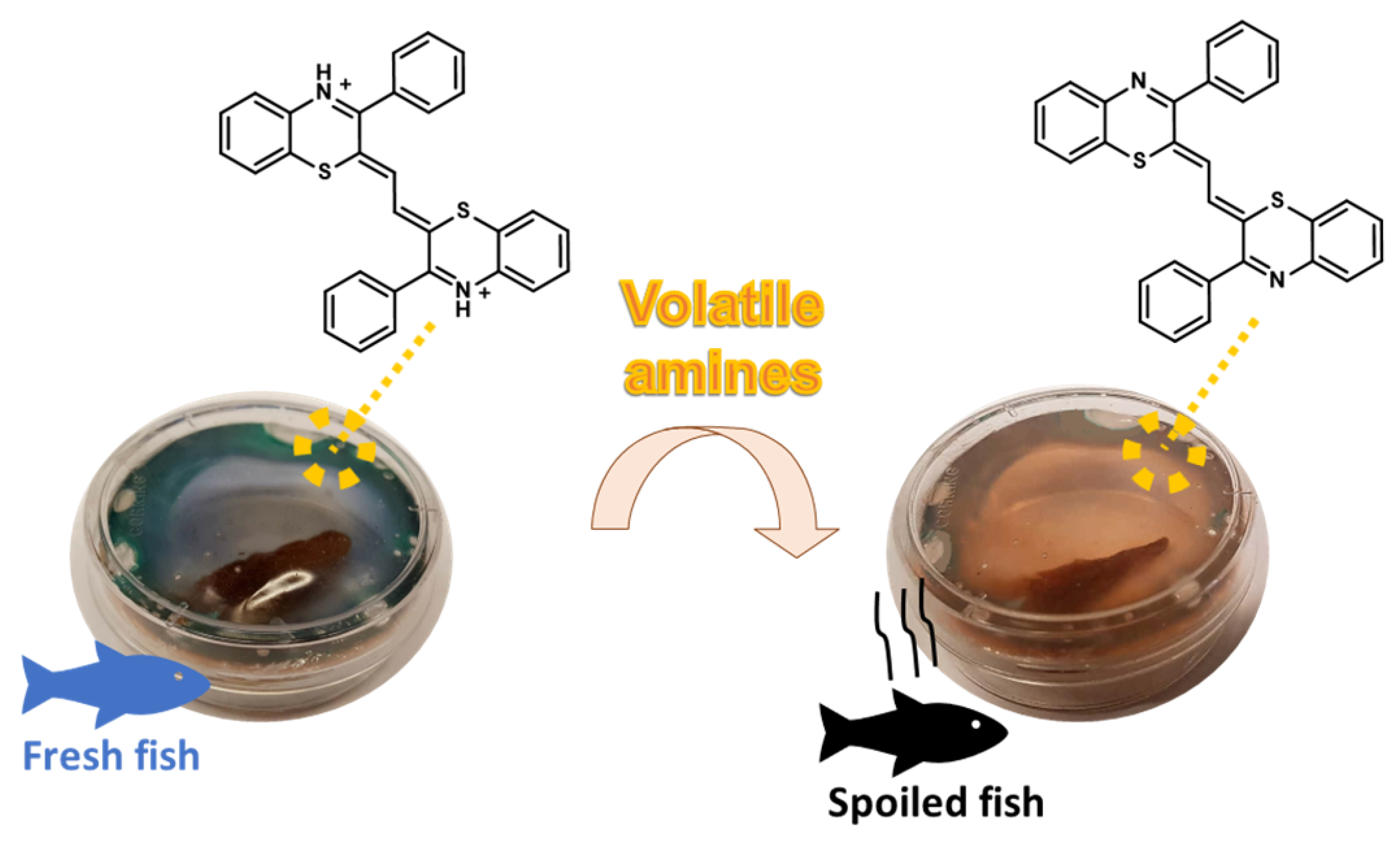
3.3. Smart Packaging
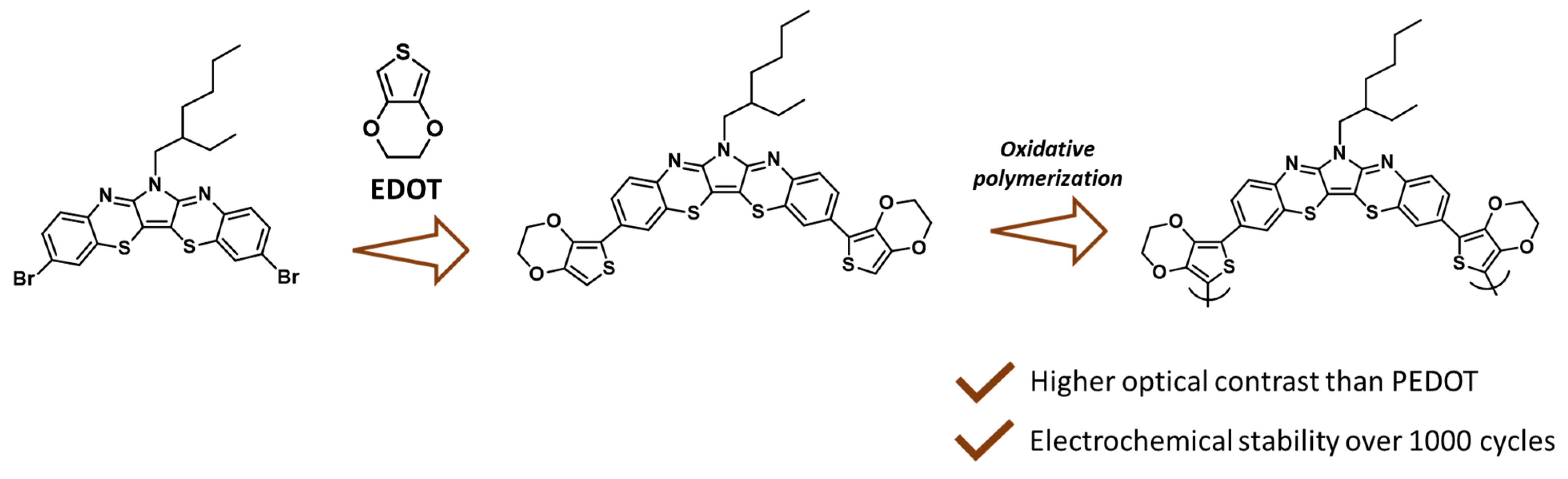
3.4. Electrochromic Device Fabrication
3.5. Bioimaging
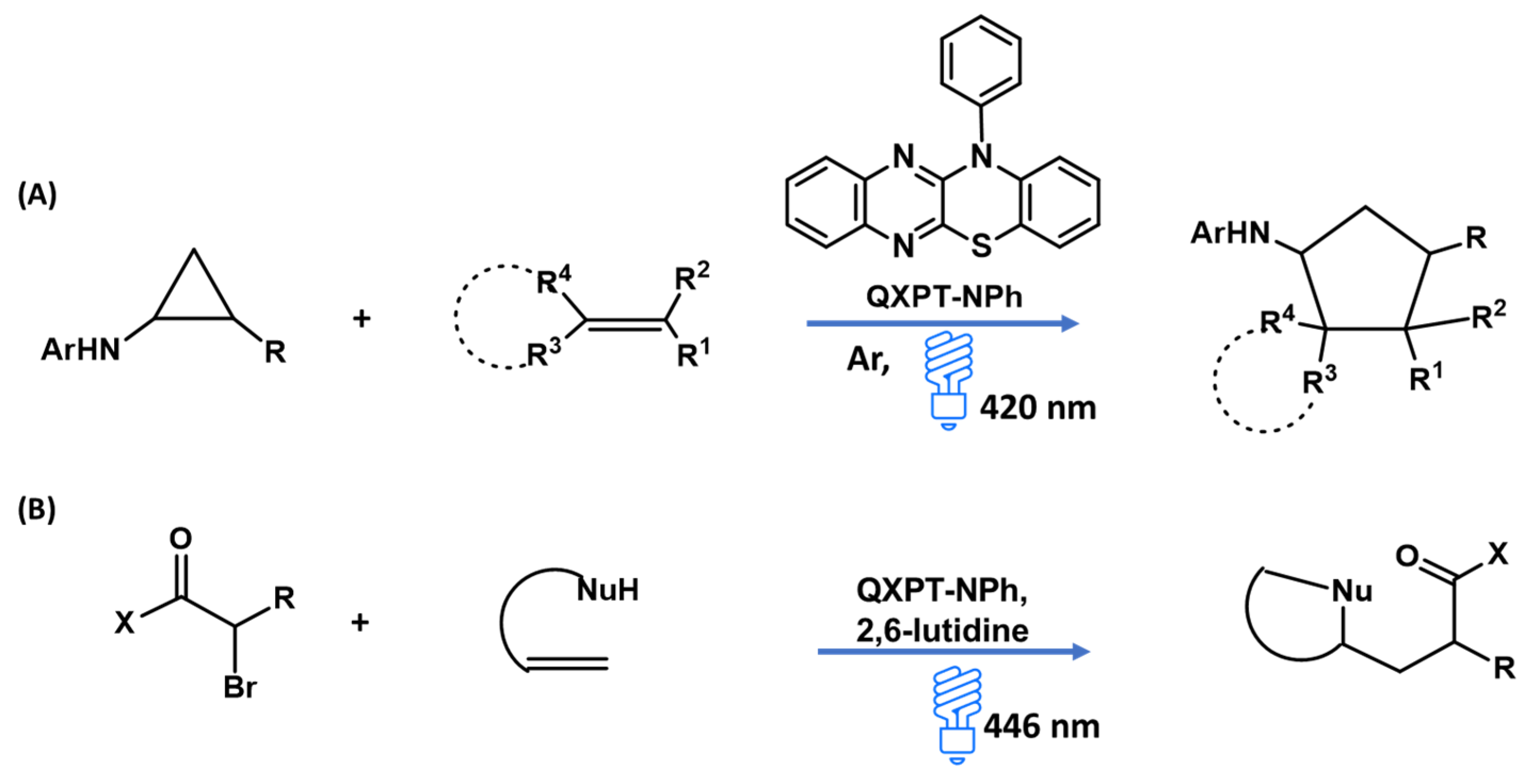
3.6. Photocatalysis
3.7. HPLC Detection Systems
4. Future Directions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Sharma, P.K.; Amin, A.; Kumar, M. A review: Medicinally important nitrogen sulphur containing heterocycles. Open Med. Chem. J. 2020, 14, 49–64. [Google Scholar] [CrossRef]
- Koperniku, A.; Garcia, A.A.; Mochly-Rosen, D. Boosting the discovery of small molecule inhibitors of glucose-6-phosphate dehydrogenase for the treatment of cancer, infectious diseases, and inflammation. J. Med. Chem. 2022, 65, 4403–4423. [Google Scholar] [CrossRef]
- Mlakić, M.; Čipor, I.; Kovačec, P.; Kragol, G.; Ratković, A.; Kovačević, T.; Zadravec, R.; Milašinović, V.; Molčanov, K.; Piantanida, I.; et al. The benzothiazine core as a novel motif for DNA-binding small molecules. Molecules 2023, 28, 4499. [Google Scholar] [CrossRef]
- Badshah, S.L.; Naeem, A. Bioactive thiazine and benzothiazine derivatives: Green synthesis methods and their medicinal importance. Molecules 2016, 21, 1054. [Google Scholar] [CrossRef]
- Choudhary, S.; Silakari, O.; Singh, P.K. Key updates on the chemistry and biological roles of thiazine scaffold: A review. Mini Rev. Med. Chem. 2018, 18, 1452–1478. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.K.; Raj, V.; Saha, S. 1,4-Benzothiazines -a biologically attractive scaffold. Mini Rev. Med. Chem. 2017, 18, 42–57. [Google Scholar] [CrossRef]
- Halloran, M.W.; Li, E.; Esguerra, K.V.N.; Lumb, J.P. A bioinspired synthesis of 1,4-benzothiazines by selective addition of sulfur nucleophiles to ortho-quinones. J. Org. Chem. 2023, 88, 2561–2569. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Crescenzi, O.; Napolitano, A.; d’Ischia, M. Nature-inspired functional chromophores from biomimetic o-quinone chemistry. Eur. J. Org. Chem. 2021, 2021, 2982–2989. [Google Scholar] [CrossRef]
- Napolitano, A.; Panzella, L.; Leone, L.; d’Ischia, M. Red hair benzothiazines and benzothiazoles: Mutation-inspired chemistry in the quest for functionality. Acc. Chem. Res. 2013, 46, 519–528. [Google Scholar] [CrossRef]
- Greco, G.; Panzella, L.; Verotta, L.; d’Ischia, M.; Napolitano, A. Uncovering the structure of human red hair pheomelanin: Benzothiazolylthiazinodihydroisoquinolines as key building blocks. J. Nat. Prod. 2011, 74, 675–682. [Google Scholar] [CrossRef]
- Greco, G.; Panzella, L.; Napolitano, A.; d’Ischia, M. The fundamental building blocks of red human hair pheomelanin are isoquinoline-containing dimers. Pigment Cell Melanoma Res. 2012, 25, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Di Donato, P.; Prota, G. New regulatory mechanisms in the biosynthesis of pheomelanins: Rearrangement vs. redox exchange reaction routes of a transient 2H-1,4-benzothiazine-o-quinonimine intermediate. Biochim. Biophys. Acta 2000, 1475, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.; Bigioni, I.; Scotto d’Abusco, A.; Baseggio Conrado, A.; Maina, S.; Francioso, A.; Mosca, L.; Fontana, M. Pheomelanin effect on UVB radiation-induced oxidation/nitration of l-tyrosine. Int. J. Mol. Sci. 2022, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Mao, H.; McCallum, N.C.; Zhou, X.; Sun, H.; Sharpe, C.; Korpanty, J.; Hu, Z.; Ni, Q.Z.; Burkart, M.D.; et al. Biomimetic pheomelanin to unravel the electronic, molecular and supramolecular structure of the natural product. Chem. Sci. 2023, 14, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhou, X.; McCallum, N.C.; Hu, Z.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the structure and function of melanin through synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Cariola, A.; Panzella, L.; Napolitano, A.; d’Ischia, M.; Valgimigli, L.; Crescenzi, O. Disentangling the puzzling regiochemistry of thiol addition to o-quinones. J. Org. Chem. 2022, 87, 4580–4589. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Amorati, R.; Cariola, A.; Valgimigli, L.; Napolitano, A. Role of sulphur and heavier chalcogens on the antioxidant power and bioactivity of natural phenolic compounds. Biomolecules 2022, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Wakamatsu, K.; Panzella, L.; Shosuke, I.; Napolitano, A.; d’Ischia, M. Isomeric cysteinyldopas provide a (photo)degradable bulk component and a robust structural element in red human hair pheomelanin. Pigment Cell Melanoma Res. 2009, 22, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and melanin-like hybrid materials in regenerative medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef]
- Panzella, L.; Szewczyk, G.; d’Ischia, M.; Napolitano, A.; Sarna, T. Zinc-induced structural effects enhance oxygen consumption and superoxide generation in synthetic pheomelanins on UVA/visible light irradiation. Photochem. Photobiol. 2010, 86, 757–764. [Google Scholar] [CrossRef]
- Ye, T.; Hong, L.; Garguilo, J.; Pawlak, A.; Edwards, G.S.; Nemanich, R.J.; Sarna, T.; Simon, J.D. Photoionization thresholds of melanins obtained from free electron laser-photoelectron emission microscopy, femtosecond transient absorption spectroscopy and electron paramagnetic resonance measurements of oxygen photoconsumption. Photochem. Photobiol. 2006, 82, 733–737. [Google Scholar] [CrossRef]
- Chedekel, M.R.; Agin, P.P.; Sayre, R.M. Photochemistry of pheomelanin: Action spectrum for superoxide production. Photochem. Photobiol. 1980, 31, 553–555. [Google Scholar] [CrossRef]
- Valgimigli, L.; Alfieri, M.L.; Amorati, R.; Baschieri, A.; Crescenzi, O.; Napolitano, A.; d’Ischia, M. Proton-sensitive free-radical dimer evolution is a critical control point for the synthesis of Δ2,2′-bibenzothiazines. J. Org. Chem. 2020, 85, 11440–11448. [Google Scholar] [CrossRef]
- Leone, L.; Crescenzi, O.; Amorati, R.; Valgimigli, L.; Napolitano, A.; Barone, V.; d’Ischia, M. Red-hair-inspired chromogenic system based on a proton-switched dehydrogenative free-radical coupling. Org. Lett. 2013, 15, 4944–4947. [Google Scholar] [CrossRef]
- Leone, L.; Crescenzi, O.; Napolitano, A.; Barone, V.; d’Ischia, M. The Δ2,2′-bi(2H-1,4-benzothiazine) structural motif of red hair pigments revisited: Photochromism and acidichromism in a unique four-state system. Eur. J. Org. Chem. 2012, 2012, 5136–5140. [Google Scholar] [CrossRef]
- Lampel, A.; McPhee, S.A.; Kassem, S.; Sementa, D.; Massarano, T.; Aramini, J.M.; He, Y.; Ulijn, R.V. Melanin-inspired chromophoric microparticles composed of polymeric peptide pigments. Angew. Chem. Int. Ed. 2021, 60, 7564–7569. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Basu, B. Greener approaches towards 1,4–benzothiazine synthesis: Recent updates and outlook. Curr. Organocatalysis, 2023; in press. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Synthesis of 1,4-benzothiazinones from acylpyruvic acids or furan-2,3-diones and o-aminothiophenol. Beilstein J. Org. Chem. 2020, 16, 2322–2331. [Google Scholar] [CrossRef]
- Kisszékelyi, P.; Peňaška, T.; Stankovianska, K.; Mečiarová, M.; Šebesta, R. Derivatives of benzo-1,4-thiazine-3-carboxylic acid and the corresponding amino acid conjugates. Beilstein J. Org. Chem. 2022, 18, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Javahershenas, R. Application of phenacyl bromide analogs as a versatile organic intermediate for the synthesis of heterocyclic compounds via multicomponent reactions. Mol. Divers. 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Singha, R.; Ghosh, P. Polyethylene glycol (PEG-200): An efficient, green and biocompatible reaction medium for the metal-free synthesis of functionalized 1,4-benzothiazines. ChemistrySelect 2023, 8, e202203780. [Google Scholar] [CrossRef]
- Goswami, M.; Dutta, A.; Paul, P.; Nongkhlaw, R. Recent developments on catalyst-free, visible-light-triggered synthesis of heterocyclic scaffolds and their mechanistic study. ChemistrySelect 2021, 6, 9684–9700. [Google Scholar] [CrossRef]
- Chatterjee, T.; Ranu, B.C. Synthesis of organosulfur and related heterocycles under mechanochemical conditions. J. Org. Chem. 2021, 86, 13895–13910. [Google Scholar] [CrossRef]
- Ataollahi, E.; Solhjoo, A.; Rezaei, Z.; Behrouz, M.; Heidari, R.; Shahbazi, M.R.; Foroozanad, R.; Zamani, L.; Khabnadideh, S.; Emami, L. Novel 1,4 benzothiazine 3-one derivatives as anticonvulsant agents: Design, synthesis, biological evaluation and computational studies. Comput. Biol. Chem. 2023, 104, 107870. [Google Scholar] [CrossRef]
- Ramzan, F.; Nabi, S.A.; Lone, M.S.; Imtiyaz, K.; Urooj, L.; Vishakha, V.; Sharma, K.; Rizvi, M.M.A.; Shafi, S.; Samim, M.; et al. Synthesis, molecular docking, and biological evaluation of a new series of benzothiazinones and their benzothiazinyl acetate derivatives as anticancer agents against MCF-7 human breast cancer cells and as anti-inflammatory agents. ACS Omega 2023, 8, 6650–6662. [Google Scholar] [CrossRef]
- Sharma, N.; Swami, S.; Shrivastava, V.; Nair, R.; Shrivastava, R. Graphene oxide and functionalized graphene oxide: Robust, 2D material as heterogeneous green catalyst for heterocyclic synthesis. Mater. Today Proc. 2021, 43, 3309–3317. [Google Scholar] [CrossRef]
- Teli, B.; Waseem, M.A.; Rashid, S.; Ganaie, B.A.; Bhat, B.A. Catalyst free synthesis of 2-aryl-2H-benzo[b][1,4]oxazines and 3-aryl-2H-benzo[b][1,4]thiazin-2-ones: An ultrasonication-assisted strategy. J. Heterocycl. Chem. 2021, 58, 1541–1545. [Google Scholar] [CrossRef]
- Borra, S.; Chae, S.; Kim, H.Y.; Oh, K. Continuous flow synthesis of 1,4-benzothiazines using ambivalent reactivity of (E)-β-chlorovinyl ketones: A point of reaction control enabled by flow chemistry. Org. Lett. 2022, 24, 5287–5292. [Google Scholar] [CrossRef]
- Qi, Y.; Gu, X.; Huang, X.; Shen, G.; Yang, B.; He, Q.; Xue, Z.; Du, M.; Shi, L.; Yu, B. Microwave-assisted controllable synthesis of 2-acylbenzothiazoles and bibenzo[b][1,4]thiazines from aryl methyl ketones and disulfanediyldianilines. Chin. Chem. Lett. 2021, 32, 3544–3547. [Google Scholar] [CrossRef]
- Mal, P.; Choudhuri, K.; Pramanik, M. Noncovalent interactions in C-S bond formation reactions. J. Org. Chem. 2020, 85, 11997–12011. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, Z.; Xu, J. Synthesis of 1,4-benzothiazines via KI/DMSO/O2-mediated three-component oxidative cyclization/coupling. Adv. Synth. Catal. 2020, 362, 1988–1992. [Google Scholar] [CrossRef]
- Gataullin, R.R. Advances in the synthesis of benzo-fused spiro nitrogen heterocycles: New approaches and modification of old strategies. Helv. Chim. Acta 2020, 103, e2000137. [Google Scholar] [CrossRef]
- Tret’yakov, N.A.; Dmitriev, M.V.; Maslivets, A.N. Synthesis of spiro[1,4-benzothiazine-2,2′-pyrroles] by the reaction of pyrrolo[2,1-c][1,4]oxazinetriones with 2-aminobenzenethiol. Russ. J. Org. Chem. 2020, 56, 935–938. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Ivanova, V.V.; Klimova, T.A.; Kolotyrkina, N.G.; Bastrakov, M.A. Synthesis of new pyrido[3,2-b][1,4]benzoxazines and -benzothiazines. Russ. Chem. Bull. 2022, 71, 126–130. [Google Scholar] [CrossRef]
- Mor, S.; Khatri, M. Convenient synthesis of benzothiazinoisoindol-11-ones and benzoindenothiazin-11-ones, and antimicrobial testing thereof. Mol. Divers. 2023, 27, 1223–1241. [Google Scholar] [CrossRef]
- de Jesus, M.P.; Echemendía, R.; Burtoloso, A.C.B. The α-alkylation of carbonyl sulfoxonium ylides: Studies and applications in the synthesis of new sulfur heterocycles. Org. Chem. Front. 2023, 10, 3577–3584. [Google Scholar] [CrossRef]
- Deeksha, N.; Sathish, E.; Kiran, N.; Singh, R. Access to sterically hindered thioethers (α-thioamides) under mild conditions using α-halohydroxamates: Application toward 1,4-benzothiazinones and 4,1-benzothiazepinones. J. Org. Chem. 2023, 88, 901–908. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Du, M.; Zhang, J.; Gu, Y.; Vaccaro, L.; Liu, P. I2/K2S2O8-Promoted ring-opening cyclizations of benzothiazoles and 3-oxo-3-arylpropanenitriles. Mol. Catal. 2022, 517, 112051. [Google Scholar] [CrossRef]
- Jiang, J.; Tuo, X.; Fu, Z.; Huang, H.; Deng, G.J. Three-component synthesis of 1,4-benzothiazines via iodide-catalyzed aerobic C–H sulfuration with elemental sulfur. Org. Biomol. Chem. 2020, 18, 3234–3238. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Crescenzi, O.; d’Ischia, M.; Napolitano, A. A cyanine-type homolog of the red hair bibenzothiazine chromophore combining reversible proton-sensing with a hydrophobic-to-hydrophilic switching response. Dyes Pigm. 2022, 197, 109872. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; d’Ischia, M.; Napolitano, A. Bioinspired heterocyclic partnership in a cyanine-type acidichromic chromophore. Molecules 2020, 25, 3817. [Google Scholar] [CrossRef]
- Ranathunge, T.A.; Curiac, C.; Green, K.A.; Kolodziejczyk, W.; Hill, G.; Morgan, S.; Delcamp, J.H.; Watkins, D.L. Heteroacene-based polymer with fast-switching visible–near infrared electrochromic behavior. ACS Appl. Mater. Interfaces 2023, 15, 7217–7226. [Google Scholar] [CrossRef] [PubMed]
- Ranathunge, T.A.; Yaddehige, M.L.; Varma, J.H.; Smith, C.; Nguyen, J.; Owolabi, I.; Kolodziejczyk, W.; Hammer, N.I.; Hill, G.; Flynt, A.; et al. Heteroacene-based amphiphile as a molecular scaffold for bioimaging probes. Front. Chem. 2021, 9, 729125. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.T.; Wang, B.; Fan, P.; Duan, R.; Cao, X.; Zhu, L.; Wang, S. A novel benzothiazine-fused coumarin derivative for sensing hypochlorite with high performance. Dyes Pigm. 2020, 182, 108675. [Google Scholar] [CrossRef]
- Brooks, J.; Everett, J.; Lermyte, F.; Tjhin, V.T.; Banerjee, S.; O’Connor, P.B.; Morris, C.M.; Sadler, P.J.; Telling, N.D.; Collingwood, J.F. Label-free nanoimaging of neuromelanin in the brain by soft X-ray spectromicroscopy. Angew. Chem. Int. Ed. 2020, 59, 11984–11991. [Google Scholar] [CrossRef]
- Luo, Z.; Cao, B.; Song, T.; Xing, Z.; Ren, J.; Wang, Z. Visible-light organophotoredox-mediated [3 + 2] cycloaddition of arylcyclopropylamine with structurally diverse olefins for the construction of cyclopentylamines and spiro[4. n] skeletons. J. Org. Chem. 2022, 87, 15511–15529. [Google Scholar] [CrossRef]
- Fischer, D.M.; Freis, M.; Amberg, W.M.; Lindner, H.; Carreira, E.M. Organophotocatalytic carbo-heterofunctionalization of unactivated olefins with pendant nucleophiles. Chem. Sci. 2023, 14, 7256–7261. [Google Scholar] [CrossRef]
- Ali, M.F.B.; Kishikawa, N.; Kuroda, N. Development of HPLC method for estimation of glyoxylic acid after pre-column fluorescence derivatization approach based on thiazine derivative formation: A new application in healthy and cardiovascular patients’ sera. J. Chromatogr. B 2020, 1143, 122054. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, G. Synthesis, structures and properties of angular cis-benzothiazinophenothiazine derivatives. ChemistrySelect 2021, 6, 4312–4318. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Wen, Z.; Feng, X.; Li, K. Influence of sulfur atoms on TADF properties from through-space charge transfer excited states. Chem. Eur. J. 2022, 28, e202202305. [Google Scholar] [CrossRef] [PubMed]
- Ajani, O.O. Functionalized 1,4-benzothiazine: A versatile scaffold with diverse biological properties. Arch. Pharm. Pharm. Med. Chem. 2012, 345, 841–851. [Google Scholar] [CrossRef]
- Fringuelli, R.; Schiaffella, F.; Vecchiarelli, A. Antifungal and immunomodulating activities of 1,4-benzothiazine azole derivatives: Review. J. Chemother. 2001, 13, 9–14. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, M.L.; Panzella, L. The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue. Molecules 2023, 28, 6237. https://doi.org/10.3390/molecules28176237
Alfieri ML, Panzella L. The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue. Molecules. 2023; 28(17):6237. https://doi.org/10.3390/molecules28176237
Chicago/Turabian StyleAlfieri, Maria Laura, and Lucia Panzella. 2023. "The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue" Molecules 28, no. 17: 6237. https://doi.org/10.3390/molecules28176237
APA StyleAlfieri, M. L., & Panzella, L. (2023). The Multifaceted Opportunities Provided by the Pheomelanin-Inspired 1,4-Benzothiazine Chromophore: A Still-Undervalued Issue. Molecules, 28(17), 6237. https://doi.org/10.3390/molecules28176237






