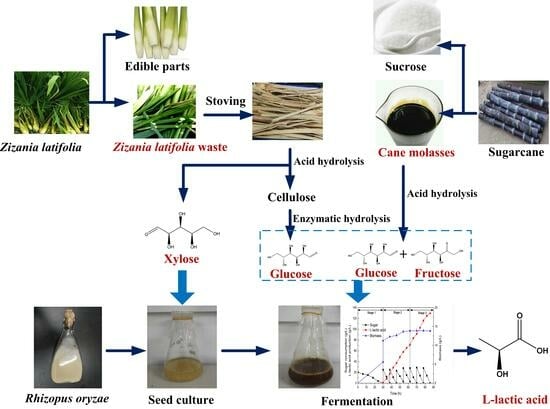
Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Different Carbon Sources on Biomass and LA Production
2.2. Z. latifolia Waste as Carbon Source for LA Fermentation
2.2.1. Effect of Z. latifolia Waste Acid Hydrolysate on Cell Growth
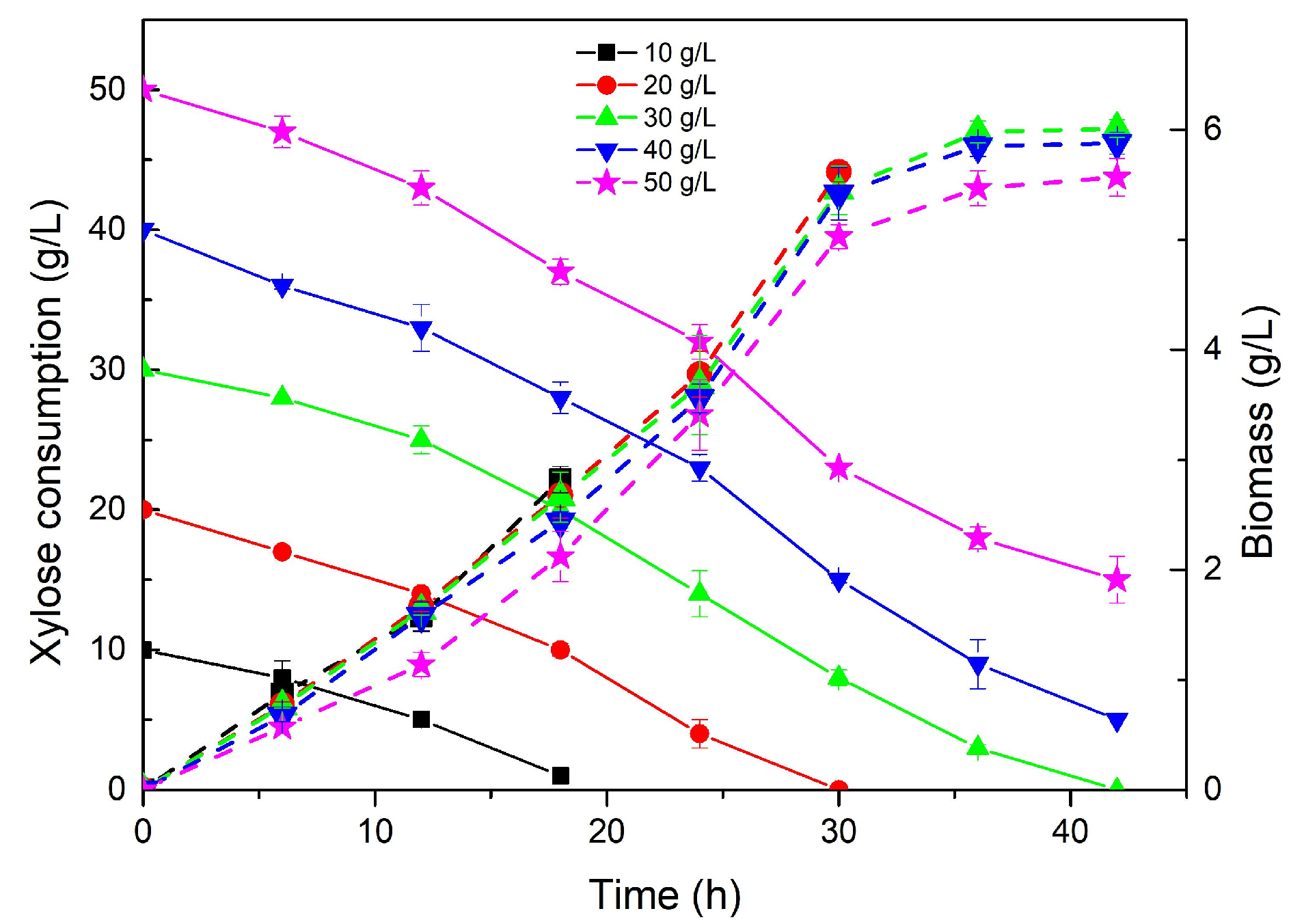
2.2.2. LA Fermentation Using Seed Cultures Grown Using Xylose from Z. latifolia Waste Acid Hydrolysate
2.2.3. Effect of Enzymatic Z. latifolia Waste Hydrolysate on LA Fermentation
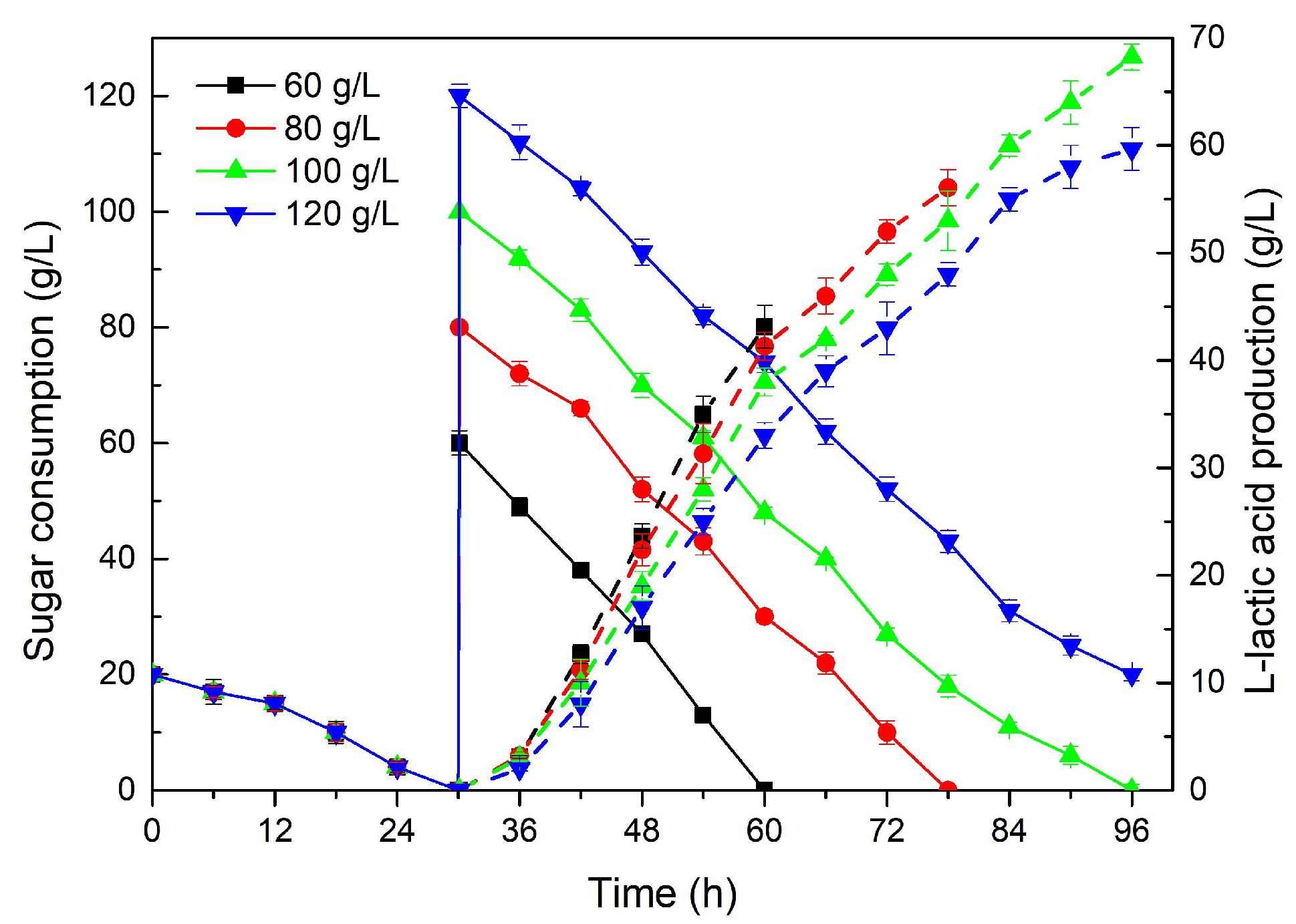
2.3. Waste Cane Molasses as Carbon Source for LA Fermentation
2.4. Multi-Stage Carbon Source Feeding Strategy
2.5. Cost Analysis of Carbon Sources
3. Materials and Methods
3.1. Microorganism
3.2. Fermentation
3.2.1. Medium
3.2.2. Fermentation Protocol
3.3. Carbon Sources and Pretreatment
3.3.1. Zizania latifolia Waste
3.3.2. Cane Molasses
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From natural environment to animal tissues: A review of microplastics(nanoplastics) translocation and hazards studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)—Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Xue, Y.F.; Yu, B.; Wang, L.M.; Zhou, C.; Ma, Y.H. A Review of the Recent Developments in the Bioproduction of Polylactic Acid and Its Precursors Optically Pure Lactic Acids. Molecules 2021, 26, 6446. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.d.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Fu, Y.-Q.; Yin, L.-F.; Zhu, H.-Y.; Jiang, R. High-efficiency l-lactic acid production by Rhizopus oryzae using a novel modified one-step fermentation strategy. Bioresour. Technol. 2016, 218, 410–417. [Google Scholar] [CrossRef]
- Shahri, S.Z.; Vahabzadeh, F.; Mogharei, A. Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioprocess Biosyst. Eng. 2020, 43, 333–345. [Google Scholar] [CrossRef]
- Zain, N.A.M.; Aziman, S.N.; Suhaimi, M.S.; Idris, A. Optimization of L (+) lactic acid production from solid pineapple waste (spw) by Rhizopus oryzae NRRL 395. J. Polym. Environ. 2021, 29, 230–249. [Google Scholar] [CrossRef]
- Rodríguez-Torres, M.; Romo-Buchelly, J.; Orozco-Sánchez, F. Effects of oxygen transfer rate on the L (+) lactic acid production by Rhizopus oryzae NRRL 395 in stirred tank bioreactor. Biochem. Eng. J. 2022, 187, 108665. [Google Scholar] [CrossRef]
- Derabli, B.; Nancib, A.; Nancib, N.; Aníbal, J.; Raposo, S.; Rodrigues, B.; Boudrant, J. Opuntia ficus indica waste as a cost effective carbon source for lactic acid production by Lactobacillus plantarum. Food Chem. 2022, 370, 131005. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Mazzoli, R. Current progress in production of building-block organic acids by consolidated bioprocessing of lignocellulose. Fermentation 2021, 7, 248. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Liu, R.; Wu, W.; Mu, H.; Han, Y.; Yang, H.; Gao, H. Ameliorating effects of water bamboo shoot (Zizania latifolia) on acute alcoholism in a mice model and its chemical composition. Food Chem. 2022, 378, 132122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Y.; He, T.; You, L.; Shen, X. Assessment of potential capability of water bamboo leaves on the adsorption removal efficiency of cationic dye from aqueous solutions. J. Polym. Environ. 2016, 24, 148–158. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Chu, C.; Shi, J.; Zhang, H.; Liu, Y.; Zhang, Z. Morphological characteristics, nutrients, and bioactive compounds of Zizania latifolia, and health benefits of its seeds. Molecules 2018, 23, 1561. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kong, J.; Wang, W.; Zhang, C.; Niu, S.; Gao, B. Study on Fe(III) and Mn(II) modified activated carbons derived from Zizania latifolia to removal basic fuchsin. Desalination 2012, 286, 268–276. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Du, Y.; Tian, H.; Zhan, W.; Zhang, T.C. Treatment of soda ash chromite ore processing residue by Waste-Molasses-Based Ball Milling: A new strategy for disposal of waste with waste. J. Clean. Prod. 2022, 374, 133981. [Google Scholar] [CrossRef]
- Yin, F.-W.; Zhu, S.-Y.; Guo, D.-S.; Ren, L.-J.; Ji, X.-J.; Huang, H.; Gao, Z. Development of a strategy for the production of docosahexaenoic acid by Schizochytrium sp from cane molasses and algae-residue. Bioresour. Technol. 2019, 271, 118–124. [Google Scholar] [CrossRef]
- Jamir, L.; Kumar, V.; Kaur, J.; Kumar, S.; Singh, H. Composition, valorization and therapeutical potential of molasses: A critical review. Environ. Technol. Rev. 2021, 10, 131–142. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef] [PubMed]
- Bulut, S.; Elibol, M.; Ozer, D. Effect of different carbon sources on l(+) -lactic acid production by Rhizopus oryzae. Biochem. Eng. J. 2004, 21, 33–37. [Google Scholar] [CrossRef]
- Taskin, M.; Esim, N.; Ortucu, S. Efficient production of l-lactic acid from chicken feather protein hydrolysate and sugar beet molasses by the newly isolated Rhizopus oryzae TS-61. Food Bioprod. Process. 2012, 90, 773–779. [Google Scholar] [CrossRef]
- Lv, X.; Song, J.; Yu, B.; Liu, H.; Li, C.; Zhuang, Y.; Wang, Y. High-throughput system for screening of high l-lactic acid-productivity strains in deep-well microtiter plates. Bioprocess Biosyst. Eng. 2016, 39, 1737–1747. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yang, S.-T.; Wang, R.; Ren, H. A novel honeycomb matrix for cell immobilization to enhance lactic acid production by Rhizopus oryzae. Bioresour. Technol. 2010, 101, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Kim, J.-H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Luo, J.; Wan, Y. Exploring the potential of lactic acid production from lignocellulosic hydrolysates with various ratios of hexose versus pentose by Bacillus coagulans IPE22. Bioresour. Technol. 2018, 261, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.H.W.; Bakker, R.R.; Eggink, G.; Weusthuis, R.A. Lactic acid production from xylose by the fungus Rhizopus oryzae. Appl. Microbiol. Biotechnol. 2006, 72, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.H.W.; Springer, J.; Eggink, G.; Weusthuis, R.A. Xylose metabolism in the fungus Rhizopus oryzae: Effect of growth and respiration on l (+)-lactic acid production. J. Ind. Microbiol. Biotechnol. 2008, 35, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Anh, P.N.; Okuda, N. Bioconversion of waste office paper to l(+)-lactic acid by the filamentous fungus Rhizopus oryzae. Bioresour. Technol. 2004, 93, 77–83. [Google Scholar] [CrossRef]
- Vially, G.; Marchal, R.; Guilbert, N. L(+) Lactate production from carbohydrates and lignocellulosic materials by Rhizopus oryzae UMIP 4.77. World J. Microbiol. Biotechnol. 2010, 26, 607–614. [Google Scholar] [CrossRef]
- Ibarruri, J.; Hernández, I. Rhizopus oryzae as fermentation agent in food derived sub-products. Waste Biomass Valorization 2018, 9, 2107–2115. [Google Scholar] [CrossRef]
- Pimtong, V.; Ounaeb, S.; Thitiprasert, S.; Tolieng, V.; Sooksai, S.; Boonsombat, R.; Tanasupawat, S.; Assabumrungrat, S.; Thongchul, N. Enhanced effectiveness of Rhizopus oryzae by immobilization in a static bed fermentor for l-lactic acid production. Process Biochem. 2017, 52, 44–52. [Google Scholar] [CrossRef]
- Ajala, E.O.; Olonade, Y.O.; Ajala, M.A.; Akinpelu, G.S. Lactic Acid Production from Lignocellulose—A Review of Major Challenges and Selected Solutions. ChemBioEng Rev. 2020, 7, 38–49. [Google Scholar] [CrossRef]
- Lu, Y.; Xing, S.; He, L.; Li, C.; Wang, X.; Zeng, X.; Dai, Y. Characterization, high-density fermentation, and the production of a directed vat set starter of Lactobacilli used in the food industry: A review. Foods 2022, 11, 3063. [Google Scholar] [CrossRef]
- Chang, H.; Wohlschlager, L.; Csarman, F.; Ruff, A.; Schuhmann, W.; Scheiblbrandner, S.; Ludwig, R. Real-Time Measurement of Cellobiose and Glucose Formation during Enzymatic Biomass Hydrolysis. Anal. Chem. 2021, 93, 7732–7738. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, M.; Yin, Z.; Zhu, W.; Liu, S.; Wang, Q. Lactic acid and animal feeds production from Sophora flavescens residues by Rhizopus oryzae fermentation. Process Biochem. 2020, 92, 401–408. [Google Scholar] [CrossRef]
- Xu, K.; Xu, P. Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresour. Technol. 2014, 153, 23–29. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Jiang, Y.; Chen, F. Molasses-based growth and production of oil and astaxanthin by Chlorella zofingiensis. Bioresour. Technol. 2012, 107, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Deng, T.T.; Cao, W.F.; Shen, F.; Liu, S.J.; Zhang, J.; Liang, X.Q.; Wan, Y.H. Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridium ljungdahlii by an Integrated Fermentation and Membrane Separation Process. Molecules 2022, 27, 954. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wang, Y.; Sun, Z. Butanol Production from Cane Molasses by Clostridium saccharobutylicum DSM 13864: Batch and Semicontinuous Fermentation. Appl. Biochem. Biotechnol. 2012, 166, 1896–1907. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Zhu, H.; Jiang, R.; Luo, X.; Yin, L. An optimized fed-batch culture strategy integrated with a one-step fermentation improves L-lactic acid production by Rhizopus oryzae. World J. Microbiol. Biotechnol. 2018, 34, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yoshida, M.; Vadlani, P.V. Biosynthesis of D-lactic acid from lignocellulosic biomass. Biotechnol. Lett. 2018, 40, 1167–1179. [Google Scholar] [CrossRef]
- Freudenberg, R.A.; Baier, T.; Einhaus, A.; Wobbe, L.; Kruse, O. High cell density cultivation enables efficient and sustainable recombinant polyamine production in the microalga Chlamydomonas reinhardtii. Bioresour. Technol. 2021, 323, 124542. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, B. L(+)-lactic acid production using sugarcane molasses and waste potato starch: An alternative approach. Int. Sugar J. 2010, 112, 17–22. [Google Scholar]
- Salvañal, L.; Clementz, A.; Guerra, L.; Yori, J.C.; Romanini, D. l-lactic acid production using the syrup obtained in biorefinery of carrot discards. Food Bioprod. Process. 2021, 127, 465–471. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zhang, J.; Pan, J. Using tobacco waste extract in pre-culture medium to improve xylose utilization for l-lactic acid production from cellulosic waste by Rhizopus oryzae. Bioresour. Technol. 2016, 218, 344–350. [Google Scholar] [CrossRef]
- Dhandapani, B.; Vishnu, D.; Murshid, S.; Ram, P.A.; Muruganandh, R.; Prasanth, D.; Sekar, S.; Senthilkumar, K. Production of lactic acid from industrial waste paper sludge using Rhizopus oryzae MTCC5384 by simultaneous saccharification and fermentation. Chem. Eng. Commun. 2021, 208, 822–830. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ajala, M.A.; Onoriemu, O.O.; Akinpelu, S.G.; Bamidele, S.H. Lactic acid production: Utilization of yam peel hydrolysate as a substrate using Rhizopus orysae in kinetic studies. Biofuels Bioprod. Biorefining 2021, 15, 1031–1045. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yong, Q.; Yang, S.-T.; Ouyang, J.; Yu, S. Simultaneous saccharification and fermentation of xylo-oligosaccharides manufacturing waste residue for l-lactic acid production by Rhizopus oryzae. Biochem. Eng. J. 2015, 94, 92–99. [Google Scholar] [CrossRef]
- Yin, F.; Sun, X.; Fu, Y.; Zheng, W.; Luo, X.; Zhang, Y.; Yin, L.; Jia, Q.; Kong, Y. A Method for Domestication of Rhizopus oryzae and Production of Lactic Acid. Chinese Patent 202011401137.X, 2 December 2020. (In Chinese). [Google Scholar]
- Ling, R.; Wei, W.; Jin, Y. Pretreatment of sugarcane bagasse with acid catalyzed ethylene glycol–water to improve the cellulose enzymatic conversion. Bioresour. Technol. 2022, 361, 127723. [Google Scholar] [CrossRef] [PubMed]



| Seed Culture | L-Lactic Acid Fermentation | |||
|---|---|---|---|---|
| Xylose Concentration (g/L) | Culture Time (h) | Biomass (g/L) | Fermentation Time (h) | L-Lactic Acid Production (g/L) |
| 10 | 18 | 2.28 | 48 | 18.25 |
| 20 | 30 | 5.62 | 42 | 23.05 |
| 30 | 42 | 6.01 | 42 | 22.78 |
| 40 | 42 | 5.88 | 42 | 20.33 |
| 50 | 42 | 5.57 | 42 | 20.12 |
| Glucose Concentration (g/L) | Glucose Consumption (g/L) | Fermentation Time (h) | L-Lactic Acid Production (g/L) | Productivity (g/L·h) | Yield (g/g) |
|---|---|---|---|---|---|
| 60 | 60 | 60 | 42.12 | 0.70 | 0.70 |
| 80 | 80 | 78 | 53.36 | 0.68 | 0.67 |
| 100 | 94 | 96 | 55.14 | 0.57 | 0.58 |
| 120 | 90 | 96 | 46.47 | 0.48 | 0.52 |
| Carbon Source | Sugar Consumption (g/L) | Biomass (g/L) | Fermentation Time (h) | L-Lactic Acid Production (g/L) | Productivity (g/L·h) | Yield (g/g) |
|---|---|---|---|---|---|---|
| Untreated molasses | 40 | 5.11 | 54 | 25.21 | 0.46 | 0.50 |
| Treated molasses | 50 | 5.89 | 54 | 36.76 | 0.68 | 0.74 |
| Sugar Concentration (g/L) | Sugar Consumption (g/L) | Fermentation Time (h) | L-Lactic Acid Production (g/L) | Productivity (g/L·h) | Yield (g/g) |
|---|---|---|---|---|---|
| 60 | 60 | 60 | 43.15 | 0.72 | 0.72 |
| 80 | 80 | 78 | 56.07 | 0.72 | 0.70 |
| 100 | 100 | 96 | 68.24 | 0.71 | 0.68 |
| 120 | 100 | 96 | 59.66 | 0.62 | 0.60 |
| Strain | Substrate | Concentration (g/L) | Yield (g/g) | Productivity (g/L·h) | References |
|---|---|---|---|---|---|
| R. arrhizus | Waste potato starch | 103.8 | - | 2.16 | [48] |
| R. arrhizus | Syrup of carrot discards | 22.18 | 0.79 | 0.31 | [49] |
| R. oryzae UMIP 4.77 | Wheat straw | 10 | 0.26 | 0.27 | [34] |
| R. oryzae | Tobacco waste water extract and glucose | 173.5 | 0.86 | 1.45 | [50] |
| R. oryzae | Cassava pulp hydrolysates | 75.28 | 0.5 | 1.05 | [36] |
| R. oryzae 3.819 | Sophora flavescens residues | 46.78 | - | 0.97 | [40] |
| R. oryzae MTCC5384 | Industrial waste paper sludge | 27 | - | - | [51] |
| R. orysae | Yam peel hydrolysate | 64.02 | 0.80 | - | [52] |
| R. oryzae NLX-M-1 | Xylo-oligosaccharides Manufacturing waste residue | 60.3 | 0.6 | 1.0 | [53] |
| R. oryzae LA-UN-1 | Zizania latifolia waste and cane molasses | 129.47 | 0.72 | 1.51 | This study |
| Pure Glucose | Z. latifolia Waste | Cane Molasses | Total Cost $ | L-Lactic Acid kg | L-Lactic Acid Cost $/t | |
|---|---|---|---|---|---|---|
| Price ($/t) | 550 | 0 | 140 | |||
| Conditional fermentation (kg) | 220 | 0 | 0 | 121 | 160 | 756.25 |
| Novel fermentation (kg) | 0 | 298 | 212 | 29.68 | 129.47 | 229.24 |
| Cost saving (%) | 69.69 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.-W.; Sun, X.-L.; Zheng, W.-L.; Yin, L.-F.; Luo, X.; Zhang, Y.-Y.; Wang, Y.-F.; Fu, Y.-Q. Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources. Molecules 2023, 28, 6234. https://doi.org/10.3390/molecules28176234
Yin F-W, Sun X-L, Zheng W-L, Yin L-F, Luo X, Zhang Y-Y, Wang Y-F, Fu Y-Q. Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources. Molecules. 2023; 28(17):6234. https://doi.org/10.3390/molecules28176234
Chicago/Turabian StyleYin, Feng-Wei, Xiao-Long Sun, Wei-Long Zheng, Long-Fei Yin, Xi Luo, Ying-Ying Zhang, Yan-Fei Wang, and Yong-Qian Fu. 2023. "Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources" Molecules 28, no. 17: 6234. https://doi.org/10.3390/molecules28176234
APA StyleYin, F.-W., Sun, X.-L., Zheng, W.-L., Yin, L.-F., Luo, X., Zhang, Y.-Y., Wang, Y.-F., & Fu, Y.-Q. (2023). Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources. Molecules, 28(17), 6234. https://doi.org/10.3390/molecules28176234