Abstract
Citrullus colocynthis (L.) Schrad. (Cucurbitaceae) is widely distributed in the desert areas of the world. The fruit bodies of C. colocynthis are recognized for their wide range of nutraceutical potential, as well as medicinal and pharmaceutical uses. The plant has been reported for various uses, such as asthma, bronchitis, cancer, colic, common cold, cough, diabetes, dysentery, and jaundice. The fruit has been extensively studied for its biological activities, which include insecticide, antitumor, and antidiabetic effects. Numerous bioactive compounds have been reported in its fruit bodies, such as essential oils, fatty acids, glycosides, alkaloids, and flavonoids. Of these, flavonoids or caffeic acid derivatives are the constituents associated with the inhibition of fungal or bacterial growth, whereas eudesmane sesquiterpenes or sesquiterpene lactones are most active against insects, mites, and nematodes. In this review, the scientific evidence for the biological activity of C. colocynthis against insecticide, cytotoxic, and antidiabetic effects is summarized.
1. Introduction
The family Cucurbitaceae, with about 123 genera and over 800 species, is found in the tropics or subtropics and is rare in temperate regions. This family of plants is generally frost-sensitive, intolerant to wet and poorly drained soils, and drought-tolerant [1,2]. The well-known members are bitter apples, cucumbers, gourds, pumpkins, and melons. Because of the increasing awareness of the health benefits of this family, their production has increased over time [3]. The genus Citrullus comprises annual or perennial herbaceous plants in the family Cucurbitaceae. This genus includes four species (C. rehmii De Winter, C. lanatus (Thunb.) Matsum. and Nakai, C. ecirrhosus Cogn., and C. colocynthis (L.) Schrad.) throughout tropical and South Africa, Southwest Asia, and the East Mediterranean region [4].
C. colocynthis (L.) Schrad., distributed in the desert areas of the world, including Sudan, Morocco, Jordan, Tunisia, and Pakistan, has nutraceutical and medicinal values [5,6,7,8,9,10,11,12]. The fruits are locally called Kattu Kattuvellari in Malayalam, colocynth/bitter in English, Pcitummatti in Tamil, Rakhal in Bengali, Anedri in Sanskrit, Indrayan in Hindi, and Hanjal in Urdu [2]. The plant is a traditional medicine used to treat asthma, jaundice, and diabetes. Recently, lots of studies have been conducted on its phytochemical compounds, pharmacology, and toxicology [13,14,15,16,17,18]. To date, there is no related review that focuses on the aspects of its insecticidal, antitumor, and antidiabetic effects. This review provides an overview of the habitat and insecticide, antitumor, and antidiabetic activities of C. colocynthis.
2. Results
2.1. Habitat
C. colocynthis is a valuable plant in the family Cucurbitaceae. This plant is widely distributed in the Arabian and Sahara deserts, Sudan, and southern parts of Asia, including India, Southern Islands, and Pakistan [19,20] (Figure 1). The fruits were introduced to Spain and Cyprus by Arabs in the middle ages [21]. C. colocynthis is a perennial herbaceous vine that produces small flowers. The stem is rough, coarsely hairy, and angular. The leaves of this plant are alternately arranged on petioles and rough to the touch, measuring 1.5–2.0 cm in width and 5.0–10.0 cm in length. C. colocynthis has pale yellow and solitary flowers. The flowers are on the axils of the leaves and are yellow. They are single, pedunculated, and monecious. Each plant produces 15–30 fruits. The fruit bodies measure 7.0–10.0 cm in diameter and are green in color with undulating yellow stripes that turn yellow when dried. The fruit is globular and bitter with a smooth texture. Also, the fruit is hard and contains approximately 250 seeds/gourd. The seeds of this plant are 6 mm long, brownish, smooth, compressed, and ovoid when ripe.
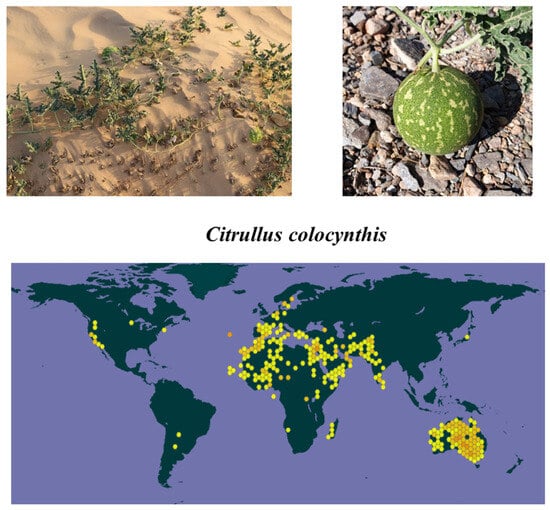
Figure 1.
The whole plant of C. colocynthis, including its fruits, as well as its geographical distribution (data from the Global Biodiversity Information Facility, ”https://www.gbif.org/”).
2.2. Traditional Uses
C. colocynthis is widely used in many parts of the world for a number of diseases including mastitis, cancer, joint pain, jaundice, bronchitis, asthma, leprosy, constipation, and diabetes [2,5,6,7]. The clinical uses have been reported in indigenous systems of medicine in tropical and subtropical countries (Sudan, Morocco, Jordan, Tunisia, and Pakistan), which include its uses in gut disorders, such as gastroenteritis, dysentery, indigestion, and colic pain, as well as diabetes, wounds, toothache, cough, and the common cold [4,20,22,23].
In Saudi Arabia, much of the local population knows that the squeezed fruit extracts are used to elicit its purgative action, which can be achieved by treading on the fruits barefoot [5,24]. The population of Northeastern Morocco has used this plant since time immemorial to treat various cardiovascular system diseases [6]. In East Africa, seed tar is applied to the skin by nomads. But, the digestion of this fruit results in acute toxic colitis, bloody diarrhea, and changes in the colon [25]. In southern Punjab, Pakistan, the dry powder of the fruits mixed with jaggery is used in a physic drench ball [7,11,19]. In Jordan, swallowing fresh seeds, locally known as Handal, is used to treat various health issues, including its use as an abortive agent, a cathartic, a diuretic, as well as arthritis and rheumatism [8,12,26]. In southern Tunisia, C. colocynthis is a useful medicine for gout, arthritis, and inflammatory disorders. But, overdosing on the plant’s immature fruit is a hazard. Intoxication manifests via cerebral congestions, hypothermia, delirium, gastrointestinal irritations, and colitis [9]. In the Sariska region, the fruits are used to treat fever, general sickness, and obstructive stomachache [27]. In Eastern, Central, and Southern Iran, the fruit is recognized as an antiepileptic, abortifacient, hair growth promoter, analgesic, antidiabetic, and purgative. Adverse events (i.e., vomiting, hematochezia, diarrhea, and colic) have been associated with the use of this plant [1,28]. In Israel, the seed oils and fruits of C. colocynthis have been used as a laxative [2].
2.3. Phytochemical Analysis
The compounds of C. colocynthis include alkaloids, flavonoids, coumarins, steroids, and phenolic acids. The names and isolated parts of the compounds are listed in Table 1, along with the analytical methods.

Table 1.
A comprehensive list of the chemical compounds from C. colocynthis.
Cucurbitacins are the main constituents of this species. The serial compounds are bitter-tasting, mainly tetracyclic, highly oxygenated, derived from skeletons [19-(10→9β)-abeo-10α-lanost-5-en]. There are 12 classes of cucurbitacins according to their structure, but not all of them are present in C. colocynthis. Yoshikawa and Zheng et al. [4,32] systematically reported the cucurbitacins (triterpenoids and their glycosides) from this species (such as cucurbitacins A–L, and cucurbitacin E 2-O-β-D-glucopyranoside). Among these cucurbitacins, cucurbitacin E is the main component in C. colocynthis fruit pulp, while compound 10 is detected as the principal cucurbitacin of the fruits [29]. Besides cucurbitacins, preliminary phytochemical screening of this species shows the presence of alkaloids, flavonoids, and phenolic acids (Figure 2). Twelve alkaloids, including quinoline, nicotinamide, uracil, 2-hydroxyquinoline, 2-methylquinoline, 4-hydroxyquinoline, 4-methylquinoline, 6-hydroxyquinoline, 6-methylquinoline, 7, 8-benzoquinoline, 8-hydroxyquinoline, and 8-methylquinoline, were detected in C. colocynthis fruits [36]. Among these, 4-methylquinoline is an effective natural insecticide for weevils in grain storage and the management of spider mites. Apart from the principal constituents, volatile compounds, ketones, epoxy compounds, hydrocarbons [39], and fatty acids [40] are also detected in C. colocynthis.
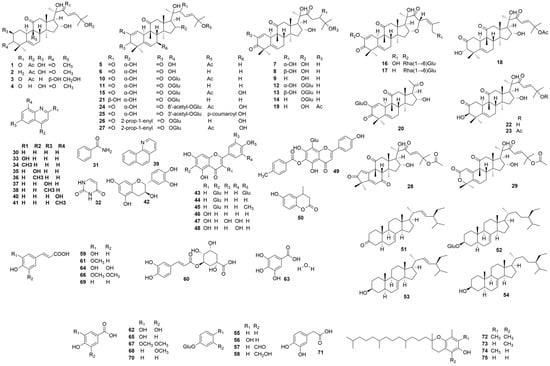
Figure 2.
Structures of the constituents in C. colocynthis.
2.4. Pharmacological Activity of C. colocynthis
2.4.1. Insecticidal Activity
C. colocynthis is used for insecticidal activities in many countries [37,41,42,43,44]. In the results of Ahmed et al., the leaf extract of C. colocynthis was exceptional at controlling Brevicoryne brassicae L. (cabbage aphid). Cucurbitacin E [45] and spinasterol [14], isolated from this species, show strong insecticidal effects against Aphis craccivora. Chawech et al. [46] reported that the ethyl acetate and pure compounds (compounds 5 and 10) showed significant larvicidal activities against Galba truncatula (mollusc gastropod) with the deterioration rate exceeding 89.2% and with no toxic effects against associated fauna (Melanoides tuberculate, Aromia moshata, Hydrophilus triangularis, and Athous haemorhoidalis). Elazab et al. [47] indicated that the methanol extracts of C. colocynthis were active against Toxoplasma gondii (an Apicomplexa intracellular protozoan) with an IC50 of 22.86 μg/mL. In Pakistan, the fruits, in combination with common or black salt, are used to treat lice infestation [48] and helminthiasis [7]. The methanol extract displays potent antimalarial activity against multidrug-resistant and chloroquine-sensitive Plasmodium falciparum strains, with no toxicity (IC50 = 6.9 and 2.01 μg/mL, respectively) [17]. The nano-extracts of C. colocynthis are efficient against Trichomonas vaginalis and safer than the drug metronidazole [49].
7,8-Benzoquinoline isolated from fruit bodies is most effective against Tetranychus urticae. Regardless of the application method, quinoline and its structural analogs show insecticidal activities (Sitophilus zeamais and S. oryzae) [36]. Ponsankar et al. [50] reported the screening of cucurbitacin E against different larval instars and analyzed the antifeedant activity using a choice-based test. Petroleum ether shows larvicidal activity against Aedes aegypti L. and Culex quinquefasciatus Say with LC50 values of 74.57 and 88.24 ppm, respectively [41,51,52]. Oleic and linoleic acids are active against Culex quinquefasciatus Say (LC50 values of 7.66 and 27.24 ppm, respectively), Anopheles stephensi Liston (LC50 values of 9.79 and 11.49 ppm, respectively), and Aedes aegypti L. (LC50 value of 8.80 and 18.20 ppm, respectively) 53]. Figure 3 briefly summarizes the medicinal sites or pure compounds with insecticide activity in C. colocynthis, and its detailed insecticide information for different species is listed in Table 2.
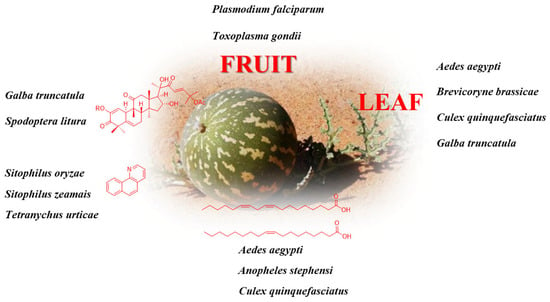
Figure 3.
The insecticide activity of C. colocynthis.

Table 2.
Insecticidal activity of extracts or pure compounds from C. colocynthis.
2.4.2. Cytotoxic Activity
In the professional literature, researchers have reported the antitumor activity of extracts and isolates from this species. Among them, antiproliferative effects have been observed via signaling pathways, including apoptotic pathways (inhibiting STAT3 function and increasing caspase-3) [54,55,56,57,58]. The plant extract increases the number of apoptotic cells and the proportion of sub-G1 cells [59]. The extract also promotes DNA damage in breast cancer cells via the ATM/CHK2/p53 signaling pathway (Figure 4). And, the antitumor activity of this species is achieved through cell cycle arrest [60]. The plant extract holds significant antitumor activity through the regulation of lipid metabolism (ELOVL2, ACSL5, HMGCLL1, and FASN) [61].
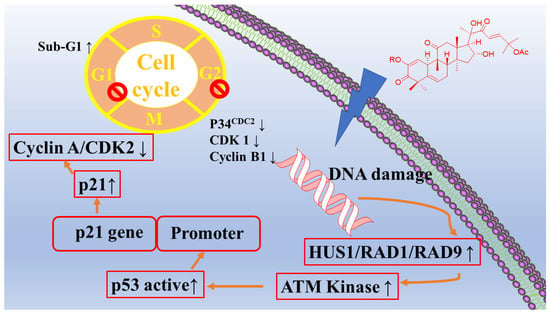
Figure 4.
Cytotoxic activity of C. colocynthis.
Ayyad et al. [23,62] reported that compounds 5 and 11 have potent inhibitory antitumor activities on HepG2, with IC50 values of 3.5 and 2.8 nmol/mL, respectively. The compounds also prolonged the normalization of the biochemical parameters, life span, and survival times of experimental mice. Two researchers showed cucurbitacin E and its analogs present significant cytotoxic activity against human colon cancer cell lines (HL-60, Caco-2, and HT29) [34,35]. An immunoblot analysis by Saeed et al. [63] highlighted that cucurbitacin E targets epidermal growth factor receptors (EGFRs). In a cell cycle analysis, compounds 2 and 10 resulted in the accumulation of breast cell lines (MDA-MB-231) at the G2/M phase [64,65]. In addition to cucurbitacin, linoleic acid, when compared to other oils, exhibits significant antitumor effects against colorectal cancer cells with IC50 values between 4 and 7 mg/mL [66]. Details of the cytotoxic activity of extracts or pure compounds from C. colocynthis are summarized in Table 3.

Table 3.
Cytotoxic activity of extracts or pure compounds from C. colocynthis.
2.4.3. Antidiabetic Activity
Diabetic diseases have side effects (peripheral vascular disease, stroke, nephropathy, neuropathy, and retinopathy) [6,16,24,68,69,70]. The fruit extracts possess insulin-enhancing activity [71]. C. colocynthis could directly reduce the formation of glycated hemoglobin (HbA1c) [13]. Benariba et al. [72,73] reported that a concentration–response correlation was observed with fruit extracts in the modulation of the insulin secretory response to D-glucose. The fruit extracts could lead to an increase in epididymal fat weight and a lesser decrease in body weight [74]. The ethanolic extract of the seeds has antioxidant and DPPH decolorization potential. It also exhibited a time-dependent decrease in blood glucose levels [75]. C. colocynthis seeds display a direct effect on endocrine pancreatic B cells [76].
Diabetes mellitus causes serious complications affecting multiple organs, and the literature reports the positive effects of C. coliformis on diabetes complications. Aqueous extracts of C. colocynthis ameliorate the toxic effects of streptozotocin. Oral administration of the plant extract reduced the plasma levels of aspartate aminotransferase (AST) and lactic dehydrogenase (LDH) significantly [77]. The fruit had a positive effect on the treatment of diabetic neuropathy, decreasing the number of demyelinated and degenerated nerve fibers [78]. The literature also showed the protective effects against cognitive impairments [79], pancreatic β-cell mass [80], liver/kidney [81], and diabetic neuropathic pain [82]. The antidiabetic activity of C. coliformis is summarized in Figure 5 and detailed in Table 4.
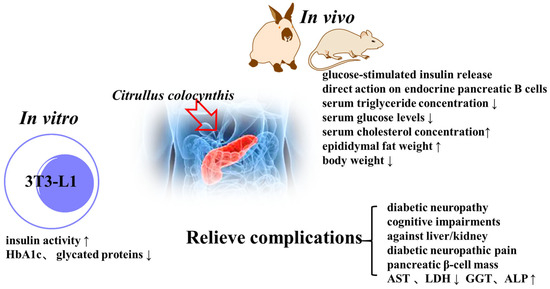
Figure 5.
Antidiabetic activity of C. colocynthis.

Table 4.
Antidiabetic activity of extracts or pure compounds from C. colocynthis.
2.5. Clinical Study
C. colocynthis could have systemic therapeutic effects on type II diabetic patients (40 patients aged 45–65) through dermal absorption. Experiments showed that the extract reduced insulin secretion and blood glucose (BG) levels. It also decreased serum urea levels, but there was no significant change in micro-albuminuria, hepatic enzymes, lipid profiles, creatinine levels, and other related indices [83]. Barghamdi et al. showed that consumption of C. colocynthis extracts in the intervention group significantly reduced mean glycosylated hemoglobin and fasting blood glucose levels and did not show any side effects (≤125 mg/day). These results indicate that the aqueous extracts had hypoglycemic effects on patients with diabetes, which was associated with their saponins and glycosidic components [84]. In the research of Huseini et al. [85], a clinical experiment was conducted on 50 type II diabetic patients for 2 months. In the research of Li’s group, thirty-two type II diabetes patients (ages from 30 to 60) were arranged for this research and distributed into four groups. Capsules of different C. colocynthis extracts were given to patients twice a day for 30 days (1 g per day dosage) and investigated for cholesterol, triglyceride, and glucose levels. C. colocynthis reduced HDL, TGL, cholesterol, and glucose levels by 5, 6, 6, and 35 percent, respectively. From a clinical experiment, it was concluded that powdered C. colocynthis possessed good antidiabetic features [86].
2.6. Toxicity
The fruit of C. colocynthis has been used as a traditional medicine, mostly for mastitis, cancer, joint pain, jaundice, bronchitis, asthma, leprosy, constipation, and diabetes. The ingestion of this fruit, however, may have many undesired effects. The biblical story of non-fatal accidental poisoning (described in The Book of 2 Kings) is a related report of the medical toxicology of C. colocynthis in the Old Testament [87]. Feeding a mixture of Nerium oleander and C. colocynthis caused more marked effects and the death of rats [21]. In 1985, a 37-year-old Saudi man was admitted to a local hospital (Riyadh Armed Forces Hospital) with one episode of vomiting, colicky abdominal pain, and severe bloody diarrhea after he had drunk the fruit extracts of C. colocynthis for self-medication for indigestion. He had fresh bleeding from his rectum, and an examination revealed extreme tenderness and slight tenderness in the lower abdomen [5]. These manifestations may be accompanied by transudate in serous cavities, epicardial fat, gelatinization of renal tissue, and entero-hepato-nephrotoxicity [88].
3. Materials and Methods
This literature review used published scientific materials collected from the Web of Science® and PubMed® databases without restriction regarding the year of publication and includes literature published through July 2023. The search term used was “Citrullus colocynthis (L.) Schrad.”. The chemical names agree with the original references.
4. Conclusions and Future Perspectives
C. colocynthis is a valuable cucurbit plant and is widely distributed in desert regions of the world. Despite its high dietary value, C. colocynthis is not widely known. In our review, we systematically reviewed the research on this traditional medicine and summarized the related data on the phytochemical structure. Phytochemical studies of the species have resulted in 75 components. Of these, cucurbitacins have previously been reported as the main constituent of this species.
Crude extracts or constituents have previously been reported to have diverse pharmacological activities, with a focus on insecticide, cytotoxic, and antidiabetic effects. These surveys provide evidence of the correlations between modern pharmacological functions and ethnomedical applications in traditional Chinese medicine (TCM). Nonetheless, the extraction or composition mechanisms are not well established and merit further investigation. Besides these activities, C. colocynthis had other pharmacological properties, such as immune-stimulatory [89], anti-allergic [32], hypolipidemic [90,91,92], anti-microbial [32,93], reproductive [94,95], gastrointestinal tract [28,29,96], anti-inflammatory [97,98], antibacterial [31,93], and antioxidative [37,55,99] activities. Toxicology experiments and representative animal models should be used to assess their potential therapeutic effects, as mentioned in TCM.
As mentioned above, C. colocynthis contains a variety of chemical components, both non-volatile and volatile. Systematic purification and identification of chemical compositions in C. colocynthis are important. LC-MS and GC-MS is currently the most commonly used characterization technique for quickly and systematically identifying possible non-volatile and volatile components in plants [100,101,102]. However, for co-eluting compounds with similar spectra, an equivocal identification may be obtained. In recent years, some new technologies, such as gas chromatography ion mobility spectrometry (GC-IMS), have been able to provide the retention time of analytes in GC columns and the separation time of ionized compounds in IMS drift tubes, as well as the amount of each ionized compound reaching the detector in the IMS drift tube, which can significantly improve the identification of co-eluting compounds with similar spectra [103]. Therefore, the combination of LC/GC and IMS may be a good solution for the systematic identification of plants with complex chemical compositions, such as C. colocynthis.
Author Contributions
Investigation and data curation, X.C., M.Q., and R.C.; writing—original draft preparation, X.C.; writing—review and editing, Q.Z. and G.C.; visualization, Y.J. and A.W.; supervision and project administration, B.L. and W.R.; funding acquisition, A.W. and W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers: 32200314 and 82071238), the Natural Science Foundation of Jiangsu Province (grant number: BK20200975), and the Nantong Science and Technology Plan Project (grant number: JC12022087).
Institutional Review Board Statement
Not Available.
Informed Consent Statement
Not available.
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Rahimi, R.; Amin, G.; Ardekani, M.R. A review on Citrullus colocynthis Schrad.: From traditional Iranian medicine to modern phytotherapy. J. Altern. Complement. Med. 2012, 18, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.; Chatha, S.A.; Sarker, S.D.; Gilani, A.H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014, 155, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Karim, S.; Wang, C.; Zhao, M.; Murtaza, G. A review on antidiabetic activity of Citrullus colocynthis Schrad. Acta Pol. Pharm. 2014, 71, 363–367. [Google Scholar] [PubMed]
- Zheng, M.; Liu, Y.; Yuan, T.; Liu, L.; Li, Z.; Huang, H. Research progress on chemical constituents of Citrullus colocynthis and their pharmacological effects. Zhongguo Zhong Yao Za Zhi 2020, 45, 816–824. [Google Scholar] [PubMed]
- Al Faraj, S. Haemorrhagic colitis induced by Citrullus colocynthis. Ann. Trop. Med. Parasitol. 1995, 89, 695–696. [Google Scholar] [CrossRef]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of hypertension and diabetes in oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef]
- Jabbar, A.; Raza, M.A.; Iqbal, Z.; Khan, M.N. An inventory of the ethnobotanicals used as anthelmintics in the southern Punjab (Pakistan). J. Ethnopharmacol. 2006, 108, 152–154. [Google Scholar] [CrossRef]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef]
- Marzouk, B.; Marzouk, Z.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef]
- Nawash, O.; Shudiefat, M.; Al-Tabini, R.; Al-Khalidi, K. Ethnobotanical study of medicinal plants commonly used by local Bedouins in the Badia region of Jordan. J. Ethnopharmacol. 2013, 148, 921–925. [Google Scholar] [CrossRef]
- Bibi, T.; Ahmad, M.; Bakhsh Tareen, R.; Mohammad Tareen, N.; Jabeen, R.; Rehman, S.U.; Sultana, S.; Zafar, M.; Yaseen, G. Ethnobotany of medicinal plants in district Mastung of Balochistan province-Pakistan. J. Ethnopharmacol. 2014, 157, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Akour, A.; Kasabri, V.; Afifi, F.U.; Bulatova, N. The use of medicinal herbs in gynecological and pregnancy-related disorders by Jordanian women: A review of folkloric practice vs. evidence-based pharmacology. Pharm. Biol. 2016, 54, 1901–1918. [Google Scholar] [CrossRef] [PubMed]
- Karimabad, M.N.; Niknia, S.; Golnabadi, M.B.; Poor, S.F.; Hajizadeh, M.R.; Mahmoodi, M. Effect of Citrullus colocynthis Extract on Glycated Hemoglobin Formation (In Vitro). Eurasian J. Med. 2020, 52, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Qin, P.; Ji, M.; An, R.; Guo, H.; Shafi, J. Spinasterol, 22,23-Dihydrospinasterol and Fernenol from Citrullus colocynthis L. with Aphicidal Activity against Cabbage Aphid Brevicoryne brassicae L. Molecules 2020, 25, 2184. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Shojaii, A.; Pur, S.A.; Motevalian, M. Anticonvulsant Activity of Hydroalcoholic Extract of Citrullus colocynthis Fruit: Involvement of Benzodiazepine and Opioid Receptors. J. Evid. Based Complement. Altern. Med. 2016, 21, NP31–NP35. [Google Scholar] [CrossRef]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Mahbodfar, H.; Zamani, Z.; Ramazani, A. Antimalarial evaluation of selected medicinal plant extracts used in Iranian traditional medicine. Iran J. Basic Med. Sci. 2017, 20, 415–422. [Google Scholar] [PubMed]
- Li, Q.Y.; Munawar, M.; Saeed, M.; Shen, J.Q.; Khan, M.S.; Noreen, S.; Alagawany, M.; Naveed, M.; Madni, A.; Li, C.X. Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): Promising Traditional Uses, Pharmacological Effects, Aspects, and Potential Applications. Front. Pharmacol. 2021, 12, 791049. [Google Scholar] [CrossRef]
- Aziz, M.A.; Adnan, M.; Khan, A.H.; Shahat, A.A.; Al-Said, M.S.; Ullah, R. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J. Ethnobiol. Ethnomedicine 2018, 14, 2. [Google Scholar] [CrossRef]
- Emami, S.; Sahebkar, A.; Javadi, B. Paresthesia: A Review of Its Definition, Etiology and Treatments in View of the Traditional Medicine. Curr. Pharm. Des. 2016, 22, 321–327. [Google Scholar] [CrossRef]
- Al-Yahya, M.A.; Al-Farhan, A.H.; Adam, S.E.I. Preliminary toxicity study on the individual and combined effects of Citrullus colocynthis and Nerium oleander in rats. Fitoterapia 2000, 71, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evid. Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Efferth, T. Medicinal Plants from Near East for Cancer Therapy. Front. Pharmacol. 2018, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Marwat, S.; Rehman, F.; Khan, E.; Khakwani, A.; Ullah, K.; Khan, K.; Khan, I.A. Useful ethnophytomedicinal recipes of angiosperms used against diabetes in South East Asian Countries (India, Pakistan & Sri Lanka). Pak. J. Pharm. Sci. 2014, 27, 1333–1358. [Google Scholar] [PubMed]
- Abdel-Hassan, I.; Abdel-Barry, J.; Tariq Mohammeda, S. The hypoglycaemic and antihyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J. Ethnopharmacol. 2000, 71, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Aburjai, T.; Hudaib, M.; Tayyem, R.; Yousef, M.; Qishawi, M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007, 110, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, B.; Singh, K.P.; Kumar, A. Ethno-veterinary uses and informants consensus factor of medicinal plants of Sariska region, Rajasthan, India. J. Ethnopharmacol. 2011, 133, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Eftekhari, Z. An ethnoveterinary study of medicinal plants in treatment of diseases and syndromes of herd dog in southern regions of Ilam province, Iran. Comp. Clin. Pathol. 2013, 22, 403–407. [Google Scholar] [CrossRef]
- Adam, S.E.I.; Al-Yahya, M.A.; Al-Farhan, A.H. Response of Najdi sheep to oral administration of Citrullus colocynthis fruits, Nerium oleander leaves or their mixture. Small Rumin. Res. 2001, 40, 239–244. [Google Scholar] [CrossRef]
- Nayab, D.; Ali, D.; Arshad, N.; Malik, A.; Choudhary, M.I.; Ahmed, Z. Cucurbitacin glucosides from Citrullus colocynthis. Nat. Prod. Res. 2006, 20, 409–413. [Google Scholar] [CrossRef]
- Gowri, S.; Priyavardhini, S.; Vasantha, K.; Umadevi, M. Antibacterial activity on Citrullus colocynthis leaf extract. Anc. Sci. Life 2009, 29, 12–13. [Google Scholar] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Kobayashi, H.; Nakamura, A.; Matsuhiri, K.; Nakamura, S.; Matsuda, H. Bioactive Saponins and Glycosides. XXVII. Structures of New Cucurbitane-Type Triterpene Glycosides and Antiallergic Constituents from Citrullus colocynthis. Chem. Pharm. Bull. 2007, 55, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Seger, C.; Sturm, S.; Mair, M.E.; Ellmerer, E.P.; Stuppner, H. 1H and 13C NMR signal assignment of cucurbitacin derivatives from Citrullus colocynthis (L.) Schrader and Ecballium elaterium L. (Cucurbitaceae). Magn. Reson. Chem. 2005, 43, 489–491. [Google Scholar] [CrossRef]
- Chawech, R.; Jarraya, R.; Girardi, C.; Vansteelandt, M.; Marti, G.; Nasri, I.; Racaud-Sultan, C.; Fabre, N. Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad. Molecules 2015, 20, 18001–18015. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Xue, J.; Wang, K.; Hua, H.; Yuan, T. Norcolocynthenins A and B, two cucurbitane 3-nor-Triterpenoids from Citrullus colocynthis and their cytotoxicity. Bioorganic Chem. 2020, 101, 104045. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Lee, H.S. Biofunctional constituent isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues show acaricidal and insecticidal efficacy. J. Agric. Food Chem. 2014, 62, 8663–8667. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Sajid, A.R.; Javeed, A.; Aslam, M.; Ahsan, T.; Hussain, D.; Mateen, A.; Li, X.; Qin, P.; Ji, M. Antioxidant, antifungal, and aphicidal activity of the triterpenoids spinasterol and 22,23-dihydrospinasterol from leaves of Citrullus colocynthis L. Sci. Rep. 2022, 12, 4910. [Google Scholar] [CrossRef]
- Nehdi, I.A.; Sbihi, H.; Tan, C.P.; Al-Resayes, S.I. Evaluation and characterisation of Citrullus colocynthis (L.) Schrad seed oil: Comparison with Helianthus annuus (sunflower) seed oil. Food Chem. 2013, 136, 348–353. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Ramanathan, T.; Satyavani, K. Characterization of Volatile Compounds from Bitter Apple (Citrullus colocynthis) Using GC-MS. Int. J. Chem. Anal. Sci. 2011, 2, 108–110. [Google Scholar]
- Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Bitter Apple (Citrullus colocynthis) An Overview of Chemical Composition and Biomedical Potentials. Asian J. Plant Sci. 2010, 9, 394–401. [Google Scholar] [CrossRef]
- Cheraghi Niroumand, M.; Farzaei, M.H.; Karimpour Razkenari, E.; Amin, G.; Khanavi, M.; Akbarzadeh, T.; Shams-Ardekani, M.R. An Evidence-Based Review on Medicinal Plants Used as Insecticide and Insect Repellent in Traditional Iranian Medicine. Iran. Red Crescent Med. J. 2016, 18, e22361. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Iqbal, K.J.; Anwer, A.; Abbas, R.Z.; Babar, W.; Ali, A.; Khan, M.K.; Javid, A.; Khan, N.; Ali, H.M.; et al. In vitro anthelmintic efficacy of Citrullus colocynthis (L.) Schrad on Haemonchus contortus. Vet. Arh. 2021, 91, 309–318. [Google Scholar] [CrossRef]
- Hyder, M.; Li, Y.; Wang, M.; Mao, J.; Zhang, L. Insecticidal Activity of Ethanol and Aqueous Extracts of Medicinal Plants against Green Peach Aphid (Myzus Persicae). Appl. Ecol. Environ. Res. 2022, 20, 2329–2342. [Google Scholar] [CrossRef]
- Mohamed, S.N.A.; Montasser, A.A.; Baioumy Ali, A.A. Acaricidal effect of Citrullus colocynthis fruit extract on the camel tick Hyalomma dromedarii (Koch, 1844). Ticks Tick-Borne Dis. 2022, 13, 101995. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Peiwen, Q.; Gu, Z.; Liu, Y.; Sikandar, A.; Hussain, D.; Javeed, A.; Shafi, J.; Iqbal, M.F.; An, R.; et al. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Sci. Rep. 2020, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Chawech, R.; Njeh, F.; Hamed, N.; Damak, M.; Ayadi, A.; Hammami, H.; Mezghani-Jarraya, R. A study of the molluscicidal and larvicidal activities of Citrullus colocynthis L. leaf extract and its main cucurbitacins against the mollusc Galba truncatula, intermediate host of Fasciola hepatica. Pest Manag. Sci. 2017, 73, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Elazab, S.T.; Soliman, A.F.; Nishikawa, Y. Effect of some plant extracts from Egyptian herbal plants against Toxoplasma gondii tachyzoites in vitro. J. Vet. Med. Sci. 2021, 83, 100–107. [Google Scholar] [CrossRef]
- Farooq, Z.; Iqbal, Z.; Mushtaq, S.; Muhammad, G.; Iqbal, M.Z.; Arshad, M. Ethnoveterinary practices for the treatment of parasitic diseases in livestock in Cholistan desert (Pakistan). J. Ethnopharmacol. 2008, 118, 213–219. [Google Scholar] [CrossRef]
- Al-Ardi, M.H. Anti-parasitic activity of nano Citrullus colocynthis and nano Capparis spinose against Trichomonas vaginalis in vitro. J. Parasit. Dis. 2021, 45, 845–850. [Google Scholar] [CrossRef]
- Ponsankar, A.; Sahayaraj, K.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Thanigaivel, A.; Petchidurai, G.; Madasamy, M.; Hunter, W.B. Toxicity and developmental effect of cucurbitacin E from Citrullus colocynthis L. (Cucurbitales: Cucurbitaceae) against Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Environ. Sci. Pollut. Res. Int. 2020, 27, 23390–23401. [Google Scholar] [CrossRef]
- Rahuman, A.A.; Venkatesan, P. Larvicidal efficacy of five cucurbitaceous plant leaf extracts against mosquito species. Parasitol. Res. 2008, 103, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Mullai, K.; Jebanesan, A. Larvicidal, ovicidal and repellent activities of the leaf extract of two cucurbitacious plants against filarial vector Culex quinquefasciatus (Say) (Diptera Culicidae). Trop. Biomed. 2007, 24, 1–6. [Google Scholar] [PubMed]
- Rahuman, A.A.; Venkatesan, P.; Gopalakrishnan, G. Mosquito larvicidal activity of oleic and linoleic acids isolated from Citrullus colocynthis (Linn.) Schrad. Parasitol. Res. 2008, 103, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, M.K.; Al-Marzoqi, A.H.; Ghasemian, A. The Anticancer Efficiency of Citrullus colocynthis toward the Colorectal Cancer Therapy. J. Gastrointest. Cancer 2020, 51, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Antioxidative, Antiproliferative and Biochemical Effects in HepG2 Cells of a Homeopathic Remedy and its Constituent Plant Tinctures Tested Separately or in Combination. Arzneimittelforschung 2003, 53, 823–830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yonbawi, A.R.; Abdallah, H.M.; Alkhilaiwi, F.A.; Koshak, A.E.; Heard, C.M. Anti-Proliferative, Cytotoxic and Antioxidant Properties of the Methanolic Extracts of Five Saudi Arabian Flora with Folkloric Medicinal Use: Aizoon canariense, Citrullus colocynthis, Maerua crassifolia, Rhazya stricta and Tribulus macropterus. Plants 2021, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Seddek, A.S.; Abdelmageed, N.; Badry, M.O.; Nishikawa, Y. Wild Egyptian medicinal plants show in vitro and in vivo cytotoxicity and antimalarial activities. BMC Complement. Med. Ther. 2022, 22, 130. [Google Scholar] [CrossRef]
- Khan, M.I.; Bouyahya, A.; Hachlafi, N.E.L.; Menyiy, N.E.; Akram, M.; Sultana, S.; Zengin, G.; Ponomareva, L.; Shariati, M.A.; Ojo, O.A.; et al. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environ. Sci. Pollut. Res. 2022, 29, 24411–24444. [Google Scholar] [CrossRef]
- Hajjar, D.; Kremb, S.; Sioud, S.; Emwas, A.H.; Voolstra, C.R.; Ravasi, T. Anti-cancer agents in Saudi Arabian herbals revealed by automated high-content imaging. PLoS ONE 2017, 12, e0177316. [Google Scholar] [CrossRef]
- Perveen, S.; Ashfaq, H.; Ambreen, S.; Ashfaq, I.; Kanwal, Z.; Tayyeb, A. Methanolic extract of Citrullus colocynthis suppresses growth and proliferation of breast cancer cells through regulation of cell cycle. Saudi J. Biol. Sci. 2021, 28, 879–886. [Google Scholar] [CrossRef]
- Perveen, S.; Ashfaq, H.; Shahjahan, M.; Manzoor, A.; Tayyeb, A. Citrullus colocynthis regulates de novo lipid biosynthesis in human breast cancer cells. J. Cancer Res. Ther. 2020, 16, 1294–1301. [Google Scholar] [PubMed]
- Ayyad, S.E.; Abdel-Lateff, A.; Alarif, W.M.; Patacchioli, F.R.; Badria, F.A.; Ezmirly, S.T. In vitro and in vivo study of cucurbitacins-type triterpene glucoside from Citrullus colocynthis growing in Saudi Arabia against hepatocellular carcinoma. Environ. Toxicol. Pharmacol. 2012, 33, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.E.M.; Boulos, J.C.; Elhaboub, G.; Rigano, D.; Saab, A.; Loizzo, M.R.; Hassan, L.E.A.; Sugimoto, Y.; Piacente, S.; Tundis, R.; et al. Cytotoxicity of cucurbitacin E from Citrullus colocynthis against multidrug-resistant cancer cells. Phytomedicine 2019, 62, 152945. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S. Search for biofunctional constituents from medicinal foods-elucidation of constituents with anti-proliferation effects and the target molecule from Citrullus colocynthis. Yakugaku Zasshi 2012, 132, 1063–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Hwaiti, M.S.; Alsbou, E.M.; Abu Sheikha, G.; Bakchiche, B.; Pham, T.H.; Thomas, R.H.; Bardaweel, S.K. Evaluation of the anticancer activity and fatty acids composition of “Handal” (Citrullus colocynthis L.) seed oil, a desert plant from south Jordan. Food Sci. Nutr. 2021, 9, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Ishola, I.O.; Ajani, I.D. Citrullus colocynthis Linn. Fruit extract ameliorates cisplatin-induced hepato-renal toxicity in rats. J. Complement. Integr. Med. 2017, 15, 20170086. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Moradi, M.T.; Mahmoodnia, L.; Alaei, S.; Asadi-Samani, F.; Luther, T. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J. Nephropathol. 2017, 6, 118–125. [Google Scholar] [CrossRef]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef]
- Drissi, F.; Lahfa, F.; Gonzalez, T.; Peiretti, F.; Tanti, J.F.; Haddad, M.; Fabre, N.; Govers, R. A Citrullus colocynthis fruit extract acutely enhances insulin-induced GLUT4 translocation and glucose uptake in adipocytes by increasing PKB phosphorylation. J. Ethnopharmacol. 2021, 270, 113772. [Google Scholar] [CrossRef] [PubMed]
- Benariba, N.; Djaziri, R.; Hupkens, E.; Louchami, K.; Malaisse, W.J.; Sener, A. Insulinotropic action of Citrullus colocynthis seed extracts in rat pancreatic islets. Mol. Med. Rep. 2013, 7, 233–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benariba, N.; Bellakdhar, W.; Djaziri, R.; Hupkens, E.; Louchami, K.; Malaisse, W.J. Protective action of Citrullus colocynthis seed extracts against the deleterious effect of streptozotocin on both in vitro glucose-stimulated insulin release from rat pancreatic islets and in vivo glucose homeostasis. Biomed. Rep. 2013, 1, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Benariba, N.; Djaziri, R.; Zerriouh, B.H.; Bellakhdar, W.; Hupkens, E.; Boucherit, Z.; Malaisse, W.J. Short- and long-term effects of various Citrullus colocynthis seed extracts in normal and streptozotocin-induced diabetic rats. Int. J. Mol. Med. 2012, 30, 1528–1536. [Google Scholar] [CrossRef][Green Version]
- Ghauri, A.O.; Ahmad, S.; Rehman, T. In vitro and in vivo anti-diabetic activity of Citrullus colocynthis pulpy flesh with seeds hydro-ethanolic extract. J. Complement. Integr. Med. 2020, 17, 20180228. [Google Scholar] [CrossRef] [PubMed]
- Nmila, R.; Gross, R.; Rchid, H.; Roye, M.; Manteghetti, M.; Petit, P.; Tijane, M.; Ribes, G.; Sauvaire, Y. Insulinotropic effect of Citrullus colocynthis fruit extracts. Planta Medica 2000, 66, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghaithi, F.; El-Ridi, M.; Adeghate, E.; Amiri, M. Biochemical effects of Citrullus colocynthis in normal and diabetic rats. Mol. Cell. Biochem. 2004, 261, 143–149. [Google Scholar] [CrossRef]
- Ostovar, M.; Akbari, A.; Anbardar, M.H.; Iraji, A.; Salmanpour, M.; Hafez Ghoran, S.; Heydari, M.; Shams, M. Effects of Citrullus colocynthis L. in a rat model of diabetic neuropathy. J. Integr. Med. 2020, 18, 59–67. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Aminizadeh, A.H.; Esmaeilpour, K.; Bejeshk, M.A.; Sadeghi, A.; Salimi, F. Investigating the effects of Citrullus colocynthis on cognitive performance and anxiety-like behaviors in STZ-induced diabetic rats. Int. J. Neurosci. 2021, 133, 343–355. [Google Scholar] [CrossRef]
- Sebbagh, N.; Cruciani-Guglielmacci, C.; Ouali, F.; Berthault, M.F.; Rouch, C.; Sari, D.C.; Magnan, C. Comparative effects of Citrullus colocynthis, sunflower and olive oil-enriched diet in streptozotocin-induced diabetes in rats. Diabetes Metab. 2009, 35, 178–184. [Google Scholar] [CrossRef]
- Tehseen, I.; Haq, T.U.; Ilahi, I.; Khan, A.A.; Attaullah, M.; Zamani, G.Y.; Zaman, S.; Ismail, I. Antidiabetic and hepato-renal protective effects of medicinal plants in STZ induced diabetic rats. Braz. J. Biol. 2022, 84, e260189. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Fang, P.F.; Xiang, D.X.; Yang, Y.Y. Topical treatments for diabetic neuropathic pain. Exp. Ther. Med. 2019, 17, 1963–1976. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Belali, R.; Bineshfar, F.; Javadzadeh, S.; Yazdanpanah, L. Evaluation of skin absorption of the Citrullus colocynthis in treatment of type II diabetic patients. J. Diabetes Metab. Disord. 2020, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Barghamdi, B.; Ghorat, F.; Asadollahi, K.; Sayehmiri, K.; Peyghambari, R.; Abangah, G. Therapeutic effects of Citrullus colocynthis fruit in patients with type II diabetes: A clinical trial study. J. Pharm. Bioallied Sci. 2016, 8, 130–134. [Google Scholar] [PubMed]
- Huseini, H.F.; Darvishzadeh, F.; Heshmat, R.; Jafariazar, Z.; Raza, M.; Larijani, B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of Type II diabetic patients: A randomized, double blind, placebo-controlled clinical trial. Phytother. Res. 2009, 23, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, M.; Zhai, X.; Huang, Y.; Khalid, A.; Malik, A.; Shah, P.; Karim, S.; Azhar, S.; Hou, X. Effect of Gymnema sylvestrs, Citrullus colocynthis and Artemisia absinthium on blood glucose and lipid profile in diabetic human. Acta Pol. Pharm. 2015, 72, 981–985. [Google Scholar] [PubMed]
- Mazokopakis, E.E.; Karagiannis, C.G. Medical toxicology in the Old Testament. The poisonous pottage. Intern. Med. J. 2017, 47, 1458–1460. [Google Scholar] [CrossRef]
- Adam, S.E.I.; Al-Farhan, A.H.; Al-Yahya, M.A. Effect of Combined Citrullus colocynthis and Rhazya Stricta Use in Najdi Sheep. Am. J. Chin. Med. 2000, 28, 385–390. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Ghorbani, A.; Shafiee-Nick, R.; Rakhshandeh, H.; Borji, A. Antihyperlipidemic effect of a polyherbal mixture in streptozotocin-induced diabetic rats. J. Lipids 2013, 2013, 675759. [Google Scholar] [CrossRef]
- Alhawiti, N.M. Antiplatelets and profibrinolytic activity of Citrullus colocynthis in control and high-fat diet-induced obese rats: Mechanisms of action. Arch. Physiol. Biochem. 2018, 124, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Birem, Z.; Tabani, K.; Lahfa, F.; Djaziri, R.; Hadjbekkouche, F. Effects of colocynth alkaloids and glycosides on Wistar rats fed high-fat diet. A biochemical and morphological study. Folia Histochem. Cytobiol. 2017, 55, 74–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaikh, J.; Shaikh, D.; Rahman, A.; Shafi, S. Antimicrobial and toxicological studies on fruit pulp of Citrullus colocynthis L. Pak. J. Pharm. Sci. 2016, 29, 9–15. [Google Scholar] [PubMed]
- Chaturvedi, M.; Mali, P.C.; Ansari, A.S. Induction of reversible antifertility with a crude ethanol extract of Citrullus colocynthis Schrad fruit in male rats. Pharmacology 2003, 68, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Qazan, W.; Almasad, M.; Daradka, H. Short and long effects of Citrullus colocynthis L. on reproductive system and fertiliity in female spague-dawley rats. Pak. J. Pharm. Sci. 2007, 10, 2699–2703. [Google Scholar]
- Goraya, K.; Iqbal, Z.; Sajid, M.; Muhammad, G.; Ain, Q.; Saleem, M. Diversity of flora used for the cure of equine diseases in selected peri-urban areas of Punjab, Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, A.; Aarab, L.; Abdel-Sattar, E. Screening of immunomodulatory activity of total and protein extracts of some Moroccan medicinal plants. Toxicol. Ind. Health 2013, 29, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Pashmforosh, M.; Rajabi Vardanjani, H.; Rajabi Vardanjani, H.; Pashmforosh, M.; Khodayar, M.J. Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats. Medicina 2018, 54, 51. [Google Scholar] [CrossRef]
- Ibrahim, T.A.; El-Hefnawy, H.M.; El-Hela, A.A. Antioxidant potential and phenolic acid content of certain cucurbitaceous plants cultivated in Egypt. Nat. Prod. Res. 2010, 24, 1537–1545. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Y.; Lu, L.; Gu, F.; Chen, H. The applications and features of liquid chromatography-mass spectrometry in the analysis of traditional Chinese medicine. Evid. Based Complement. Altern. Med. 2016, 2016, 3837270. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Chen, S.; Liu, X.; Zhou, Y.; Zhang, X.; Xu, X. Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography-mass spectrometry. J. Pharm. Biomed. A 2013, 72, 267–291. [Google Scholar] [CrossRef]
- Ye, J. Application of gas chromatography-mass spectrometry in research of traditional Chinese medicine. Chem. Pap. 2009, 63, 506–511. [Google Scholar] [CrossRef]
- Rodriguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).