Enhancement of Bottle Gourd Oil Activity via Optimized Self-Dispersing Lipid Formulations (SDLFs) to Mitigate Isoproterenol-Evoked Cardiac Toxicity in Rats via Modulating BNP, MMP2, and miRNA-21 and miRNA-23a Genes’ Expression
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Design and Statistical Analysis
Effect of Formulation Variables on BG-SDLFs Characteristics
2.2. Formulation Optimization
2.3. Characterization of the Optimized BG-SDLF
2.3.1. Visual Inspection, Drug Content, and Thermodynamic Stability
2.3.2. Robustness to Dilution and Phase Separation
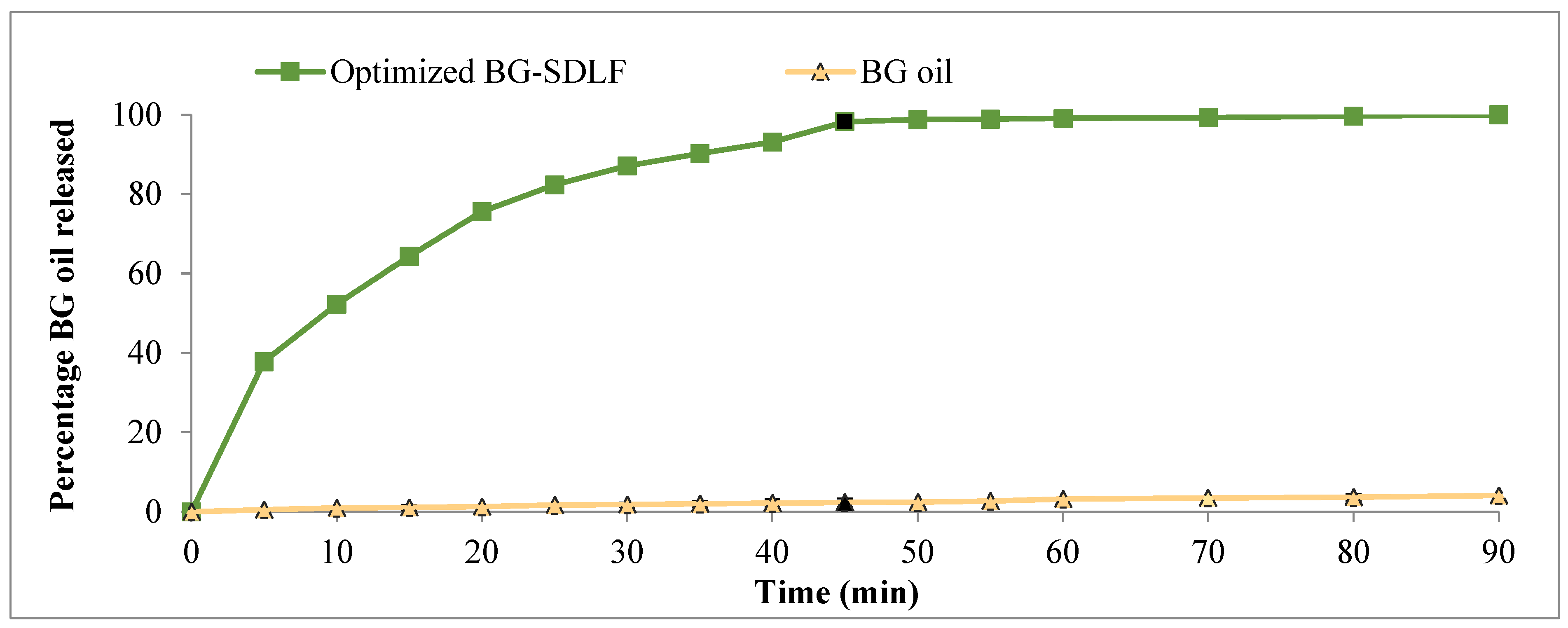
2.3.3. In Vitro Dissolution Studies
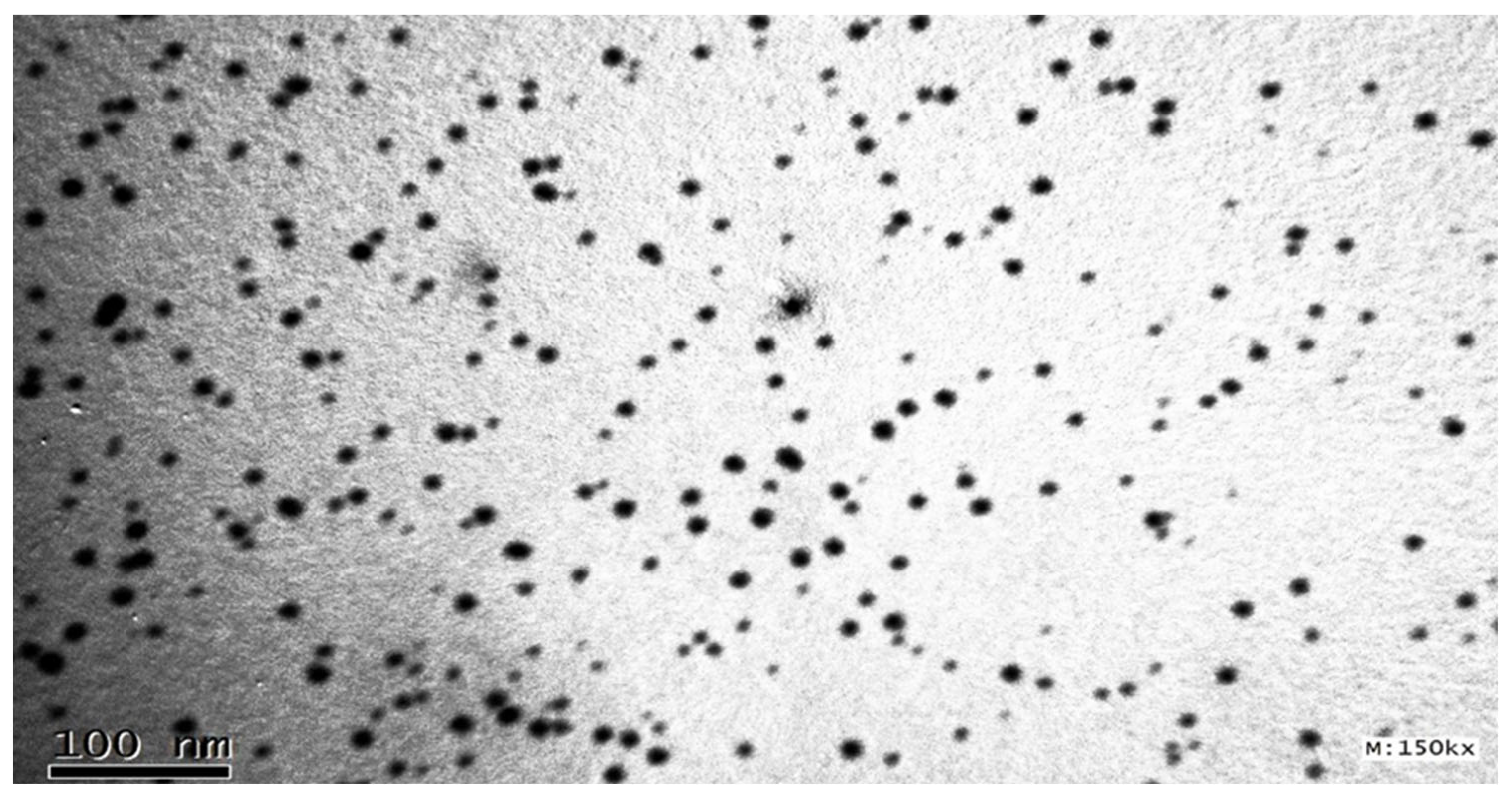
2.3.4. Determination of Surface Morphology by TEM
2.4. Biochemical Investigation of Cardioprotective Effect of BG Oil, the Optimized BG-SDLF, and Omega 3 in ISO-Treated Rats
2.4.1. Effect of BG Oil, the Optimized BG-SDLF, and Omega 3 on Plasma BNP and NT-pro-BNP
2.4.2. Effect of BG Oil, the Optimized BG-SDLF, and Omega 3 on Plasma Cystatin C, Galectin-3, Lp-PLA2, and MMP2 Levels
2.4.3. Effect of BG Oil, the Optimized BG-SDLF, and Omega 3 on Plasma cTnI and cTnT Levels
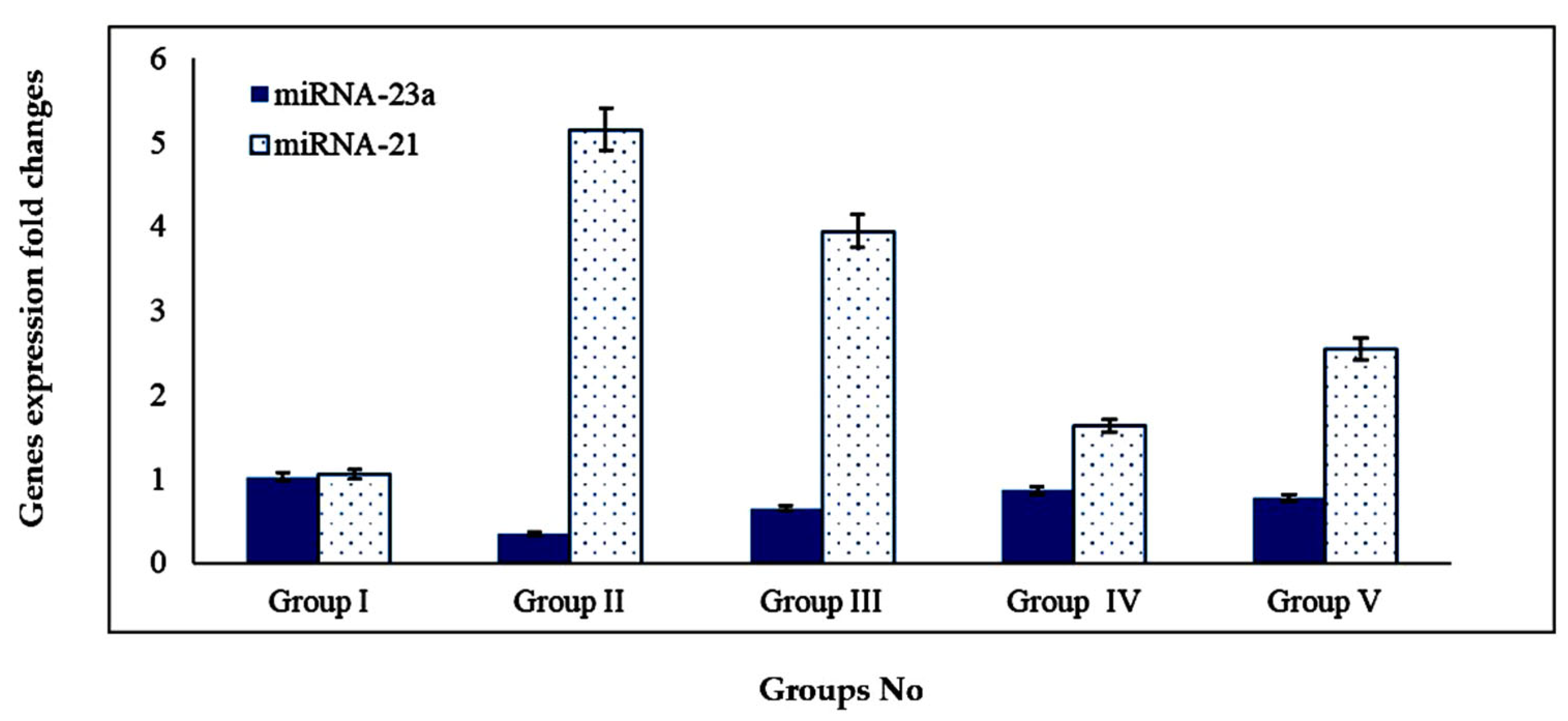
2.4.4. Effect of BG Oil, the Optimized BG-SDLF and Omega 3 on Plasma miRNA-21 and miRNA-23a Genes
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of BG Oil Self-Dispersing Lipid Formulations (BG-SDLFs)
3.2.2. Characterization of the Prepared BG-SDLFs
Droplet Size Analysis (DS) and Poly Dispersity Index (PDI) Determination
Emulsification Efficiency Determination
3.2.3. Optimization of BG-SDLFs
3.2.4. Characterization of the Optimum BG-SDLF
Visual Inspection, Drug Content Measurement, and Thermodynamic Stability Assessment
Robustness to Dilution
Surface Morphology via Transmission Electron Microscopy [TEM]
In Vitro Dissolution of BG-SDLF Formula
3.2.5. In Vivo Assessment
Experimental Design
Determination of BNP, NT-proBNP, cTnI, and cTnT Levels
Determination of Cystatin C, Galectin-3, Lp-PLA2, and MMP2 Levels
Quantitative Real-Time PCR for the Determination of miRNA-21 and miRNA-23a
- -
- miRNA-21 primer sequence: F: 5′-ACGTTGTGTAGCTTATCAGACTG-3′
- -
- miRNA-23a primer sequence: F: 5′-GGGGGGGGATCACATTGCCA-3′
- -
- U6 (qRT-PCR internal control): F: 5′-GCTTCGGCAGCACATATACTAAAAT-3′
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Cardiovascular Diseases [CVDs]. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-[cvds] (accessed on 17 March 2023).
- Ravassa, S.; González, A.; Bayés-Genís, A.; Lupón, J.; Díez, J. Myocardial Interstitial Fibrosis in the Era of Precision Medicine. Biomarker-Based Phenotyping for a Personalized Treatment. Rev. Esp. Cardiol. 2020, 73, 248–254. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, M.; Cao, Y.; Zhang, Z.; Jiang, B.; Tian, F.; Feng, J.; Dou, Y.; Gorospe, M.; Zheng, M.; et al. HuR Regulates Phospholamban Expression in Isoproterenol-Induced Cardiac Remodelling. Cardiovasc. Res. 2020, 116, 944–955. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.A.; Mahmoud, A.Z.; Sayed, A.A.; Abd El Latif, M.A. Physiochemical Properties and Phytochemical Characteristics of Bottle Gourd (Lagenaria siceraria) Seed Oil. Egypt J. Chem. 2022, 65, 269–277. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, M.S.; Amir, K.; Bi, J.B.; Asif, M.; Madni, A.; Kamboh, A.A.; Manzoor, Z.; Younas, U.; Chao, S. Lagenaria Siceraria Fruit: A Review of Its Phytochemistry, Pharmacology, and Promising Traditional Uses. Front. Nutr. 2022, 9, 927361. [Google Scholar] [CrossRef]
- Dhakad, G.; Tambe, K.; Shirsat, S.; Jaiswal, N. Review on Study of Bottle Gourd on Human Health. Res. J. Pharmacol. Pharmacodyn. 2022, 14, 174–178. [Google Scholar] [CrossRef]
- Deshpande, J.R.; Choudhari, A.A.; Mishra, M.R.; Meghre, V.S.; Wadodkar, S.G.; Dorle, A.K. Beneficial Effects of Lagenaria Siceraria [Mol.] Standley Fruit Epicarp in Animal Models. Indian J. Exp. Biol. 2008, 46, 234–242. [Google Scholar]
- Panchal, C.V.; Sawale, J.A.; Poul, B.N.; Khandelwal, K.R. Hepatoprotective Activity of Lagenaria Siceraria [Molina] Standley Fruits Against Paracetamol Induced Hapatotoxicity in Mice. Int. J. Pharm. Sci. Res. 2013, 4, 371–377. [Google Scholar] [CrossRef]
- Saboo, S.S.; Thorat, P.K.; Tapadiya, G.G.; Khadabadi, S.S. Ancient and Recent Medicinal Uses of Cucurbitaceae Family. Int. J. Ther. Appl. 2013, 9, 11–19. [Google Scholar]
- Gershanik, T.; Benita, S. Self-Dispersing Lipid Formulations for Improving Oral Absorption of Lipophilic Drugs. Eur. J. Pharm. Biopharm. 2000, 50, 179–188. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar Gupta, S.; Kumar Sharma, P. Self-Emulsifying Drug Delivery Systems [SEDDS] for Oral Delivery of Lipid Based Formulations. African J. Basic Appl. Sci. 2012, 4, 7–11. [Google Scholar] [CrossRef]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent Advances in Improving the Bioavailability of Hydrophobic/Lipophilic Drugs and Their Delivery via Self-Emulsifying Formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- Ujilestari, T.; Martien, R.; Ariyadi, B.; Danar Dono, N.; Zuprizal. Self-nanoemulsifying Drug Delivery System [SNEDDS] of Amomum Compactum Essential Oil: Design, Formulation, and Characterization. J. Appl. Pharm. Sci. 2018, 8, 14–21. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; Sheta, N.M.; Boshra, S.A. Isolation, Formulation, and Efficacy Enhancement of Morin Emulsified Carriers Against Lung Toxicity in Rats. AAPS PharmSciTech 2018, 19, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- El-Mancy, S.S.; El-Haddad, A.E.; Alshareef, W.A.; Saadeldeen, A.M.; El-Emam, S.Z.; Elnahas, O.S. Enhancement of Antimicrobial and Antiproliferative Activities of Standardized Frankincense Extract Using Optimized Self-Nanoemulsifying Delivery System. Sci. Pharm. 2021, 89, 36. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing Spanlastics for Improving the Ocular Delivery of Clotrimazole: In Vitro Characterization, Ex vivo Permeability, Microbiological Assessment and In vivo Safety Study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef]
- Cuiné, J.F.; Charman, W.N.; Pouton, C.W.; Edwards, G.A.; Porter, C.J.H. Increasing the Proportional Content of Surfactant [Cremophor EL] Relative to Lipid in Self-emulsifying Lipid-based Formulations of Danazol Reduces Oral Bioavailability in Beagle Dogs. Pharm. Res. 2007, 24, 748–757. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M.S. Design and evaluation of self-nanoemulsifying drug delivery systems [SNEDDS] for cefpodoxime proxetil. Int. J. Pharm. 2007, 329, 166–172. [Google Scholar] [CrossRef]
- Elsayed, I.; El-Dahmy, R.M.; Elshafeey, A.H.; El Gawad, N.A.A.; El Gazayerly, O.N. Tripling the Bioavailability of Rosuvastatin Calcium Through Development and Optimization of An In-Situ Forming Nanovesicular System. Pharmaceutics 2019, 11, 275. [Google Scholar] [CrossRef]
- Taha, E.; Samy, A.; Kassem, A.; Khan, M. Response Surface Methodology for the Development of Self-Nanoemulsified Drug Delivery System [SNEDDS] of All-Trans-Retinol Acetate. Pharm. Dev. Technol. 2005, 10, 363–370. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Chen, J.; Eastoe, J.; Li, X. Design and optimization of a new self-nanoemulsifying drug delivery system. J. Colloid. Interface Sci. 2009, 330, 443–448. [Google Scholar] [CrossRef]
- Fayez, S.M.; Elnahas, O.S.; Fayez, A.M.; El-Mancy, S.S. Coconut Oil Based Self-Nano Emulsifying Delivery Systems Mitigate Ulcerogenic Nsaids Side Effect and Enhance Drug Dissolution: Formula Optimization, In-Vitro, and In-Vivo Assessments. Int. J. Pharm. 2023, 634, 122666. [Google Scholar] [CrossRef] [PubMed]
- Sanka, K.; Suda, D.; Bakshi, V. Optimization of Solid-Self Nanoemulsifying Drug Delivery System for Solubility and Release Profile of Clonazepam Using Simplex Lattice Design. J. Drug Deliv. Sci. Technol. 2016, 33, 114–124. [Google Scholar] [CrossRef]
- Balata, G.F.; Essa, E.A.; Shamardl, H.A.; Zaidan, S.H.; Abourehab, M.A.S. Self-Emulsifying Drug Delivery Systems as a Tool to Improve Solubility and Bioavailability of Resveratrol. Drug Des. Devel. Ther. 2016, 10, 117–128. [Google Scholar] [CrossRef]
- Maalouf, R.; Bailey, S. A Review on B-type Natriuretic Peptide Monitoring: Assays and Biosensors. Hear. Fail. Rev. 2016, 21, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Jerie, P. Assessing the Benefits of Natriuretic Peptides-Guided Therapy in Chronic Heart Failure. Cardiol. J. 2015, 22, 5–11. [Google Scholar] [CrossRef]
- Palmiere, C.; Tettamanti, C.; Bonsignore, A.; De Stefano, F.; Vanhaebost, J.; Rousseau, G.; Scarpelli, M.P.; Bardy, D. Cardiac Troponins and NT-Probnp in The Forensic Setting: Overview of Sampling Site, Postmortem Interval, Cardiopulmonary Resuscitation, and Review of The Literature. Forensic. Sci. Int. 2018, 282, 211–218. [Google Scholar] [CrossRef]
- Michaud, K.; Augsburger, M.; Donzé, N.; Sabatasso, S.; Faouzi, M.; Bollmann, M.; Mangin, P. Evaluation of Postmortem Measurement of NT-Probnp as a Marker for Cardiac Function. Int. J. Legal. Med. 2008, 122, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Huelsman, M.; Strecker, K.; Bojic, A.; Moser, P.; Stanek, B.; Pacher, R. B-Type Natriuretic Peptide Predicts Sudden Death in Patients with Chronic Heart Failure. Circulation 2002, 105, 2392–2397. [Google Scholar] [CrossRef]
- De Groote, P.; Dagorn, J.; Soudan, B.; Lamblin, N.; McFadden, E.; Bauters, C. B-type Natriuretic Peptide and Peak Exercise Oxygen Consumption Provide Independent Information for Risk Stratification in Patients with Stable Congestive Heart Failure. J. Am. Coll. Cardiol. 2004, 43, 1584–1589. [Google Scholar] [CrossRef]
- Ibarrola, J.; Arrieta, V.; Sádaba, R.; Martinez-Martinez, E.; Garcia-Peña, A.; Alvarez, V.; Fernández-Celis, A.; Gainza, A.; Santamaría, E.; Fernández-Irigoyen, J.; et al. Galectin-3 Down-Regulates Antioxidant Peroxiredoxin-4 in Human Cardiac Fibroblasts: A New Pathway to Induce Cardiac Damage. Clin. Sci. 2018, 132, 1471–1485. [Google Scholar] [CrossRef]
- Ibarrola, J.; Sádaba, R.; Garcia-Peña, A.; Arrieta, V. A Role for Fumarate Hydratase in Mediating Oxidative Effects of Galectin-3 in Human Cardiac Fibroblasts. Int. J. Cardiol. 2018, 258, 217–223. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Farnworth, S.L.; Hodkinson, P.S.; Henderson, N.C.; Atkinson, K.M.; Leffler, H.; Nilsson, U.J.; Haslett, C.; Forbes, S.J.; Sethi, T. Regulation of Alternative Macrophage Activation by Galectin-3. J. Immunol. 2008, 180, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.R.; Nascimento, L.D.; Gerlach, R.F.; Rodrigues, K.E.; Prado, A.F. Matrix Metalloproteinase 2 as a Pharmacological Target in Heart Failure. Pharmaceuticals 2022, 15, 920. [Google Scholar] [CrossRef]
- Ge, P.C.; Chen, Z.H.; Pan, R.Y.; Ding, X.Q.; Liu, J.Y.; Jia, Q.W.; Liu, Z.; He, S.Z.; An, F.H.; Li, L.H.; et al. Synergistic Effect of Lipoprotein-Associated Phospholipase A2 with Classical Risk Factors on Coronary Heart Disease: A Multi-Ethnic Study in China. Cell. Physiol. Biochem. 2016, 40, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Lassus, J.; Harjola, V.; Siirilawaris, K.; Sund, R.; Melin, J.; Pulkki, K.; Peuhkurinen, K.; Nieminen, M.S. 643 Prognostic Value of Cystatin C in Acute Heart Failure in Relation to Other Markers of Renal Function and NT-proBNP. Eur. J. Hear. Fail. Suppl. 2006, 5, 150. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Shah, A.S.V.; Mcallister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Hayward, C.; Padmanabhan, S.; Welsh, C.; et al. Comparison between High-Sensitivity Cardiac Troponin T and Cardiac Troponin I in a Large General Population Cohort. Clin. Chem. 2018, 64, 1607–1616. [Google Scholar] [CrossRef]
- Neumann, J.T.; Havulinna, A.S.; Zeller, T.; Appelbaum, S.; Kunnas, T.; Nikkari, S.; Jousilahti, P.; Blankenberg, S.; Sydow, K.; Salomaa, V. Comparison of Three Troponins as Predictors of Future Cardiovascular Events—Prospective Results from the FINRISK and BiomaCaRE Studies. PLoS ONE 2014, 9, e90063. [Google Scholar] [CrossRef] [PubMed]
- Sekuklu, S.D.; Donoghue, M.T.A.; Spillane, C. miR-21 As A Key Regulator of Oncogenic Processes. Biochem. Soc. Trans. 2009, 37, 918–925. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, R.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNAs are Aberrantly Expressed in Hypertrophic Heart: Do They Play a Pole in Cardiac Hypertrophy? Am. J. Pathol. 2007, 170, 1831–1840. [Google Scholar] [CrossRef]
- Suárez, Y.; Fernández-Hernando, C.; Pober, J.S.; Sessa, W.C. Dicer Dependent MicroRNAs Regulate Gene Expression and Functions in Human Endothelial Cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA Expression Signature and Antisense-Mediated Depletion Reveal an Essential Role of Microrna in Vascular Neointimal Lesion Formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef]
- Mandal, C.C.; Ghosh-Choudhury, T.; Dey, N.; Choudhury, G.G.; Ghosh-Choudhury, N. miR-21 is Targeted by Omega-3 Polyunsaturated Fatty Acid to Regulate Breast Tumor CSF-1 Expression. Carcinogenesis 2012, 33, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xu, C.; Guan, J.; Ping, Y.; Fan, H.; Li, Y.; Zhao, H.; Li, X. Discovering Dysfunction of Multiple MicroRNAs Cooperation in Disease by a Conserved MicroRNA Co-Expression Network. PLoS ONE 2012, 7, e32201. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Hussain, S.-R.A.; Biswas, S.; Azad, A.; Rink, C.; Gnyawali, S.; Shilo, S.; Nuovo, G.J.; Sen, C.K. MicroRNA Expression in Response to Murine Myocardial Infarction: Mir-21 Regulates Fibroblast Metalloprotease-2 via Phosphatase and Tensin Homologue. Cardiovasc. Res. 2009, 82, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R. Intracellular Targets of Matrix Metalloproteinase-2 in Cardiac Disease: Rationale and Therapeutic Approaches. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Viappiani, S.; Nicolescu, A.; Holt, A.; Sawicki, G. Activation and modulation of 72 kDa matrix metalloproteinase-2 by Peroxynitrite and Glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lv, Z.; Zhuang, Y. MicroRNA-23a is involved in tumor necrosis factor-α induced apoptosis in Mesenchymal Stem Cells and Myocardial Infarction. Exp. Mol. Pathol. 2014, 97, 23–30. [Google Scholar] [CrossRef]
- Li, S.; Ren, J.; Sun, Q. The Expression of MicroRNA-23a Regulates Acute Myocardial Infarction in Patients and In Vitro Through Targeting PTEN. Mol. Med. Rep. 2018, 17, 6866–6872. [Google Scholar] [CrossRef]
- Nazeam, J.A.; Ragab, G.M.; El-Gazar, A.A.; El-Mancy, S.S.; Jamil, L.; Fayez, S.M. Topical Nano Clove/Thyme Gel against Genetically Identified Clinical Skin Isolates: In Vivo Targeting Behavioral Alteration and IGF-1/pFOXO-1/PPAR γ Cues. Molecules 2021, 26, 5608. [Google Scholar] [CrossRef]
- Badawi, A.A.; ABD EL-Aziz, N.; Amin, M.M.; Sheta, N.M. Topical Benzophenone-3 Microemulsion-Based Gels: Preparation, Evaluation and Determination of Microbiological UV Blocking Activity. Int. J. Pharm. Pharma. Sci. 2014, 6, 562–570. Available online: https://journals.innovareacademics.in/index.php/ijpps/article/view/1975 (accessed on 18 August 2023).
- Batool, A.; Arshad, R.; Razzaq, S.; Nousheen, K.; Kiani, M.H.; Shahnaz, G. Formulation and Evaluation of Hyaluronic Acid-Based Mucoadhesive Self Nanoemulsifying Drug Delivery System [SNEDDS] of Tamoxifen for Targeting Breast Cancer. Int. J. Biol. Macromol. 2020, 152, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-Nanoemulsifying Drug Delivery System for Adefovir Dipivoxil: Design, Characterization, In Vitro and Ex Vivo Evaluation. Colloids Surfaces a Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Soliman, S.M.; Sheta, N.M.; Ibrahim, B.M.M.; El-Shawwa, M.M.; Abd El-Halim, S.M. Novel Intranasal Drug Delivery: Geraniol Charged Polymeric Mixed Micelles for Targeting Cerebral Insult as a Result of Ischaemia/Reperfusion. Pharmaceutics 2020, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; El-Megraba, N.; Abdallaha, M. Self-Emulsifying Drug Delivery Formulations. Zagazig J. Pharm. Sci. 2018, 27, 1–21. [Google Scholar] [CrossRef]
- Atta, N.; Ismaiel, G.H.Z. Usage of Oil and Powder of Bottle Gourd and Pumpkin Seeds in Production of High Nutritive Value Biscuit. Egypt J. Nutr. Health 2020, 15, 39–53. [Google Scholar] [CrossRef]
- Boshra, S.A. Resveratrol Modulates miR-34a in Cardiotoxicity Induced by Isoproterenol. J. Med. Food. 2020, 23, 593–599. [Google Scholar] [CrossRef]
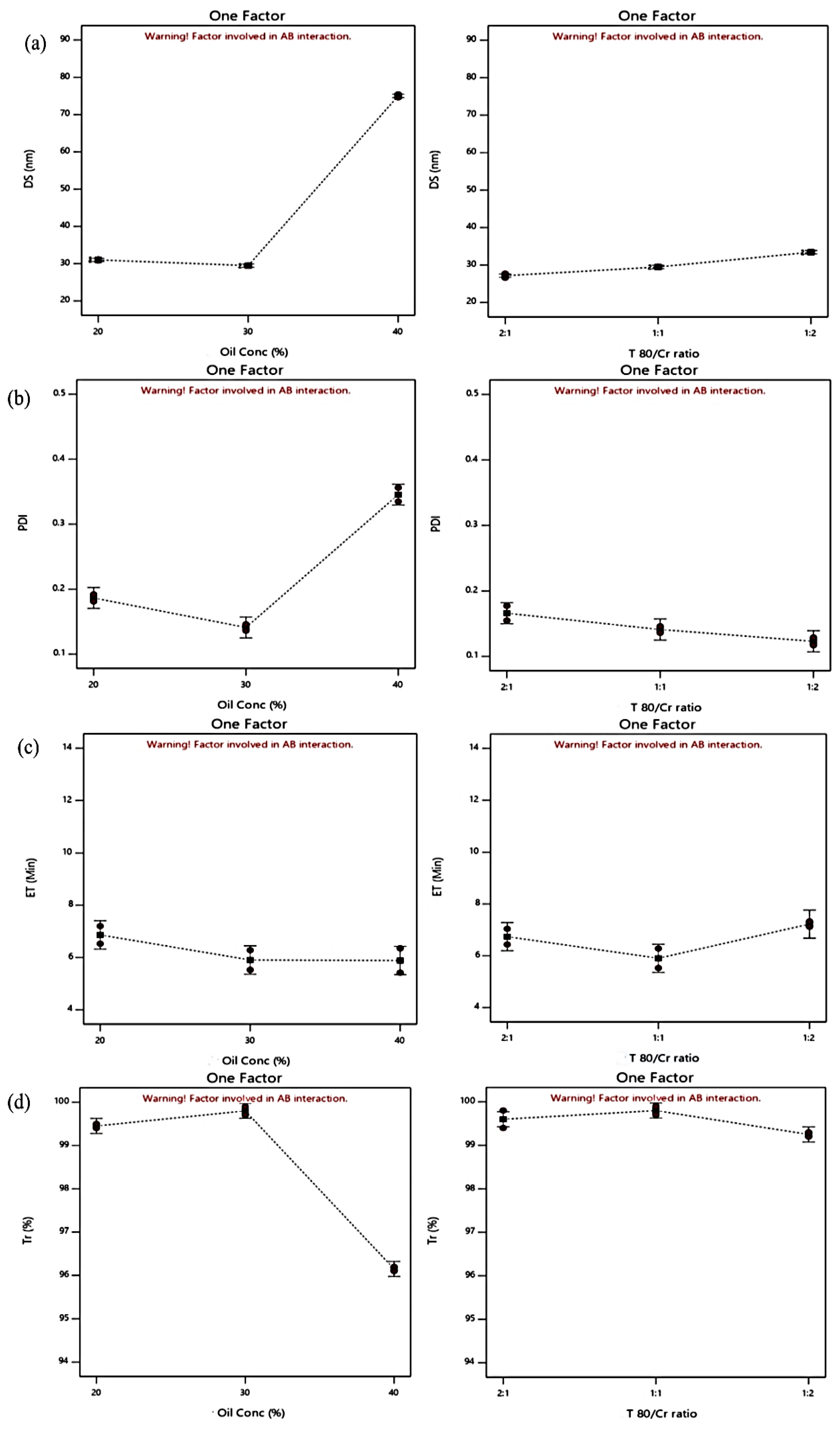
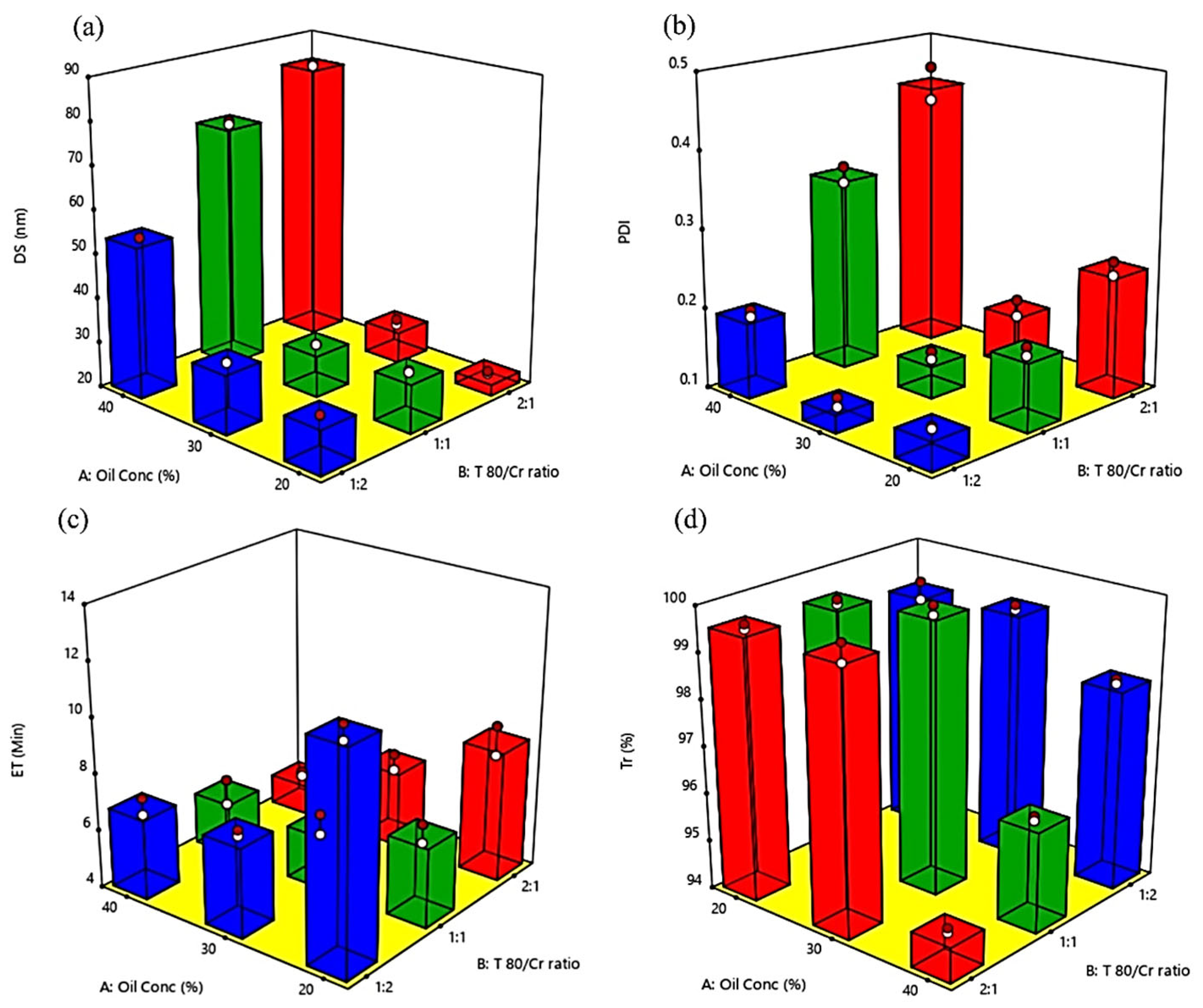
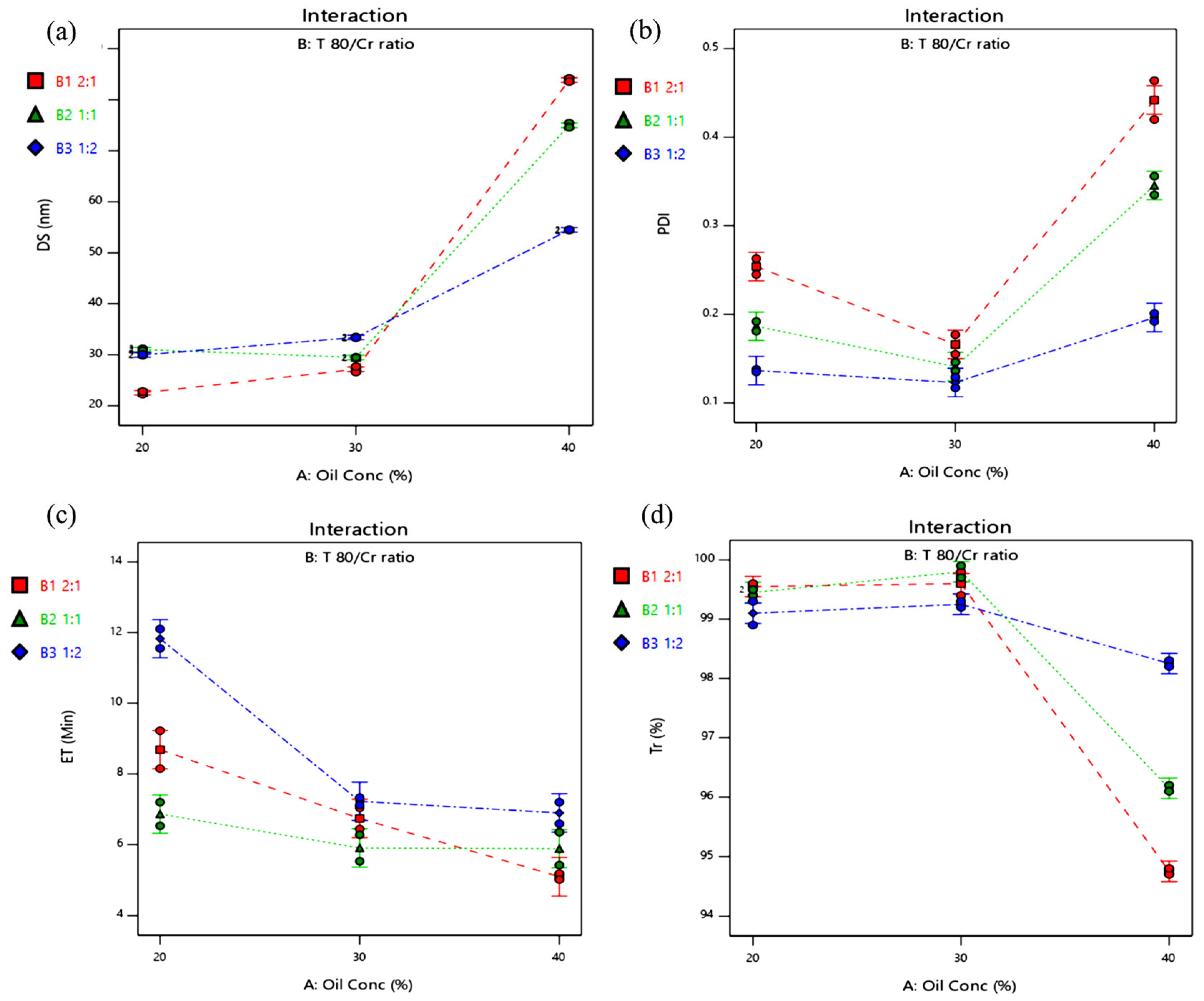
| Formula Code | BG-SDLFs Compositions * | Responses * | |||||
|---|---|---|---|---|---|---|---|
| IPM (% w/w) | Smix (% w/w) | T 80/Cr ratio | DS (nm) | PDI | ET (min) | Tr (%) | |
| F1 | 10 | 80 | 2:1 | 22.6 ± 0.3 | 0.254 ± 0.009 | 8.8 ± 0.8 | 99.5 ± 0.1 |
| F2 | 10 | 80 | 1:1 | 31.2 ± 0.4 | 0.192 ± 0.010 | 7.2 ± 0.5 | 99.5 ± 0.1 |
| F3 | 10 | 80 | 1:2 | 29.9 ± 0.1 | 0.138 ± 0.006 | 12.0 ± 0.2 | 99.1 ± 0.3 |
| F4 | 20 | 70 | 2:1 | 27.1 ± 0.6 | 0.177 ± 0.044 | 6.9 ± 0.2 | 99.6 ± 0.3 |
| F5 | 20 | 70 | 1:1 | 29.4 ± 0.1 | 0.136 ± 0.010 | 6.2 ± 0.4 | 99.8 ± 0.1 |
| F6 | 20 | 70 | 1:2 | 33.5 ± 0.3 | 0.129 ± 0.018 | 7.4 ± 0.2 | 99.3 ± 0.1 |
| F7 | 30 | 60 | 2:1 | 84.3 ± 0.8 | 0.435 ± 0.025 | 5.2 ± 0.3 | 94.8 ± 0.1 |
| F8 | 30 | 60 | 1:1 | 75.4 ± 1.5 | 0.335 ± 0.037 | 6.3 ± 0.8 | 96.2 ± 0.1 |
| F9 | 30 | 60 | 1:2 | 54.4 ± 0.1 | 0.201 ± 0.009 | 7.2 ± 0.2 | 98.3 ± 0.1 |
| Group No. | Group Description | BNP (pg/mL) | NT-pro-BNP (pg/mL) |
|---|---|---|---|
| I | Normal control | 85.77 ± 7.30 a | 21.43 ± 2.19 a |
| II | Isoproterenol (85 mg/kg) | 177.84 ± 9.92 b | 79.83 ± 5.7 b |
| III | Isoproterenol + BG oil (100 mg/kg) | 128.50 ± 6.86 c | 43.24 ± 5.46 c |
| IV | Isoproterenol + the optimized BG-SDLF (100 mg/kg) | 109.36 ± 16.61 d | 29.17 ± 4.80 d |
| V | Isoproterenol + omega 3 (100 mg/kg) | 102.47 ± 10.54 d | 34.08 ± 3.53 e |
| Group No. | Group Description | Cystatin C (mg/L) | Galectin-3 (ng/mL) | Lp-PLA2 (ng/mL) | MMP2 (mg/mL) |
|---|---|---|---|---|---|
| I | Normal control | 0.84 ± 0.02 a | 42.71 ± 3.53 a | 327.20 ± 22.75 a | 4.90 ± 0.30 a |
| II | Isoproterenol (85 mg/kg) | 2.37 ± 0.21 b | 80.34 ± 5.35 b | 485.90 ± 30.63 b | 20.56 ± 1.86 b |
| III | Isoproterenol + BG oil (100 mg/kg) | 1.56 ± 0.08 c | 62.80 ± 6.13 c | 402.97 ± 20.75 c | 13.37 ± 0.57 c |
| IV | Isoproterenol + optimized BG-SDLF (100 mg/kg) | 1.24 ± 0.19 c | 44.89 ± 2.41 a | 355.82 ± 27.48 d | 7.26 ± 0.37 d |
| V | Isoproterenol + omega 3 (100 mg/kg) | 1.49 ± 0.06 c | 53.21 ± 3.64 d | 368.31 ± 30.21 e | 11.10 ± 1.46 c |
| Group No. | Group Description | cTnI (ng/mL) | cTnT (ng/mL) |
|---|---|---|---|
| I | Normal control | 0.34 ± 0.05 a | 0.56 ± 0.06 a |
| II | Isoproterenol (85 mg/kg) | 0.72 ± 0.06 b | 1.10 ± 0.09 b |
| III | Isoproterenol + BG oil (100 mg/kg) | 0.52 ± 0.04 c | 0.79 ± 0.07 c |
| IV | Isoproterenol + optimized BG-SDLF (100 mg/kg) | 0.36 ± 0.02 d | 0.62 ± 0.03 d |
| V | Isoproterenol + omega 3 (100 mg/kg) | 0.38 ± 0.03 d | 0.69 ± 0.04 e |
| Factors (Independent Variables) | Levels | Optimized Level * | ||
|---|---|---|---|---|
| X1: Oil concentration (%) | 20 | 30 | 40 | 30 |
| X2: T 80: Cr RH40 ratio | 2:1 | 1:1 | 1:2 | 1:1 |
| Responses (Dependent variables) | Formulation optimization Desirability constraints | Predicted values | ||
| Y1: DS (nm) | Minimize | 29.5 | ||
| Y2: PDI | Minimize | 0.141 | ||
| Y3: ET (min) | Minimize | 5.9 | ||
| Y4: Tr (%) | Maximize | 99.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mancy, S.S.; Boshra, S.A.; Elnahas, O.S.; Fayez, S.M.; Sheta, N.M. Enhancement of Bottle Gourd Oil Activity via Optimized Self-Dispersing Lipid Formulations (SDLFs) to Mitigate Isoproterenol-Evoked Cardiac Toxicity in Rats via Modulating BNP, MMP2, and miRNA-21 and miRNA-23a Genes’ Expression. Molecules 2023, 28, 6168. https://doi.org/10.3390/molecules28166168
El-Mancy SS, Boshra SA, Elnahas OS, Fayez SM, Sheta NM. Enhancement of Bottle Gourd Oil Activity via Optimized Self-Dispersing Lipid Formulations (SDLFs) to Mitigate Isoproterenol-Evoked Cardiac Toxicity in Rats via Modulating BNP, MMP2, and miRNA-21 and miRNA-23a Genes’ Expression. Molecules. 2023; 28(16):6168. https://doi.org/10.3390/molecules28166168
Chicago/Turabian StyleEl-Mancy, Shereen S., Sylvia A. Boshra, Osama S. Elnahas, Sahar M. Fayez, and Nermin M. Sheta. 2023. "Enhancement of Bottle Gourd Oil Activity via Optimized Self-Dispersing Lipid Formulations (SDLFs) to Mitigate Isoproterenol-Evoked Cardiac Toxicity in Rats via Modulating BNP, MMP2, and miRNA-21 and miRNA-23a Genes’ Expression" Molecules 28, no. 16: 6168. https://doi.org/10.3390/molecules28166168
APA StyleEl-Mancy, S. S., Boshra, S. A., Elnahas, O. S., Fayez, S. M., & Sheta, N. M. (2023). Enhancement of Bottle Gourd Oil Activity via Optimized Self-Dispersing Lipid Formulations (SDLFs) to Mitigate Isoproterenol-Evoked Cardiac Toxicity in Rats via Modulating BNP, MMP2, and miRNA-21 and miRNA-23a Genes’ Expression. Molecules, 28(16), 6168. https://doi.org/10.3390/molecules28166168









