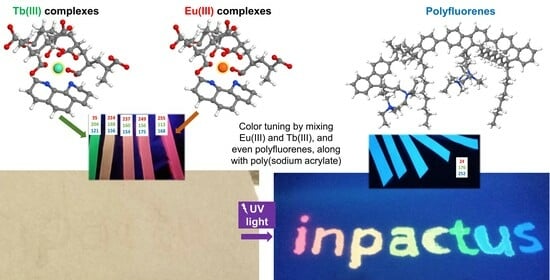
Luminescent Papers with Asymmetric Complexes of Eu(III) and Tb(III) in Polymeric Matrices and Suggested Combinations for Color Tuning
Abstract
:1. Introduction
2. Results
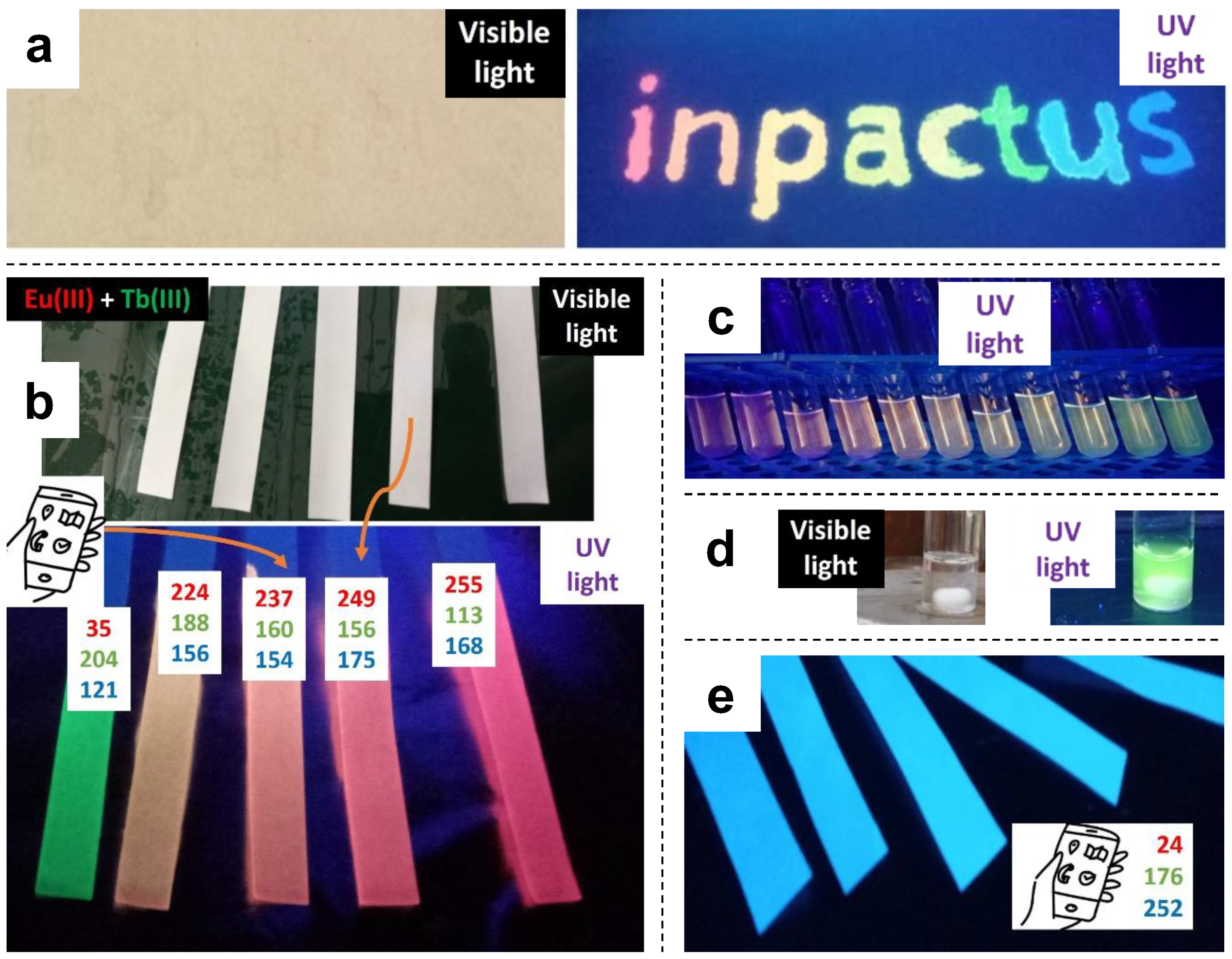
2.1. General View on the Usability for Authentication Purposes
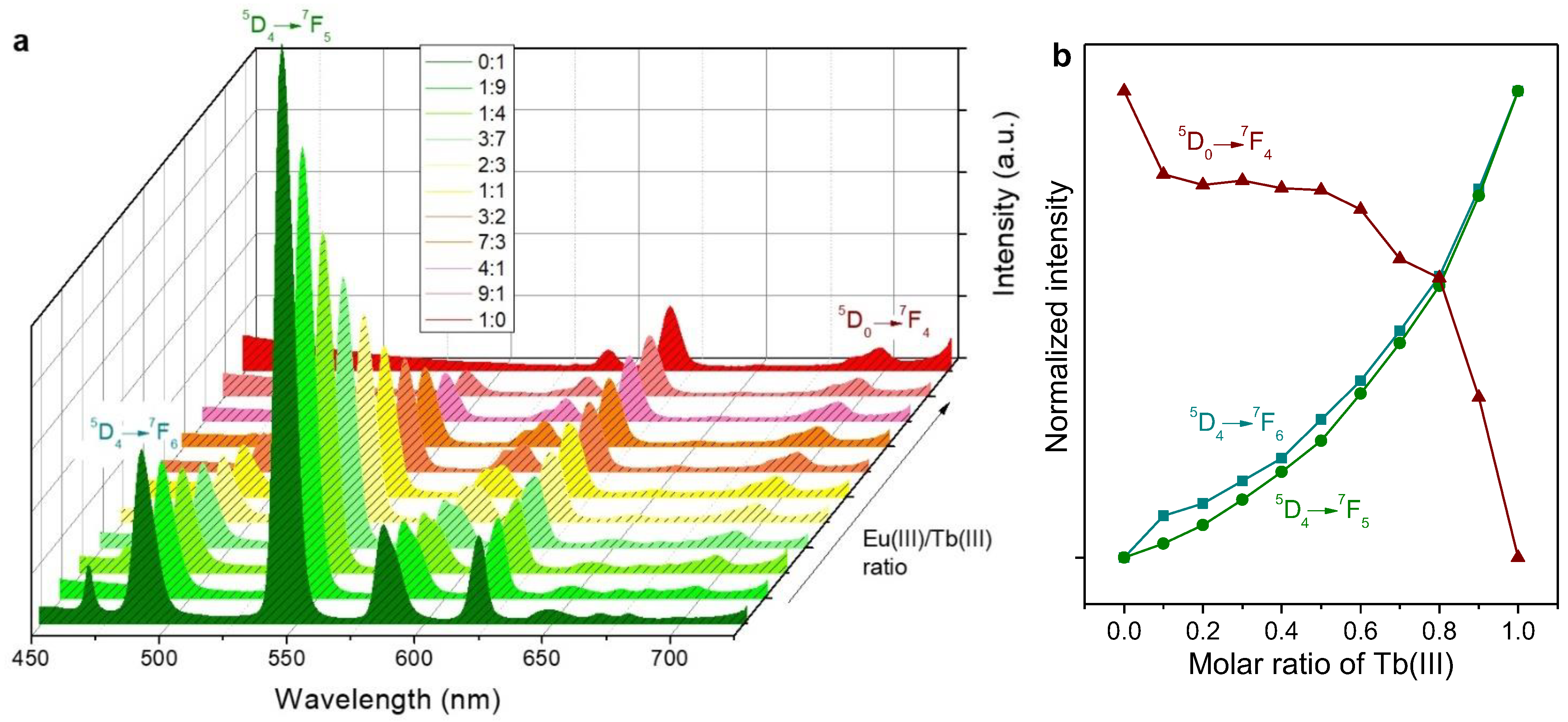
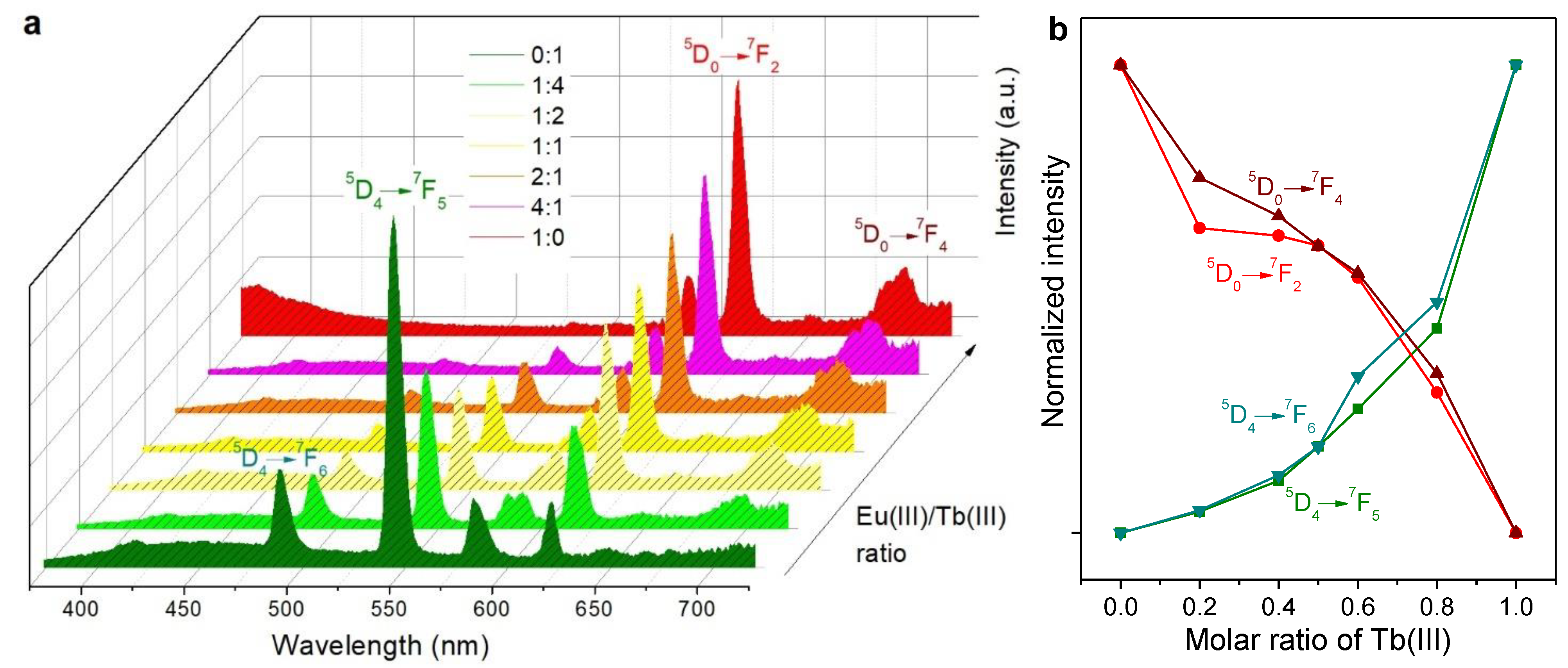
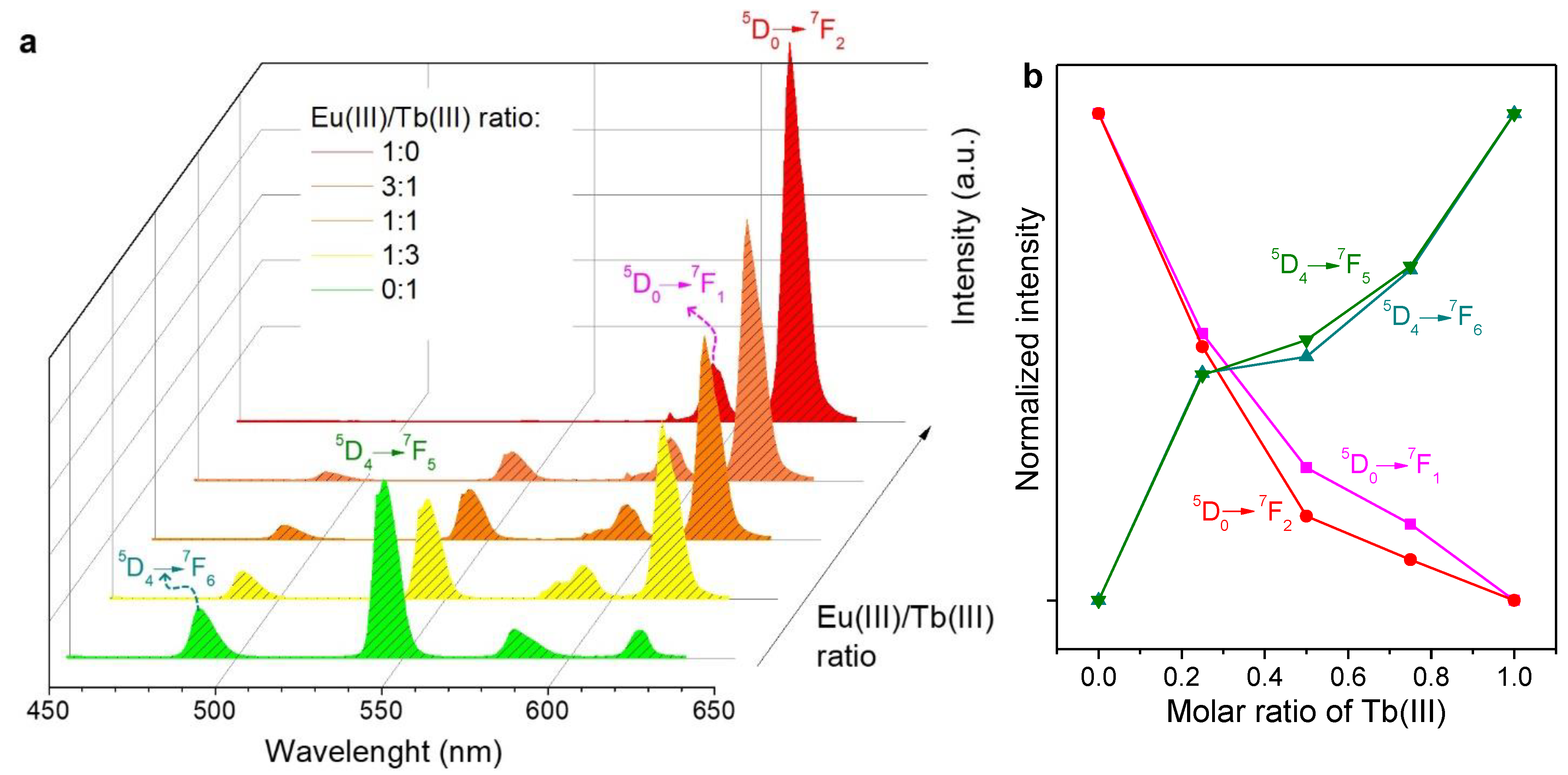
2.2. Emission Spectra in Solution
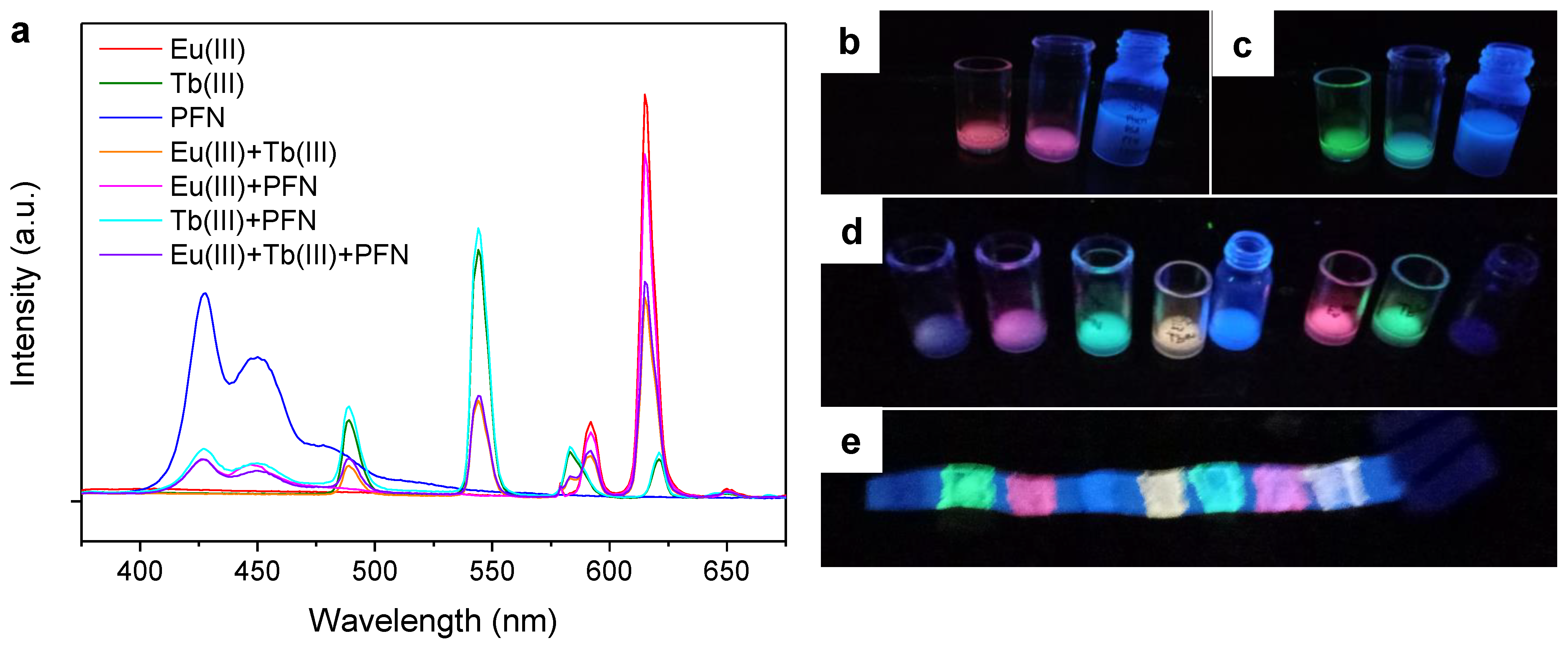
2.3. Emission Spectra of Coated Papers
2.4. Further Color Tuning with Polyfluorenes
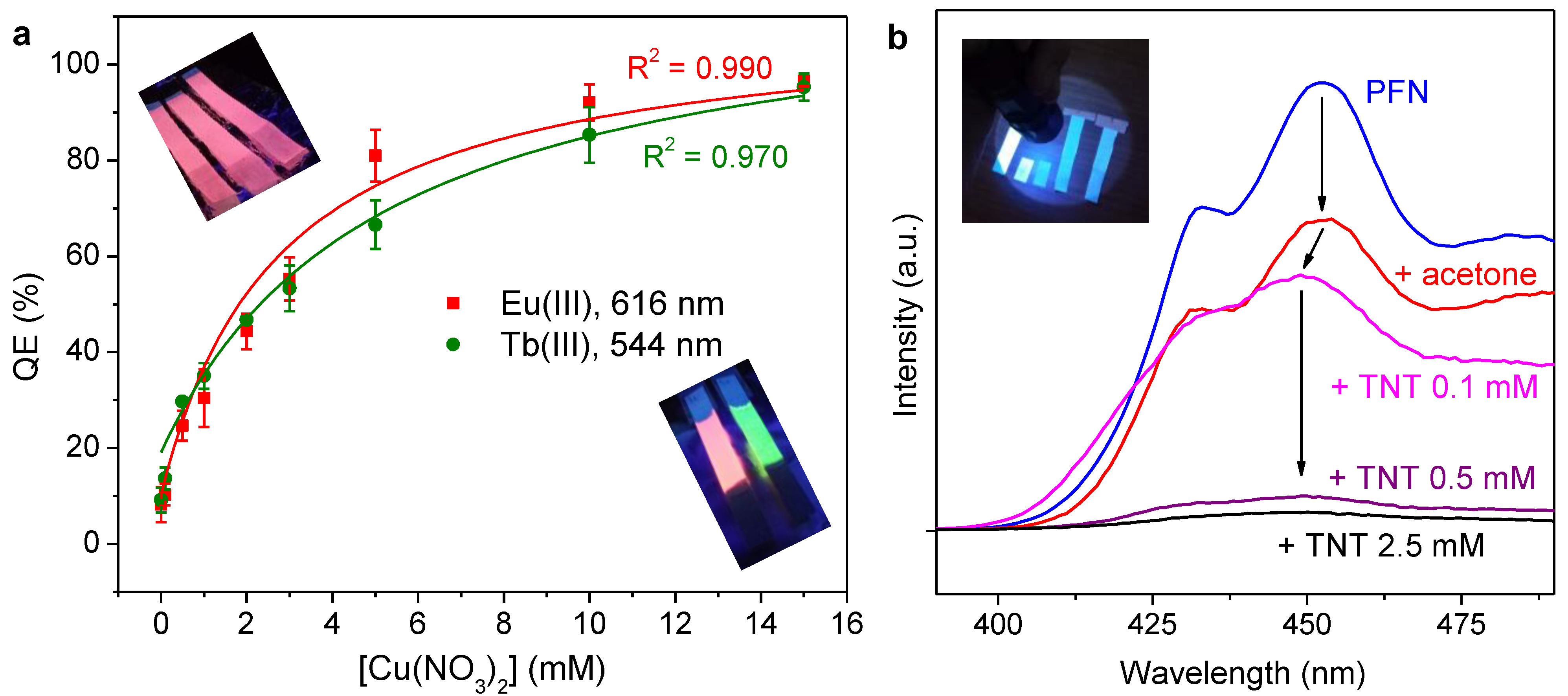
2.5. Quenching of Luminescence
3. Discussion
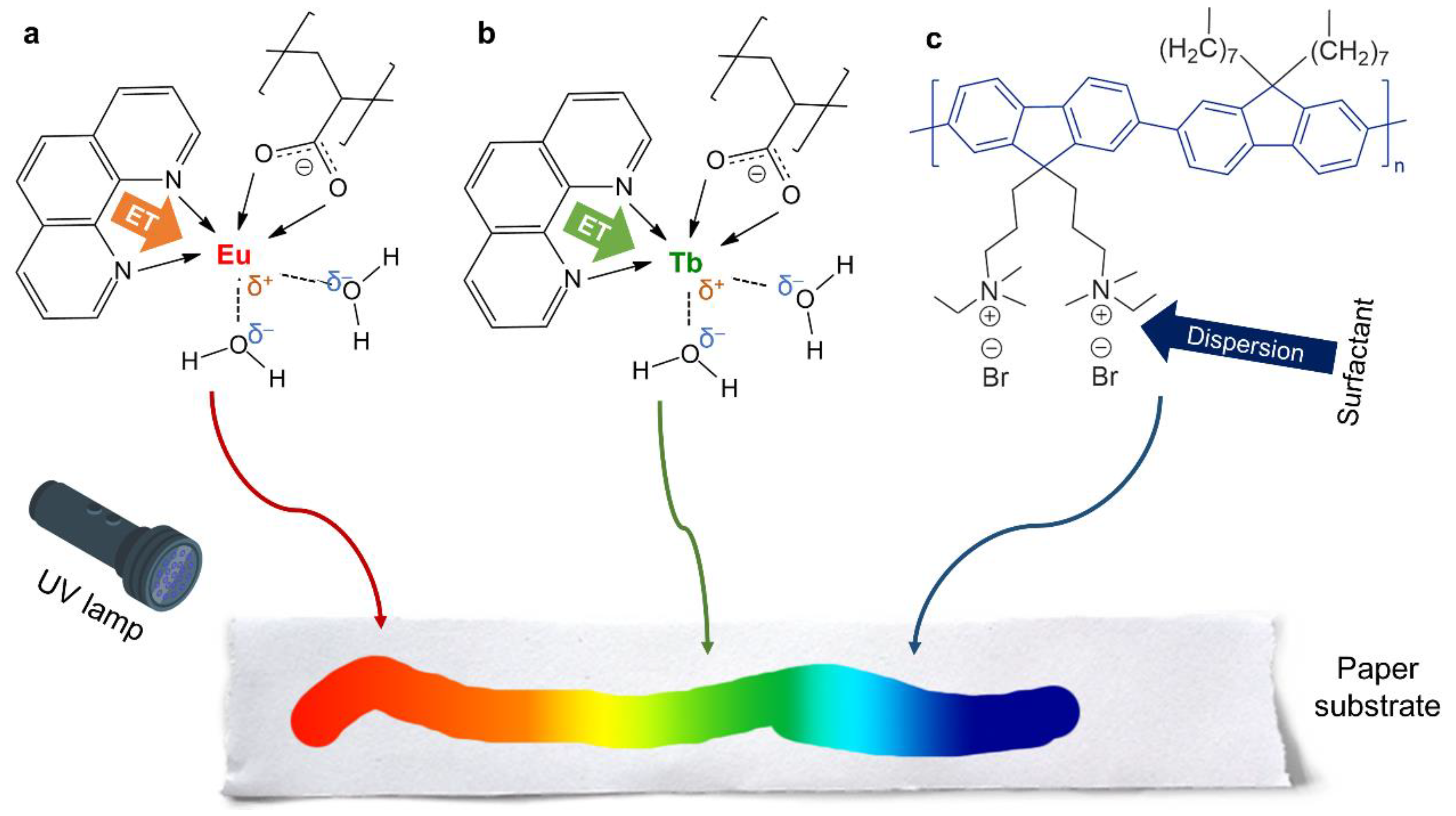
3.1. Insights into the Antenna Effect and Wet/Dry Differences
3.2. On Luminescence Quenching
4. Materials and Methods
4.1. Materials
4.2. Preparation of Inks
4.3. Dip Coating
4.4. Spectrofluorometry
4.5. Quenching Assays
5. Conclusions
- Mixtures of Tb(III)/PSA and Eu(III)/PSA resulted in luminescent solutions with tunable color in the green-to-red range, including yellow and orange, but with low emission intensity.
- The antenna effect, essentially consisting of energy transfer from Phen to the metal center of the complex (either europium or terbium), greatly increased luminescence but favored Eu(III) over Tb(III).
- Adding a polyfluorene, stabilized in an aqueous medium with SDS, and avoiding the use of co-solvents that would result in the precipitation of PFN, allowed us to attain a blue–red color range, including cyan and magenta.
- Impregnated papers, once dry, required shorter wavelengths of UV radiation for proper excitation than their aqueous counterparts.
- Color tuning possibilities, by themselves, allowed for anti-counterfeiting applications, as papers that look white under natural light can display complex full-color information under UV radiation.
- Although Cu(II) was proven an effective quencher for Ln(III)-coated papers, attempts to implement this feature in paper-based analytical devices faces the limitation of a high limit of detection. Instead, responsiveness towards Cu(II) can also be taken advantage of for anti-counterfeiting purposes.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cruz-Navarro, A.; Hernández-Romero, D.; Flores-Parra, A.; Rivera, J.M.; Castillo-Blum, S.E.; Colorado-Peralta, R. Structural Diversity and Luminescent Properties of Coordination Complexes Obtained from Trivalent Lanthanide Ions with the Ligands: Tris((1H-Benzo[d]Imidazol-2-Yl)Methyl)Amine and 2,6-Bis(1H-Benzo[d]Imidazol-2-Yl)Pyridine. Coord. Chem. Rev. 2021, 427, 213587. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of Lanthanide Complexes: From Fundamental to Prospective Approaches Related to Water- and Molecular-Stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Mumtaz, F.; Jaffari, G.H.; Khan, G.S. Correlation between the Unit Cell Parameters and Electronic Transitions in Bi1-XEuxFeO3 Thin Films. Mater. Chem. Phys. 2023, 300, 127541. [Google Scholar] [CrossRef]
- Qin, T.; Shi, Z.; Zhang, W.; Dong, X.; An, N.; Sakiyama, H.; Muddassir, M.; Srivastava, D.; Kumar, A. 2D Isostructural Ln(III)-Based Coordination Polymer Derived from Imidazole Carboxylic Acid: Synthesis, Structure and Magnetic Behavior. J. Mol. Struct. 2023, 1282, 135220. [Google Scholar] [CrossRef]
- Matsushita, A.F.Y.; Tapia, M.J.; Pais, A.A.C.C.; Valente, A.J.M. Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism. Polymers 2020, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Romanova, J.; Lyapchev, R.; Kolarski, M.; Tsvetkov, M.; Elenkova, D.; Morgenstern, B.; Zaharieva, J. Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands. Molecules 2023, 28, 4113. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A.; Salinas-Castillo, A. Fluorescent Conjugated Polymers for Chemical and Biochemical Sensing. TrAC Trends Anal. Chem. 2011, 30, 1513–1525. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(Vinyl Alcohol) as Versatile Biomaterial for Potential Biomedical Applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Knaapila, M.; Winokur, M.J. Structure and Morphology of Polyfluorenes in Solutions and the Solid State BT. In Polyfluorenes; Scherf, U., Neher, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 227–272. ISBN 978-3-540-68734-4. [Google Scholar]
- Quintero-Jaime, A.F.; Hafed-Khatiri, S.; Huerta, F.; Quijada, C.; Montilla, F. Dynamics and Coherence of Photoexcited States in Polyfluorene Films with Ordered Chain Phases. J. Mater. Chem. C 2022, 10, 11801–11809. [Google Scholar] [CrossRef]
- Andres, J.; Hersch, R.D.; Moser, J.E.; Chauvin, A.S. A New Anti-Counterfeiting Feature Relying on Invisible Luminescent Full Color Images Printed with Lanthanide-Based Inks. Adv. Funct. Mater. 2014, 24, 5029–5036. [Google Scholar] [CrossRef]
- Aguado, R.; Santos, A.R.M.G.; Vallejos, S.; Valente, A.J.M. Paper-Based Probes with Visual Response to Vapors from Nitroaromatic Explosives: Polyfluorenes and Tertiary Amines. Molecules 2022, 27, 2900. [Google Scholar] [CrossRef] [PubMed]
- Aguado, R.; Murtinho, D.; Valente, A.J.M. A Broad Overview on Innovative Functionalized Paper Solutions. Nord. Pulp Pap. Res. J. 2019, 34, 395–416. [Google Scholar] [CrossRef]
- Tian, X.; Peng, H.; Li, Y.; Yang, C.; Zhou, Z.; Wang, Y. Highly Sensitive and Selective Paper Sensor Based on Carbon Quantum Dots for Visual Detection of TNT Residues in Groundwater. Sens. Actuators B Chem. 2017, 243, 1002–1009. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Jinyang, S.; Wu, X.; Sun, Y.; Pan, X.; Li, D. Miniature Fluorescent Sensor for Chloride Ion Concentration Determination Based on Modified Stern–Volmer Equation. Measurement 2013, 46, 3982–3987. [Google Scholar] [CrossRef]
- Matsushita, A.F.Y.; Pais, A.A.C.C.; Valente, A.J.M. Energy Transfer and Multicolour Tunable Emission of Eu,Tb(PSA)Phen Composites. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 569, 93–101. [Google Scholar] [CrossRef]
- Hill, B.; Roger, T.; Vorhagen, F.W. Comparative Analysis of the Quantization of Color Spaces on the Basis of the CIELAB Color-Difference Formula. ACM Trans. Graph. 1997, 16, 109–154. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Sang, X.; Hu, H.; Li, S.; Zhang, D.; Liu, C.; Wang, Q.; Zhang, B.; Wang, W.; et al. Modulated Luminescence of Lanthanide Materials by Local Surface Plasmon Resonance Effect. Nanomaterials 2021, 11, 1037. [Google Scholar] [CrossRef]
- Zhou, W.-L.; Chen, Y.; Lin, W.; Liu, Y. Luminescent Lanthanide–Macrocycle Supramolecular Assembly. Chem. Commun. 2021, 57, 11443–11456. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Mechanisms and Dynamics of Fluorescence Quenching BT. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 331–351. ISBN 978-0-387-46312-4. [Google Scholar]
- Tapia, M.J.; Burrows, H.D. Cation Polyelectrolyte Interactions in Aqueous Sodium Poly(Vinyl Sulfonate) as Seen by Ce3+ to Tb3+ Energy Transfer. Langmuir 2002, 18, 1872–1876. [Google Scholar] [CrossRef]
- Costa, D.; Miguel, M.G.; Lindman, B. Effect of Additives on Swelling of Covalent DNA Gels. J. Phys. Chem. B 2007, 111, 8444–8452. [Google Scholar] [CrossRef]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactant–Polymer Systems. In Surfactants and Polymers in Aqueous Solution; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 277–303. ISBN 9780470856420. [Google Scholar]
- Guembe-García, M.; González-Ceballos, L.; Arnaiz, A.; Fernández-Muiño, M.A.; Sancho, M.T.; Osés, S.M.; Ibeas, S.; Rovira, J.; Melero, B.; Represa, C.; et al. Easy Nitrite Analysis of Processed Meat with Colorimetric Polymer Sensors and a Smartphone App. ACS Appl. Mater. Interfaces 2022, 14, 37051–37058. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Tao, J.; Zhang, M.; Pan, H.; Xu, X.; Tang, R. Surface Modification of Hydroxyapatite Nanocrystallite by a Small Amount of Terbium Provides a Biocompatible Fluorescent Probe. J. Phys. Chem. C 2008, 112, 12219–12224. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Zheng, Y.; Jia, G.; Zhang, L.; Huang, Y.; Yang, M.; Song, Y.; Zhang, H. Facile Shape-Controlled Synthesis of Luminescent Europium Benzene-1,3,5-Tricarboxylate Architectures at Room Temperature. CrystEngComm 2009, 11, 2622–2628. [Google Scholar] [CrossRef]
- Huang, F.; Wu, H.; Wang, D.; Yang, W.; Cao, Y. Novel Electroluminescent Conjugated Polyelectrolytes Based on Polyfluorene. Chem. Mater. 2004, 16, 708–716. [Google Scholar] [CrossRef]
- Dong, W.; Fei, T.; Scherf, U. Conjugated Polymers Containing Tetraphenylethylene in the Backbones and Side-Chains for Highly Sensitive TNT Detection. RSC Adv. 2018, 8, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, C.A.; Brito, H.F.; Malta, O.L.; Serra, O.A. Luminescence and Energy Transfer of the Europium (III) Tungstate Obtained via the Pechini Method. J. Lumin. 2003, 101, 11–21. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J. Perspective on Europium Activated Fine-Grained Metal Molybdate Phosphors for Solid State Illumination. J. Mater. Chem. 2011, 21, 3788–3795. [Google Scholar] [CrossRef]
- Alexander, D.; Joy, M.; Thomas, K.; Sisira, S.; Biju, P.R.; Unnikrishnan, N.V.; Sudarsanakumar, C.; Ittyachen, M.A.; Joseph, C. Efficient Green Luminescence of Terbium Oxalate Crystals: A Case Study with Judd-Ofelt Theory and Single Crystal Structure Analysis and the Effect of Dehydration on Luminescence. J. Solid State Chem. 2018, 262, 68–78. [Google Scholar] [CrossRef]
- Grüll, F.; Voitkiv, A.B.; Müller, C. Interatomic-Distance Dependence of Resonant Energy-Transfer Phenomena. Phys. Rev. Res. 2020, 2, 33303. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Chen, Y.; Gong, M. Energy Transfer and Multicolor Tunable Emission in Single-Phase Tb3+, Eu3+ Co-Doped Sr3La(PO4)3 Phosphors. Ceram. Int. 2016, 42, 13919–13924. [Google Scholar] [CrossRef]
- Ding, M.; Chen, D.; Ma, D.; Dai, J.; Li, Y.; Ji, Z. Highly Enhanced Upconversion Luminescence in Lanthanide-Doped Active-Core/Luminescent-Shell/Active-Shell Nanoarchitectures. J. Mater. Chem. C 2016, 4, 2432–2437. [Google Scholar] [CrossRef]
- Ding, M.; Dong, B.; Lu, Y.; Yang, X.; Yuan, Y.; Bai, W.; Wu, S.; Ji, Z.; Lu, C.; Zhang, K.; et al. Energy Manipulation in Lanthanide-Doped Core–Shell Nanoparticles for Tunable Dual-Mode Luminescence toward Advanced Anti-Counterfeiting. Adv. Mater. 2020, 32, 2002121. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Yuan, Y.; Ding, M.; Bai, W.; Wu, S.; Ji, Z. Efficient Dual-Mode Luminescence from Lanthanide-Doped Core-Shell Nanoarchitecture for Anti-Counterfeiting Applications. Nanotechnology 2020, 31, 365705. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Muthukumaran, J.; Jain, M.; Tariq, M.; Al-Lohedan, H.A.; Al-Sanea, A.S.S. Detailed Experimental and In Silico Investigation of Indomethacin Binding with Human Serum Albumin Considering Primary and Secondary Binding Sites. Molecules 2023, 28, 2979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pei, J.; Li, G. Lanthanide Ternary Complex as a Fluorescent Probe for Highly Sensitive and Selective Detection of Copper Ions Based on Selective Recognition and Photoinduced Electron Transfer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122287. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Q.; Li, G.-P.; Liu, W.-C.; Li, Q.-L.; Li, B.-H.; Gable, R.W.; Hou, L.; Batten, S.R. Two Unusual Nanocage-Based Ln-MOFs with Triazole Sites: Highly Fluorescent Sensing for Fe3+ and Cr2O72−, and Selective CO2 Capture. Chempluschem 2016, 81, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Habib, F.; Qazi, M.J.; Ganayee, M.A.; Ahmad, Z.; Yatoo, M.A. A Short Review Article on Conjugated Polymers. J. Polym. Res. 2023, 30, 115. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Q.; Liu, L.; Wang, S. Conjugated Polymers for Sensitive Chemical Sensors. Prog. Chem. 2011, 23, 1993–2002. [Google Scholar]
- Pina, J.; de Castro, C.S.; Delgado-Pinar, E.; Sérgio Seixas de Melo, J. Characterization of 4-Methylesculetin and of Its Mono- and Di-Methoxylated Derivatives in Water and Organic Solvents in Its Ground, Singlet and Triplet Excited States. J. Mol. Liq. 2019, 278, 616–626. [Google Scholar] [CrossRef]
| System | In Solution | On Paper |
|---|---|---|
| Ln(III)/PSA | Eu(III): UV-A; Tb(III): UV-B/UV-A | UV-B/UV-C |
| Ln(III)/PSA/Phen | UV-A/UV-B. Max. ~ 336–348 nm | UV-B/UV-C. Max. < 270 nm |
| PFN/SDS | Visible–UV-B. Max. ~ 413 nm | UV-A–UV-C. Max. ~ 370 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado, R.J.; Gomes, B.O.; Durães, L.; Valente, A.J.M. Luminescent Papers with Asymmetric Complexes of Eu(III) and Tb(III) in Polymeric Matrices and Suggested Combinations for Color Tuning. Molecules 2023, 28, 6164. https://doi.org/10.3390/molecules28166164
Aguado RJ, Gomes BO, Durães L, Valente AJM. Luminescent Papers with Asymmetric Complexes of Eu(III) and Tb(III) in Polymeric Matrices and Suggested Combinations for Color Tuning. Molecules. 2023; 28(16):6164. https://doi.org/10.3390/molecules28166164
Chicago/Turabian StyleAguado, Roberto J., Beatriz O. Gomes, Luisa Durães, and Artur J. M. Valente. 2023. "Luminescent Papers with Asymmetric Complexes of Eu(III) and Tb(III) in Polymeric Matrices and Suggested Combinations for Color Tuning" Molecules 28, no. 16: 6164. https://doi.org/10.3390/molecules28166164
APA StyleAguado, R. J., Gomes, B. O., Durães, L., & Valente, A. J. M. (2023). Luminescent Papers with Asymmetric Complexes of Eu(III) and Tb(III) in Polymeric Matrices and Suggested Combinations for Color Tuning. Molecules, 28(16), 6164. https://doi.org/10.3390/molecules28166164