Exploring the Influence of Chemical Conditions on Nanoparticle Graphene Oxide Adsorption onto Clay Minerals
Abstract
:1. Introduction
2. Results and Discussion
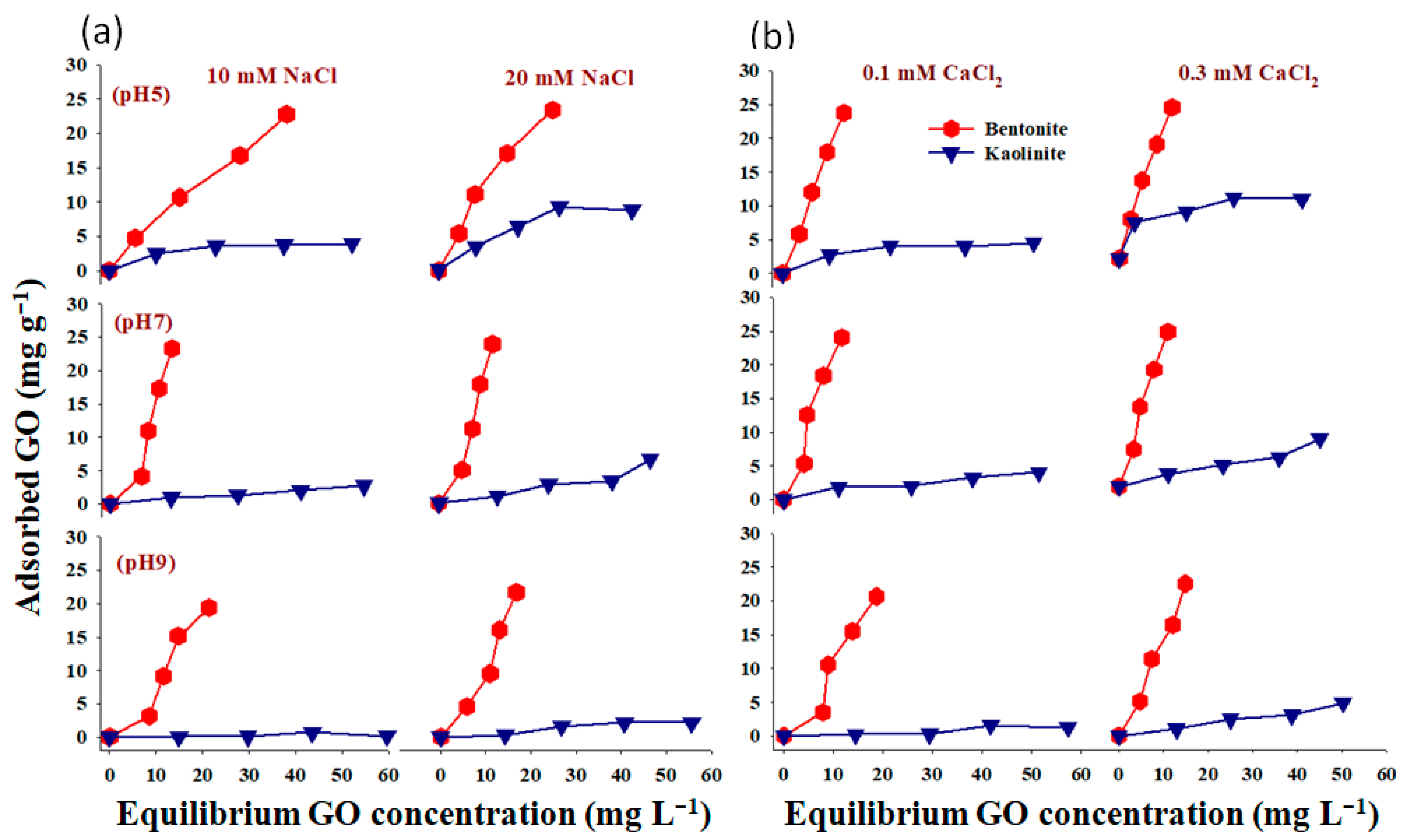
2.1. Influence of Concentrations on GO Nanoparticle Adsorption
2.2. Influence of Clay Minerals on GO Nanoparticles Adsorption
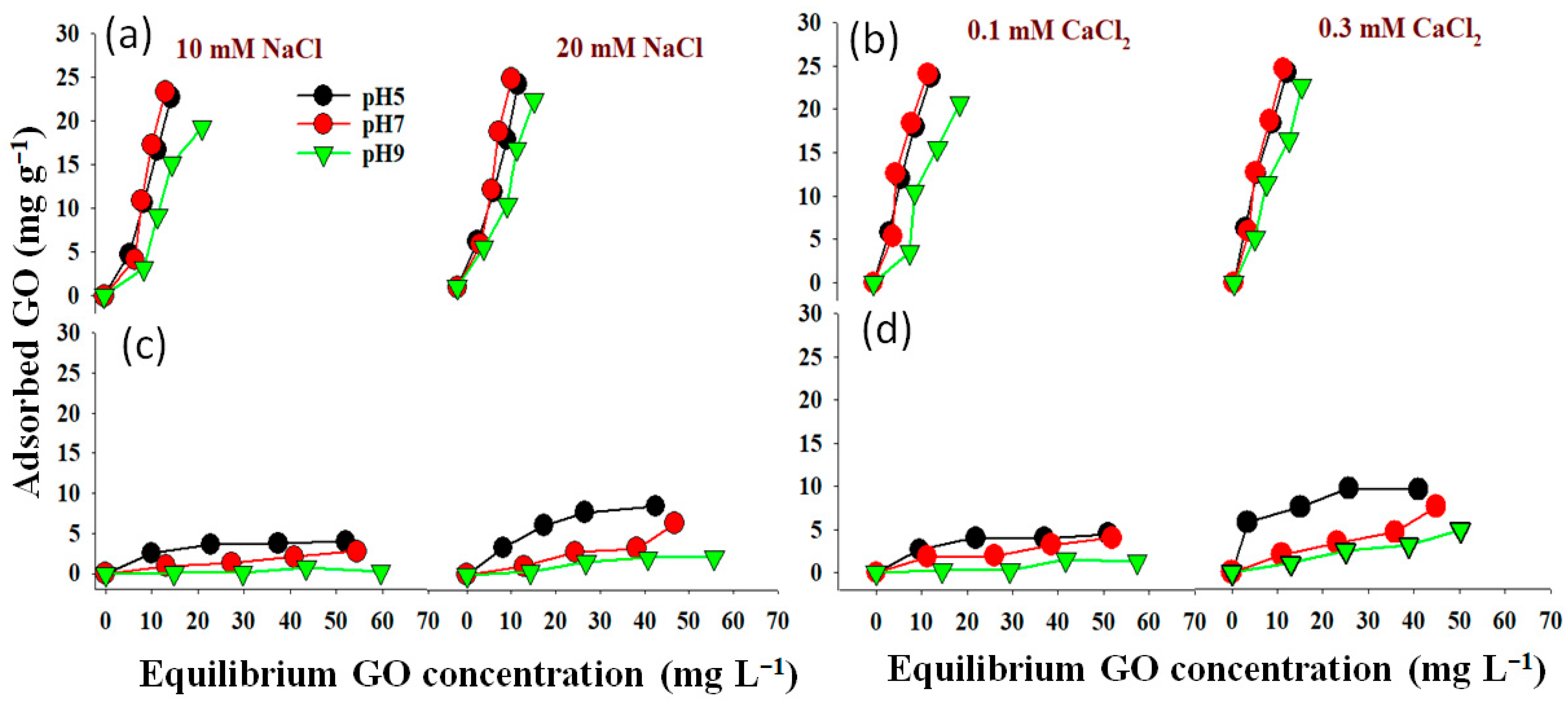
2.3. Influence of pH on GO Nanoparticles Adsorption
2.4. The Relationship between Ionic Strength and GO Nanoparticle Adsorption
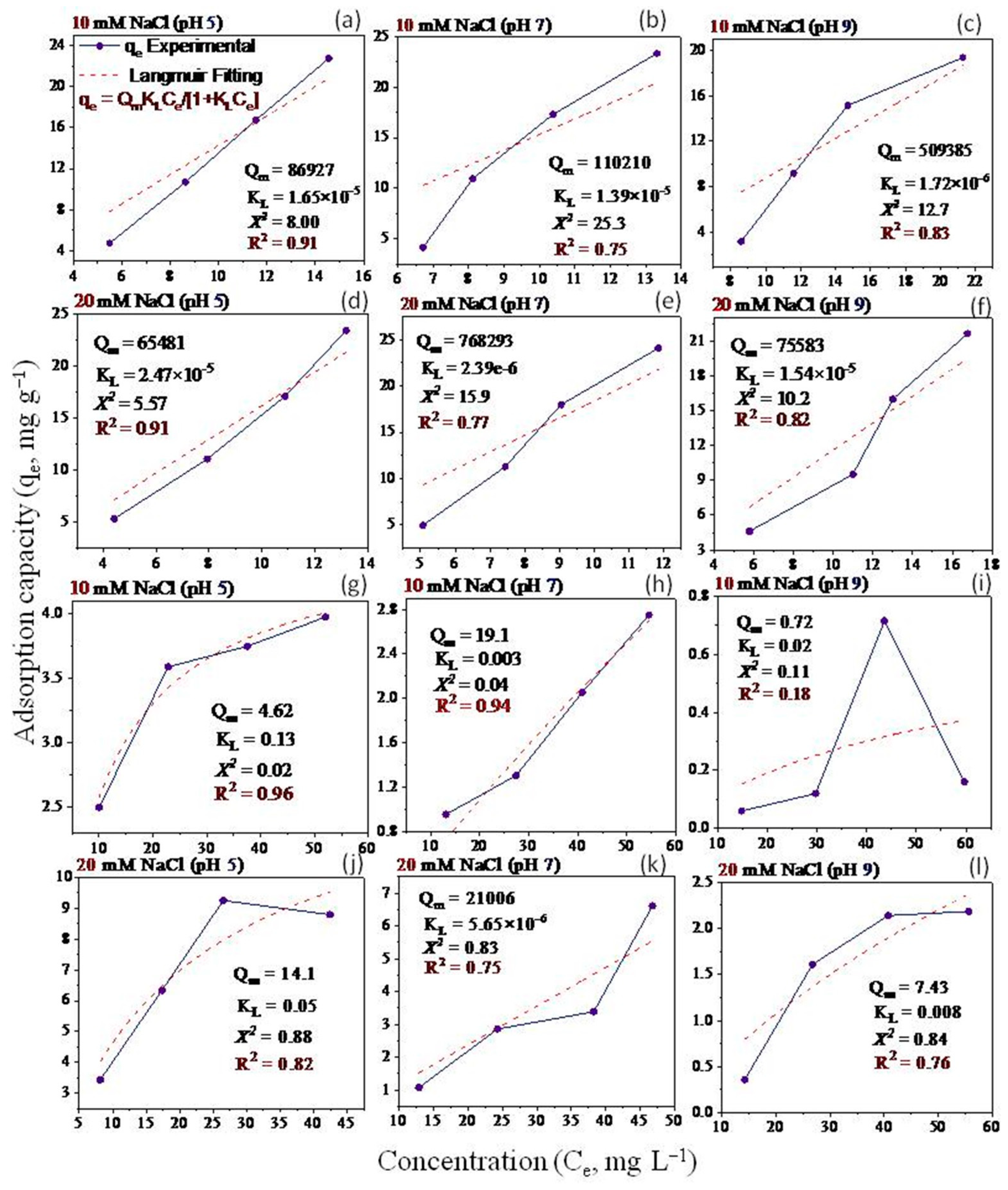
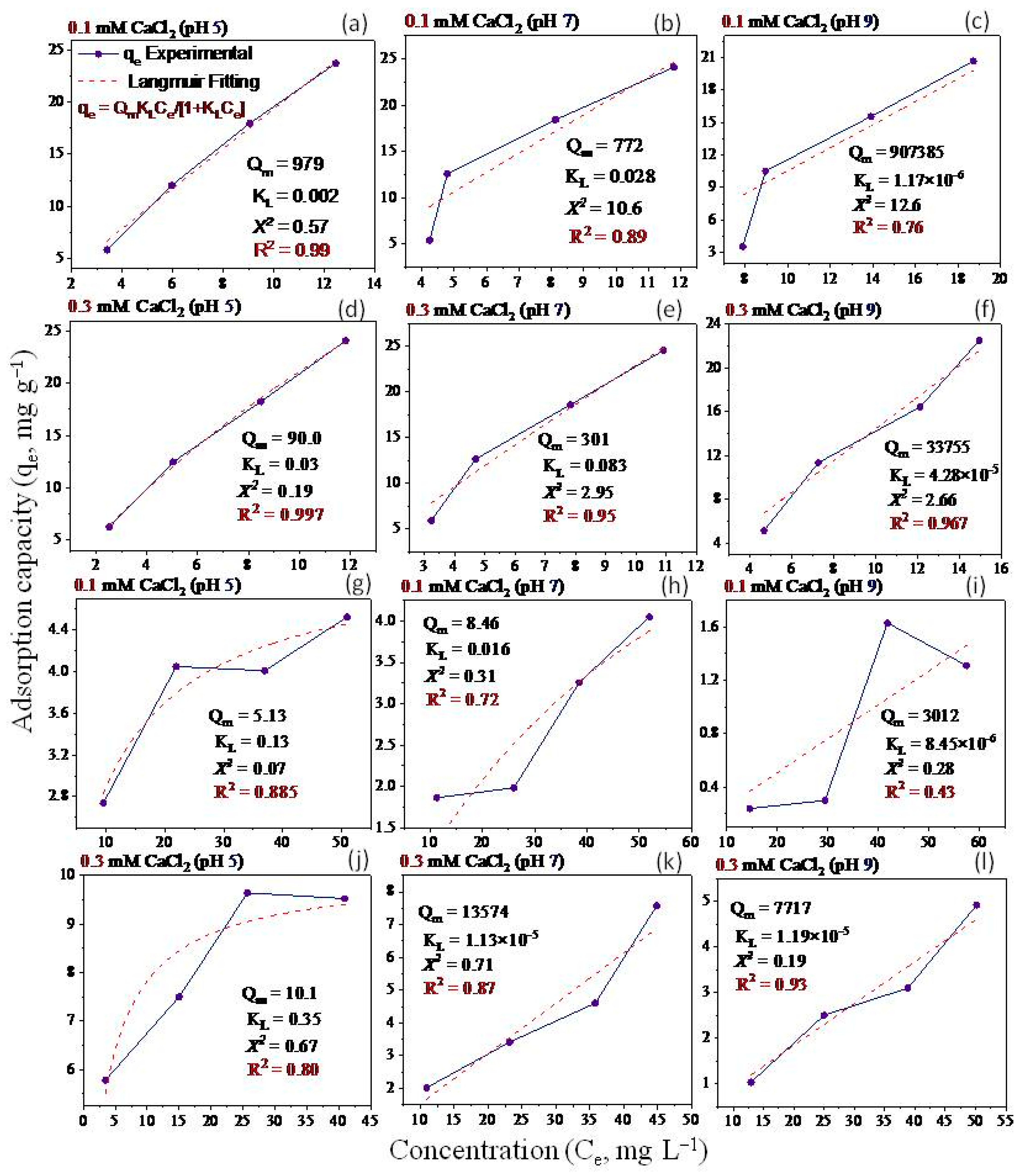
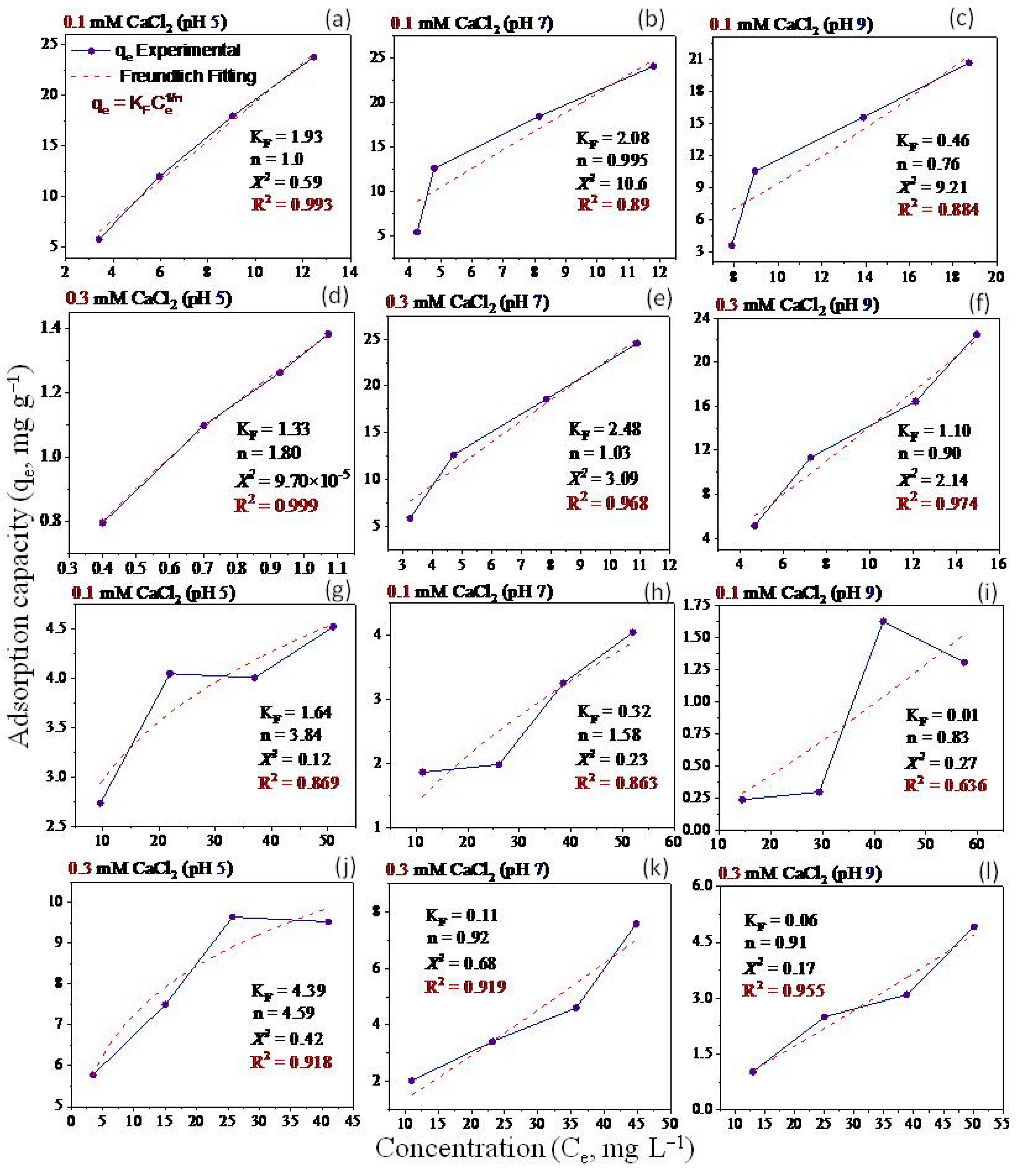
2.5. Adsorption Isotherms
2.6. Environmental Implications
2.7. The Limitations of the Study
3. Materials and Methods
3.1. Synthetic of GO Nanoparticles
3.2. Adsorption of GO Nanoparticles on Clay Minerals under Different Chemical Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shen, C.; Lazouskaya, V.; Zhang, H.; Li, B.; Jin, Y.; Huang, Y. Influence of surface chemical heterogeneity on attachment and detachment of microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 14–29. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, R.; Liu, B.; Wang, J.; Wang, S.; Han, M.Y.; Zhang, Z. Π-conjugated carbon radicals at graphene oxide to initiate ultrastrong chemiluminescence. Angew. Chem. Int. Ed. 2014, 53, 10109–10113. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wang, L.; Fan, Q.; Pan, D.; Li, J.; Chi, F.; Xie, Y.; Yu, S.; Xiao, C. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China Chem. 2019, 62, 933–967. [Google Scholar] [CrossRef]
- Kong, Q.; Preis, S.; Li, L.; Luo, P.; Wei, C.; Li, Z.; Hu, Y.; Wei, C. Relations between metal ion characteristics and adsorption performance of graphene oxide: A comprehensive experimental and theoretical study. Sep. Purif. Technol. 2020, 232, 115956. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Pang, H.; Yu, S.; Ai, Y.; Ma, X.; Song, G.; Hayat, T.; Alsaedi, A.; Wang, X. Effect of graphene oxide surface modification on the elimination of Co (II) from aqueous solutions. Chem. Eng. J. 2018, 344, 380–390. [Google Scholar] [CrossRef]
- He, J.-Z.; Li, Z.-Z.; Wang, D.-J.; Zhou, D.-M. Biofilms and extracellular polymeric substances mediate the transport of graphene oxide nanoparticles in saturated porous media. J. Hazard. Mater. 2015, 300, 467–474. [Google Scholar]
- Mazive, P.A.; Hu, B.; Zhu, H.; He, W.; Mazive, A.M. Graphene Oxide-Based Nanomaterials: The Preparation, Application, and Factors that Affect the Adsorption Capacity on Drinking Water Treatment-Review. J. Nanotechnol. Res. 2020, 2, 060–091. [Google Scholar]
- Lanphere, J.D.; Rogers, B.; Luth, C.; Bolster, C.H.; Walker, S.L. Stability and transport of graphene oxide nanoparticles in groundwater and surface water. Environ. Eng. Sci. 2014, 31, 350–359. [Google Scholar] [CrossRef]
- Zhou, D.; Jiang, X.; Lu, Y.; Fan, W.; Huo, M.; Crittenden, J. Cotransport of graphene oxide and Cu (II) through saturated porous media. Sci. Total Environ. 2016, 550, 717–726. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Jaisi, D.P. Cotransport of hydroxyapatite nanoparticles and hematite colloids in saturated porous media: Mechanistic insights from mathematical modeling and phosphate oxygen isotope fractionation. J. Contam. Hydrol. 2015, 182, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, Y.-n.; Zhu, M.; Li, X.; Keller, A.A.; Wang, T.; Li, F. Heteroaggregation of engineered nanoparticles and kaolin clays in aqueous environments. Water Res. 2015, 80, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Marouf, R.; Dali, N.; Boudouara, N.; Ouadjenia, F.; Zahaf, F. Study of adsorption properties of bentonite clay. In Montmorillonite Clay; IntechOpen: London, UK, 2021. [Google Scholar]
- Sotirelis, N.P.; Chrysikopoulos, C.V. Heteroaggregation of graphene oxide nanoparticles and kaolinite colloids. Sci. Total Environ. 2017, 579, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, L.; Kang, H.; Li, N.; Wang, W.; Jiang, P. Study on Adsorption Properties and Mechanism of Graphene Oxide (GO) by Kaolin. Nat. Environ. Pollut. Technol. 2022, 21, 1357–1365. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Wu, K.; Wei, X.L.; Zhang, Y.; Chen, J.; Deng, C.X.; Jin, J.; Yu, Z.M.; Wang, L. Rectorite-supported nano-Fe3O4 composite materials as catalyst for P-chlorophenol degradation: Preparation, characterization, and mechanism. Appl. Clay Sci. 2019, 176, 66–77. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- He, G.; Wang, C.; Cao, J.; Fan, L.; Zhao, S.; Chai, Y. Carboxymethyl chitosan-kaolinite composite hydrogel for efficient copper ions trapping. J. Environ. Chem. Eng. 2019, 7, 102953. [Google Scholar] [CrossRef]
- Lu, X.; Lu, T.; Zhang, H.; Shang, Z.; Chen, J.; Wang, Y.; Li, D.; Zhou, Y.; Qi, Z. Effects of solution chemistry on the attachment of graphene oxide onto clay minerals. Environ. Sci. Process. Impacts 2019, 21, 506–513. [Google Scholar] [CrossRef]
- Lu, T.; Xia, T.; Qi, Y.; Zhang, C.; Chen, W. Effects of clay minerals on transport of graphene oxide in saturated porous media. Environ. Toxicol. Chem. 2017, 36, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Interaction between viruses and clays in static and dynamic batch systems. Environ. Sci. Technol. 2010, 44, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V.; Sotirelis, N.P.; Kallithrakas-Kontos, N.G. Cotransport of graphene oxide nanoparticles and kaolinite colloids in porous media. Transp. Porous Media 2017, 119, 181–204. [Google Scholar] [CrossRef]
- Amar, A.; Loulidi, I.; Kali, A.; Boukhlifi, F.; Hadey, C.; Jabri, M. Physicochemical Characterization of Regional Clay: Application to Phenol Adsorption. Appl. Environ. Soil Sci. 2021, 2021, 8826063. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, L.; Wang, F.; Hou, L.; Chen, W. Factors controlling transport of graphene oxide nanoparticles in saturated sand columns. Environ. Toxicol. Chem. 2014, 33, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, L.; Chen, W. Transport of graphene oxide nanoparticles in saturated sandy soil. Environ. Sci. Process. Impacts 2014, 16, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, L.; Wang, L.; Kan, A.T.; Chen, W.; Tomson, M.B. Transport of fullerene nanoparticles (n C60) in saturated sand and sandy soil: Controlling factors and modeling. Environ. Sci. Technol. 2012, 46, 7230–7238. [Google Scholar] [CrossRef]
- Ryan, J.N.; Elimelech, M. Colloid mobilization and transport in groundwater. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Feng, Y.; Huynh, K.A.; Xie, Z.; Liu, G.; Gao, S. Heteroaggregation and sedimentation of graphene oxide with hematite colloids: Influence of water constituents and impact on tetracycline adsorption. Sci. Total Environ. 2019, 647, 708–715. [Google Scholar] [CrossRef]
- Tetteh, S.; Zugle, R.; Ofori, A.; Adotey, J.P.K. Kinetics and equilibrium thermodynamic studies of the adsorption of phenolphthalein and methyl orange onto muscovite clay. Front. Chem. Res. 2020, 2, 33–37. [Google Scholar]
- Olaofe, O.; Olagboye, S.; Akanji, P.; Adamolugbe, E.; Fowowe, O.; Olaniyi, A. Kinetic studies of adsorption of heavy metals on clays. Int. J. Chem. 2015, 7, 48. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Giannadakis, G.I.; Chrysikopoulos, C.V. Interaction of graphene oxide nanoparticles with quartz sand and montmorillonite colloids. Environ. Technol. 2020, 41, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Nas, M.S. The investigation of thermodynamics parameters and adsorption kinetic of the maxilon blue 5G dye on Turkey green clay. J. Inst. Sci. Technol. 2019, 9, 749–758. [Google Scholar] [CrossRef]
- Ahmat, A.M.; Thiebault, T.; Guégan, R. Phenolic acids interactions with clay minerals: A spotlight on the adsorption mechanisms of Gallic Acid onto montmorillonite. Appl. Clay Sci. 2019, 180, 105188. [Google Scholar] [CrossRef]
- Li, N.; Yan, X.; Dai, W.; Lv, B.; Wang, W. Adsorption properties and mechanism of sepiolite to graphene oxide in aqueous solution. Arab. J. Chem. 2023, 16, 104595. [Google Scholar] [CrossRef]
- Li, N.; Fang, J.; Jiang, P.; Li, C.; Kang, H.; Wang, W. Adsorption properties and mechanism of attapulgite to graphene oxide in aqueous solution. Int. J. Environ. Res. Public Health 2022, 19, 2793. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, F.; Wang, Z.; Cao, X.; Xing, B. Heteroaggregation of graphene oxide with minerals in aqueous phase. Environ. Sci. Technol. 2015, 49, 2849–2857. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Chen, W.; Lu, T.; Yang, H.; Wang, X.; Lu, M.; Qi, Z.; Li, D. Graphene oxide nanoparticles and hematite colloids behave oppositely in their co-transport in saturated porous media. Chemosphere 2021, 265, 129081. [Google Scholar] [CrossRef]
- Xia, T.; Lin, Y.; Guo, X.; Li, S.; Cui, J.; Ping, H.; Zhang, J.; Zhong, R.; Du, L.; Han, C. Co-transport of graphene oxide and titanium dioxide nanoparticles in saturated quartz sand: Influences of solution pH and metal ions. Environ. Pollut. 2019, 251, 723–730. [Google Scholar] [CrossRef]
- Adam, V.; Loyaux-Lawniczak, S.; Labille, J.; Galindo, C.; Del Nero, M.; Gangloff, S.; Weber, T.; Quaranta, G. Aggregation behaviour of TiO2 nanoparticles in natural river water. J. Nanopart. Res. 2016, 18, 13. [Google Scholar] [CrossRef]
- Dong, F.; Zhou, Y. Distinct mechanisms in the heteroaggregation of silver nanoparticles with mineral and microbial colloids. Water Res. 2020, 170, 115332. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Z.; Xu, Q.; Mao, H.; Zhang, D.; Ghosh, S.; Pradhan, N.R.; Pan, B.; Xing, B. Suspended state heteroaggregation kinetics of kaolinite and fullerene (nC60) in the presence of tannic acid: Effect of π-π interactions. Sci. Total Environ. 2020, 713, 136559. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, E.; Zhang, M.; Peijnenburg, W.J.; Liu, Y.; Song, L.; Cao, X.; Zhao, L.; Qiu, H. Interactions of CeO2 nanoparticles with natural colloids and electrolytes impact their aggregation kinetics and colloidal stability. J. Hazard. Mater. 2020, 386, 121973. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.T.; Tombacz, E. Surface chemistry of soil minerals. Soil Miner. Environ. Appl. 2002, 7, 37–67. [Google Scholar]
- Sotirelis, N.P.; Chrysikopoulos, C.V. Interaction between graphene oxide nanoparticles and quartz sand. Environ. Sci. Technol. 2015, 49, 13413–13421. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.A.; Torkzaban, S. Colloid adhesive parameters for chemically heterogeneous porous media. Langmuir 2012, 28, 13643–13651. [Google Scholar] [CrossRef] [PubMed]
- Omurlu, C.; Pham, H.; Nguyen, Q. Interaction of surface-modified silica nanoparticles with clay minerals. Appl. Nanosci. 2016, 6, 1167–1173. [Google Scholar] [CrossRef]
- Angioi, S.; Polati, S.; Roz, M.; Rinaudo, C.; Gianotti, V.; Gennaro, M. Sorption studies of chloroanilines on kaolinite and montmorillonite. Environ. Pollut. 2005, 134, 35–43. [Google Scholar] [CrossRef]
- Wang, H.; Adeleye, A.S.; Huang, Y.; Li, F.; Keller, A.A. Heteroaggregation of nanoparticles with biocolloids and geocolloids. Adv. Colloid Interface Sci. 2015, 226, 24–36. [Google Scholar] [CrossRef]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metals. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Song, L.; Johnson, P.R.; Elimelech, M. Kinetics of colloid deposition onto heterogeneously charged surfaces in porous media. Environ. Sci. Technol. 1994, 28, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ko, C.-H.; Bhattacharjee, S.; Elimelech, M. Role of spatial distribution of porous medium surface charge heterogeneity in colloid transport. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 3–15. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, B.; Wu, L.; Muñoz-Carpena, R.; Huang, Q. Effect of solution chemistry on multi-walled carbon nanotube deposition and mobilization in clean porous media. J. Hazard. Mater. 2012, 231, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-J.; Wang, Q.H.; Lin, S.; Park, K.-C.; Jin, Z.; Strano, M.S.; Blankschtein, D. Breakdown in the wetting transparency of graphene. Phys. Rev. Lett. 2012, 109, 176101. [Google Scholar] [CrossRef] [PubMed]
- Idris, O.A.; Ahmed, H.S. Phosphorus sorption capacity as a guide for phosphorus availability of selected Sudanese soil series. Afr. Crop Sci. J. 2012, 20, 59–65. [Google Scholar]
- Chrysikopoulos, C.V.; Aravantinou, A.F. Virus attachment onto quartz sand: Role of grain size and temperature. J. Environ. Chem. Eng. 2014, 2, 796–801. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Syngouna, V.I. Attachment of bacteriophages MS2 and ΦX174 onto kaolinite and montmorillonite: Extended-DLVO interactions. Colloids Surf. B Biointerfaces 2012, 92, 74–83. [Google Scholar] [CrossRef]
- Subramanyam, B.; Das, A. Linearized and non-linearized isotherm models comparative study on adsorption of aqueous phenol solution in soil. Int. J. Environ. Sci. Technol. 2009, 6, 633–640. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Zhou, D.; Abdel-Fattah, A.I.; Keller, A.A. Clay particles destabilize engineered nanoparticles in aqueous environments. Environ. Sci. Technol. 2012, 46, 7520–7526. [Google Scholar] [CrossRef]
- He, Y.; Hu, R.; Zhong, Y.; Zhao, X.; Chen, Q.; Zhu, H. Graphene oxide as a water transporter promoting germination of plants in soil. Nano Res. 2018, 11, 1928–1937. [Google Scholar] [CrossRef]
- Kabiri, S.; Degryse, F.; Tran, D.N.; da Silva, R.C.; McLaughlin, M.J.; Losic, D. Graphene oxide: A new carrier for slow release of plant micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325–43335. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.; Ikhtiari, R.; Fugetsu, B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon 2011, 49, 3907–3919. [Google Scholar] [CrossRef]
- He, Y.; Qian, L.; Zhou, K.; Hu, R.; Huang, M.; Wang, M.; Zhao, G.; Liu, Y.; Xu, Z.; Zhu, H. Graphene oxide promoted cadmium uptake by rice in soil. ACS Sustain. Chem. 2019, 7, 10283–10292. [Google Scholar] [CrossRef]
- Forstner, C.; Orton, T.G.; Skarshewski, A.; Wang, P.; Kopittke, P.M.; Dennis, P.G. Effects of graphene oxide and graphite on soil bacterial and fungal diversity. Sci. Total Environ. 2019, 671, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Elrys, A.S.; Raza, S.; Elnahal, A.S.; Na, M.; Ahmed, M.; Zhou, J.; Chen, Z. Do soil property variations affect dicyandiamide efficiency in inhibiting nitrification and minimizing carbon dioxide emissions? Ecotoxicol. Environ. Saf. 2020, 202, 110875. [Google Scholar] [CrossRef]
- Shaarawy, H.; Hussein, H.; Kader, E.A.; Hussien, N.H.; Hawash, S. Adsorption performance of coated bentonite via graphene oxide. Bull. Natl. Res. Cent. 2020, 44, 53. [Google Scholar] [CrossRef]
- Annan, E.; Nyankson, E.; Agyei-Tuffour, B.; Armah, S.K.; Nkrumah-Buandoh, G.; Hodasi, J.A.M.; Oteng-Peprah, M. Synthesis and characterization of modified kaolin-bentonite composites for enhanced fluoride removal from drinking water. Adv. Mater. Sci. Eng. 2021, 2021, 6679422. [Google Scholar] [CrossRef]
- Jadid, A.; Shahsavari, S.; Seifkordi, A.; Yazdi, A.V. Adsorption of Phenol in Wastewater Using Nano Grapheme Oxide-Chitosan-Bentonite Absorbent. Depiction Health 2021, 11, 368–380. [Google Scholar] [CrossRef]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A.; Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. The equilibrium studies in the adsorption of hazardous substances using clay minerals. In Clay Materials for Environmental Remediation; Springer: Cham, Switzerland, 2015; pp. 57–91. [Google Scholar]
- Liang, L.; Xiong, J.; Liu, X.; Luo, D. An investigation into the thermodynamic characteristics of methane adsorption on different clay minerals. J. Nat. Gas Sci. Eng. 2016, 33, 1046–1055. [Google Scholar] [CrossRef]
- He, H.; Zhu, J. Analysis of organoclays and organic adsorption by clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 310–342. [Google Scholar]
- Zafar, S.; Khan, M.I.; Rehman, H.U.; Fernandez-Garcia, J.; Shahida, S.; Prapamonthon, P.; Khraisheh, M.; Rehman, A.U.; Ahmad, H.B.; Mirza, M.L. Kinetic, equilibrium, and thermodynamic studies for adsorptive removal of cobalt ions by rice husk from aqueous solution. Desalination Water Treat. 2020, 204, 285–296. [Google Scholar] [CrossRef]
- Raya, I.; Widjaja, G.; Mahmood, Z.H.; Kadhim, A.J.; Vladimirovich, K.O.; Mustafa, Y.F.; Kadhim, M.M.; Mahmudiono, T.; Husein, I.; Kafi-Ahmadi, L. Kinetic, isotherm, and thermodynamic studies on Cr (VI) adsorption using cellulose acetate/graphene oxide composite nanofibers. Appl. Phys. A 2022, 128, 167. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Utilization of oil palm biodiesel solid residue as renewable sources for preparation of granular activated carbon by microwave induced KOH activation. Bioresour. Technol. 2013, 130, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Dai, W.; Kang, H.; Lv, B.; Jiang, P.; Wang, W. Study on the adsorption performance and adsorption mechanism of graphene oxide by red sandstone in aqueous solution. Adsorpt. Sci. Technol. 2022, 2022, 2557107. [Google Scholar] [CrossRef]
- Deepthi Rani, R.; Sasidhar, P. Sorption of cesium on clay colloids: Kinetic and thermodynamic studies. Aquat. Geochem. 2012, 18, 281–296. [Google Scholar] [CrossRef]
- Jahan, N.; Roy, H.; Reaz, A.H.; Arshi, S.; Rahman, E.; Firoz, S.H.; Islam, M.S. A comparative study on sorption behavior of graphene oxide and reduced graphene oxide towards methylene blue. Case Stud. Chem. Environ. Eng. 2022, 6, 100239. [Google Scholar] [CrossRef]
- Freundlich, H. New conception in colloidal chemistry, colloid and capillary chemistry. Methuen 1926, 45, 970–984. [Google Scholar]
- Isa, M.H.; Lang, L.S.; Asaari, F.A.; Aziz, H.A.; Ramli, N.A.; Dhas, J.P.A. Low cost removal of disperse dyes from aqueous solution using palm ash. Dye Pigment. 2007, 74, 446–453. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.I.M.; Awad, E.A.M.; Dahdouh, S.M.M.; El-Etr, W.M.T.; Marey, S.A.; Hatamleh, A.A.; Mahmood, M.; Elrys, A.S. Exploring the Influence of Chemical Conditions on Nanoparticle Graphene Oxide Adsorption onto Clay Minerals. Molecules 2023, 28, 6162. https://doi.org/10.3390/molecules28166162
Ibrahim MIM, Awad EAM, Dahdouh SMM, El-Etr WMT, Marey SA, Hatamleh AA, Mahmood M, Elrys AS. Exploring the Influence of Chemical Conditions on Nanoparticle Graphene Oxide Adsorption onto Clay Minerals. Molecules. 2023; 28(16):6162. https://doi.org/10.3390/molecules28166162
Chicago/Turabian StyleIbrahim, Marwa I. M., Elsayed A. M. Awad, Salah M. M. Dahdouh, Wafaa M. T. El-Etr, Samy A. Marey, Ashraf Atef Hatamleh, Mohsin Mahmood, and Ahmed S. Elrys. 2023. "Exploring the Influence of Chemical Conditions on Nanoparticle Graphene Oxide Adsorption onto Clay Minerals" Molecules 28, no. 16: 6162. https://doi.org/10.3390/molecules28166162
APA StyleIbrahim, M. I. M., Awad, E. A. M., Dahdouh, S. M. M., El-Etr, W. M. T., Marey, S. A., Hatamleh, A. A., Mahmood, M., & Elrys, A. S. (2023). Exploring the Influence of Chemical Conditions on Nanoparticle Graphene Oxide Adsorption onto Clay Minerals. Molecules, 28(16), 6162. https://doi.org/10.3390/molecules28166162




