cRGD-Conjugated GdIO Nanoclusters for the Theranostics of Pancreatic Cancer through the Combination of T1–T2 Dual-Modal MRI and DTX Delivery
Abstract
1. Introduction
2. Results and Discussion
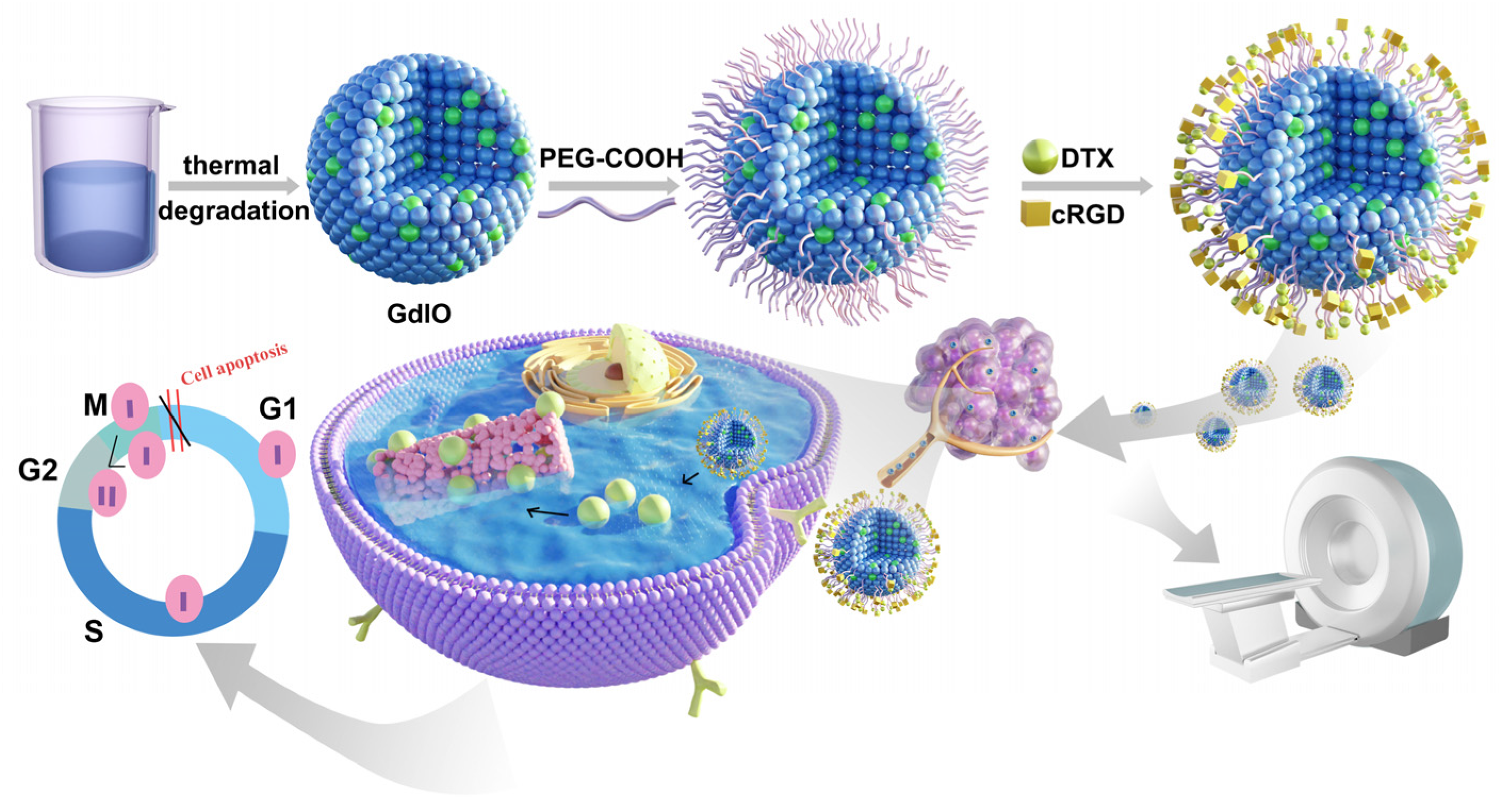
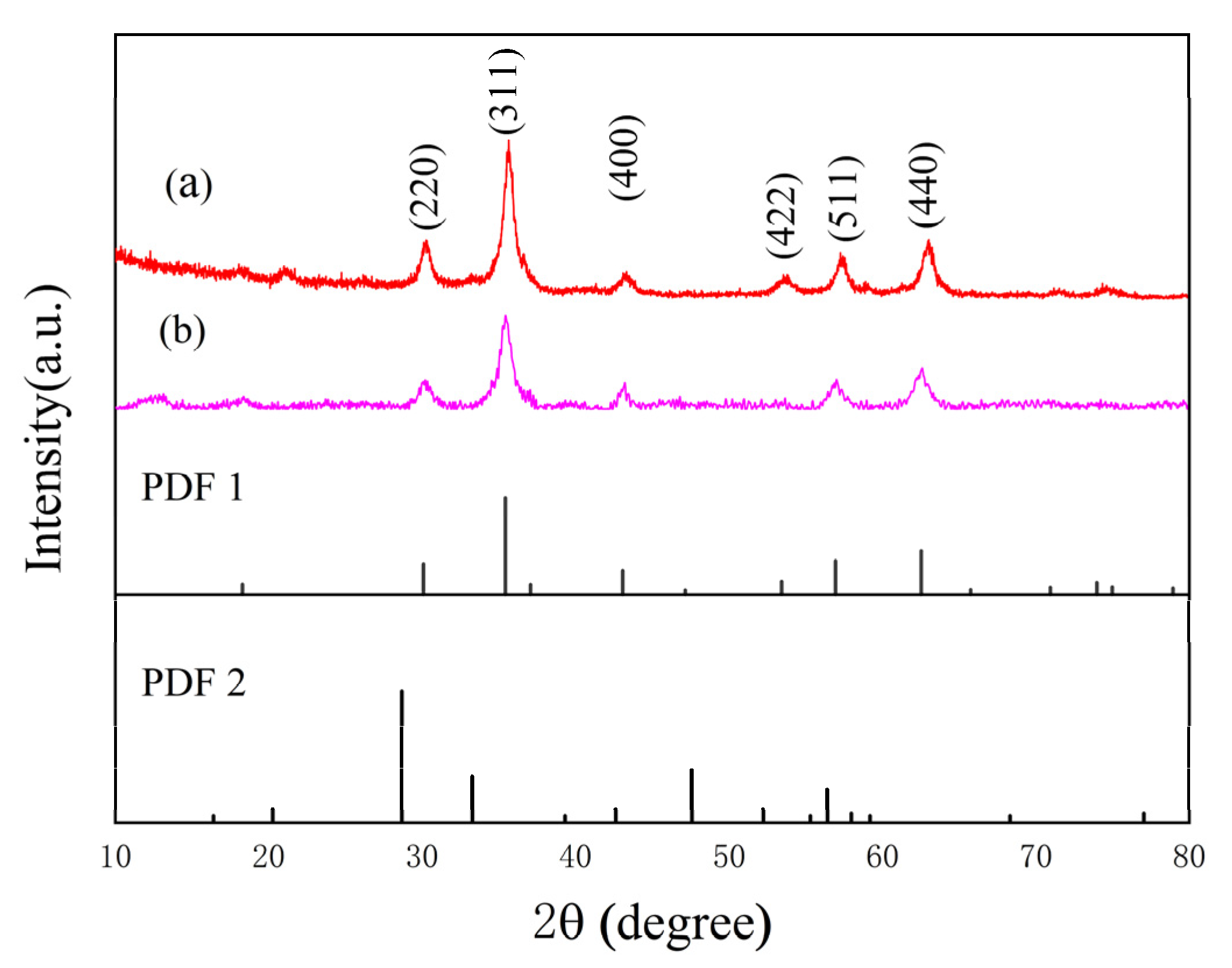
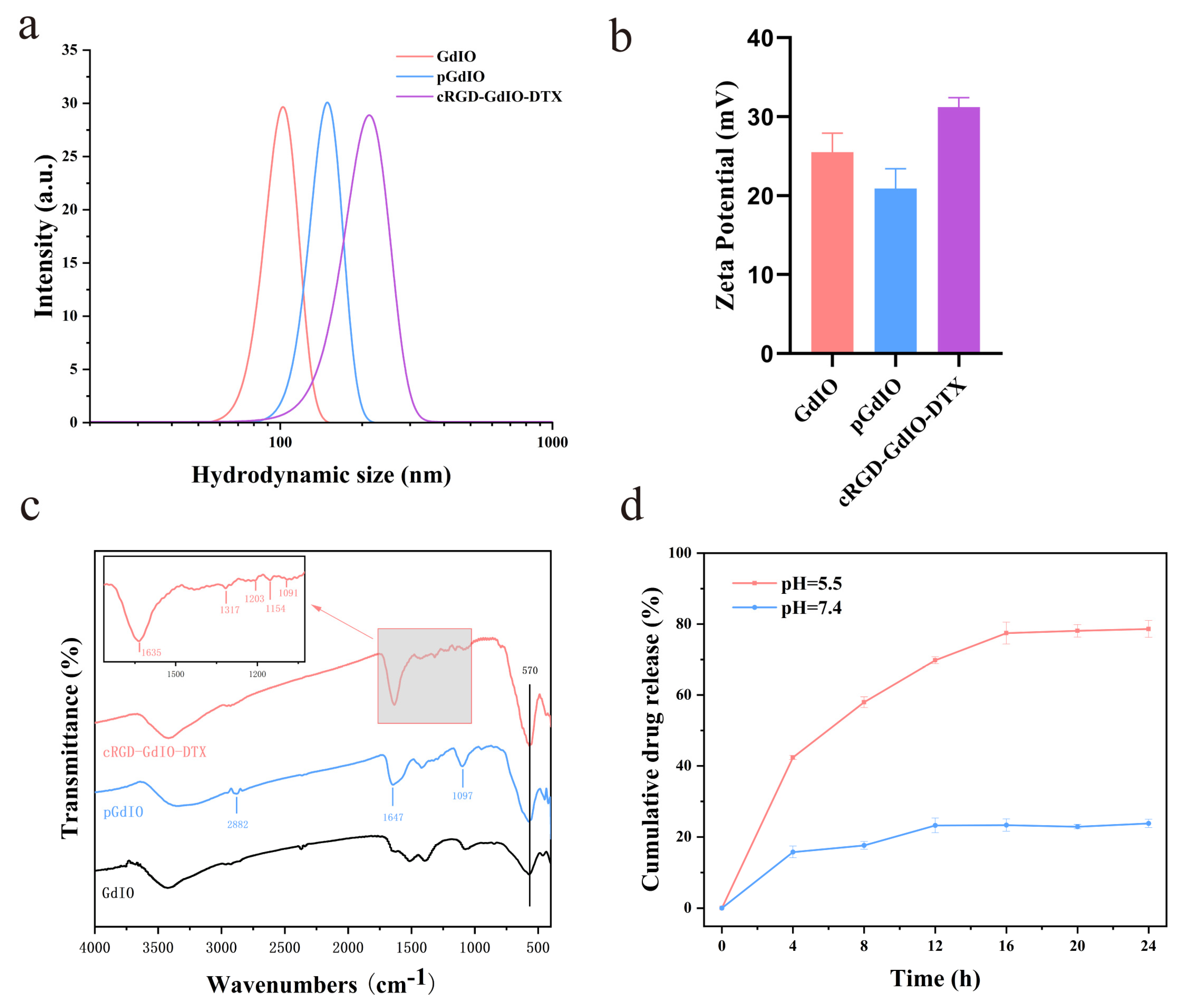
2.1. Synthesis and Characterization
2.2. Drug Loading and Release Behavior
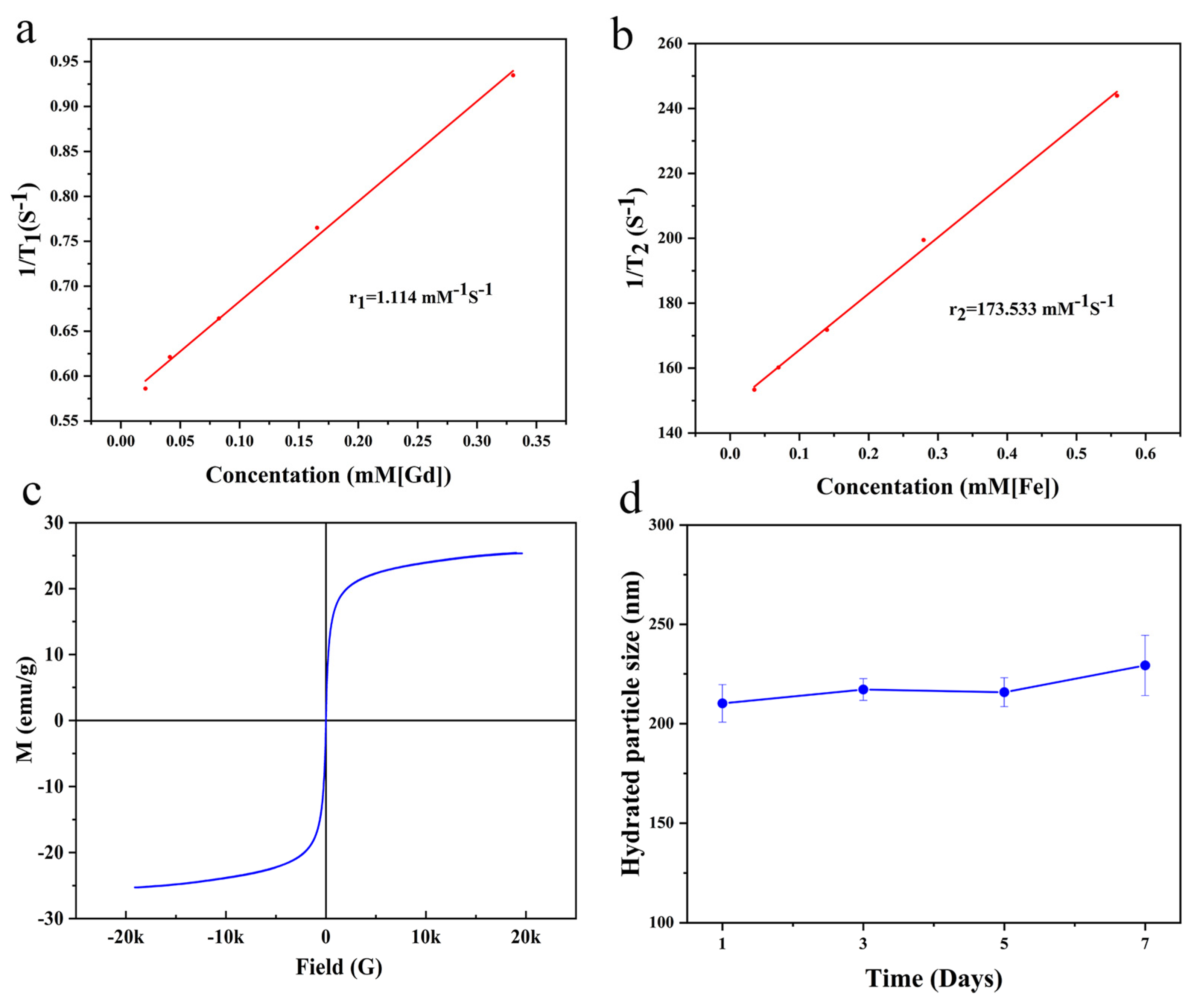
2.3. Magnetic Properties and Relaxivity of the Nanoclusters
2.4. Biocompatibility and Biotoxicity
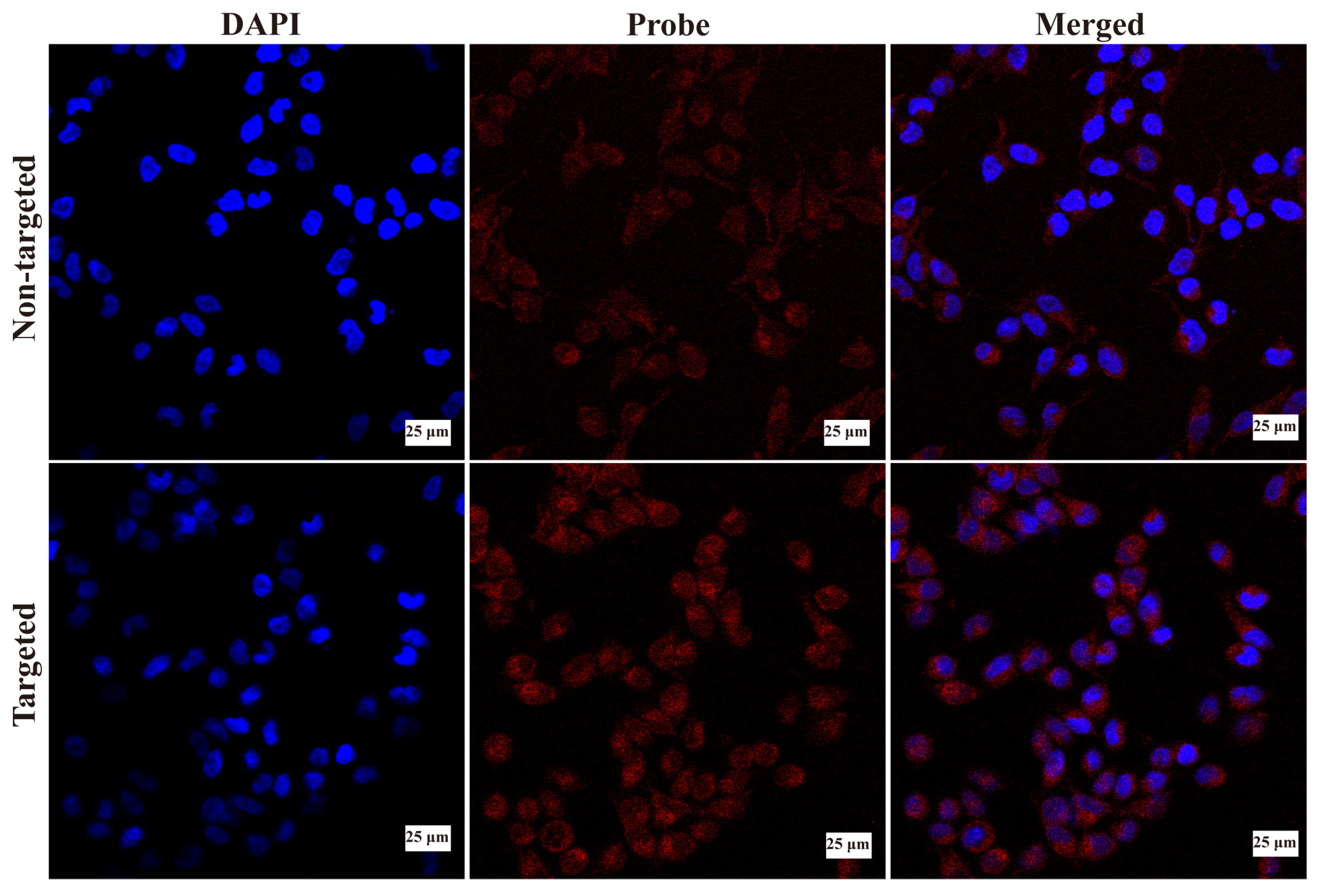
2.5. Cellular Uptake of the Nanoclusters In Vitro
2.6. Antitumor Effect of the Nanoclusters In Vitro
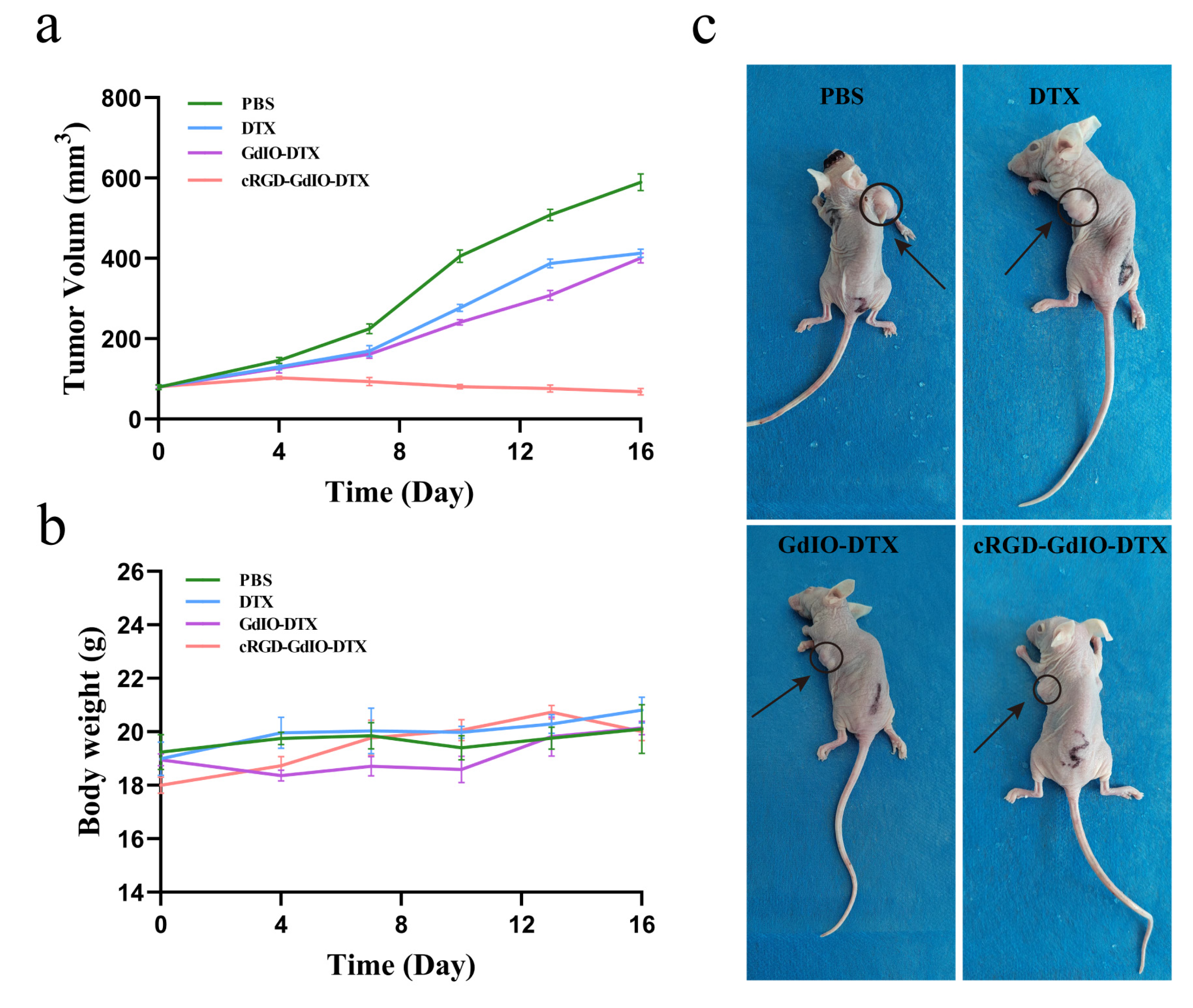
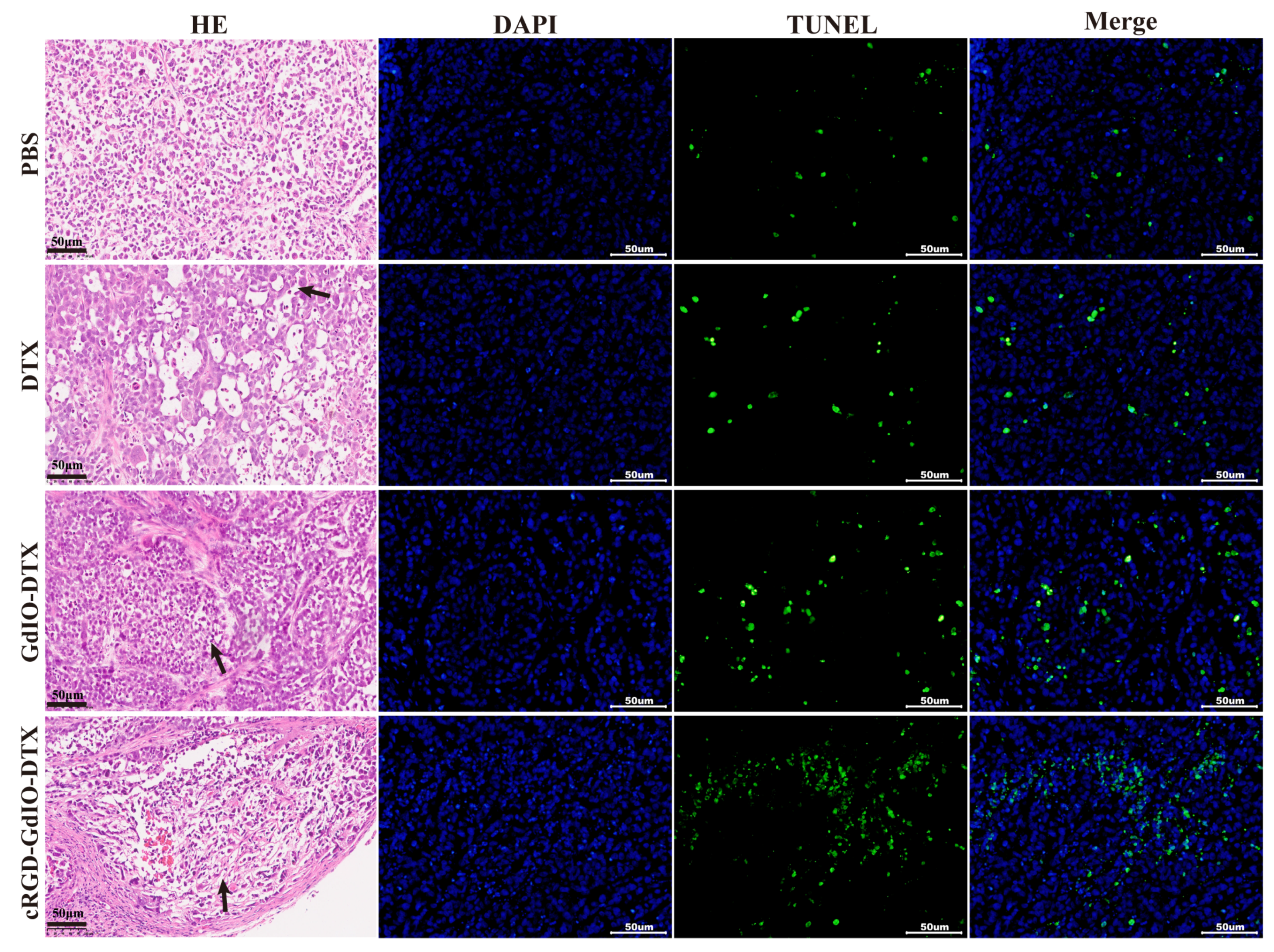
2.7. Antitumor Effect of the Nanoclusters In Vivo
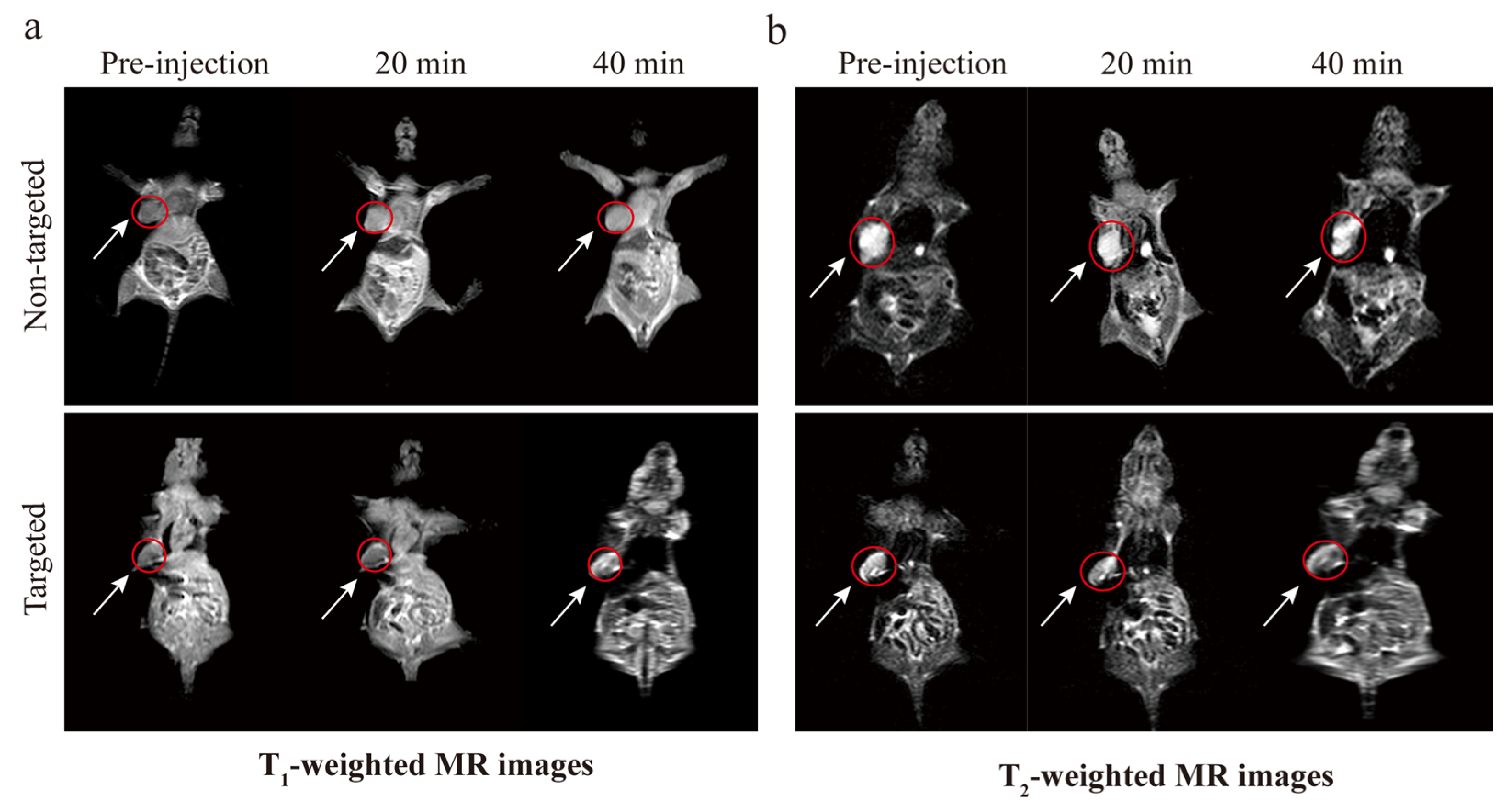
2.8. MR Imaging In Vivo
3. Materials and Methods
3.1. Materials
3.2. Synthesis of RGD-GdIO-DTX
3.2.1. Synthesis of GdIO Nanoclusters
3.2.2. Synthesis of pGdIO Nanoclusters
3.2.3. Synthesis of cRGD-GdIO-DTX
3.3. Characterization
3.4. Drug Loading and Release Behavior
3.5. Magnetic Properties and Relaxivity of the Nanoclusters
3.6. Biocompatibility and Biotoxicity
3.7. Cellular Uptake of the Nanoclusters In Vitro
3.8. Antitumor Effect of the Nanoclusters In Vitro
3.9. Antitumor Effect of the Nanoclusters In Vivo
3.10. MR Imaging In Vivo
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA A Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, Y.; Qin, J.; Nie, G.; Lei, B.; Xiao, Y.; Zheng, M.; Rong, J. Fabrication of reduced graphene oxide and sliver nanoparticle hybrids for Raman detection of absorbed folic acid: A potential cancer diagnostic probe. ACS Appl. Mater. Interfaces 2013, 5, 4760–4768. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, L.; Si, Y.; Li, Q.; Xiao, J.; Wang, B.; Liang, C.; Wu, Z.; Tian, G. Oxygenenriched Fe3O4/Gd2O3 nanopeanuts for tumor-targeting MRI and ROS-triggered dual-modal cancer therapy through platinum (IV) prodrugs delivery. Chem. Eng. J. 2020, 388, 124269. [Google Scholar] [CrossRef]
- Kim, H.; Jin, S.; Choi, H.; Kang, M.; Park, S.G.; Jun, H.; Cho, H.; Kang, S. Target-switchable Gd(III)-DOTA/protein cage nanoparticle conjugates with multiple targeting affibody molecules as target selective T1 contrast agents for high-field MRI. J. Control. Release Off. J. Control. Release Soc. 2021, 335, 269–280. [Google Scholar] [CrossRef]
- Fu, S.; Cai, Z.; Ai, H. Stimulus-Responsive Nanoparticle Magnetic Resonance Imaging Contrast Agents: Design Considerations and Applications. Adv. Healthc. Mater. 2021, 10, e2001091. [Google Scholar] [CrossRef]
- Yin, Q.; Qi, G.; Wang, S.; Tian, H.; Gao, X.; Zhang, Z.; Hao, L. Magnetic resonance/fluorescence dual-modality contrast agents targeting αvβ6-overexpressing tumors based on A20FMDV2 peptide as a ligand. Biochem. Biophys. Res. Commun. 2023, 664, 86–93. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Yang, J.; Xu, Y.; Zhang, C.; Wang, Z.; Shi, X.; Zhang, G. Facile Synthesis of Folic Acid-Modified Iron Oxide Nanoparticles for Targeted MR Imaging in Pulmonary Tumor Xenografts. Mol. Imaging Biol. 2016, 18, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Mohs, A.M.; Lu, Z.R. Gadolinium(III)-based blood-pool contrast agents for magnetic resonance imaging: Status and clinical potential. Expert Opin. Drug Deliv. 2007, 4, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, J.; Zhang, C.; Wang, P.; Li, W.; Wang, Y.; Ran, L.; Qin, X.; Yan, M.; Qian, J.; et al. PDGF-B conjugating mesoporous IO/GdO nanocomposites for accurate diagnosis of orthotopic prostatic cancer through T1-T2 dual-modal MRI contrast enhancement. Mater. Adv. 2022, 16, 100278. [Google Scholar] [CrossRef]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Yang, M.; Gao, L.; Liu, K.; Luo, C.; Wang, Y.; Yu, L.; Peng, H.; Zhang, W. Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta 2015, 131, 661–665. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Qian, J.; Zhong, K.; Yao, J.; Cai, D.; Cheng, Z.; Wu, Z. Magnetic Relaxation Switch Detecting Boric Acid or Borate Ester through One-Pot Synthesized Poly(vinyl alcohol) Functionalized Nanomagnetic Iron Oxide. ACS Appl. Mater. Interfaces 2015, 7, 16837–16841. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wang, R.; Zhao, P.; Gu, W.; Ye, L. Albumin-mediated synthesis of fluoroperovskite KMnF3 nanocrystals for T1–T2 dual-modal magnetic resonance imaging of brain gliomas with improved sensitivity. Chem. Eng. J. 2020, 395, 125066. [Google Scholar] [CrossRef]
- Zhou, Z.; Bai, R.; Munasinghe, J.; Shen, Z.; Nie, L.; Chen, X. T1–T2 Dual-Modal Magnetic Resonance Imaging: From Molecular Basis to Contrast Agents. ACS Nano 2017, 11, 5227–5232. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, D.; Bao, J.; Chen, Q.; Liu, G.; Chen, Z.; Chen, X.; Gao, J. A synergistically enhanced T1–T2 dual-modal contrast agent. Adv. Mater. 2012, 24, 6223–6228. [Google Scholar] [CrossRef]
- Li, J.; You, J.; Wu, C.; Dai, Y.; Shi, M.; Dong, L.; Xu, K. T1–T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents. Int. J. Nanomed. 2018, 13, 4607–4625. [Google Scholar] [CrossRef]
- Nie, J.; Cheng, W.; Peng, Y.; Liu, G.; Chen, Y.; Wang, X.; Liang, C.; Tao, W.; Wei, Y.; Zeng, X.; et al. Co-delivery of docetaxel and bortezomib based on a targeting nanoplatform for enhancing cancer chemotherapy effects. Drug Deliv. 2017, 24, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, W.; Tang, G.; Wang, H.; Wu, M.; Yu, W.; Zhou, Z.; Mou, Y.; Liu, X. Targeted Codelivery of Docetaxel and Atg7 siRNA for Autophagy Inhibition and Pancreatic Cancer Treatment. ACS Appl. Bio Mater. 2019, 2, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Rath, O.; Kozielski, F. Kinesins and cancer. Nature reviews. Cancer 2012, 12, 527–539. [Google Scholar]
- Di Costanzo, F.; Gasperoni, S.; Rotella, V.; Di Costanzo, F. Targeted delivery of albumin bound paclitaxel in the treatment of advanced breast cancer. OncoTargets Ther. 2009, 2, 179–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Cole, S.P.; Cai, T.; Cai, Y.U. Applications of nanoparticle drug delivery systems for the reversal of multidrug resistance in cancer. Oncol. Lett. 2016, 12, 11–15. [Google Scholar] [CrossRef]
- Andisheh, F.; Oroojalian, F.; Shakour, N.; Ramezani, M.; Shamsara, J.; Khodaverdi, E.; Nassirli, H.; Hadizadeh, F.; Alibolandi, M. Docetaxel encapsulation in nanoscale assembly micelles of folate-PEG-docetaxel conjugates for targeted fighting against metastatic breast cancer in vitro and in vivo. Int. J. Pharm. 2021, 605, 120822. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Oudot, A.; Moreau, M.; Boidot, R.; Chassagnon, R.; Saïd, N.M.; Roux, S.; Mirjolet, C.; Millot, N. Titanate Nanotubes Engineered with Gold Nanoparticles and Docetaxel to Enhance Radiotherapy on Xenografted Prostate Tumors. Cancers 2019, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhang, J.; Lei, S.; Yan, M.; Xie, W.; Qi, X.; Wang, H.; Xiao, J.; Chen, S.; Li, S.; et al. Atomically precise multi-domain GdxFe3-xO4 nanoclusters with modulated contrast properties for T2-weighted magnetic resonance imaging of early orthotopic cancer. Chem. Eng. J. 2022, 429, 132255. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.J.; Guo, Z.; Wang, H.B.; Wang, D.D.; Zhou, J.J.; Chen, Q.W. pH-responsive iron manganese silicate nanoparticles as T1–T2* dual-modal imaging probes for tumor diagnosis. ACS Appl. Mater. Interfaces 2015, 7, 5373–5383. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y.P.; Jin, Y.; et al. Appropriate Size of Magnetic Nanoparticles for Various Bioapplications in Cancer Diagnostics and Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.; Santosh, L.; Gawali, B.K.; Barick, K.C.; Barick, P.D.; Babu, B.N.; Pandey, K.I.; Priyadarsini, P.A.; Hassan, P.A. PEG mediated shape-selective synthesis of cubic Fe3O4 nanoparticles for cancer therapeutics. J. Alloys Compd. 2018, 737, 347–355. [Google Scholar] [CrossRef]
- Lu, C.; Quan, Z.-S.; Sur, J.C.; Kim, S.-H.; Lee, C.H.; Chai, K.Y. Oen-pot fabrication of carboxyl-functionalized biocompatible magnetic nanocrystals for conjugation with targeting agents. New J. Chem. 2010, 34, 2040–2046. [Google Scholar] [CrossRef]
- Lu, C.; Bhatt, L.R.; Jun, H.Y.; Park, S.H.; Chai, K.Y. Carboxyl-polyethylene glycol-phosphoric acid: A ligand for highly stabilized iron oxide nanoparticles. J. Mater. Chem. 2012, 22, 19806–19811. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Ma, J.; Yuan, H.; Liang, H.; Wu, L.; Chai, K.Y.; Li, S. Facile synthesis of superparamagnetic magnetite nanoflowers and their applications in cellular imaging. RSC Adv. 2016, 6, 42649–42655. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Undzys, E.; Aman, A.; Press, B.; Li, S.D. A docetaxel-carboxymethylcellulose nanoparticle outperforms the approved taxane nanoformulation, Abraxane, in mouse tumor models with significant control of metastases. J. Control. Release 2012, 162, 575–581. [Google Scholar] [CrossRef]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; McFarlane, J.D. Extracellular pH distribution in human tumours. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 1995, 11, 211–216. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, H.Y.; Perrett, S.; Zhao, Y.; Tang, Z.; Nie, G. Chirality of glutathione surface coating affects the cytotoxicity of quantum dots. Angew. Chem. 2011, 50, 5860–5864. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, G.; Wang, D.; Zhang, C.; Yang, C.; Bai, G.; Qian, J.; Chen, Q.; Zhang, Z.; Wu, Z.; et al. Nanostructure Enhanced Water Interaction to Increase the Dual-Mode MR Contrast Performance of Gadolinium-doped Iron Oxide Nanoclusters. Chem. Eng. J. 2019, 360, 289–298. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, K.; Fu, C.; Liu, H.; Lei, M.; Bao, J.; Fu, A.; Yu, Y.; Zhang, W. Interfacial engineered gadolinium oxide nanoparticles for magnetic resonance imaging guided microenvironment-mediated synergetic chemodynamic/photothermal therapy. Biomaterials 2019, 219, 119379. [Google Scholar] [CrossRef]
- Furman, G.B.; Goren, S.D.; Meerovich, V.M.; Sokolovsky, V.L. Anisotropy of spin-spin and spin-lattice relaxation times in liquids entrapped in nanocavities: Application to MRI study of biological systems. J. Magn. 2016, 263, 71–78. [Google Scholar] [CrossRef]
- Khawaja, A.Z.; Cassidy, D.B.; Al Shakarchi, J.; McGrogan, D.G.; Inston, N.G.; Jones, R.G. Revisiting the risks of MRI with Gadolinium based contrast agents-review of literature and guidelines. Insights Imaging 2015, 6, 553–558. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Williamson, E.E.; Eckel, L.J. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology 2017, 285, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, X.; Qi, Y.; Yu, B.; Xu, F.J. Reduction-responsive multifunctional hyperbranched polyaminoglycosides with excellent antibacterial activity, biocompatibility and gene transfection capability. Biomaterials 2016, 106, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Kong, S.; Ouyang, Q.; Li, C.; Hou, T.; Chen, Y.; Li, S. Chitosan-Gentamicin Conjugate Hydrogel Promoting Skin Scald Repair. Mar. Drugs 2020, 18, 233. [Google Scholar] [CrossRef]
- Torcasio, S.M.; Oliva, R.; Montesi, M.; Panseri, S.; Bassi, G.; Mazzaglia, A.; Piperno, A.; Coulembier, O.; Scala, A. Three-armed RGD-decorated starPLA-PEG nanoshuttle for docetaxel delivery. Biomater. Adv. 2022, 140, 213043. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Jackson, N.; Eaton, S.; Santos, N.D.; Barta, I.; Zaifman, J.; Chen, S.; Tam, Y.Y.C.; Krishnan, S.; Chithrani, D.B. Lipid-Nanoparticle-Mediated Delivery of Docetaxel Prodrug for Exploiting Full Potential of Gold Nanoparticles in the Treatment of Pancreatic Cancer. Cancers 2022, 14, 6137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, C.; Liu, J.; Hu, S.; Yu, J.; Yin, Q.; Tian, H.; Ding, Z.; Qi, G.; Wang, L.; et al. Aptamer-mediated hollow MnO2 for targeting the delivery of sorafenib. Drug Deliv. 2023, 30, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zeng, D.; Tian, Y.; Chen, F.; Feng, J.; Shi, J. Core/shell structured hollow mesoporous nanocapsules: A potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano 2010, 4, 6001–6013. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhao, Y.; Sun, L. pH-responsive drug release and real-time fluorescence detection of porous silica nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 19–26. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Qi, G.; Zhang, M.; Hao, L. pH-Responsive Drug Delivery and Imaging Study of Hybrid Mesoporous Silica Nanoparticles. Molecules 2022, 27, 6519. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Qi, G.; Zhang, Z.; Yin, Q.; Li, N.; Li, Z.; Shi, G.; Hu, H.; Hao, L. cRGD-Conjugated GdIO Nanoclusters for the Theranostics of Pancreatic Cancer through the Combination of T1–T2 Dual-Modal MRI and DTX Delivery. Molecules 2023, 28, 6134. https://doi.org/10.3390/molecules28166134
Wang S, Qi G, Zhang Z, Yin Q, Li N, Li Z, Shi G, Hu H, Hao L. cRGD-Conjugated GdIO Nanoclusters for the Theranostics of Pancreatic Cancer through the Combination of T1–T2 Dual-Modal MRI and DTX Delivery. Molecules. 2023; 28(16):6134. https://doi.org/10.3390/molecules28166134
Chicago/Turabian StyleWang, Shengchao, Guiqiang Qi, Zhichen Zhang, Qiangqiang Yin, Na Li, Zhongtao Li, Guangyue Shi, Haifeng Hu, and Liguo Hao. 2023. "cRGD-Conjugated GdIO Nanoclusters for the Theranostics of Pancreatic Cancer through the Combination of T1–T2 Dual-Modal MRI and DTX Delivery" Molecules 28, no. 16: 6134. https://doi.org/10.3390/molecules28166134
APA StyleWang, S., Qi, G., Zhang, Z., Yin, Q., Li, N., Li, Z., Shi, G., Hu, H., & Hao, L. (2023). cRGD-Conjugated GdIO Nanoclusters for the Theranostics of Pancreatic Cancer through the Combination of T1–T2 Dual-Modal MRI and DTX Delivery. Molecules, 28(16), 6134. https://doi.org/10.3390/molecules28166134






