Abstract
The functionalisation of C–H bonds has been an enormous achievement in synthetic methodology, enabling new retrosynthetic disconnections and affording simple synthetic equivalents for synthons. Hydrogen atom transfer (HAT) is a key method for forming alkyl radicals from C–H substrates. Classic reactions, including the Barton nitrite ester reaction and Hofmann–Löffler–Freytag reaction, among others, provided early examples of HAT. However, recent developments in photoredox catalysis and electrochemistry have made HAT a powerful synthetic tool capable of introducing a wide range of functional groups into C–H bonds. Moreover, greater mechanistic insights into HAT have stimulated the development of increasingly site-selective protocols. Site-selectivity can be achieved through the tuning of electron density at certain C–H bonds using additives, a judicious choice of HAT reagent, and a solvent system. Herein, we describe the latest methods for functionalizing C–H/Si–H/Ge–H bonds using indirect HAT between 2018–2023, as well as a critical discussion of new HAT reagents, mechanistic aspects, substrate scopes, and background contexts of the protocols.
1. Introduction
The ability to use C–H bonds as de facto functional handles has streamlined the synthesis of complex organic molecules and changed how chemists approach retrosynthesis [1,2,3,4,5]. Using C–H bonds as functional handles instead of pre-functionalised substrates, the yields’ various benefits include lower step counts in multistep synthesis and an improved atom economy of reactions [3,6,7,8]. Furthermore, the diversity of transformations available to C–H bonds potentially enables access to a wide array of functionality to be introduced into a common core [7,9,10]. Broadly speaking, C–H functionalisation is achieved by generating reactive intermediates from C–H bonds to subsequently harness their reactivity. This can be achieved through organometallic C–H activation [6,11,12,13,14], carbene/nitrene C–H insertion [2,11,15,16,17], enzymatic C–H functionalisation [18], or hydrogen atom transfer (HAT) [11,19,20]. However, site-selective C–H functionalisation is challenging due to the minimal differences between C–H bonds in organic molecules [6,21,22,23]. HAT generates alkyl radicals from C–H bonds through the radical abstraction of hydrogen atoms [19]. Alkyl radicals are highly reactive intermediates that are relatively insensitive to steric crowding and do not form aggregates [24]. Alkyl radicals react chemoselectively with radical traps or couple with other radicals, even with substrates that contain N-heterocycles as well as polar and acidic functional groups [20,24,25,26,27,28]. Additionally, HAT processes can be fine-tuned towards specific C–H bonds through choice of HAT reagent, change of solvent, or addition of certain additives [29,30].
Developments in HAT have previously been reviewed [7,19,20,31,32,33,34,35,36,37]. However, due to the rapid pace of protocols published in this field, this review will overlap minimally with those reviews, while works covered previously were omitted unless deemed critical for providing a coherent narrative. Metal hydride-mediated hydrogen atom transfer (MHAT), direct HAT, and indirect HAT mediated by halogen radicals are not covered in this review [31,38,39,40,41,42,43,44,45,46,47,48]. Direct HAT describes HAT where an excited photocatalyst directly carries out HAT [19]. For instance, triplet state ketones [49,50,51,52,53,54,55,56,57,58], decatungstate photocatalysts [48,59,60,61,62,63,64,65,66,67,68,69,70,71], and nitroarenes [72] are direct HAT reagents. Indirect HAT describes protocols where a radical H-atom abstractor is generated in situ [19,73].
1.1. HAT Background and Mechanism
Hydrogen atom transfer is a one-step process that transfers a hydrogen atom (proton and electron) from one species to another (Scheme 1) [74,75]. However, in the context of synthesis, HAT is used for C–H functionalisation by harnessing the reactivity of the alkyl radical with various radical traps [19].

Scheme 1.
Generic HAT process.
The bond dissociation energy (BDE) is the key driving force for HAT [76,77,78]. Accordingly, the BDE of A–H in Compound 1.3 (A–H) should be greater than the C–H bond being abstracted (B–H) to favour product formation [75]. Fortunately, BDE values are well-documented in the chemical literature [76,79,80]. BDE values can also be matched to ensure the desired hydrogen atom is abstracted. For example, thiyl radicals undergo HAT with relatively weak C–H bonds to form the corresponding alkyl radical and a thiol [alkyl thiols BDES–H ≈ 87 kcal/mol] [81]. Hamashima and co-workers developed an arylation of benzylamine 2.1 C(sp3)–H bonds, which proceeded through regio- and chemo-selective HAT of the benzylic C(sp3)–H using a thiyl radical derived from thiobenzoic acid 2.3 [N,N-dimethylbenzylamine 2.1 BDEC–H = 84.9 kcal/mol versus thiobenzoic acid 2.3 BDES–H = 87.4 kcal/mol] (Scheme 2) [82].
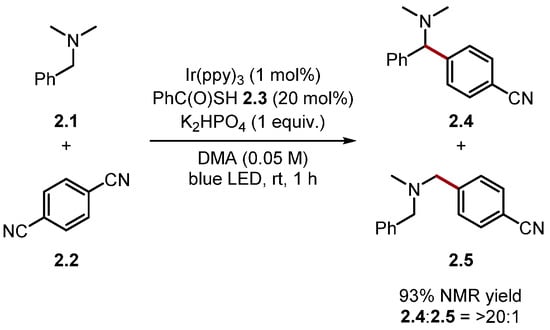
Scheme 2.
Benzylamine C–H arylation using a thiyl radical formed from thiobenzoic acid.
Conversely, stronger HAT reagents can be used to abstract unactivated C(sp3)–H bonds of alkanes [76]. Knowles and co-workers reported the alkylation of cyclohexane 3.1 through HAT with amidyl radical 3.3• [amide 3.3 BDEN–H = 107 kcal/mol versus cyclohexane 3.1 BDEC–H = 99.5 kcal/mol] (Scheme 3) [83]. The high BDE value of N–H bonds in amides allows for HAT of unactivated C(sp3)–H bonds of alkanes. Hence, BDE values of the HAT reagent and the substrate should be matched carefully to ensure the HAT process is thermodynamically spontaneous and selective [76].
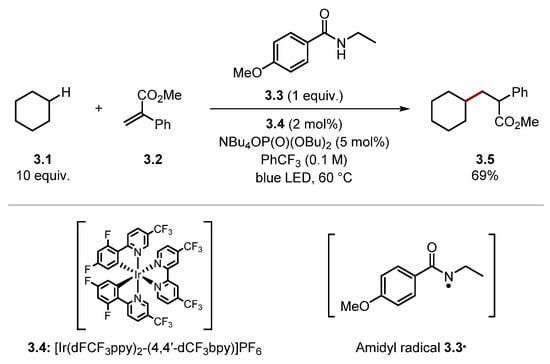
Scheme 3.
C(sp3)–H alkylation using amide 3.3 as a HAT reagent.
Despite radicals being electronically neutral species, electronic factors in transition states of radical reactions greatly influence their rate and selectivity [84,85,86,87]. In this context, polar effects describe the effect the charge transfer has on the activation energy, and HAT is strongly influenced by such electronic factors. Usually, polarity-matched HAT describes the tendency of electrophilic HAT reagents to abstract electron-rich (“hydridic”) hydrogen atoms and nucleophilic HAT reagents to abstract electron-poor (“protic”) hydrogen atoms (Scheme 4) [84,88].
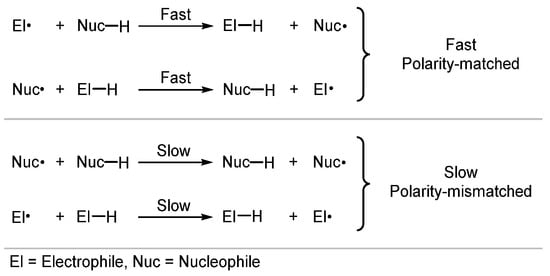
Scheme 4.
Polarity effects in HAT processes.
Most HAT reagents are electrophilic and selectively abstract electron-rich hydrogen atoms [30]. As a result, HAT normally occurs adjacent to an electron-donating group (EDG) or another stabilizing functional group [19,48]. Bietti and co-workers have extensively studied the reaction rates of HAT [29,30,80,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. Recently, Bietti studied the rates of HAT for saturated N-containing heterocycles and tetramethyl urea 5.1 using dicumyl peroxide 5.2 (Scheme 5) [89].

Scheme 5.
Transition state (‡) of HAT with cumyloxyl radical.
The HAT transition state can be described as developing a partial positive charge at the C–atom, along with a partial negative charge on the abstracting radical (cumyloxyl radical in this case) [29,77,78]. Functional groups such as amides stabilise the partial positive charge on the incipient radical atom through an orbital overlap of the σ* of the α-C–H (developing SOMO) with a heteroatom lone pair or a π-system [89]. This transition state model has been probed through experimental observations, Hammett plot analysis, and computational studies [78,88,90,103]. HAT processes are dictated by an electron density at different C–H bonds meaning a change in solvent, an addition of H-bond donor/acceptors, or Brønsted/Lewis-acid/base additives can alter the rates of HAT [29,30]. For instance, strong H-bonding solvents [such as hexafluoroisopropanol (HFIP)] are used to supress undesired HAT adjacent to H-bond acceptors (e.g., heteroatoms) [72,92,104,105,106]. However, H-bonding solvents are also known to accelerate HAT at cyclohexane and 1,4-cyclohexadiene through H-bonding to oxyl-radicals [29,107,108,109].
The abstraction of protic hydrogen atoms is difficult as most HAT-capable radicals are inherently electrophilic heteroatom-centred radicals or radical cations [30]. However, the abstraction of protic hydrogens can be accomplished through a polarity reversal catalysis (PRC) [84]. PRC can generate a nucleophilic HAT reagent through an initial polarity-matched HAT step (Scheme 6) [110,111]. For example, electrophilic alkoxyl radical 6.2 reacts with amino boranes 6.3 to form a nucleophilic amine boryl radical 6.5, which abstracts protic hydrogen atoms selectively, such as acetonitrile 6.6 α-C(sp3)–H to generate electrophilic alkyl radical 6.7.
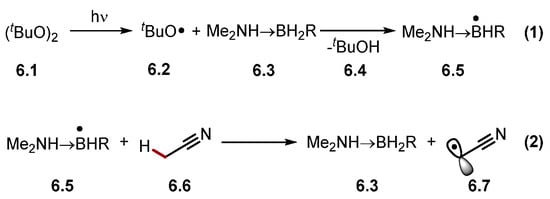
Scheme 6.
HAT of protic hydrogen atom from MeCN using PRC.
1.2. Indirect HAT
As mentioned before, indirect HAT describes protocols where a radical H-atom abstractor is generated in situ [19,73]. Radicals capable of HAT are typically formed in the following ways (Scheme 7).
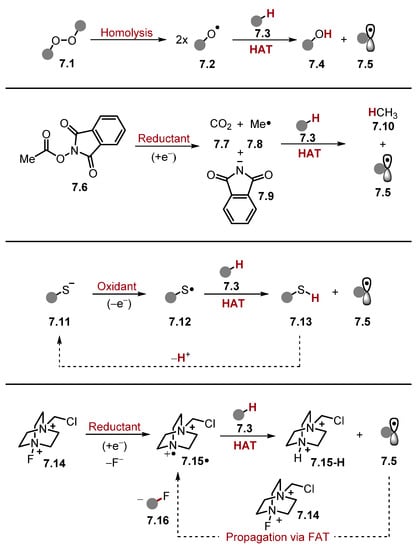
Scheme 7.
Methods of generating a HAT-capable species in situ.
(1) Homolytic/Heterolytic cleavage of a weak bond. The weak O–O bond in peroxides 7.1 undergoes homolysis to form two oxygen-centred radicals 7.2 capable of HAT [tBuCH2O–OCH2tBu BDEO–O = 36.4 kcal/mol] [79,112]. The peroxide 7.1 can also undergo heterolysis with a reducing agent/acid to form one equivalent of O-centred radical 7.2 [112].
(2) Mesolytic cleavage of a radical ion. For instance, a redox active ester (RAE) 7.6 can be reduced to a radical anion [113,114,115,116]. The radical anion undergoes mesolytic cleavage forming CO2 7.7, phthalimide anion 7.9, and a methyl radical 7.8, which is a competent HAT reagent [117].
(3) Oxidation of a heteroatom or an anion using photoredox catalysis or (less commonly) electrochemistry [118]. For example, a thiolate 7.11 can be oxidised to a thiyl radical 7.12, which can abstract a hydrogen atom to form a thiol 7.13 and alkyl radical 7.5 [119]. Deprotonation of the thiol 7.13 allows the turnover of the HAT reagent to make the process catalytic.
(4) Radical propagation steps can continuously regenerate the HAT reagent (otherwise known as chain transfer). In the provided example, a fluorine atom transfer (FAT) between alkyl radical 7.5 and Selectfluor 7.14 affords the fluorinated product 7.16 and generates an equivalent of TEDA2+· 7.15· for further HAT [120]. Notably, chain transfer can also be a contributing pathway in reactions where Methods (1), (2), and (3) are the main pathways with widely varying degrees of chain contribution. In photoredox chemistry, the contribution of the chain transfer to the reaction mechanism can be investigated using quantum-yield measurements or “light/dark experiments” [121]. However, reactions that utilise chain propagation as the major pathway typically use a sub-stoichiometric amount of initiator to initiate the process [122,123,124,125,126].
Each method can form a radical species capable of HAT, therefore facilitating the generation of radicals from corresponding C–H bonds, as well as X–H bonds (where X = heteroatom). Harnessing the high reactivity of radicals allows for a multitude of transformations [28,58,59,76,127,128].
2. C–H Functionalisation Using HAT Chemistry
The functionalisation of C–H bonds through radical mechanisms has been a subject of intense research in recent years [19,33,76]. Accessing alkyl radicals can be accomplished through oxidation–deprotonation pathways. However, this approach requires C–H substrates that are easily oxidised substrates and/or requires strongly oxidizing photoredox catalysts [76,129,130,131,132,133]. However, hydrogen atom transfer relies on the abstraction of weak/activated C–H bonds to generate the corresponding alkyl radical [7,19,48].
2.1. Nitrogen-Based HAT Reagents
Nitrogen-centred radicals that participate in HAT chemistry are typically highly electrophilic, and their N–H derivatives possess a range of N–H bond strengths [79,134]. However, due to the high BDE of quinuclidine-type species (such as TEDA2+-H 7.15-H) and amide N–H 3.3 and the electrophilic character of their corresponding N-centred radicals, these species are used for the abstraction of strong hydridic C–H bonds.
2.1.1. Quinuclidine and DABCO-Style HAT Reagents
Quinuclidine and DABCO-type HAT reagents are amongst the most popular reagents for HAT (especially in tandem catalytic protocols with photoredox catalysts) [118,135]. They remain a popular option especially for HAT of strong hydridic C–H bonds due to their strong N+–H bonds [quinuclidine 8.4-H BDEN+–H = 100 kcal/mol] [136]. Additionally, the high electrophilicity of quinuclidine radical cation 8.4· has been exploited in highly chemo/regio-selective HAT protocols where additives increase electron density at certain hydrogen atoms [137,138]. In some instances, quinuclidine 8.4 has shown a dependence on water. Hence, some protocols use water as a co-solvent/additive [139,140,141]. This is likely caused by water aiding the solubility of the inorganic base (e.g., Na2CO3 or NaHCO3) in the reaction medium, which accelerates the turnover of quinuclidine 8.4 by deprotonating the protonated quinuclidinium intermediate 8.4-H. Martin recently demonstrated the arylation of C(sp3)–O bonds in 7/8-membered cyclic acetals (Scheme 8) [142]. This reaction was initiated by HAT of acetal 8.1 α-C–H with bromine radical 8.6 or quinuclidine radical cation 8.4· and subsequent β-scission of radical 8.7 to form alkyl radical 8.8. Alkyl radical 8.8 is trapped by nickel complex 8.10. The subsequent reductive elimination of a Ni(III) complex delivers product 8.5. Control experiments showed the reaction proceeded in a 20% lower yield in the absence of quinuclidine 8.4, suggesting that bromine radical 8.6 is a competing HAT reagent. Halogen radicals (such as Cl· and Br·) can form through photolysis of metal halide bonds triggered by ligand-to-metal charge transfer (LMCT) [31,143,144,145,146]. The general protocol displayed an excellent functional group tolerance with functionalised acetal rings, ketones and pyridines, and heterocycles reacting well (see products 8.13–8.16). A vinyl bromide and an alkyl bromide (product 8.16) were also competent electrophilic coupling partners. In 2021, Wang developed a general difluoroallylation protocol using photoredox and HAT tandem catalysis (Scheme 9) [147]. Quinuclidine 9.5 was the optimal HAT reagent for this protocol, as found in preceding literature using identical C–H substrates [137,148]. Alkyl radicals were trapped with 2-trifluoromethylstyrenes 9.1 affording gem-difluoroalkene products 9.6 or 9.7.
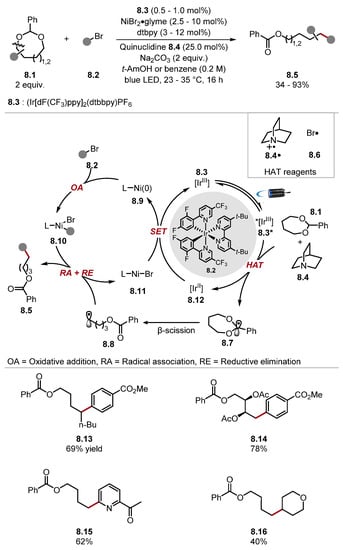
Scheme 8.
C-C bond formation through C(sp3)-O bond scission in cyclic acetals through HAT with quinuclidine.
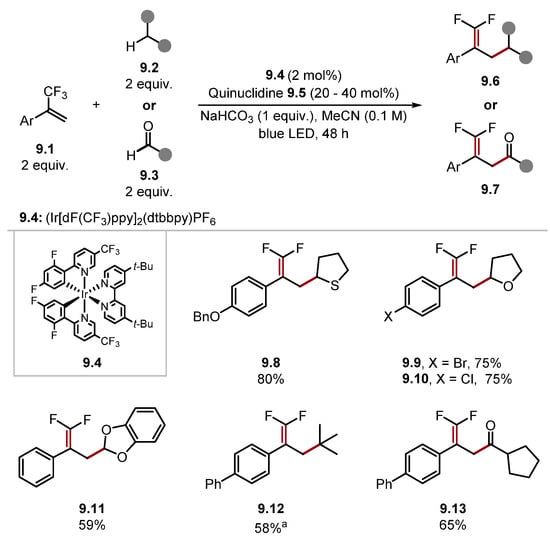
Scheme 9.
C-H difluoroallylation with photoredox HAT tandem catalysis using quinuclidine. aProduct 9.12 was formed from pivaldehyde.
This protocol displayed an excellent functional group tolerance and was able to utilise numerous C–H substrates as radical precursors. For instance, in addition to amides and carbamates, thioether, ether and acetal products 9.8–9.11 were prepared, while alkyl aldehydes formed ketone products, such as 9.13. The method did not tolerate aryl aldehydes or alkyl aldehydes containing benzene rings. However, it is worth noting that acetonitrile was the solvent. Solvent effects are important in HAT processes, and the HAT of formyl C–H bonds is known to proceed more efficiently in less-polar solvents such as dioxane or isooctane [98,148]. Notably, alkyl aldehydes with a tertiary alkyl group adjacent to the aldehyde afforded decarbonylated products, as seen in product 9.12, which was formed from pivaldehyde. This is due to the fast rate of decarbonylation of the corresponding acyl radicals [149]. The method was also highly tolerant of various functional groups on the styrene, and the method was showcased on numerous pharmaceuticals. In 2022, Jing used a similar protocol for hydrosilane 10.2 Si–H difluoroallylation-forming products 10.4 (Scheme 10) [150]. Quinuclidine radical cation 10.3· is known to abstract hydrogen from Si–H bonds [Et3Si–H BDESi–H = 95.1 kcal/mol versus quinuclidine 10.3 BDEN+–H = 100 kcal/mol] [79,136,151]. In 2018, Molander reported an example of a radical/polar annulation reaction (RPAR) to form a cyclopropyl product 11.4 proceeding through HAT with 3-acetoxyquinuclidine 11.3 (Scheme 11) [152]. The product was obtained in a moderate yield. However, this could probably be improved by using a photocatalyst, which is stronger in its reduced form (PC•−) as benzyl radicals are known to be reduced slowly by 4CzIPN•− [153].
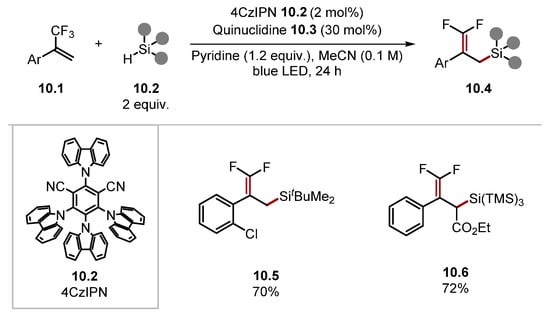
Scheme 10.
Si-H difluoroallylation with photoredox HAT tandem catalysis using quinuclidine.
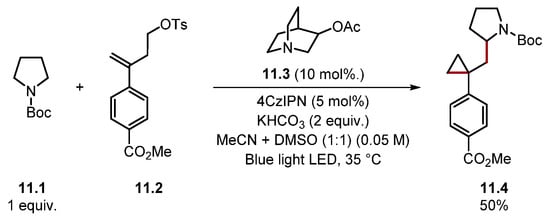
Scheme 11.
Radical/polar annulation reaction (RPAR) forming a cyclopropyl product 11.4 proceeding through HAT with 3-acetoxyquinuclidine.
In 2022, Xia used quinuclidine 12.3 for cyclobutylation of α-oxy C(sp3)–H bonds using a photoredox HAT tandem catalysis manifold (Scheme 12) [154]. An α-oxyalkyl radical, formed through HAT with a quinuclidine radical cation, attacks electron-poor bicyclo [1.1.0]butane 12.2 to form the functionalised cyclobutane product 12.4 after the reduction and protonation. Alcohols 12.5, acetals 12.6, and ethers 12.7 were formed from the appropriate substrates. However, N-Boc pyrrolidine, secondary amines, and 1,4-dioxane were unsuccessful. The derivatisation of aldehydes, amides, and thioethers was not attempted.
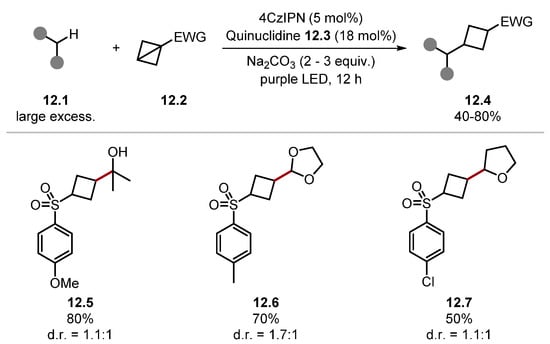
Scheme 12.
α-Oxyalkyl C(sp3)–H cyclobutylation via photoredox HAT tandem catalysis with quinuclidine.
As mentioned before, polar effects are important in HAT chemistry (Section 1.1). These effects make it possible to promote, or deter, HAT processes by either increasing or decreasing electron density at specific H atoms, respectively. Selectivity has been achieved by adding H-bonding and Brønsted/Lewis acid/base additives [137,138,139,155,156]. Methods for promoting H-atom abstractions at alcohol α-C–H bonds are now widespread [138,157,158,159,160]. In 2022, Suárez used phenylboronic acid 13.4 to promote HAT at alcohol α-C–H bonds on protected α-amino alcohols 13.1 with quinuclidine 13.3 (Scheme 13) [161]. This protocol provides access to γ-oxo-δ-amino acids 13.6 after an oxidation step with IBX [162]. Boc was the optimal protecting group for the amine. However, Cbz was also used. The protocol tolerated a wide range of functionality and used various Giese acceptors. For example, an α,β-unsaturated amide formed product 13.7 and acrylonitrile formed product 13.8; α,β-unsaturated esters and ketones also reacted well. The protocol proceeded in moderate-to-good yields even in the presence of other weak C–H bonds, as seen in product 13.9. Overall, this method further expands the utility of alcohols with α-C–H bonds as radical precursors and complements the existing methods [155].
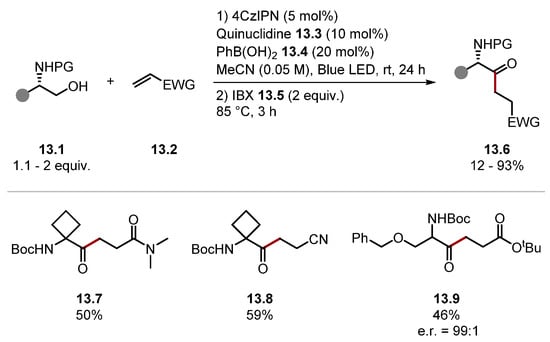
Scheme 13.
Alcohol α-C(sp3)–H alkylation using quinuclidine for HAT provides ketones upon oxidation with IBX.
In contrast to the abundant literature describing the promotion of HAT at alcohol α-C–H bonds, using unprotected amines remains difficult [163]. In 2022, Kanai used a germanium catalyst 14.5 to promote HAT at primary amine 14.1 α-C–H bonds with quinuclidine 14.4 (Scheme 14) [164]. This was used in a Giese protocol with α,β-unsaturated esters 14.2, which provide lactams 14.6 upon cyclisation. Computational studies showed that the addition of germanium catalyst lowered the BDE of amine α-C–H bonds [ethylamine BDEα-C–H = 94.0 kcal/mol] by 1.3–7.0 kcal/mol depending on whether an aminogermane (neutral 4-coordinate germanium) or aminogermate (anionic 5-coordinate germanate) species is formed in situ [79]. The use of base was vital for the success of the reaction. This effect is probably due to the faster turnover of quinuclidinium 14.4-H, as well as the prevention of the amine from being protonated, which is known to suppress HAT pathways [29]. This method tolerated a range of functionality, such as alcohols, nitriles, ethers, and acetals (see products 14.7, 14.8). The use of germanium catalyst 14.5 supressed HAT at weak benzylic positions and acetal α-C–H bonds [diphenylmethane BDEbenzylic C–H = 84.5 kcal/mol and 1,1-dimethoxyethane BDEC–H = 88.2 kcal mol] [79]. These results parallel other methods that use additives to increase electron density at C–H bonds to promote their abstraction even in the presence of weaker bonds through polar effects [137,138,139,155]. The mechanism of the reaction proceeds through the coordination of a germanium catalyst 14.10 to amine 14.1 forming an amino complex 14.11. The amino complex 14.11 undergoes HAT faster than a primary amine 14.1. Hence, the amino α-C–H bond in complex 14.11 is abstracted with quinuclidine radical cation 14.4· to deliver α-aminoalkyl radical 14.12, which is trapped with a Giese acceptor 14.2 that forms a radical adduct 14.13. The radical 14.13 is rapidly reduced and protonated to deliver the Giese product 14.15, which can cyclise to form a lactam 14.6 turning over the germanium catalyst 14.10.
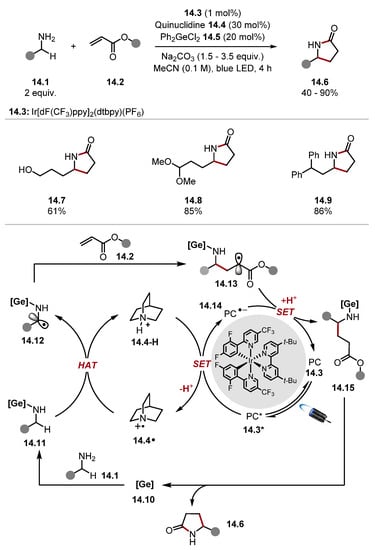
Scheme 14.
Promotion of HAT at amine α-C–H bonds with a Ge catalyst using quinuclidine.
DABCO 15.1 is different from quinuclidine 15.2 as the radical cation 15.1· and DABCO–H 15.1-H are stabilised through a 1-spin-4-non-bonded-electron orbital interaction [165]. As a result, DABCO 15.1 forms weaker N+–H bonds compared with quinuclidine 15.2, which is not stabilised [DABCO 15.1-H BDEN+–H = 91.3 kcal/mol and quinuclidine–H 15.2-H BDEN+–H = 100.0 kcal/mol] [136,165]. On that account, a recent trend in DABCO-type HAT reagents has involved removing this stabilizing interaction through the quaternisation of one nitrogen atom, resulting in stronger N+–H bonds (for instance, Compound 15.3) [166]. In several reports, such species are formed through the reduction of Selectfluor 15.4 to form TEDA2+· radical 15.5· (Scheme 15) [20,57,167,168,169,170,171]. To the best of our knowledge, no BDE value is known for N+–H in TEDA2+–H 15.5-H. However, it is assumed to be around 100 kcal/mol due to its ability to activate alkanes [171].
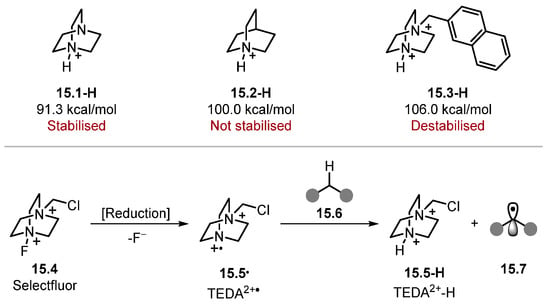
Scheme 15.
Comparison of DABCO, quinuclidine, and quaternised DABCO.
In 2022, Pieber reported a benzylic C(sp3)–H fluorination of phenylacetic acids 16.1 using Selectfluor 16.2 and DMAP 16.3 (Scheme 16) [120].
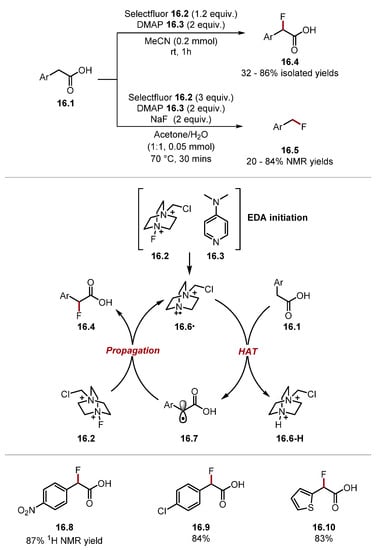
Scheme 16.
Fluorination of phenylacetic acid α-C–H bonds with Selectfluor.
Conducting the reaction in a mixture of acetone and water led to a decarboxylative fluorination product 16.5. However, using acetonitrile as a solvent formed fluorinated phenylacetic acid derivatives 16.4. The initiation step is thought to proceed through an EDA complex between Selectfluor 16.2 and DMAP 16.3, which forms TEDA2+· radical 16.6·. The authors did not perform any experiments elucidating the initiation. However, a similar mechanistic pathway has been previously investigated by Van Humbeck and Baran [126,172]. In addition, Selectfluor 16.2 has a low reduction potential (Selectfluor 16.2 E1/2 = +0.33 V vs. SCE in MeCN) [173]. TEDA2+· radical 16.6· is a strong HAT reagent and can abstract a benzylic C–H to form benzylic radical 16.7. The benzylic radical 16.7 can propagate the chain reaction by abstracting a fluorine atom from Selectfluor 16.2, forming the fluorinated product 16.4 and TEDA2+· 16.6· [Selectfluor 16.2 BDEN–F = 64.0 kcal/mol versus (fluoromethyl)benzene BDEC–F = 97.6 kcal/mol] [79,174]. In 2020, Lectka and Dudding reported a site-selective fluorination of ketals using Selectfluor 17.2 and xanthone under irradiation with visible light (Scheme 17) [175].
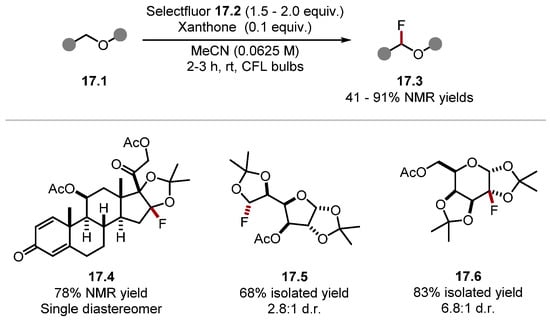
Scheme 17.
Site-selective fluorination of ketals with Selectfluor.
This system worked best on molecules with rigid C–H bonds, most notably on cyclic acetals and ethers. This is likely due to the increased stabilisation of HAT pathways through hyperconjugation in cyclic systems [89]. Numerous polycyclic systems were amenable to the fluorination method. For instance, the precurosor to steroid 17.4 was fluorinated at the ketal α-C(sp3)–H bond in a high yield in the presence of a weak allylic C–H bond. Compound 17.5 was also formed in a good yield in the presence of numerous ethereal and acetal C–H bonds. Hence, the site-selectivity observed was not entirely dependent on BDE values as seen with galactose diacetonide 17.6 where five ethereal C–H bonds were present. Computational studies showed that intermolecular interactions in the transition state caused the observed regioselectivity. In a subsequent work, Lectka and Dudding showed carbonyl groups can also direct C(sp3)–H fluorination [176]. In 2022, Lectka developed a similar method using alcohols 18.1, which directed fluorination to the γ-C(sp3)–H bonds, forming products 18.3 (Scheme 18) [177]. In addition, 5,6,7-membered rings fluorinated the γ-C(sp3)–H bond, as seen in products 18.4 and 18.5. Notably, the tertiary carbon was prefered to the methylene in product 18.5. The weaker benzylic position was left unreacted in product 18.6. In the absence of the hydroxyl group, the fluorination occured on the benzylic C(sp3)–H. This demonstrates how the intermolecular interactions in the transition state could alter the outcome of a HAT reaction. Similarly to the group’s previous work, more rigid C–H bonds in cyclic systems reacted in preference to linear ones, as seen in Product 18.7.
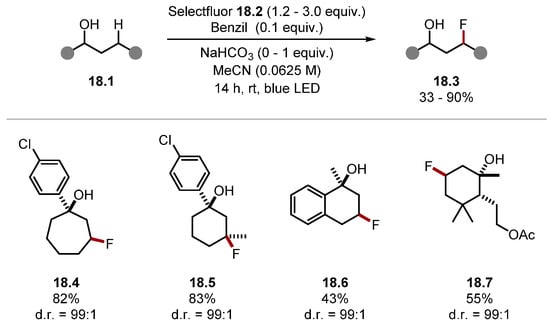
Scheme 18.
Hydroxy-directed fluorination of C(sp3)–H bonds using Selectfluor.
Maruoka developed cationic DABCO-type Catalysts 19.4 and 19.5 for C–H alkylation through a Giese pathway (Scheme 19) [166]. DFT studies showed the DABCO-derived cationic catalysts formed N+–H BDE values between 104–109 kcal/mol [19.4-H BDEN+–H = 106 kcal/mol]. The low oxidation potential of the reduced form of the acridinium catalyst 19.3 meant only easily reducible alkenes were tolerated, as seen in products 19.7, 19.8, and 19.11 (E1/2 (Acr 19.3/Acr+) = −0.56 V vs. SCE in MeCN versus E1/2 (·CH2CO2Et/−CH2CO2Et) = −0.63 V versus SCE in MeCN) [130,178,179,180]. The HAT reagent formed from 19.4 was effective in the alkylation of various C–H substrates forming cycloalkanes 19.7, alcohols 19.8, aldehydes 19.9, toluene 19.10, and ethers 19.11 and 19.12. Furthermore, where multiple abstractable C–H bonds were present, the regioselectivity could be refined by using a substituted HAT reagent 19.5. This effect can be seen on ethers 19.11 and 19.12. The general method was also showcased on biologically active and complex molecules.
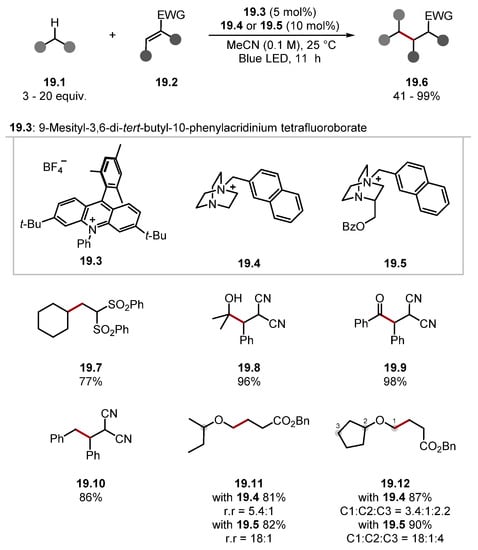
Scheme 19.
Cationic DABCO-based HAT catalyst used in Giese protocol.
DABCO 20.4 has also been applied to H-atom abstraction from formate anion 20.5 to form a radical anion of CO2 20.10 (CO2•−) (BDE) [181]. CO2•− 20.10 is a highly nucleophilic radical that can behave as a strong reductant (CO2•− E1/2 = −2.21 V vs. SCE in DMF) [182,183,184]. Generally, substrates with reduction potentials greater than −2.1 V versus SCE undergo SET reaction pathways, while those with reduction potentials lower than this value undergo radical addition pathways [184].
Li used DABCO and potassium formate to access CO2•− 20.10 for an arylative carboxylation of styrenes 20.1 (Scheme 20) [185]. This reaction proceeds through the oxidation of DABCO 20.4 to form DABCO radical cation 20.4·. DABCO radical cation 20.4· can abstract a hydrogen atom from formate 20.5 to form CO2•− 20.10 [formate HCO2− BDEC–H = 86 kcal/mol versus DABCO BDEN+–H = 91.3 kcal/mol] [136,186]. CO2•− 20.10 can reduce aryl halides 20.2 to access aryl radical 20.11 ((CO2•−) E1/2 = −2.21 V versus SCE in DMF versus (4-bromobenzotrifluoride) E1/2 = −2.18 V vs. SCE in DMF) [187]. The aryl radical 20.11 then adds to styrene 20.1 to form a benzylic radical 20.12, which is subsequently reduced to the benzylic anion 20.13 by the photocatalyst. The benzylic anion 20.13 traps CO2 to form a carboxylate, which is methylated in a subsequent step to form the 1,2-difunctionalised product 20.6. Electronically diverse aryl bromides and iodides reacted in good yields. However, only electron-poor aryl chlorides were reacted, and no unsuccessful examples were shown.
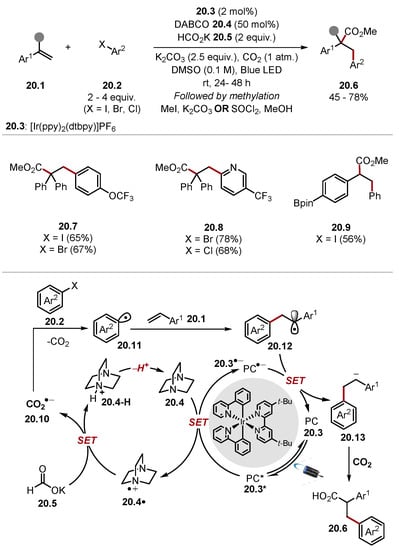
Scheme 20.
Carboxylative arylation of styrenes 20.1 with CO2•−.
Zhu and Guo accessed succinic acid products 21.5 from alkenes 21.1 using a combination of DABCO 21.3 and sodium formate 21.4 under an atmosphere of CO2 with photoredox catalysis (Scheme 21) [188].
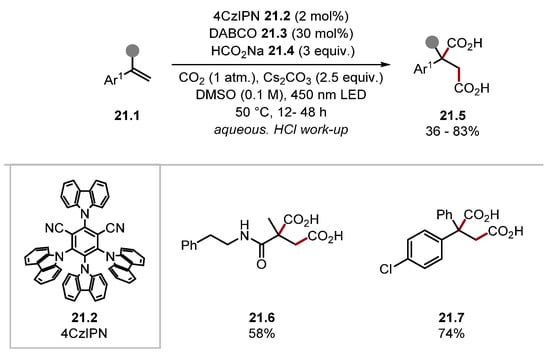
Scheme 21.
Succinic acid synthesis through dicarboxylation of alkenes with CO2•−.
Mita used a similar combination of DABCO 22.3 and formate salt 22.4 to form CO2•− for the addition of heteroaromatics to form products such as benzofurans 22.6, benzothiophenes 22.7, and indoles 22.8 and 22.9) (Scheme 22) [189]. In the case of benzofurans 22.6, 6-membered lactone by-products were also observed in yields of 3–32%. Various thiols are also used for HAT from formate salts to form CO2•− (see Section 2.2.1).

Scheme 22.
Dearomative carboxylation of heteroarenes 22.1 with CO2•−.
2.1.2. Amide HAT Reagents
Amidyl radicals form amides upon HAT, which have very strong N–H bonds [amide BDEN–H = 97–111 kcal/mol] (Scheme 3) [79]. This makes amidyl radicals powerful HAT abstractors, capable of abstracting unactivated C(sp3)–H bonds. In recent years, 1,5-HAT intramolecular protocols using amidyl radicals have been popular, drawing from the classic Hofmann–Löffler–Freytag and Barton reactions [190]. However, the use of amidyl radicals for intermolecular HAT is less explored, and, herein, we cover recent developments in this area [83].
In 2021, Hu used amidyl radicals formed by the reduction of N-fluorosulfonamide 23.3 or N-fluoroamide 23.11 for the functionalisation of strong C(sp3)–H bonds and carboxylic acids through decarboxylation (Scheme 23) [191]. Fluorosulfonamide 23.3 and fluorocarboxamide 23.11 were used as radical precursors to access amidyl radicals 23.18. Numerous C–H substrates were used in the general protocol. For example, THF formed product 23.5, Boc-protected piperidine formed product 23.6, and cycloalkanes were also derivatised to form products 23.7–23.9. The authors also developed a decarboxylation protocol from carboxylic acids 23.10, which, upon decarboxylation and trapping of the radical, yields products 23.12. This protocol required blue LEDs, and the authors suggest that this could trigger a homolysis of the N–F bonds, as well as forming Cu(I) species from Cu(II) under light irradiation [192,193]. Hence, the authors suggest a HAT step between the O–H of the carboxylic acid and the amidyl radical derived from 23.11. Using the general protocol, various decarboxylated products were obtained; for instance, Boc-piperidine 23.13, ketone 23.14, benzylic thioether 23.15, and ibuprofen derivative 23.16. Moreover, using different aryl sulfone radical traps allowed various functional groups to be introduced, including a range of thioethers as well as alkene, alkyne 23.9, nitriles, trifluoromethylthioether 23.16, azide, and halogens. The reaction occurs through a reduction of fluoroamides 23.3 or 23.11 with copper (I) 23.17, forming a copper (II) fluoride salt 23.19 and amidyl radical 23.18 [194,195]. Amidyl radical 23.18 then abstracts a hydrogen atom from the C–H substrate 23.1 or oxidises carboxylic acid 23.10 to form an alkyl radical 23.21. Alkyl radical 23.21 can be trapped with a multitude of SOMO-philes (radical traps) to form functionalised Product 23.4 or 23.12 and sulfonyl radical 23.22. Sulfonyl radical 23.22 and copper (II) fluoride 23.19 react forming a sulfonyl fluoride 23.23 and reforming a copper (I) catalyst 23.17 [196]. In contrast to previously reported protocols [197,198,199,200,201], using fluoroamides 23.3 and 23.11 allowed for a general C(sp3)–H functionalisation method capable of introducing various functional groups.
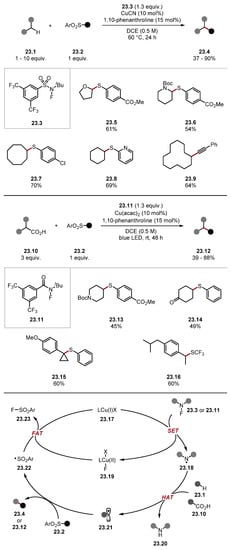
Scheme 23.
General method for C–H functionalisation using fluoroamides 23.3 and 23.11 as amidyl radical precursors.
Niu used fluorosulfonamide 24.3 for allylation and alkynylation of unactivated C(sp3)–H bonds (Scheme 24) [202]. The reaction occurs through a pathway akin to Hu’s protocol (Scheme 23). This method showed good functional group tolerance and incorporated a range of functional groups through numerous SOMO-philes 24.2. For instance, allylic sulfones formed products 24.5, 24.6, and 24.9, and alkynyl sulfones formed products 24.7 and 24.8.
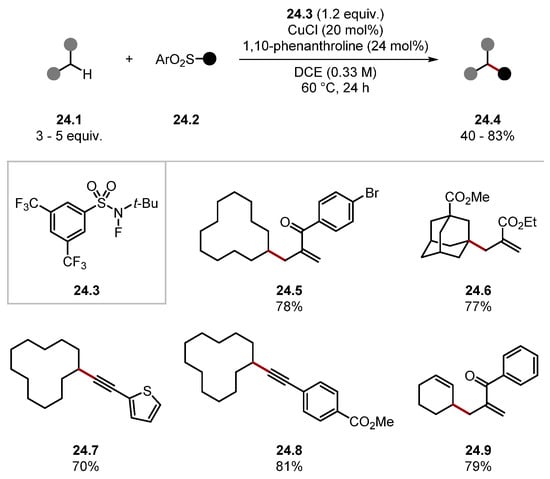
Scheme 24.
General method for C–H functionalisation using fluoroamide 24.3 as amidyl radical precursor.
In 2022, Alexanian and Leibfarth developed a general method of aliphatic C(sp3)–H functionalisation through HAT using Amide 25.2 (Scheme 25) [203]. This method was an adaptation of Alexanian’s and Leibfarth’s previous works, which used similar amides [197,198,200,204]. However, this work used a HAT reagent precursor 25.2, allowing different radical traps 25.3 to be used. This allowed various functional groups to be introduced into C–H bonds. Moreover, the C–H substrate was the limiting reagent. The reaction is believed to proceed through a chain propagation mechanism where amide 25.2 undergoes initiation through homolysis or chain transfer (CT) with radical 25.7 forming amidyl radical 25.5. Amidyl radical 25.5 can abstract hydrogen from unactivatived aliphatic C–H bonds. The alkyl radical 25.6 reacts with a radical trap 25.3 to form C–H functionalised product 25.4 and radical 25.7. The authors elegantly matched the inherent electrophilicity of the expelled radical 25.7 to trap it with the electron-rich alkene on HAT reagent 25.2 for chain propagation. Cleavage of the resulting radical adduct expels a ketone 25.8 and amidyl radical 25.5, thus propagating the chain. Various aliphatic compounds were functionalised, and, more importantly, various functional groups were introduced into C(sp3)–H bonds. All radical traps outlined were used with cyclooctane forming products in yields of 44–100% NMR/GC yields; for instance, nitrile 25.9 and azide 25.10. Ibuprofen methyl ester was fluorinated on the benzylic position to form product 25.11. The iodination product 25.12 was also accessed. Thiolated products 25.13 and 25.14 were also obtained in good yields. Further derivatisations of products were demonstrated, and the protocol was used to introduce functionality into waste-stream aliphatic polymers. This general protocol has enormous potential as various other radical traps may also be used in this manner to introduce an even greater array of functionality into C(sp3)–H bonds [76].

Scheme 25.
General C–H functionalisation method using amidyl radical precursor 25.2.
Alexanian recently built on this work again using HAT reagent 26.3 for a C–H heteroarylation protocol (Scheme 26) [205]. The method used aryl sulfones 26.2 as radical traps, which imposed regioselectivity, negating a common drawback in Minisci-type reactions [127]. Various C–H substrates were derivatised, and the method was selective for the most hydridic C–H bonds, as seen in product 26.6. Notably, several complex scaffolds were derived regioselectively and diastereoselectively, showcasing the method’s potential for LSF applications. Reagent 26.3 was also recently applied to the functionalisation of B–H bonds in icosahedral carboranes [206]. Ooi has developed a zwitterionic 1,2,3-triazolium amidate HAT catalyst 27.4 [207]. These HAT reagents work on a similar basis to Knowles’s and Alexanian’s works describing amidyl radicals as HAT reagents [83,199,203,204,208]. Amide HAT catalysts, such as 27.4, form very strong N–H bonds [amide 27.4–H BDEN–H = 100 kcal/mol], making 27.4 a HAT reagent capable of oxidising strong C–H bonds similar to quinuclidine. Ooi’s previous work shows HAT catalyst 27.4 readily abstracts hydrogen atoms adjacent to carbamates, ethers, aldehydes, and alcohols [207]. In 2022, Ooi used the amidate HAT pre-catalyst 27.4 for a dehydrogenative cross-coupling of various C–H substrates and enamides or 1,1-diarylethenes under irradiation by blue LEDs (Scheme 27) [209].
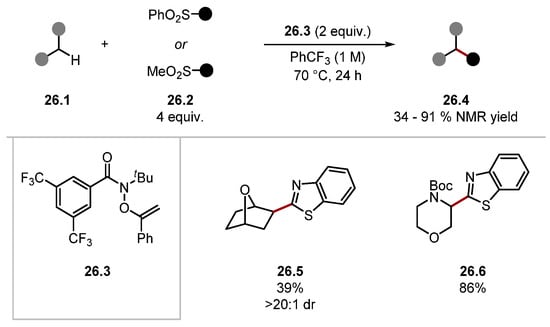
Scheme 26.
Heteroarylation of C(sp3)–H bonds via HAT using amidyl radical precursor 26.3.
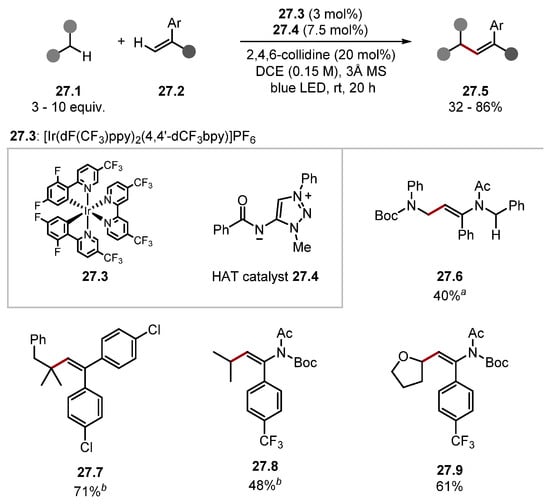
Scheme 27.
HAT-mediated dehydrogenative cross-coupling using amidyl radical precursor 27.4. aNo 3A MS, bDecarbonylated product made from the corresponding aldehyde.
Carbamates and ethereal α-C(sp3)–H were readily alkenylated, forming products 27.6 and 27.9, respectively. Aldehydes resulted in decarbonylated products such as 27.7 and 27.8. Various enamide substrates were used in the protocol. Notably, a benzyl protecting group 27.6 was tolerated. Various functionalities were incorporated into arene fragments, such as chloride 27.7 and trifluoromethyl groups 27.8 and 27.9. Ooi has used HAT pre-catalyst 28.3 for a C(sp3)–H alkylation of benzylic fluorides 28.1 (Scheme 28) [210]. The method showed good functional group tolerance with substrates containing halides, ethers, and esters forming products in good yields. Ooi has also recently developed a diphenylphosphinyl amidate HAT catalyst similar to 28.3, which was used in a Giese protocol with substituted alkanes and cycloalkanes [211].
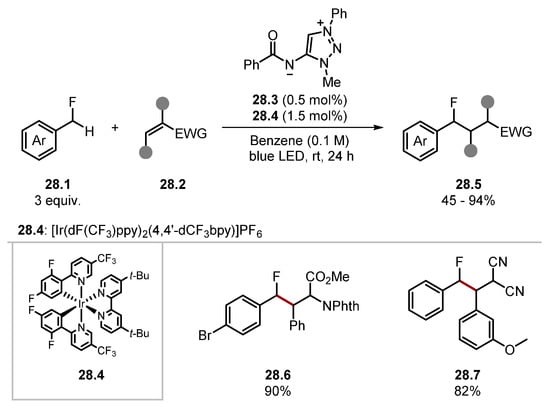
Scheme 28.
HAT-mediated C(sp3)–H alkylation of benzylic fluorides 28.1 with amidyl radical precursor 28.3.
2.1.3. Azidyl Radical as a HAT Reagent
The azidyl radical 29.7 has previously been used as an oxidant and HAT reagent [212,213]. In recent years, it has mainly been used in the context of primary amine α-C–H HAT. The azidyl radical 29.7 is usually formed through oxidation of its anion 29.10, although access through homolytic pathways is known. The azidyl radical is inherently electrophilic and abstracts hydridic hydrogen atoms to form hydrazoic acid [hydrazoic acid 29.8 BDEN–H = 92.7 kcal/mol] [214]. Hydrazoic acid 29.8 is easily deprotonated to regenerate the azide anion 29.10. Due to the facile oxidation of the azide anion to azidyl radical (nBu4NN3 29.4 E1/2 = +0.87 V vs. SCE in MeCN), the reagent can be made catalytic through the use of photoredox catalysis or electrochemistry for oxidation [153,215,216,217]. While the concentration of hydrazoic acid in such reactions is small, it is worth being mindful of hydrazoic acid’s high toxicity and explosive risk [218].
In 2020, Cresswell used tetrabutylammonium azide 29.4 (which forms azidyl radical 29.7 upon oxidation) for α-C–H alkylation of unprotected amines 29.1 (Scheme 29, top) [215]. When α,β-unsaturated esters were used as Giese acceptors, a separate cyclisation step afforded γ-lactams, such as 29.13. Previous work sought to develop amine α-C–H alkylation protocols by protecting amines in situ [163]. However, using azidyl radical 29.7 as a HAT catalyst allows unprotected amines 29.1 to be used directly. This reaction proceeds through a HAT and photoredox dual catalysis manifold. The excitation of 4CzIPN 29.3 by blue light produces photoexcited 4CzIPN* 29.3* (E1/2 (PC*/PC•−) = +1.43 V vs. SCE), which can oxidise azide anion 29.10 (Ered = +0.87 V vs. SCE in MeCN) to form 4CzIPN•− 29.6 and azidyl radical 29.7. Azidyl radical 29.7 abstracts an amino α-C–H from 29.1 to form α–aminoalkyl radical 29.9 [cyclohexylamine BDEα-C–H = 91.1 kcal/mol] [80]. The alkyl radical 29.9 is trapped with a Giese acceptor 29.2 to afford Giese radical adduct 29.11. The intermediate 29.11 is rapidly reduced by 4CzIPN•− 29.6 (E1/2 = (PC/PC•−) −1.24 V vs. SCE in MeCN versus E1/2 (•CH2CO2Et/−CH2CO2Et) = −0.63 V versus SCE in MeCN) and subsequently protonated to form product 29.5. The low quantum yield (Φ = 0.04) of the reaction suggests that a chain contribution is insignificant. The general protocol showed good chemoselectivity with selectivity for amine α-C(sp3)–H bonds, even in the presence of weak carbamate α-C–H bonds 29.13 and benzylic C(sp3)–H bonds. Alcohols 29.14, thioethers, sulfones, and esters (among other functional groups) were tolerated. Numerous Giese acceptors were also used such as 2-vinylpyridine and 4-vinylpyridine. A separate dialkylation protocol was developed for primary amines with two α-C–H bonds. Several derivatisations of primary amines were demonstrated, including reductive amination and amidations. Later that year, Cresswell showed the formation of γ-amino phosphonates 29.17 through the same pathway (Scheme 29, bottom) [216]. The method showed a functional group tolerance akin to the group’s previous reports with cyclobutylamine 29.18, alcohols 29.19, esters 29.20, carbamates, and compounds with benzylic C–H bonds reacting in moderate-to-high yields.
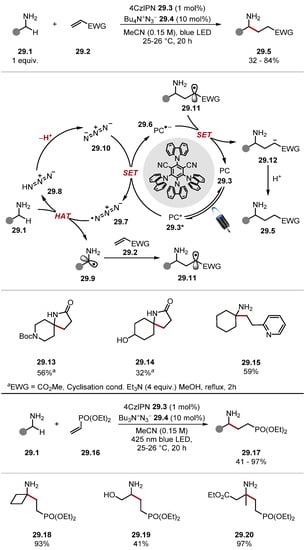
Scheme 29.
α-C–H alkylation of unprotected primary amines via HAT with azidyl radical.
In 2021, Cresswell used tetrabutylammonium azide 30.4 for α-C–H alkylation of unprotected amines 30.1 with styrenes 30.2 (Scheme 30) [153]. The protocol afforded Giese products sluggishly when 4CzIPN was used as a photocatalyst. This was due to the higher reduction potential of benzylic radicals (formed upon radical addition to styrenes, such as 30.2), compared with radicals with adjacent EWGs (E1/2 (•CH2Ph/−CH2Ph) = −1.43 V versus SCE in MeCN compared to E1/2 (•CH2CO2Et/−CH2CO2Et) = −0.63 V versus SCE in MeCN). Hence, when a more strongly reducing photocatalyst 3DPA2FBN 30.3 was used, the product formed in a high yield (E1/2 (PC/PC•−) = −1.92 V versus SCE in DCM) [135]. The functional group tolerance was excellent on both the amine substrates and the styrene substrates. For instance, silanes, heterocycles 30.7, nitriles 30.6, Bpin 30.8, and halides were tolerated. The general protocol was showcased in a one-step synthesis of Fingolimod 30.10 using a flow set-up. In 2023, Sneha and Orr–Ewing investigated the mechanism of these protocols showing an equivalent of azidyl radical (•N3), which rapidly reacts with N3− to form a cyclic dimer N6•−, which acts as a reservoir of azidyl radical (•N3), which carries out the HAT step [219].
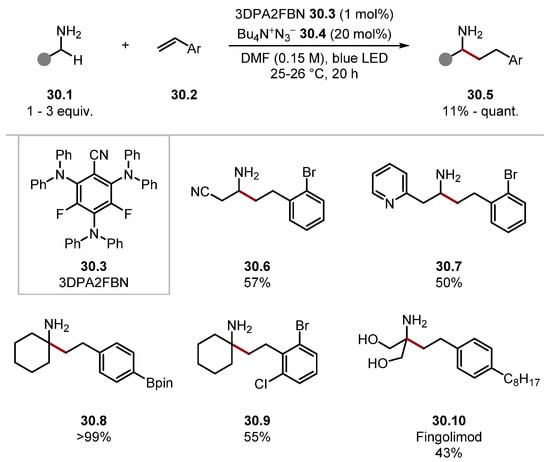
Scheme 30.
α-C–H alkylation of primary amines 30.1 with styrenes 30.2 through HAT with azidyl radical.
In 2022, Park showed tetrabutylammonium azide 31.3 could be used catalytically under anodic oxidation to generate α-amino radicals for the alkylation of γ-lactams 31.1, and one δ–lactam example, through HAT with azidyl radical (Scheme 31) [217]. This reaction proceeds through HAT of an α-amino C–H bond with an anodically generated azidyl radical. The radical is subsequently trapped with Giese-acceptors 31.2 to form alkylated products 31.4. The procedure showed good chemo- and site-selectivity, even in the presence of weaker C–H bonds, as seen in product 31.6, which contains an allylic C–H bond. Numerous Giese acceptors were used; for instance, α,β-unsaturated sulfonamides 31.6, α,β-unsaturated sulfones, and α,β-unsaturated phosphonates, among many others. Notably, benzylamines have been problematic substrates in other HAT protocols [153,215].
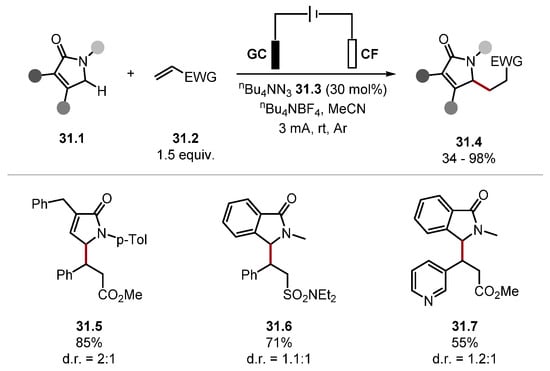
Scheme 31.
C(sp3)–H functionalisation of γ-lactams based on HAT with azidyl radical.
2.2. Sulfur-Based HAT Agents
Thiyl radicals are commonly used in HAT procedures [19,32]. S-centred radicals are less electronegative than their O/N-centred counterparts. However, due to their greater polarisability and the low pKa of thiols 7.14 (and thio-acids), thiyl radicals 7.13 are readily accessible through the oxidation of thiolates 7.12 (Scheme 7). Thiyl radicals form S–H bonds (thiols) upon HAT [aliphatic thiols BDES–H ≈ 87 kcal mol−1] [81]. This makes them excellent reagents for HAT from weak (highly activated) C–H bonds, such as α-amino, benzylic, and allylic C–H bonds, as well as weak heteroatom–hydrogen bonds, such as Si–H and Ge–H. Thiols can also be used to close catalytic cycles/reactions through HAT. However, this application is not covered by this review [220,221,222,223,224,225].
2.2.1. Thiols and Thioacid HAT Reagents
MacMillan and co-workers demonstrated the arylation of benzylic ether 32.5, and allylic 32.8 C(sp3)–H bonds proceeding through a coupling of an alkyl radical and a persistent arene radical anion (Scheme 32) [119,226].
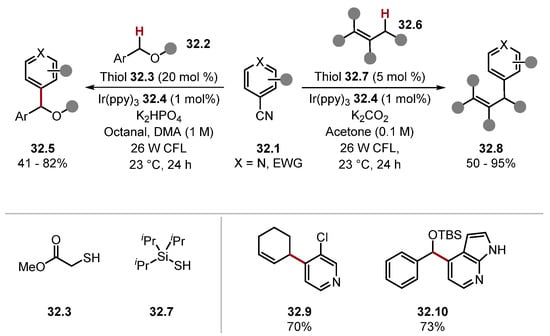
Scheme 32.
Arylation of benzylic ethers and allylic species with thiyl radicals.
These protocols displayed excellent functional group tolerance with respect to all reactants. For instance, alcohols, N/S/O-containing heterocycles 32.9 and 32.10, and halogen substituents were tolerated. Moreover, in both protocols, only monoarylated products were observed. These works showed that thiyl radicals can abstract hydrogen atoms from allylic and benzylic C(sp3)–H bonds, inspiring countless protocols proceeding through similar mechanistic pathways with thiyl radicals. The Hamashima group found that benzylamines 33.1 were arylated through a coupling between radical and radical anion akin to that of the MacMillan group (Scheme 33) [82]. Thiobenzoic acid was used as a HAT reagent precursor as it is readily deprotonated (thioacetic acid, pKa = 3.2) [227] and can subsequently be oxidised even in the presence of amines (PhC(O)SK E1/2 +0.80 V versus Ag/AgCl in DMA versus N,N-dimethylbenzylamine 33.11 E1/2 = +1.25 V vs. Ag/AgCl in DMA). The method was amenable to late-stage C(sp3)–H arylation of several pharmaceuticals and showed outstanding functional group tolerance as heterocycles and primary amines and alcohols (among others) were tolerated (see products 33.6 and 33.7).
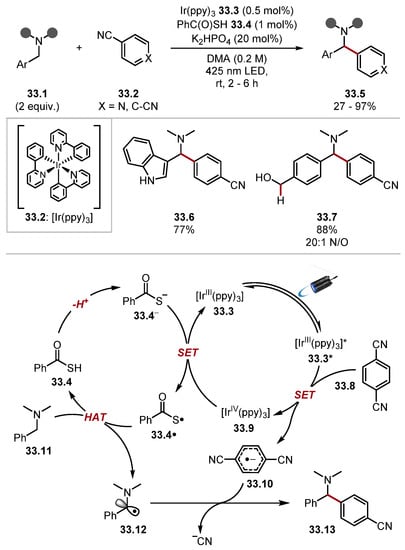
Scheme 33.
Arylation of benzylamine C(sp3)–H though HAT with thiobenzoic acid 33.4.
The reaction proceeds though photoexcitation of Ir(ppy)3 33.3 by 425 nm blue light, forming a strong reductant [IrIII (ppy)3]* 33.3* (E1/2 (IrIV/*IrIII) = −1.73 V vs. SCE in MeCN), which reduces Terephthalonitrile 33.8 (E1/2 = −1.61 V versus SCE in MeCN) to form aryl radical anion 33.10. The HAT catalytic cycle proceeds by deprotonation of thiobenzoic acid 33.4 (thioacetic acid, pKa = 3.2) [227]. The thiobenzoate anion 33.4− is then oxidised by [IrIV(ppy)3] 33.9 (E1/2 (IrIV/IrIII) = +0.77 V vs. SCE in MeCN) to form the S-centred radical 33.4·, which can abstract a hydrogen atom from N-benzylamine 33.11 [thiobenzoic acid 33.4 BDES–H = 87.4 kcal/mol versus benzylamine 33.11 BDEα-C–H = 84.9 kcal/mol]. The benzylic radical 33.12 then undergoes a radical-radical anion coupling with 33.10 to form product 33.13 [26,228]. The Hamashima group subsequently developed a photocatalyst-free arylation of benzylamine 34.1 C(sp3)–H bonds with thiobenzoic acid 34.3 as a HAT reagent (Scheme 34) [229]. In this work, donor-acceptor complex 34.5 is believed to initiate the formation of S-centred radical 34.3, as well as arene radical anion 34.10 upon excitation by visible light [230,231,232]. Control experiments showed that the addition of N,N-dimethylbenzylamine to PhC(O)SK caused an absorption of visible light around 400–450 nm. The authors do not elucidate the EDA complex, which initiates the reaction and a UV–VIS spectrum of PhC(O)SK and terephthalonitrile; another electron acceptor was not investigated. S-centred radical 34.3· would undergo a HAT process with benzylamine 34.1 to generate an α-aminoalkyl radical 34.6. The α-aminoalkyl radical 34.6 could then combine with arene radical anion 34.7, affording the product 34.4.
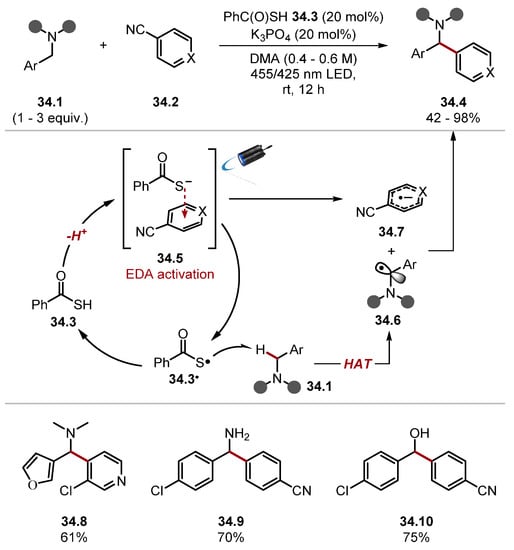
Scheme 34.
Benzylamine C(sp3)–H arylation through EDA-initiated HAT using thiobenzoic acid.
Liu used thiol HAT catalysts to abstract allylic and benzylic hydrogen atoms to form alkyl radicals for radical–radical anion couplings with ketyl radicals/ketyl radical anions to form tertiary alcohol products (Scheme 35) [233]. Two general protocols were developed; one used TIPS–SH 35.4, and the other used thioacetate salt 35.5. Both diaryl ketones and alkyl aryl ketones were suitable substrates. Aliphatic ketones did not react. Numerous heterocycles were tolerated, including benzothiazole, as seen in product 35.8. The protocol mainly functionalised allylic and benzylic C–H bonds, with one example of aldehyde C–H functionalisation. The mechanism of the reaction is believed to occur through reductive SET to ketone 35.17 by Ir(ppy)3 35.3 to form a ketyl radical anion 35.18 (benzophenone E1/2 = −1.66 V vs. Ag/AgCl versus E1/2 (IrIV/IrIII) = −1.73 V versus SCE) [234,235]. Ir(IV)(ppy)3− 35.12 is sufficiently strong to oxidise thiolate 35.16 to thiyl radical 35.13, which is capable of abstracting weak allylic or benzylic C(sp3)–H bonds to form alkyl radical 35.14. The alkyl radical 35.14 subsequently undergoes radical–radical anion coupling with the ketyl radical anion 35.18 to form tertiary alcohols 35.19. Thiyl radicals have also been used for the functionalisation of Si–H bonds [151,236].
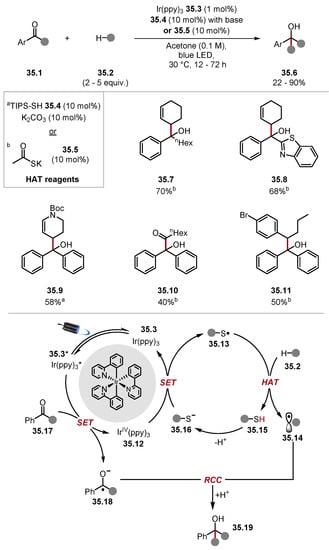
Scheme 35.
Ketone carbonyl alkylation through radical–radical anion coupling mediated by photoredox catalysis and HAT with triisopropylsilanethiol and thioacetic acid. a TIPSH 35.4 and K2CO3 were used. b Thioacetate salt 35.5 was used.
In 2022, Lin developed an arylsilylation of styrenes 36.2 with hydrosilanes 36.1 and cyanoarenes 36.3 under irradiation with blue LEDs (Scheme 36) [237]. The reaction proceeds through HAT of a silane 36.1 Si–H with thiyl radical 36.5 to form silyl radical 36.8 [thiols BDES–H ≈ 87 kcal/mol versus Ph3SiH BDESi–H = 86.4 kcal/mol] [79,81]. The silyl radical 36.8 can add into a styrene 36.2 to form a benzylic radical adduct 36.9. The benzylic radical adduct 36.9 then undergoes a radical-radical anion cross-coupling, with radical anion 36.10 expelling a cyanide to form the arylsilylated product 36.6. There are two competing initiation mechanisms in this reaction: In one, the radical anion 36.10 and thiyl radical 36.5• can form through EDA complex 36.11 [238]. Alternatively, the process is initiated by 4CzIPN 36.4, although it should be noted 4CzIPN•− (36.4•−) is not sufficiently strong to reduce 4-cyanopyridine to its radical anion 36.10 (E1/2 (PC/PC•−) = −1.24 V versus SCE in MeCN versus 4-cyanopyridine E1/2 = −1.86 V vs. SCE in MeCN) [135,239]. Moreover, reactions in which 4-cyanopyridine or 1,4-dicyanobenzene are reduced to radical anions feature more strongly reducing photoredox catalysts [119,226]. Additionally, control experiments showed that the reaction occurred in 64% yield in the absence of photocatalyst, compared with 92% for optimal conditions, suggesting initiation through an EDA complex is the major pathway. Hence, the mechanistic pathway of this protocol is not fully understood. The protocol showed good functional group tolerance towards all three reactants with products featuring amines 36.12, amides, ethers 36.13, and halides 36.14, among other functionalities being prepared in fair-to-excellent yields. Silanes containing alkyl substituents provide the lowest yields.

Scheme 36.
Arylsilylation of styrenes 36.2 with hydrosilanes 36.1 and cyanoarenes 36.3 proceeding through HAT with triisopropylsilanethiol.
In 2021, Schoenebeck used iPr3SiSH 37.4 to abstract hydrogen from Ge–H bonds to facilitate a hydrogermylation of alkenes (Scheme 37) [240]. Notably, organogermanes have shown enormous potential as functional handles [241]. Various olefins were tolerated with the procedure being relatively insensitive to the electronic nature of the olefin. The good yield of product 37.8 from 4-bromostyrene and product 37.7 from an unactivated alkene may indicate an innate chain propagation as benzylic radicals are reduced slowly by 4CzIPN•− [153]. However, no quantum yields were measured. DFT studies showed an abstraction of hydrogen from Et3Ge–H, while TIPS–S· was thermodynamically favourable [Et3Ge–H BDEGe–H = 86.0 kcal/mol versus alkyl thiol BDES–H ≈ 87 kcal mol−1] [79,81].

Scheme 37.
Hydrogermylation of olefins via HAT of H–GeEt3 37.2 with triisopropylsilanethiol.
Huang and Rueping developed an allylic C(sp3)–H alkylation protocol proceeding through photoredox HAT dual catalysis using triisopropylsilanethiol 38.3 as HAT reagent (Scheme 38) [242]. The assessment of the substrate scope for this general protocol showed a good functional group tolerance with heterocycles (thiophene and pyridine) and pinacolborane, as seen in products 38.6, esters 38.7, as well as halogens and ethers tolerated on the imine substrate. The general conditions were also used to functionalise natural products, as seen in the formation of compound 38.8. The Ooi group developed a similar protocol for silyl enol ethers 39.1, where a β-C(sp3)–H Mannich-type alkylation of imines 39.2 is accomplished using TIPS–SH 39.3 (Scheme 39) [243]. This protocol also displayed a wide functional group tolerance with ethers, thioethers 39.7, and various heterocycles being tolerated. The reactions also proceeded well in the presence of a weak benzyl ether C(sp3)–H bond, as seen in the formation of 39.6. The quantum yield of this reaction (average Φ = 0.092) shows that chain contribution to the reaction is not significant, adding evidence to the mechanism previously suggested by Huang and co-workers [242].

Scheme 38.
Allylic C(sp3)–H alkylation through HAT with triisopropylthiol and photoredox catalysis.

Scheme 39.
Silyl enol ether β-C(sp3)–H Mannich-type alkylation through HAT with triisopropylthiol and photoredox catalysis.
In 2021, Rovis and Schoenebeck developed a site-selective α-C–H alkylation of trialkylamines 40.1 through a Giese addition by establishing a reversible HAT step with triphenylsilanethiol 40.4 (Scheme 40) [133]. Establishing an equilibrium in the HAT step allowed for both the less and more substituted radicals to form as their BDE vales are almost identical. However, the more nucleophilic (more substituted) α-amino radical undergoes Giese addition faster [85]. Hence, the selectivity of this protocol is guided by the Curtin–Hammett principle. 13C NMR was used to predict the regioselectivity of the alkylation as α-amino C atoms, with more downfield shifts reacted more favourably. The evaluation of the substrate scope for the protocol showed numerous functional groups were tolerated, such as alcohols, ketones, and amides (see product 40.10), among many others. Site-selectivity for the more substituted alkyl group ranged from 1.3:1 to 27:1 (where ratio is mentioned), and there were notable exceptions in attainable site selectivity. N-Ethylpiperidine was alkylated on the ring, as seen in product 40.6. Additionally, where the Giese acceptor had substituents on the terminal carbon site-selectivity had reversed, as seen by product 40.8 [85]. The method was also showcased on 12 pharmaceuticals, such as Dextromethorphan 40.9 and Lidocaine 40.10, demonstrating potential for late-stage functionalisation. It is worth noting the contrast in selectivity compared with similar C–H functionalisation methods [244,245].
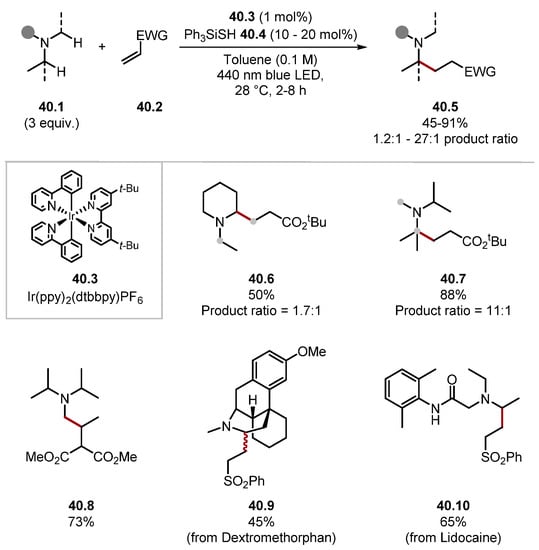
Scheme 40.
Site selective α-C–H alkylation of trialkylamines through a reversible HAT step with triphenylsilanethiol.
Wendlandt used Ph3SSiH 41.4 to reversibly abstract alcohol α-C(sp3)–H bonds to establish an equilibrium, which leads to stereochemical editing of vicinal diols through thermodynamic control (Scheme 41) [246].
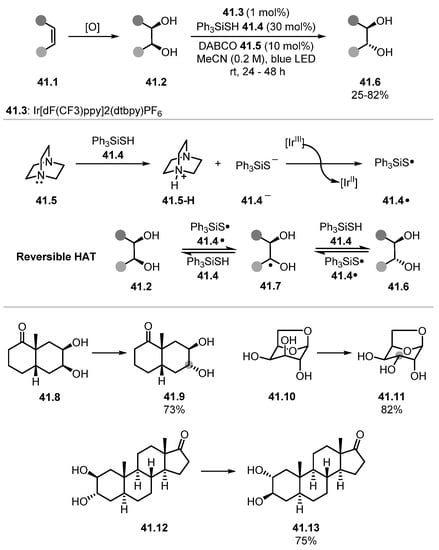
Scheme 41.
Reversible HAT for trans-selective stereochemical editing of vicinal diols with triphenylsilanethiol.
This protocol changes stereochemistry of vicinal diols from cis-diols 41.2 to trans-diols 41.6. DABCO 41.5 serves as a base, deprotonating Ph3SiSH 41.4 to a thiolate 41.4−, which is oxidised to a thiyl radical 41.4· by the photoredox catalyst 41.3. The thiyl radical 41.4· can reversibly abstract the alcohol α-C(sp3)–H atom where this equilibrium will favour the trans-isomer 41.6. Thiyl radicals have been used in previous efforts to edit stereochemistry through reversible HAT steps [247]. Various diols were edited using this method affording the products in low-to-excellent yields. Despite lower yields for certain substrates, the authors note that traditional means of changing stereochemistry of diols can take numerous steps. The method was also highly chemoselective, with substrates containing sensitive functional groups like ketones and acetals reacting in high yields, as seen in products 41.9 and 41.11. Complex structures like trans-diaxial diastereomer 41.12 were converted at both α-hydroxy C–H bonds to form product 41.13.
In 2021, the Dilman group demonstrated an example of a thiyl radical activating unactivated alkanes’ C(sp3)–H bonds for thiolation (Scheme 42) [248]. The disulfide 42.1 undergoes a homolysis of the S–S bond to generate two thiyl radicals capable of HAT. Screening experiments showed diphenyl disulfide provided no product, while ((C6F5)S)2 provided less than a 5% yield of the product. ((Pyf)S)2 42.1 was able to thiolate even unactivated C(sp3)–H bonds of alkanes to form products such as 42.5 and 42.6. Various saturated heterocycles formed products in good yields (such as product 42.7), and benzylic C(sp3)–H bonds were also amenable (42.8).

Scheme 42.
Thiolation of unactivated C(sp3)–H bonds using (SPyf)2 42.1.
Thiols have also been used as HAT reagents in the context of CO2•− formation via HAT from formate salts [181]. In 2018, Jui developed a defluorinative alkylation of trifluorotoluenes 43.1 (Scheme 43) [249]. Mechanistically, this reaction worked through reductive SET of trifluorotoluenes 43.1 with photoexcited N-phenylphenothiazine (PTH*) 43.4* to form radical anion 43.13. The radical anion 43.13 would then expel a fluoride to form a difluorobenzylic radical 43.14, which is trapped by an olefin to afford radical 43.15. Cyclohexanethiol 43.6 would quench radical 43.15 to form the product 43.7. The resulting thiyl radical 43.6· abstracts a hydrogen atom from the formate 43.3 to form CO2•−. CO2•− completes the catalytic cycle by restoring photocatalyst 43.11 and releasing CO2. This paper was a landmark for both CO2•− and trifluorotoluene defluorinative reactions. However, the scope of trifluorotoluenes 43.1 was limited to activated trifluorotoluenes with additional EWGs. In 2019, Jui built on his previous work by developing a similar protocol, which tolerated substrates containing electron-donating groups (Scheme 44) [250]. Further optimisation found Miyake’s phenoxazine 44.4 as a photocatalyst, thiophenol 44.5 as an HAT catalyst, and an elevated temperature of 100 °C to be optimal. General defluorinative alkylation and hydrodefluorination protocols were developed. While BDE values between formate C–H and thiophenol [thiophenol BDES–H = 83.3 kcal/mol versus formate HCO2− BDEC–H = 86 kcal/mol] are not matching, the elevated temperature and potential for competing initiation through formate oxidation in DMSO mean that CO2•− can form through several pathways [251,252]. Subsequent work by Jui found a similar protocol has a quantum yield (Φ) of 2.63, indicating a radical chain contribution [184]. Additionally, in similar studies by Wickens, Stern–Volmer studies showed methyl thiosalicylate-quenched excited state 4DPAIPN at a faster rate than a formate salt [252]. In 2022, Zhu, Guo, and Zhu described a similar protocol for defluorinative alkylation of trifluoromethylbenzimidazoles 45.1 (Scheme 45) [253].
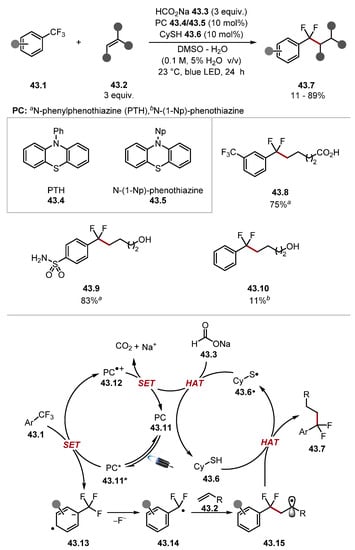
Scheme 43.
Defluorinative alkylation of trifluorotoluenes 43.1.

Scheme 44.
Defluorinative alkylation and hydrodefluorination of trifluorotoluenes 44.1.

Scheme 45.
Defluorinative alkylation of trifluoromethylbenzimidazoles.
Thiols have also been used to promote CO2•− formation via HAT from formate salts in elegant protocols by Molander, Glorius, and Wickens [183,252,254,255,256,257,258,259]. However, due to mechanistic complexities associated with initiation and the similarity with the HAT chemistry already mentioned, these works have been omitted.
2.2.2. BINOL-Derived Thiophosphoric Acids
In recent years, thiyl radicals formed from BINOL-derived thiophosphoric acids have been used for HAT processes. Such reagents have been shown to effectively abstract hydrogen atoms from C–H bonds up to 96 kcal/mol [260]. This has allowed them to be used in a wider range of HAT processes than traditional thiyl radicals covered in Section 2.2.1.
In 2020, the Kanai group reported the use of thiophosphoric acid 46.4 as a HAT reagent in a catalytic acceptorless dehydrogenation (CAD) of secondary alcohols 46.11 to ketones 46.13 (Scheme 46) [260]. Initial studies investigated thiophosphoric acid 46.4 in combination with an acridinium photocatalyst 46.3 for a HAT-photoredox tandem catalysis system in a Giese pathway using benzylidene malononitrile 46.2. This system successfully alkylated various hydridic C–H bonds. Namely, formyl C(sp2)–H bonds (see product 46.6), benzylic C(sp3)–H bonds (46.7), and ethereal C(sp3)–H bonds (46.8) were alkylated effectively [benzaldehyde BDEC–H = 88.7 kcal/mol and toluene BDEC–H = 89.3 kcal/mol and THF BDEC–H = 92.1 kcal/mol] [79]. Alkylation of α-alcohol C(sp3)–H was less effective (46.9) [methanol BDEα-C–H = 96.2 kcal/mol] and strong aliphatic C(sp3)–H bonds of cyclohexane 46.10 were abstracted slowly [cyclohexane BDEC–H = 99.5 kcal/mol]. Overall, this study showed thiophosphoric acid 46.4 and related binol-derived thiophosphoric acid HAT reagents are capable of abstracting stronger C–H bonds than standard thiol HAT catalysts (Section 2.2.1). Kanai also demonstrated a ternary catalytic system for the CAD of secondary alcohol 46.11, combining HAT, photoredox, and nickel catalysis. The general protocol provided moderate-to-quantitative yields and possessed a good chemoselectivity profile. The mechanism of the reaction is believed to proceed through HAT of the alcohol α-C–H bond to form an alkyl radical that is captured by the nickel catalyst, which undergoes a reductive SET and subsequent β-hydride elimination to form an enol (ketone). The β-hydride elimination step was probed through the scrambling of deuterium in product 46.19, as well as substrates without β-hydrogens undergoing the process in yields around 10%. The system was also extended to the oxidation of aldehydes to esters. Kanai and coworkers also reported a ternary catalysis method for the allylation of aldehydes 47.1 proceeding through the HAT of allylic C(sp3)–H bonds with thiophosphoric imide (TPI) 47.5 (Scheme 47) [261].
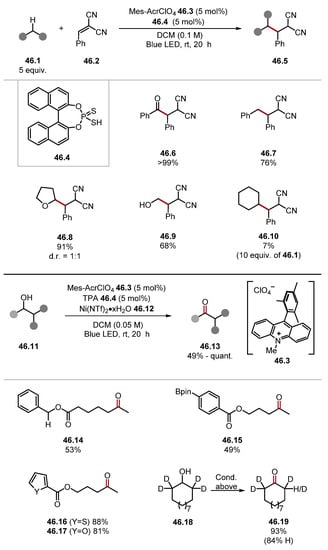
Scheme 46.
Thiophosphoric acid 46.4 as a HAT catalyst and CAD of secondary alcohols.
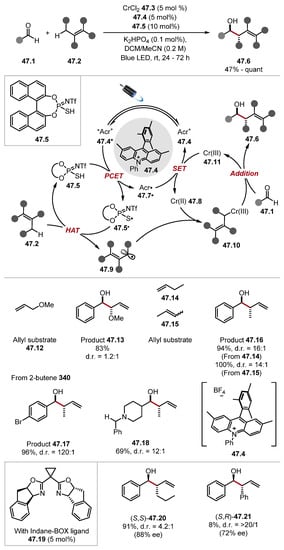
Scheme 47.
Allylation of aldehydes through ternary catalysis with thiophosphoric imide 47.5.
Mechanistically, this method proceeds through the photoexcitation of acridinium photocatalyst 47.4 to generate the strongly oxidising photoexcited catalyst 47.4* (E1/2 = +2.12 V vs. SCE in MeCN) [262]. The excited photocatalyst 47.4* can oxidise TPI 47.5, which also loses a proton to form TPI radical 47.5·. The TPI radical 47.5· can abstract a hydrogen atom from a weak allylic C–H bond in 47.2 to generate allyl radical 47.9, which is intercepted by Cr(II) 47.8 to form Cr(III) complex 47.10. The allylic Cr(III) complex 47.10 reacts with an aldehyde 47.1, and the subsequent species undergoes hydrolysis to form anti-product 47.6. A screening of HAT reagents showed that other thiols resulted in no desired product. Notably, the allylic substrates reacted to produce branched products as opposed to linear ones; for instance, product 47.16. Allyl ether 47.12 reacted to form product 47.13. The chemoselectivity of the protocol was exceptional with allylic C(sp3)–H bonds being functionalised even in the presence of benzylic amines (47.18) and benzylic ethers, among other species known to undergo HAT processes. The addition of a chiral INDANE-box ligand 47.19 to the reaction resulted in the formation of the products in high ee values e.g., products 47.20 (88% ee) and 47.21 (72% ee). Subsequently, Kanai used HAT reagent 48.4 for a hydroxyalkylation of N-heteroaromatics 48.1, with aldehydes 48.2 to form hydroxyalkylated products 48.5 (Scheme 48) [263].

Scheme 48.
Hydroxyalkylation of N-heteroaromatics with aldehydes using thiophosphoric acid 48.4.
The authors suggested a plausible mechanism for the reaction proceeding through the oxidation of 48.4 with photoexcited MesAcr 48.3* to form a thiyl radical 48.4·, which abstracts a formyl hydrogen atom [benzaldehyde BDEC–H = 88.7 kcal/mol] to form radical 48.7. Radical 48.7 undergoes a Minisci-type addition to form intermediate 48.9, which rapidly undergoes a spin centre shift (SCS) step to form radical 48.10 [264,265,266,267]. The final product 48.5 is delivered upon SET and protonation or PCET. The general protocol tolerated a wide range of functionality on both the aldehyde and N-heteroaromatic substrates. Notably, aliphatic aldehydes (product 48.11) and aromatic aldehydes (product 48.12) were amenable to this transformation, and common functional groups such as esters, amides, ketones, and halides were tolerated. In 2022, the Glorius group reported an arylation of allylic C(sp3)–H bonds proceeding through a triple tandem catalysis protocol combining photoredox and HAT catalysis with nickel-catalysed cross-coupling (Scheme 49) [268]. This methodology showed a good functional group tolerance with respect to both the aryl bromide and allylic substates. Impressively, TPI 49.3 was selective for allylic C(sp3)–H bonds over benzyl ether C(sp3)–H bonds, as previously noted in Kanai’s work (Scheme 47) [261]. This protocol afforded linear olefin products, rather than branched ones, resulting from a lower energy transition state that was required for the reductive elimination of the linear product. This linear selectivity has been noted in a similar protocol by the Rueping group [144]. The chemoselectivity of the protocol was outstanding, with esters (see product 49.6), amides, sulfonamides (49.7), nitriles, ketones, and N-containing heterocycles, including tetrazole among others. Nitro groups were not tolerated. However, low-valent nickel species usually do not tolerate nitro groups due to competitive reductive pathways forming nitroso compounds and inhibiting the catalyst [269,270,271]. The mechanism of this reaction was studied using DFT studies and is believed to progress through an oxidative addition of Ni(I) complex 49.11 with an aryl bromide 49.2 to form a Ni(III) complex 49.12. This is supported by experimental work by Doyle, who isolated an Ni(III) complex formed by oxidative addition of the Ni(I)-bypyridine complex with aryl bromides [272]. Complex 49.12 is subsequently reduced by MesAcr· 49.9 to a Ni(II) complex 49.13, which traps allyl radical 49.10 to form Ni(III) complex 49.14. The reductive elimination of a linear product is more energetically favourable than a branched product due to steric effects and hyperconjugation in the transition state. Hence, the linear olefin 49.5 is obtained.
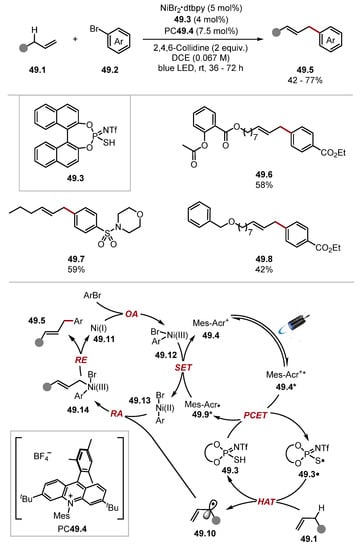
Scheme 49.
Allylic C(sp3)–H arylation through ternary catalysis with thiophosphoric imide 49.3.
To date, no studies have extensively screened acridinium photocatalysts for the oxidation of BINOL-derived thiophosphoric acids. The decomposition of acridinium photocatalysts containing mesitylene rings through HAT pathways has previously been proposed by Nicewicz and can potentially be alleviated through the use of other acridinium photocatalysts, which were not explored in any works covering BINOL-derived thiophosphoric acid HAT reagents [178,273,274]. Moreover, PC48.3 and other acridinium photocatalysts without substitution at the core are known to work well for intramolecular processes, but they can decompose through competing radical addition pathways in intermolecular processes [262,275]. BINOL-derived HAT reagents have also been used in processes initiated through EDA complexes. In 2022, the Melchiorre group developed a benzylation of allylic C(sp3)–H, which proceeded through HAT using phosphorodithioic acid reagent 50.3 (Scheme 50, top) [276].
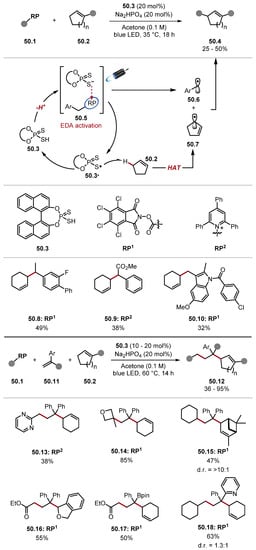
Scheme 50.
Benzylation of allylic C–H bonds through EDA initiation and HAT using phosphorodithioic acid reagent.
The reaction is initiated through EDA complex 50.5 between a thiolate and tetrachlorophthalimides (RP1) or Katritzky salts (RP2) [230,277]. Upon irradiation, the EDA complex undergoes an intracomplex SET and rapidly fragments to form a benzyl radical 50.6 and thiyl radical 50.3·, alongside CO2 and tetrachlorophthalimide anion (with RP1) or 2,4,6-triphenylpyridine (with RP2). The thiyl radical 50.3· can subsequently abstract an allylic hydrogen atom to form an allyl radical 50.7, which couples with benzylic radical 50.6. Radical homocoupling products were observed in the reactions supporting this mechanism. The first protocol described in this work focused on directly reacting benzylic radicals with allylic radical to benzylate allylic C(sp3)–H bonds. This protocol resulted in the desired products in low-to-moderate yields. However, it showed a good chemoselectivity profile and tolerated both radical precursors RP1 and RP2.
The method was amenable to LSF of pharmaceuticals; for instance, product 50.10 was formed from indomethacin. Another version of this protocol was developed to use kinetically unstable alkyl radicals, which did not react in the initial protocol (Scheme 50, bottom). This was accomplished by trapping the unstable alkyl radical with styrenes 50.11 to form a more stable benzylic radical, which subsequently coupled with an allylic radical 50.7 to form products 50.12. The second protocol was assessed with a large substrate scope evaluating radical precursors, alkyl radical, styrene species, and allylic species. Pyrimidine benzylic radical formed product 50.13, and an oxetane tertiary radical formed Product 50.14. Likewise, a range of allylic precursors and styrene acceptors derived products such as pinene derivative 50.15 and benzyl ether 50.16, pinacol boronic ester 50.17, and pyridine 50.18. The method was also showcased on several natural products and pharmaceuticals, further demonstrating the applicability of this procedure. This work was promptly followed by the Kanai group describing an EDA organocatalytic system that forms an HAT-active thiyl radical upon irradiation by visible light (Scheme 51) [278]. The EDA organocatalytic system was capable of several transformations for proceeding via HAT without an exogenous photosensitiser. This work built upon an observation that the hydroxyalkylation of N-heteroaromatics with aldehydes proceeded partially in the absence of a photocatalyst in the group’s previous work (Scheme 48) [278]. Hence, it was assumed that the irradiation of the EDA complex provided a thiyl radical, which can activate C–H bonds. A general protocol for the hydroxyalkylation of N-heteroaromatics was described. The protocol provided good-to-excellent yields and improved upon the yields of the previous study [263]. Following this, alcohols were used as alkylating agents for N-heteroaromatics in good-to-excellent yields. Interestingly, THF and ambroxide 51.12-afforded ring opened product 51.11. The electron-acceptor catalyst (EAC) 51.15 was used to activate HAT reagent 51.3 in situ without a photocatalyst. EAC 51.15 was used for an imine alkylation protocol. The EAC complex was also used for a CAD of secondary alcohols to ketones. This protocol provided the ketones in good yields and proceeded in the presence of weak benzylic ether C(sp3)–H bonds.

Scheme 51.
EDA organocatalytic system for HAT processes.
Melchiorre subsequently developed a heteroarylation of allylic C–H bonds through EDA initiation (Scheme 52) [279]. Mechanistically, the reaction is initiated through an EDA complex 52.8 between the phosphorodithioate of reagent (S)-52.3 and heteroarene 52.1. The irradiation of complex 52.8 forms pyridyl radical 52.10 and thiyl radical 52.3·. The thiyl radical 52.3· abstracts an allylic C–H from 52.11 to form 52.12. Radical-radical coupling between pyridyl radical 52.10 and allylic radical 52.12 forms intermediate 52.13, which forms desired product 52.14. Usually, C4 selectivity at the pyridine substrate was achieved due to the pyridyl radical 52.10 having greater spin density (SOMO) at C4 (as found by DFT and EPR hyperfine splitting). The protocol yielded C6 products when bulky substituents were present in position 3 (e.g., esters and amides).
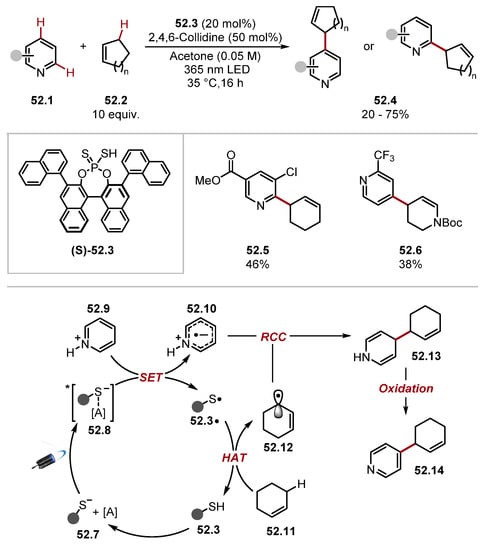
Scheme 52.
Heteroarylation of allylic C–H bonds through EDA initiation and HAT with phosphorodithioic acid reagent 52.3.
2.3. Oxygen-Based HAT Reagents
As mentioned in Section 2.2 thiols can be deprotonated and oxidised to generate thiyl radicals capable of abstracting weak H atoms (Section 1.2). In contrast, oxygen-centred radicals are typically formed through the homolysis/reduction of weak O–O bonds of peroxides [tBuCH2O–OCH2tBu BDEO–O = 36.4 kcal mol−1] [79] or the oxidation of oxyanions (Section 2.3.1). Oxygen-centred radicals capable of HAT can also be accessed through the excitation of carbonyl compounds with light. In particular, ketones and 1,2-diketones form triplet states capable of HAT [49,53,280,281]. However, this is a method of direct HAT [19,48]. This section describes methods to access O-centred radicals in indirect HAT.
2.3.1. Pyridinium N-Oxide HAT Reagents
Recently, pyridine N-oxides have been used as precursors to HAT reagents. Deng and Nicewicz independently reported the use of pyridine N-oxides as catalytic HAT reagents with acridinium photoredox catalysts simultaneously (Scheme 53) [282,283]. Pyridine N-oxides can act as HAT reagents through the oxidation of pyridine N-oxide 53.1 to N-oxide cation radical 53.1· [284,285,286], which form a protonated N-oxide 53.1–H upon HAT [287]. Computational studies by Deng found BDE values in protonated N-oxides used in their study ranged from 97.7–111.1 kcal/mol [282], and Nicewicz found that they range from 93–101 kcal/mol [283]. Protonated pyridine N-oxides are acidic (N-hydroxy-4-methylpyridine pKa = 2.43 in DMSO) [288], meaning that pyridine N-oxides 53.1 can be used catalytically under basic conditions, similar to other HAT reagents covered within this review. Pyridine N-oxides were prone to deactivation by deoxygenation side reactions [283]. However, substituents on pyridine N-oxides can be adjusted to suppress this pathway.
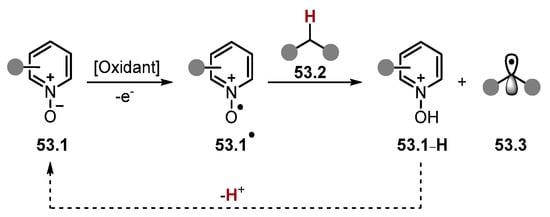
Scheme 53.
HAT with pyridine N-oxides.
Nicewicz accessed alkyl radicals 54.12 through HAT, with oxyl radicals derived from pyridine N-oxides 54.5 and 54.6 for Giese and Minisci pathways (Scheme 54) [283]. Extensive screening identified methods A and B for the alkylation of C–H bonds. Method B requires a higher catalyst loading due to the decomposition of HAT catalyst 54.6 through deoxygenation. For example, method B (HAT catalyst 54.6) improved the yield of product 54.7 from 12% to 85% yield. Several Giese acceptors were tolerated. However, the substrate scope is limited to easily reducible alkenes due to the reduced form of MesAcrBF4 54.3 having a low oxidation potential. The scope of C–H substrates was wide as products derived from alkanes (54.7, 54.8, and 54.9), amides, esters, ethers, alcohols, and aldehydes were readily formed. The functional group tolerance was good. For instance, halides 54.8 and carboxylic acid 54.9 provided good yields. The mechanism occurs through the oxidation of pyridine N-oxide (E1/2 = +1.84 V vs. SCE in MeCN) with photoexcited acridinium photocatalyst 54.3* (E1/2 = +2.08 V vs. SCE in MeCN) forming N-oxyl radical cation 54.11·, which is a strong HAT reagent capable of oxidizing strong C–H bonds [pyridine N-oxide 54.6 BDEO–H = 99 kcal/mol−1 versus cyclohexane BDEC–H = 99 kcal/mol−1]. The resulting alkyl radical 54.12 is trapped with a Giese acceptor 54.2 forming a radical adduct 54.13. The radical adduct is reduced to an anion by Acr• 54.3 (MesAcr 54.3 E1/2 (PC/PC•−) = −0.59 V versus SCE in MeCN versus E1/2 (•CH2CO2Et/−CH2CO2Et) = −0.63 V versus SCE in MeCN) and subsequently protonated [179,180]. Nicewicz also used pyridine N-oxide 54.6 for Minisci-type reactions. The functional group tolerance of this reaction was similar to the alkylation protocol with heteroarene products arising from amides (54.18), toluene (54.19), and cyclohexane (54.20) were formed products in moderate-to-good yields.

Scheme 54.
Pyridine N-oxide radical cations as HAT reagents 1.
Deng showed the functionalisation of various C–H substrates using pyridinium N-oxide 55.4 (Scheme 55) [282]. Products were successfully derived from aldehydes (55.6), amides (55.7), alcohols, ethers, and benzylic substrates, which were derivatised successfully. Impressively using one equivalent of C–H substrate only led to a minor decrease in yield. Moreover, various radical traps were deployed in this process to form 55.10 and 55.11, with diisopropyl azodicarboxylate (DIAD) forming 55.12.
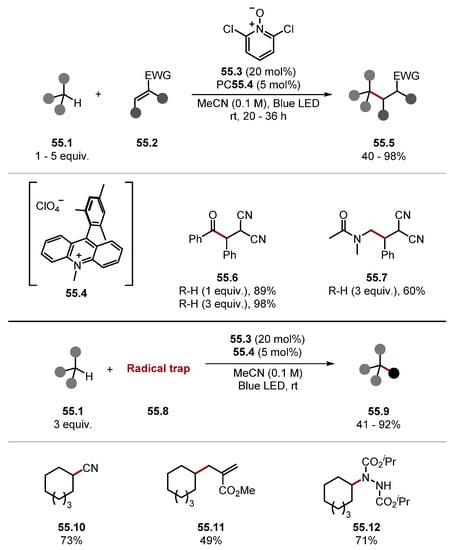
Scheme 55.
Pyridine N-oxide radical cations as HAT reagents 2.
Gryko showed that an EDA complex 56.8 between pyridine N-oxides 56.3 and Brønsted or Lewis acid-activated azines can generate pyridine N-oxide cation radicals 56.10· for a subsequent HAT process (Scheme 56) [289]. The radicals generated through HAT were harnessed in Minisci-style reactions with various heteroarenes 56.2 activated under acidic conditions [127]. Various HAT substrates reacted well; for instance, cycloalkanes, alkenes, ethers, amides, and carbamates, as seen in products 56.5–56.7. The method also tolerated numerous heterocycles. Notably, halides 56.6 and esters 56.7 reacted in moderate yields. The reaction is believed to be initiated through an EDA complex 56.8, which upon irradiation by visible light provides reduced heteroarene 56.9 and N-oxide cation radical 56.10·. N-oxide cation radical 56.10· can abstract hydrogen from strong C–H bonds to form alkyl radical 56.14. The alkyl radical is trapped by an acid-activated heteroarene 56.13 to form radical adduct 56.15. The radical adduct 56.15 delivers the protonated product 56.16 after formally losing a hydrogen atom.
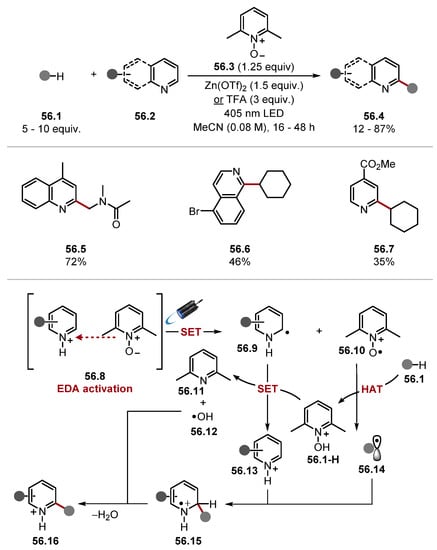
Scheme 56.
Pyridine N-oxide cation radicals as HAT reagents in a Minisci-style reaction.
2.3.2. Peroxide HAT Reagents
Peroxides have historically been used as oxidants and continue to be a reliable and robust option for indirect HAT and oxidation [112,290,291,292,293]. Alkyl peroxides function under acidic conditions, and peroxides with higher degrees of substitution are thermally stable [112]. Peroxides generate O-centred radicals through the homolysis of weak O–O bonds typically induced by light, heat, or a photosensitiser [tBuCH2O–OCH2tBu BDEO–O = 36.4 kcal mol−1] [79,112]. Alternatively, peroxides can generate O-centred radicals through the reduction of O–O bonds mediated by a metal complex or photosensitiser to form a oxyanion and oxyradical (Scheme 7) [112]. The resulting O-centred radicals can abstract hydrogen atoms to form moderate-to-strong O–H bonds depending on the peroxide used [tBuOOH BDEO–H = 89.4 kcal/mol and tBuOH BDEO–H = 105.1 kcal/mol and MeC(O)O-H BDEO–H = 106.4 kcal/mol] [79].
In 2021, Stahl used peroxides (dicumyl peroxide or di-tert-butyl peroxide) to methylate C–H bonds in the presence of a nickel catalyst and ligand with visible light (Scheme 57) [294]. In this method, peroxides filled two roles: the HAT reagent and a source of methyl radical (after β-methyl scission) [295]. To this end, a 4-to-6-fold excess of peroxide reagent was used, and polar solvents were used (TFE or MeCN). Polar solvents produced superior results due to faster β-methyl scission, which likely arises from the increased solvation of the ketone by-product [296,297]. The nickel catalyst mediated the coupling of two alkyl radicals. In its absence, greater amounts of side-products were formed, and selective radical-radical coupling did not occur. The method afforded methylated products in low-to-fair yields. However, these are yields of monomethylated products after purification. Therefore, in the context of late-stage functionalisation, this is an excellent result.
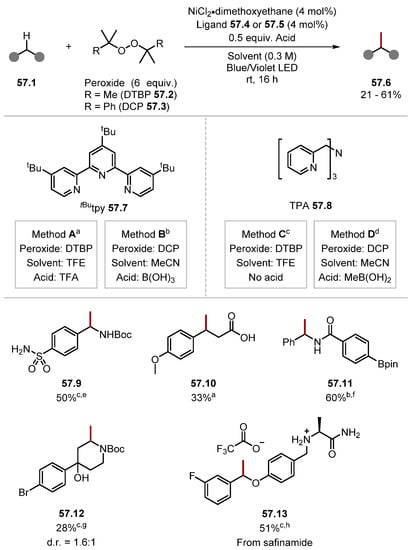
Scheme 57.
Methylation of C–H bonds through a combination of HAT using oxyl radicals and Ni-catalysed cross-coupling. a Method A was used. b Method B was used. c Method C was used. d Method D was used. e 0.5 equiv. MeB(OH)2 used as acid. f 1:1 MeCN:DMSO used as solvent. g 0.15 M concentration. h 4 equiv. of DCP, and MeCN was used as solvent.
The scope of the reaction was wide, and numerous functional groups were tolerated; for instance, sulfonamides (57.9), carboxylic acids (57.10), amides (57.11), alcohols (57.12), esters, ketones and amines, and carbamates (57.12). Notably, the abstraction at amine α-C–H bonds could be suppressed under acidic conditions as seen in safinamide derivative 57.13 [29]. Overall, this method is a landmark in terms of synthetic tools available for medicinal chemistry to explore the magic methyl effect [9,298,299]. Phipps recently disclosed an enantioselective Minisci reaction with alcohols and chiral phosphoric acid proceeding through HAT with dicumyl peroxide 58.3 under blue-light irradiation (Scheme 58) [300]. Notably, no photocatalyst was used, echoing Phipps’s previous hydrogen atom transfer-driven enantioselective Minisci reaction with amides [281]. DFT studies showed the enantioselectivity in this protocol arises from a complex of the protonated azine with a chiral phosphoric acid (CPA), which forms H-bonds with the incoming alcohol similarly to the group’s previous work [281,301]. Despite the protocol yielding products in only moderate yields (up to 60%), the enantiomeric excesses were excellent, with up to 94% ee. Moreover, the functional group tolerance of the protocol was good with numerous alcohols and azines reacting in moderate yields with excellent ee values. Kramer reported an enantioselective amidation and amination of benzylic C–H bonds using copper with BOX ligand 59.4 and photoredox tandem catalysis with di-tert-butyl peroxide (DTBP) 59.6 (Scheme 59) [302]. Amides and carbamates reacted in good yields. The protocol showed good functional group tolerance, and the enantioselectivity could be changed by changing the ligand’s stereochemistry (59.9 and 59.10). The deprotection of Boc-protected benzylic amine 59.11 allows for a two-step C–H amination. Zhou developed a similar transformation under thermal conditions using DTBP 60.4 and a Cu catalyst with BOX ligand 60.3 (Scheme 60) [303]. Only amides were showcased in the protocol, but both aryl and alkyl amides reacted in good yields.
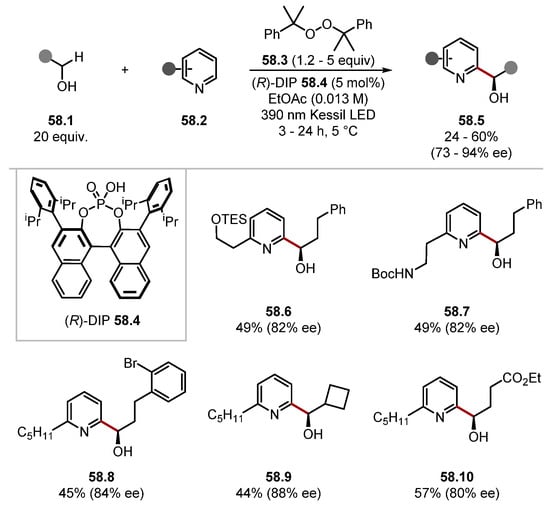
Scheme 58.
Enantioselective Minisci reaction with alcohols through HAT with dicumyl peroxide.

Scheme 59.
Enantioselective amidation using di-tert-butyl peroxide 59.6 for HAT with photoredox and copper-tandem catalysis.
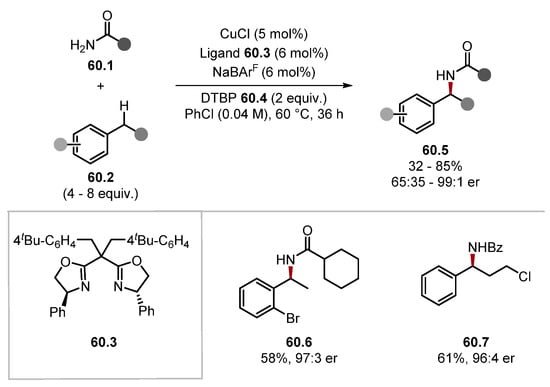
Scheme 60.
Enantioselective amination of benzylic C–H positions using di-tert-butyl peroxide and a Cu-catalyst.
Stahl used a tert-butyl peroxybenzoate (TBPB) 61.2 with a photoactive copper catalyst for a benzylic C–H esterification (Scheme 61) [304]. The hydrolysis of the products allowed for a two-step C–H hydroxylation drawing a parallel with a recent direct hydroxylation of C–H bonds with nitroarene by Parasram [72].

Scheme 61.
Esterification of benzylic C–H bonds using a photoactive Cu-catalyst and tert-butyl peroxybenozate 61.2.
Doyle used TBHP 62.3 to form diazoacyl radicals 62.13 from α-diazo esters 62.1. Diazoacyl radicals 62.13 react with styrenes 62.2 to form pyrazolines 62.4 (Scheme 62) [305]. Only styrenes 62.2 and α-diazo esters 62.1 were used as substrates. However, the functional group tolerance of the reaction was good with products formed bearing nitro groups (62.5), 1,3-benzodioxole (62.6), and alkynes (62.7) in good yields. The reaction occurs through reductions of TBHP 62.3, with an iron (II) complex 62.8 to form oxyl-radical 62.9 and an iron (III) complex 62.10. TBHP 62.3 can form the peroxyl-radical 62.12 upon the oxidation of TBHP 62.3 with Fe(III) 62.10. HAT to tert-butoxy radical 62.9 from TBHP 62.3 also delivered a peroxyl radical 62.12 [tBuOH BDEO–H = 105.1 kcal/mol versus tBuOOH BDEO–H = 89.4 kcal/mol] [79]. Peroxyl-radical 62.12 can abstract a hydrogen atom from α-diazo ester 62.1 to form diazoacyl radicals 62.13, which undergo a Giese addition with Styrenes 62.2 to form a benzylic radical adduct 62.14. The benzylic radical adduct 62.14 rapidly cyclises to form radical 62.15, which is quenched by gaining a hydrogen atom via HAT or through a reduction and subsequent protonation affording an intermediate 62.16. Intermediate 62.16 yields the pyrazoline 62.4 final product after tautomerisation. Musacchio used TBPB 63.14 and N-alkoxypyridinium salts 63.13 in a C–H azolation of benzylic and allylic C–H bonds (Scheme 63) [306]. N-alkoxypyridinium salts 63.13 are known to generate alkoxyl radicals in situ upon reduction [307]. N-alkoxypyridinium salts have been used to trigger HAT events from C–H, P–H, and Si–H bonds [308,309,310,311,312,313]. In this protocol, alkoxyl radicals were used to abstract benzylic C–H bonds to form a benzylic radical. The benzylic radical was further oxidised to a carbocation, which reacted with numerous azoles. Secondary benzylic positions were derivatised using the C–H substrate as a limiting reagent. However, the functionalisation of tertiary positions used a three-fold excess of the C–H substrate. Musacchio has developed mechanistically similar protocols incorporating a range of nucleophiles into C–H bonds (e.g., F, C, O, N, Br, Cl) [314,315]. Gong described an asymmetric 1,2-oxidative alkylation of conjugated dienes 64.2 to form allylic esters 64.6 (Scheme 64) [316]. This method used tert-butyl peroxybenzoate 64.3 as a HAT reagent to form oxyl radical 64.11 through a reduction with Cu(I) 64.9. Oxyl radical 64.11 abstracts hydrogen from various C–H substrates to form alkyl radical 64.12. Alkyl radicals are rapidly trapped by dienes to form allylic radicals 64.13 [317]. The allylic radical 64.13 then reacts with Cu(II) complex 64.10 to form the desired product 64.6, reforming Cu(I) catalyst 64.9.

Scheme 62.
Formation of pyrazolines from styrenes 62.2, and diazoacyl radical 62.13 formed through HAT with peroxyl radical 62.12.
Scheme 63.
Azolation of benzylic C–H bonds through a ORPC using N-alkoxypyridinium salts or tert-butyl peroxybenozate for HAT.

Scheme 64.
Asymmetric 1,2-oxidative alkylation of conjugated dienes mediated by HAT with tert-butyl peroxybenozate and Cu-catalysis.
2.3.3. Miscellaneous Oxygen HAT Reagents
Many other O-centred radicals can also be used for HAT. Persulfates are also common HAT reagents [318,319,320]. In 2021, Leonori used ammonium persulfate 65.3 to form aminoboryl radical 65.12 for a Minisci-style borylation of azines 65.2 with aminoborane 65.1 (Scheme 65) [321]. The protocol was amenable to many azines and showed excellent functional group tolerance with alcohols 65.6, halides 65.7, and nitriles, among others reacting well. The protocol was also showcased on pharmaceuticals; for instance, Voriconazole was borylated to product 65.8. The reaction mechanism occurs through the oxidation of persulfate 65.9 to the O-centred radical 65.11. The O-centred radical 65.11 is electrophilic and can abstract a hydridic hydrogen atom from a B–H bond in amino borane 65.1 to form an aminoboryl radical 65.12·. Aminoboryl radical 65.12· is a highly nucleophilic radical and rapidly adds to azine 65.2-H through a Minisci-style pathway to form radical adduct 64.13, which formally loses a hydrogen atom through chain contribution or PCET with the oxidised photocatalyst to form a borylated product 65.5-H.
Scheme 65.
Borylation of azines 65.2 using amino boranes 65.1. aReaction without acid, instead using K2CO3 (2 equiv.). bReaction used Ru(bpy)3(PF6)2 (2 mol%) and AcOH (10 equiv.).
In recent years, N-hydroxyphthalimide (NHPI) 66.1 has been explored extensively as a radical initiator, oxidant, and/or HAT reagent [322,323,324]. Phthalimide-N-oxyl radical (PINO) 66.1· can abstract hydrogen from weak, typically benzylic or allylic, C–H bonds to form NHPI 66.1 [NHPI BDEO–H = 88.1 kcal/mol compared with cyclohexene BDEC–H = 81.0 kcal/mol or toluene BDEC–H = 88.5 kcal/mol] [79,325]. N-hydroxyphthalimide 66.1 can formally lose a hydrogen atom to form phthalimide-N-oxyl radical 66.1·. Practically, this can be accomplished through oxidation followed by deprotonation. The oxidation potential of N-hydroxyphthalimide 66.1− is quite high (E1/2 = +1.44 V vs. SCE in MeCN). However, the addition of pyridine lowers the oxidation potential significantly (E1/2 = +0.78 V versus SCE in MeCN, with two equivalents of pyridine) [326,327]. In 2022, Stahl generated phthalimide-N-oxyl radical 66.1· anodically from NHPI 66.1 in the presence of two equivalents of pyridine for a PINOylation of methylarenes 66.2 (Scheme 66) [328]. The PINOylated products 66.3 were readily converted to benzylic alcohols or benzaldehydes under photocatalytic conditions in subsequent steps. Interestingly, a mixture of C–O 66.3 and C–N 66.4 coupled products was formed. However, the use of more hindered secondary radicals resulted in almost no C–N product forming. The method showed good functional group tolerance forming products bearing ketones, halides, amides 66.5, and numerous heterocycles, such as product 66.6. product 66.7 was formed in a 7:1 ratio with its regioisomer. Additionally, a robustness screening had shown the method developed to be quite insensitive to many different functional groups and heterocycles. Diphenyl phosphate 67.4, and other phosphates, are seldomly used in HAT protocols but remain a viable option due to the strength of O–H bonds [diphenyl phosphate 67.4 BDEO–H = 102.4 kcal/mol] and low pKa (pKa = 2.4) [329,330]. However, the relatively high oxidation potential of dialkyl/diaryl hydrogen phosphates limits wider relevance (67.4 E1/2 ((RO)2P(O)O·/(RO)2P(O)O−) = +1.50 V vs. SCE in MeCN) [330]. In 2022, Wu and Deng developed a Minisci-style cross-dehydrogenative coupling between heteroarenes and various C–H substrates under photoredox–HAT conditions in a stop-flow microtubing reactor (Scheme 67) [331]. The protocol used strongly oxidised MesAcrClO4 67.3 as a photocatalyst (E1/2 = +2.06 V versus SCE in MeCN) to oxidise a phosphate anion 67.4− (E1/2 ((PhO)2P(O)O·/(PhO)2P(O)O−) = +1.59 V versus SCE in MeCN) [331,332]. This forms the reduced 67.6 and phosphatyl radical 67.4·, which abstracts a hydrogen from substrate 67.1 to form alkyl radical 67.7 and reform phosphate 67.4. The alkyl radical was trapped by a protonated azine heterocycle 67.2 to form radical adduct 67.8. The radical adduct 67.8 can then be reduced to form Intermediate 67.9 (and reform PC67.3), which rapidly loses H2 to rearomatise and form the functionalised heteroarene 67.10. The prepared products from numerous C–H substrates, including ethers, alcohols, ethane, aldehydes, and cycloalkanes, such as cyclohexane product 67.11. Aldehydes with secondary and tertiary substitution on α-carbon atoms formed decarbonylated products, such as product 67.12 from pivaldehyde. This is due to fast rates of decarbonylation for the corresponding acyl radicals [149]. The functional group tolerance of the reaction was good, with products being formed including numerous heterocycles, halides (see product 67.11), amines (67.13), and ketones, among others reacting well. The method was showcased on numerous drug molecules, such as Fasudil 67.13.

Scheme 66.
PINOlylation of methylarenes 66.2, with NHPI 66.1 under cathodic oxidation.
Scheme 67.
Minisci-style cross-dehydrogenative coupling between heteroarenes 67.2 and C–H substrates 67.1 through HAT with diphenyl phosphate 67.4.
2.4. Carbon and Boron HAT Agents
2.4.1. Carbon HAT Reagents
C-centred radicals are commonly used in intramolecular HAT processes, but they are rare in intermolecular HAT processes [333]. There are several reasons for this. The first reason is the historical difficulties associated with forming C-centred radicals [24]. In recent years, this limitation has been overcome with modern methods of radical generation and the use of various radical precursors [45,48,76,143,275,277,334,335,336,337,338]. Another reason is an inherently poor polarity match in the transition state between a C-centred radical and C–H bond [333], compared with the HAT of hydridic hydrogen atoms using N/S/O-centred radicals [32,87]. The limited examples of intermolecular HATs with C-centred radicals possess very favourable thermodynamic effects, as species that form bonds with very high C–H BDE values are used, or hydrogen atoms are abstracted from weak C–H bonds. Additionally, C(sp2) radicals are typically used as they possess greater electrophilic character than C(sp3) radicals [339]. These trends are clear looking at examples from the literature using highly reactive aryl radical [benzene BDEC–H = 112.9 kcal/mol] or methyl radical [methane BDEC–H = 105.0 kcal/mol] for HAT processes [79,340,341,342,343,344]. Other reported C-centred HAT reagents are electrophilic. Hence, they provide a better polarity match in the transition state and/or take place intramolecularly [19,333].
Miyake showed that aryl halides 68.1 and thiophenols 68.2 formed C–S cross-coupled product 68.3 under basic conditions and irradiation by visible light (Scheme 68) [232]. Mechanistic studies suggested that this reaction occurred through an EDA complex 68.6, generating an aryl radical 68.7 and S-radical 68.8. These species subsequently underwent a radical–radical cross-coupling [230,231]. The mechanistic implications of Miyake’s findings inspired several groups to investigate aryl halides as precursors to aryl radicals for HAT processes.
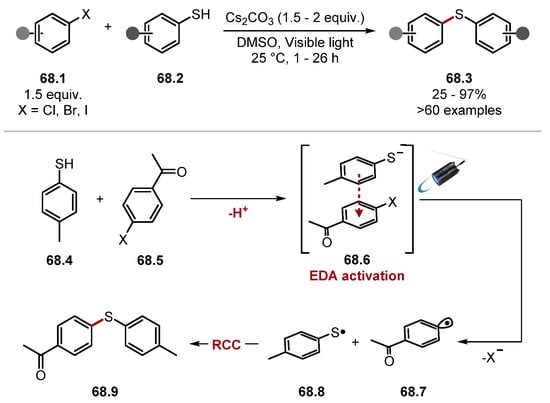
Scheme 68.
C–S cross-coupling through EDA activation and visible light.
In 2021, Akiyama used aryl halides 69.3 and 69.4 as pro-radicals in a HAT-driven cross-coupling of C–H substrate 69.9 (such as THF 69.1) and thiophenol 69.2 for a C–S (Scheme 69) [345]. The mechanism of the reaction occurs through the formation of the EDA complex 69.6 upon deprotonation of thiophenol 69.2. Upon irradiation with blue LEDs, the EDA complex fragments expel a bromide ion, thiophenyl radical 69.8, and aryl radical 69.7. As mentioned previously, aryl radicals 69.7 abstract hydrogen atoms to form alkyl radical 69.11, which can selectively react with S-radical 69.8 to form cross-coupled product 69.12. p-Bromoacetophenone 69.3 and p-bromobenzophenone 69.4 were used as aryl radical precursors. The protocol displayed good chemoselectivity as halides, while alcohol 69.13, amine 69.14, and pyridine 69.15 reacted well. Benzylic thiol 69.16 was tolerated. However, alkyl thiols were inactive in the reaction, which was likely due to the requirement for π–π interactions in the EDA complex. Numerous substrates reacted well under adapted conditions, forming products such as tetrahydrothiophene (69.18), urea (69.19), and cyclohexane (69.20). Shortly after, Xia published a similar method utilizing iodoarene 70.3 as an aryl pro-radical (Scheme 70) [346]. An assessment of the substrate scope showed excellent functional group tolerance. Several heterocycles, such as indole 70.5, thiophene, furan, and pyridine were prepared. The reaction was also tolerant of steric hindrance, as seen in mesityl product 70.6. An adjusted version of the method was used to assess the scope of C–H substrate. Again, the method was competent on numerous substrates, forming products such as carbamates (70.10), benzylic product (70.11), and allylic substrates (70.12), among many others. In contrast to Akiyama’s approach, the pro-radical iodoarene 70.3 contains electron-donating groups. Notably, less sterically encumbered iodoarenes provided greater amounts of the C–S cross-coupled product, as per Miyake’s work (Scheme 68). Using iodoarene 70.3, only 5% of the C–S product (such as 68.3) was seen. By comparison, around 14% of a similar side-product was seen in Akiyama’s screening experiments. In both works, this side product was easily removed by column chromatography.

Scheme 69.
C–S cross-coupling through EDA activation to form aryl radicals. a Additional step to reduce residual ketone with NaBH4 and MeOH. b 1 mL of C–H substrate (0.1 M w.r.t thiol).

Scheme 70.
C–S cross-coupling through EDA activation to form aryl radicals for HAT.
In 2022, Akiyama developed another EDA complex strategy to access aryl radicals for the oxidation of C–H bonds in 71.1 using 4-tert-butylphenol 71.3 and aryl iodides 71.4 or 71.5 (Scheme 71) [347]. Electron-poor arenes 71.2 were used as radical traps in a base-promoted homolytic substitution [348,349,350]. Several aromatic compounds were functionalised, including cyanoarene (71.7), benzothiazole (71.8), and isoquinoline (71.9). Several C–H substrates were also amenable to this protocol, such as ethers (71.7, 71.8, 71.9), acetals, amide (71.10), and trioxane (71.11). Trioxane 71.11 was deprotected to the corresponding aldehyde in a 96% yield constituting a two-step formylation of a C–H bond. The aryl radical 71.14 was generated by the irradiation of a halogen-bond-assisted EDA complex 71.12 with blue LEDs [351]. The aryl radical 71.14 can oxidise the C–H bond in 71.1 to form an alkyl radical 71.16. This radical is trapped by electron-poor arene 71.2 to form radical adduct 71.17. The deprotonation and SET or HAT of radical adduct 71.17 delivers the desired product 71.6.
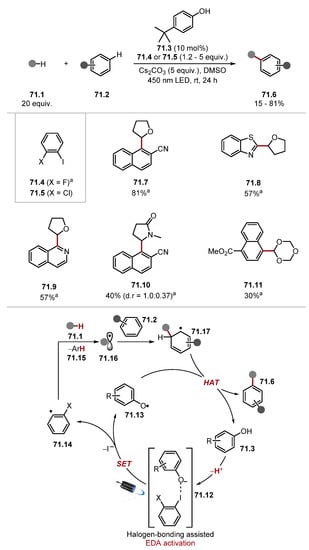
Scheme 71.
C–H arylation through halogen-bond-assisted EDA activation to form aryl radicals for HAT. a Aryl iodide 71.4 was used as a HAT reagent precursor.
In 2023, Gevorgyan developed an allylic C–H amination using the Pd-catalysis in tandem with HAT under blue-light irradiation (Scheme 72) [352]. The oxidative addition of aryl bromide 72.3 with Pd(0) 72.7 under blue-light irradiation results in a formal SET process, which forms Pd(I) 72.8 and aryl radical 72.9. The aryl radical 72.9 can then abstract an allylic C–H atom from 72.2 to form allyl radical 72.11. Allyl radical 72.11 combines with Pd(I) 72.8 to form Pd allyl complex 72.12, which intercepts an amine 72.1 to form the allylic amine product 72.4 and reforms the Pd(0) catalyst 72.7. Aryl bromides 72.5 and 72.6 were used as aryl radical precursors. Notably, using (R)-BINAP as ligand instead of xantphos afforded enantiopure products. The method formed products from a wide variety of amines, including cyclic 72.13 and linear amines 72.18, as well as protected amino acids 72.17 and 72.18. Various alkenes were also used, including α,β-unsaturated ester 72.19 and vinyl silane 72.20.

Scheme 72.
Amination of allylic C–H bonds through HAT with aryl radicals and Pd-catalysis.
In 2023, Rovis used the expulsion of aryl radicals from a nickel oxidative addition complex for a N-demethylation of trialkylamines 73.1 using nickel and photoredox tandem catalysis (Scheme 73) [353]. The reaction occurs through the oxidative addition of Ni(0) to alkenyl/aryl bromide 73.2. A subsequent anion exchange from nickel bromide to nickel pivalate facilitates the expulsion of an aryl radical upon an energy transfer step involving the photocatalyst. HAT on trialkylamine 73.1 yields an α-amino radical that can be captured with Ni(I) and form an alkyl Ni(II) complex. This complex exists in equilibrium with an Ni(0)-iminium complex. Hydrolysis of this complex delivers the demethylated product and the Ni(0) catalyst. Products were isolated as Boc-protected amines for the ease of isolation. The protocol was showcased on a number of pharmaceuticals containing a trialkylamine motif, forming products such as 73.12a, 73.13a, and 73.14a. The expulsion of an aryl radical from metal centres through LCMT runs parallel to the expulsion of halogen radicals (e.g., Cl·, Br·) where these radical species have also been used in the context of HAT [31,142,143].
Scheme 73.
N-Demethylation of trialkyl amines using a combination of nickel and photoredox catalysis. a Conditions 1 were used. b Conditions 2 were used. c Conditions 3 were used. d Conditions 4 were used. e Conditions 5 were used. f Isolated yields after a one pot boc protection with NEt3 and (Boc)2O.
In 2021, Doyle used a methyl radical 74.17 for HAT in an oxidative radical polar crossover (ORPC) protocol, which functionalised C–H bonds with nucleophiles (Scheme 74) [117]. The scope of C–H substrates was assessed using Et3N.3HF 74.3 as a source of F−. Generally, the methyl radical precursor 74.2 was used as the limiting reagent. However, low-to-excellent yields were also achieved by using C–H substrate as a limiting reagent with two equivalents of HAT reagent 74.2 with a 6-fold excess of Et3N.3HF 74.3. The scope of the fluorination was general and displayed a good functional group tolerance with ketones 74.6, esters, ethers, heterocycles, and even carboxylic acids 74.7 reacting well. Various C–H substrates were used; for instance, alkenes with allylic C–H bonds form products such as 74.8, while benzylic species form 74.6 and 74.7. The methyl radical 74.17 also abstracted weak H-atoms adjacent to EWGs such as ketones. Numerous nucleophiles were competent in an adjusted protocol, such as water, electron-rich arenes 74.12, thiols 74.13, and alcohols 74.14. Hammett plot analysis showed HAT with the methyl radical 74.17 was less affected by polar effects than a methoxyl radical, explaining the abstraction of hydrogen atoms adjacent to EWGs preferably. Notably, there has been plenty of historical debate around the philicity of a methyl radical [86]. However, it should be noted that C-centred radicals are not as adept at abstracting protic hydrogen atoms as their isoelectronic amino–boryl radical counterparts as they possess less nucleophilic character [321].

Scheme 74.
C–H functionalisation through HAT with methyl radical and radical-polar crossover.
The mechanism proceeds by oxidative quenching of the photoexcited Ir(p-F-ppy)3 74.4 (E1/2 [IrIV/*IrIII] = −1.60 V versus SCE in MeCN) [354] with (74.2 E1/2 = −1.24 V versus SCE in DMF) [117]. The resulting radical anion rapidly undergoes mesolytic cleavage forming phthalimide anion 74.16, CO2, and methyl radical 74.17 [355]. The methyl radical 74.17 then abstracts a hydrogen atom from C–H substrate 74.1 [diphenylmethane BDEC–H = 85.3 kcal/mol] to form an alkyl radical 74.20 and methane 74.19 [methane BDEC–H = 105.0 kcal/mol] [79]. The alkyl radical 74.20 is then oxidised with Ir(p-F-ppy)3 74.15 (E1/2 (IrIV/IrIII) = +0.97 V versus SCE in MeCN) [354], forming carbocation 74.21, which traps a nucleophile forming the desired product 74.22.
2.4.2. Boron HAT Reagents
Chemistry involving boron-centred radicals (boryl radicals) has been popular in recent years [356]. In terms of HAT chemistry, boryl radicals have been used for the selective abstraction of protic H-atoms, making them very unique [84]. Hence, the application of modern technologies of radical generation (photoredox and electrochemistry) to boron-centred radicals are welcome additions in the literature. In 2022, Ye reported the selective abstraction of protic hydrogen atoms from 1,3-dicarbonyls and similar species 75.1 to generate electrophilic radicals for the hydroalkylation of electron-neutral olefins 75.2 (Scheme 75) [357]. This protocol represents an umpolung of classic enol reactivity as species like Meldrum’s acid and dimethyl malonate in products 75.13 and 75.15 generate electrophilic radicals upon HAT. As mentioned previously, the abstraction of protic hydrogen atoms is known to occur with boryl radicals [84]. Amino-borane 75.4 was found to generate a boryl radical 75.4· upon PCET with a photoredox catalyst 75.3. The boryl radical 75.4· selectively abstracted a protic hydrogen from compound 75.1 [75.4 BDEB–H = 100 kcal/mol versus dimethyl malonate BDEC–H = 93.9 kcal/mol]. This generates an electrophilic alkyl radical 75.10, which undergoes radical addition with electron-neutral olefin 75.2 to form radical adduct 75.12. The radical adduct 75.12 undergoes HAT with thiophenol 75.9 to deliver product 75.6. Various 1,3-dicarbonyl, and related species, including Meldrum’s acid (75.13), acetoacetanilide (75.14), dimethylmalonate (75.15), and amide (75.16) reacted in excellent yields. Primary and secondary amides are problematic in classic enolate chemistry due to the acidity of amide protons [358,359,360]. The olefin substrate was also able to accommodate a wide variety of functionality, including pyridine (75.14), Boc-piperidine (75.15), halogens, and tosylates. The method was also showcased on numerous drugs and complex molecules. This work is a landmark for HAT chemistry as the radical reactivity of enols demonstrated here represents a unique opportunity for the expansion of classic enol C–H functionalisation into the radical sphere. Other methods for generating electrophilic radicals require pre-functionalised substrates (e.g., α-haloketones) or involve the oxidation of enolates [24,361,362,363,364]. Consequently, methods that proceed through HAT of protic hydrogen atoms are of interest for decreasing step counts, improving chemoselectivity and atom economy (oxidative enolate coupling requires stoichiometric amounts of oxidants). Wu recently reported HAT of protic hydrogen atoms in acetonitrile was a competing pathway in a reaction driven by halogen atom transfer with a tertiary amine borane complex [365].
Scheme 75.
HAT for abstraction of protic hydrogen atoms with amino-borane 75.4 and subsequent hydroalkylation of unactivated olefins.
3. Conclusions and Closing Remarks
As demonstrated in this review, the field of HAT has experienced tremendous growth due to facile access to radical species enabled by photoredox catalysis and electrochemistry. The increasing understanding of mechanistic intricacies in HAT chemistry has enabled the design of increasingly selective protocols. This can be seen, for example, in fine-tuning the electron density of C–H bonds with additives, as well as the methodical selection and alterations made to HAT reagents. This trend is likely to continue in the coming years with increasingly selective and efficient protocols for HAT chemistry becoming available. The selective functionalisation of specific bonds in saturated heterocycles (for instance, β/γ-C–H bonds in piperidine or β-C–H bonds in pyrrolidine) would constitute a notable milestone in HAT chemistry. This challenge has been addressed with direct HAT using decatungstate [59]. However, indirect HAT methods are limited to one example [294]. Another current challenge is the use of a C–H substrate as the limiting reagent to make HAT protocols viable in LSF, although notable recent advances have been made in this area (Section 2.1.2 and Section 2.3.1). Finally, the introduction of stereocentres at C–H bonds is an outstanding challenge that has received attention in recent years [50,302,303]. The parallel developments in photoredox catalysis, electrochemistry, or other methods of radical generation will enable further development of HAT chemistry. A great example of this is the use of EDA initiation in tandem with HAT chemistry (Section 2.2.2 and Section 2.4.1). In our opinion, the end goal of C–H functionalisation is for specific C–H bonds to be acknowledged as functional handles and widely used as such. In this sense, the wide range of radical traps available, including metal catalysts, and the body of HAT reagents to oxidise them will allow an ever-increasing range of functional groups to be incorporated into C–H bonds.
Author Contributions
Conceptualisation, F.S.M. and J.A.M.; Investigation, F.S.M.; writing—original draft preparation, F.S.M. and J.A.M.; writing—review and editing, F.S.M. and J.A.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge Jonathan. D. Bell and Yubiao Tian for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| (TRIPS)2 | Bis(2,4,6-triisopropylphenyl) disulfide |
| 4CzIPN | 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene |
| Acr | Acridinium |
| Ar | Aryl |
| Bn | Benzyl |
| Boc | tert-butyloxycarbonyl |
| BOX | Bis(oxazoline) (ligands) |
| Bz | Benzoyl |
| CAD | Catalytic Acceptorless Dehydrogenation |
| Cbz | Carboxybenzyl |
| CT | Chain transfer |
| Cz | Carbazolyl |
| DABCO | Diazabicyclooctane |
| DCE | 1,2-Dichloroethane |
| DFT | Density-functional theory |
| DMA | Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| dr | Diastereomeric ratio |
| Dtbbpy | Di–tert-butylbipyridyl |
| EAC | Electron acceptor catalyst |
| EDA | Electron–donor–acceptor |
| ee | Enantiomeric excess |
| er | Enantiomeric ratio |
| EWG | Electron-withdrawing group |
| HAT | Hydrogen atom transfer |
| HFIP | Hexafluoroisopropanol |
| IBX | 2-Iodoxybenzoic acid |
| iPr | Isopropyl |
| LED | Light-emitting diode |
| LSF | Late-stage functionalisation |
| MesAcr | Mesityl acridinium |
| MLCT | Metal–ligand charge transfer |
| OA | Oxidation addition |
| PCET | Proton-coupled electron transfer |
| PFTB | Perfluoro-tert-butanol |
| PRC | Polarity reversal catalysis |
| PTH | N-phenylphenothiazine |
| Pyf | Tetrafluoropyridinyl |
| RE | Reductive elimination |
| SCE | Saturated calomel electrode |
| SCS | Spin-centred-shift |
| SET | Single electron transfer |
| SFL | Sulfolane |
| SOMO | Singly occupied molecular orbital |
| TBAB | Tetrabutylammonium bromide |
| tBu | tert-butyl |
| TEDA2+ | Selectfluor Radical Dication |
| TIPS | Triisopropylsilane |
| TMS | Trimethylsilyl |
| TMS | Trimethylsilyl group |
| TPI | Thiophosphoric imide |
References
- Lam, N.Y.S.; Wu, K.; Yu, J.-Q. Advancing the Logic of Chemical Synthesis: C–H Activation as Strategic and Tactical Disconnections for C–C Bond Construction. Angew. Chem. Int. Ed. 2021, 60, 15767–15790. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.C.K.; Rovis, T. Complementary Strategies for Directed C(sp3)–H Functionalisation: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed. 2018, 57, 62–101. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.J.; Provencher, P.A.; Sorensen, E.J. Recent applications of C–H functionalisation in complex natural product synthesis. Chem. Soc. Rev. 2018, 47, 8925–8967. [Google Scholar] [CrossRef]
- Yoshioka, S.; Nagatomo, M.; Inoue, M. Application of Two Direct C(sp3)–H Functionalisations for Total Synthesis of (+)-Lactacystin. Org. Lett. 2015, 17, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Falcone, N.A.; Bosse, A.T.; Park, H.; Yu, J.-Q.; Davies, H.M.L.; Sorensen, E.J. A C–H Functionalization Strategy Enables an Enantioselective Formal Synthesis of (–)-Aflatoxin B2. Org. Lett. 2021, 23, 9393–9397. [Google Scholar] [CrossRef] [PubMed]
- Rogge, T.; Kaplaneris, N.; Chatani, N.; Kim, J.; Chang, S.; Punji, B.; Schafer, L.L.; Musaev, D.G.; Wencel-Delord, J.; Roberts, C.A.; et al. C–H activation. Nat. Rev. Methods Primers 2021, 1, 43. [Google Scholar] [CrossRef]
- Capaldo, L.; Quadri, L.L.; Ravelli, D. Photocatalytic hydrogen atom transfer: The philosopher’s stone for late-stage functionalisation? Green Chem. 2020, 22, 3376–3396. [Google Scholar] [CrossRef]
- Barham, J.P.; John, M.P.; Murphy, J.A. Contra-thermodynamic Hydrogen Atom Abstraction in the Selective C–H Functionalisation of Trialkylamine N-CH3 Groups. J. Am. Chem. Soc. 2016, 138, 15482–15487. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The medicinal chemist’s toolbox for late stage functionalisation of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Candish, L.; Collins, K.D.; Cook, G.C.; Douglas, J.J.; Gómez-Suárez, A.; Jolit, A.; Keess, S. Photocatalysis in the Life Science Industry. Chem. Rev. 2022, 122, 2907–2980. [Google Scholar] [CrossRef]
- Karimov, R.R.; Hartwig, J.F. Transition-Metal-Catalyzed Selective Functionalisation of C(sp3)–H Bonds in Natural Products. Angew. Chem. Int. Ed. 2018, 57, 4234–4241. [Google Scholar] [CrossRef]
- Britton, L.; Docherty, J.; Sklyaruk, J.; Cooney, J.; Nichol, G.S.; Dominey, A.; Thomas, S.P. Iron-catalysed Alkene and Heteroarene H/D Exchange by Reversible Protonation of Iron-hydride Intermediates. Chem. Sci. 2022, 13, 10291–10298. [Google Scholar] [CrossRef]
- Britton, L.; Skrodzki, M.; Nichol, G.S.; Dominey, A.P.; Pawluć, P.; Docherty, J.H.; Thomas, S.P. Manganese-Catalyzed C(sp2)–H Borylation of Furan and Thiophene Derivatives. ACS Catal. 2021, 11, 6857–6864. [Google Scholar] [CrossRef]
- Britton, L.; Docherty, J.H.; Dominey, A.P.; Thomas, S.P. Iron-Catalysed C(sp2)–H Borylation Enabled by Carboxylate Activation. Molecules. 2020, 25, 905. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, Z.; Wu, K.; Ma, J.; Zhou, Y.-G.; Yu, Z. Recent advances in transition-metal-catalyzed carbene insertion to C–H bonds. Chem. Soc. Rev. 2022, 51, 2759–2852. [Google Scholar] [CrossRef]
- Han, F.; Choi, P.H.; Ye, C.-X.; Grell, Y.; Xie, X.; Ivlev, S.I.; Chen, S.; Meggers, E. Cyclometalated Chiral-at-Ruthenium Catalyst for Enantioselective Ring-Closing C(sp3)–H Carbene Insertion to Access Chiral Flavanones. ACS Catal. 2022, 12, 10304–10312. [Google Scholar] [CrossRef]
- Peil, S.; Gutiérrez González, A.; Leutzsch, M.; Fürstner, A. C–H Insertion via Ruthenium Catalyzed gem-Hydrogenation of 1,3-Enynes. J. Am. Chem. Soc. 2022, 144, 4158–4167. [Google Scholar] [CrossRef]
- Romero, E.; Jones, B.S.; Hogg, B.N.; Rué Casamajo, A.; Hayes, M.A.; Flitsch, S.L.; Turner, N.J.; Schnepel, C. Enzymatic Late-Stage Modifications: Better Late Than Never. Angew. Chem. Int. Ed. 2021, 60, 16824–16855. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071. [Google Scholar] [CrossRef]
- Golden, D.L.; Suh, S.-E.; Stahl, S.S. Radical C(sp3)–H functionalisation and cross-coupling reactions. Nat. Rev. Chem. 2022, 6, 405–427. [Google Scholar] [CrossRef]
- Newhouse, T.; Baran, P.S. If C-H Bonds Could Talk: Selective C-H Bond Oxidation. Angew. Chem. Int. Ed. 2011, 50, 3362–3374. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Quevedo, R.E.; Kohrt, J.T.; Oderinde, M.S.; Reilly, U.; White, M.C. Late-stage oxidative C(sp3)–H methylation. Nature. 2020, 580, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Luan, Y.-X.; Lam, N.Y.S.; Li, J.-F.; Li, Y.; Ye, M.; Yu, J.-Q. A directive Ni catalyst overrides conventional site selectivity in pyridine C–H alkenylation. Nat. Chem. 2021, 13, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Lo, J.C.; Edwards, J.T.; Baran, P.S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. [Google Scholar] [CrossRef] [PubMed]
- Crespi, S.; Fagnoni, M. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 2020, 120, 9790–9833. [Google Scholar] [CrossRef] [PubMed]
- Leifert, D.; Studer, A. The Persistent Radical Effect in Organic Synthesis. Angew. Chem. Int. Ed. 2020, 59, 74–108. [Google Scholar] [CrossRef]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef]
- Le Vaillant, F.; Waser, J. Alkynylation of radicals: Spotlight on the “Third Way” to transfer triple bonds. Chem. Sci. 2019, 10, 8909–8923. [Google Scholar] [CrossRef]
- Bietti, M. Activation and Deactivation Strategies Promoted by Medium Effects for Selective Aliphatic C–H Bond Functionalisation. Angew. Chem. Int. Ed. 2018, 57, 16618–16637. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, M.; Salamone, M.; Bietti, M. Electronic control over site-selectivity in hydrogen atom transfer (HAT) based C(sp3)–H functionalisation promoted by electrophilic reagents. Chem. Soc. Rev. 2022, 51, 2171–2223. [Google Scholar] [CrossRef]
- Bonciolini, S.; Noël, T.; Capaldo, L. Synthetic Applications of Photocatalyzed Halogen-radical mediated Hydrogen Atom Transfer for C–H Bond Functionalisation. Eur. J. Org. Chem. 2022, 2022, e202200417. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Li, J.; Li, C.-J. Photocatalytic C(sp3) radical generation via C–H, C–C, and C–X bond cleavage. Chem. Sci. 2022, 13, 5465–5504. [Google Scholar] [CrossRef] [PubMed]
- Holmberg-Douglas, N.; Nicewicz, D.A. Photoredox-Catalyzed C–H Functionalisation Reactions. Chem. Rev. 2022, 122, 1925–2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wang, S.; An, Q.; Liu, L.; Wang, H.; Li, Y.; Feng, K.; Zuo, Z. Resurgence and advancement of photochemical hydrogen atom transfer processes in selective alkane functionalisations. Chem. Sci. 2023, 14, 6841–6859. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lin, Y.-M.; Gong, L. The Merger of Photocatalyzed Hydrogen Atom Transfer with Transition Metal Catalysis for C–H Functionalisation of Alkanes and Cycloalkanes. Eur. J. Org. Chem. 2021, 2021, 5545–5556. [Google Scholar] [CrossRef]
- Zhang, S.; Findlater, M. Electrochemically Driven Hydrogen Atom Transfer Catalysis: A Tool for C(sp3)/Si–H Functionalisation and Hydrofunctionalisation of Alkenes. ACS Catal. 2023, 13, 8731–8751. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Rubio, Á.; Martínez-Balart, P.; Álvarez-Constantino, A.M.; Fañanás-Mastral, M. C–C bond formation via photocatalytic direct functionalisation of simple alkanes. Chem. Commun. 2023, 59, 9424–9444. [Google Scholar] [CrossRef]
- Turner, O.J.; Murphy, J.A.; Hirst, D.J.; Talbot, E.P.A. Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles. Chem. Eur. J. 2018, 24, 18658–18662. [Google Scholar] [CrossRef]
- Turner, O.J.; Hirst, D.J.; Murphy, J.A. Hydrogen Atom Transfer-Mediated Domino Cyclisation Reaction to Access (Spiro)Quinazolinones. Chem. Eur. J. 2020, 26, 3026–3029. [Google Scholar] [CrossRef]
- Obradors, C.; Martinez, R.M.; Shenvi, R.A. Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc. 2016, 138, 4962–4971. [Google Scholar] [CrossRef]
- Matos, J.L.M.; Vásquez-Céspedes, S.; Gu, J.; Oguma, T.; Shenvi, R.A. Branch-Selective Addition of Unactivated Olefins into Imines and Aldehydes. J. Am. Chem. Soc. 2018, 140, 16976–16981. [Google Scholar] [CrossRef] [PubMed]
- Crossley, S.W.M.; Obradors, C.; Martinez, R.M.; Shenvi, R.A. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalisations of Olefins. Chem. Rev. 2016, 116, 8912–9000. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Matos, J.L.M.; Yagi, A.; Shenvi, R.A. Branch-Selective Hydroarylation: Iodoarene–Olefin Cross-Coupling. J. Am. Chem. Soc. 2016, 138, 12779–12782. [Google Scholar] [CrossRef]
- Iwasaki, K.; Wan, K.K.; Oppedisano, A.; Crossley, S.W.M.; Shenvi, R.A. Simple, Chemoselective Hydrogenation with Thermodynamic Stereocontrol. J. Am. Chem. Soc. 2014, 136, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Crossley, S.W.M.; Matos, J.L.M.; Vásquez-Céspedes, S.; Shevick, S.L.; Shenvi, R.A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res. 2018, 51, 2628–2640. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Huffman, T.R.; McCourt, R.O.; van der Puyl, V.; Shenvi, R.A. Hydroalkylation of Olefins to form Quaternary Carbons. J. Am. Chem. Soc. 2019, 141, 7709–7714. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Z. Metal-hydride hydrogen atom transfer (MHAT) reactions in natural product synthesis. Org. Chem. Front. 2021, 8, 7050–7076. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C–H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. [Google Scholar] [CrossRef]
- Gu, Y.; Yin, H.; Wakeling, M.; An, J.; Martin, R. Defunctionalisation of sp3 C–Heteroatom and sp3 C–C Bonds Enabled by Photoexcited Triplet Ketone Catalysts. ACS Catal. 2022, 12, 1031–1036. [Google Scholar] [CrossRef]
- Xu, S.; Ping, Y.; Li, W.; Guo, H.; Su, Y.; Li, Z.; Wang, M.; Kong, W. Enantioselective C(sp3)–H Functionalisation of Oxacycles via Photo-HAT/Nickel Dual Catalysis. J. Am. Chem. Soc. 2023, 145, 5231–5241. [Google Scholar] [CrossRef]
- Sanjosé-Orduna, J.; Silva, R.C.; Raymenants, F.; Reus, B.; Thaens, J.; de Oliveira, K.T.; Noël, T. Dual role of benzophenone enables a fast and scalable C-4 selective alkylation of pyridines in flow. Chem. Sci. 2022, 13, 12527–12532. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, Y.; Martin, R. sp3 C–H Arylation and Alkylation Enabled by the Synergy of Triplet Excited Ketones and Nickel Catalysts. J. Am. Chem. Soc. 2018, 140, 12200–12209. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.W.; Yuan, M.; Polites, V.C.; Gutierrez, O.; Molander, G.A. Photochemical C–H Activation Enables Nickel-Catalyzed Olefin Dicarbofunctionalisation. J. Am. Chem. Soc. 2021, 143, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Prakash, G.; Teja, C.; Lahiri, G.K.; Maiti, D. Metal-free photoinduced hydrogen atom transfer assisted C(sp3)–H thioarylation. Green Chem. 2023, 25, 3431–3436. [Google Scholar] [CrossRef]
- Masuda, Y.; Tsuda, H.; Murakami, M. C1 Oxidation/C2 Reduction Isomerisation of Unprotected Aldoses Induced by Light/Ketone. Angew. Chem. Int. Ed. 2020, 59, 2755–2759. [Google Scholar] [CrossRef]
- Dewanji, A.; Krach, P.E.; Rueping, M. The Dual Role of Benzophenone in Visible-Light/Nickel Photoredox-Catalyzed C–H Arylations: Hydrogen-Atom Transfer and Energy Transfer. Angew. Chem. Int. Ed. 2019, 58, 3566–3570. [Google Scholar] [CrossRef]
- Xia, J.-B.; Zhu, C.; Chen, C. Visible Light-Promoted Metal-Free C–H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination. J. Am. Chem. Soc. 2013, 135, 17494–17500. [Google Scholar] [CrossRef]
- Pulcinella, A.; Bonciolini, S.; Lukas, F.; Sorato, A.; Noël, T. Photocatalytic Alkylation of C(sp3)–H Bonds Using Sulfonylhydrazones. Angew. Chem. Int. Ed. 2023, 62, e202215374. [Google Scholar] [CrossRef]
- Sarver, P.J.; Bacauanu, V.; Schultz, D.M.; DiRocco, D.A.; Lam, Y.-h.; Sherer, E.C.; MacMillan, D.W.C. The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem. 2020, 12, 459–467. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Zhu, B.; Liang, Y.-J.; Guan, W.; Su, Z.-M. Origin and Regioselectivity of Direct Hydrogen Atom Transfer Mechanism of C(sp3)–H Arylation by [W10O32]4–/Ni Metallaphotoredox Catalysis. Inorg. Chem. 2021, 60, 18706–18714. [Google Scholar] [CrossRef]
- Capaldo, L.; Bonciolini, S.; Pulcinella, A.; Nuño, M.; Noël, T. Modular allylation of C(sp3)–H bonds by combining decatungstate photocatalysis and HWE olefination in flow. Chem. Sci. 2022, 13, 7325–7331. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wen, Z.; Laudadio, G.; Capaldo, L.; Lammers, R.; Rincón, J.A.; García-Losada, P.; Mateos, C.; Frederick, M.O.; Broersma, R.; et al. Accelerated and Scalable C(sp3)–H Amination via Decatungstate Photocatalysis Using a Flow Photoreactor Equipped with High-Intensity LEDs. ACS Cent. Sci. 2022, 8, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, D.; Pulcinella, A.; Bovy, L.; Broersma, R.; Noël, T. Rapid and Direct Photocatalytic C(sp3)–H Acylation and Arylation in Flow. Angew. Chem. Int. Ed. 2021, 60, 21277–21282. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Maheshwari, A.; Sambiagio, C.; Deng, Y.; Laudadio, G.; Van Aken, K.; Sun, Y.; Gemoets, H.P.L.; Noël, T. Optimisation of a Decatungstate-Catalyzed C(sp3)–H Alkylation Using a Continuous Oscillatory Millistructured Photoreactor. Org. Process Res. Dev. 2020, 24, 2356–2361. [Google Scholar] [CrossRef]
- Wan, T.; Capaldo, L.; Laudadio, G.; Nyuchev, A.V.; Rincón, J.A.; García-Losada, P.; Mateos, C.; Frederick, M.O.; Nuño, M.; Noël, T. Decatungstate-Mediated C(sp3)–H Heteroarylation via Radical-Polar Crossover in Batch and Flow. Angew. Chem. Int. Ed. 2021, 60, 17893–17897. [Google Scholar] [CrossRef]
- Laudadio, G.; Deng, Y.; van der Wal, K.; Ravelli, D.; Nuño, M.; Fagnoni, M.; Guthrie, D.; Sun, Y.; Noël, T. C(sp(3))–H functionalisations of light hydrocarbons using decatungstate photocatalysis in flow. Science 2020, 369, 92–96. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Decatungstate as Direct Hydrogen Atom Transfer Photocatalyst for SOMOphilic Alkynylation. Org. Lett. 2021, 23, 2243–2247. [Google Scholar] [CrossRef]
- Jorea, A.; Bassetti, B.; Gervasoni, K.; Protti, S.; Palmieri, A.; Ravelli, D. More Chips to Nitroolefins: Decatungstate Photocatalysed Hydroalkylation Under Batch and Flow Conditions. Adv. Synth. Catal. 2023, 365, 722–727. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Shih, Y.-L.; Wu, Y.-K.; Ryu, I. Site-Selective C(sp3)–H Alkenylation Using Decatungstate Anion as Photocatalyst. Adv. Synth. Catal. 2022, 364, 1039–1043. [Google Scholar] [CrossRef]
- Fukuyama, T.; Yamada, K.; Nishikawa, T.; Ravelli, D.; Fagnoni, M.; Ryu, I. Site-selectivity in TBADT-photocatalyzed C(sp3)–H Functionalisation of Saturated Alcohols and Alkanes. Chem. Lett. 2018, 47, 207–209. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I. Site-Selective C–H Functionalisation by Decatungstate Anion Photocatalysis: Synergistic Control by Polar and Steric Effects Expands the Reaction Scope. ACS Catal. 2018, 8, 701–713. [Google Scholar] [CrossRef]
- Paolillo, J.M.; Duke, A.D.; Gogarnoiu, E.S.; Wise, D.E.; Parasram, M. Anaerobic Hydroxylation of C(sp3)–H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes. J. Am. Chem. Soc. 2023, 145, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.J.; Rosen, B.R.; Chen, Y.; Tang, J.; Chen, K.; Eastgate, M.D.; Baran, P.S. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 2016, 533, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M. Understanding Hydrogen Atom Transfer: From Bond Strengths to Marcus Theory. Acc. Chem. Res. 2011, 44, 36–46. [Google Scholar] [CrossRef]
- Lai, W.; Li, C.; Chen, H.; Shaik, S. Hydrogen-Abstraction Reactivity Patterns from A to Y: The Valence Bond Way. Angew. Chem. Int. Ed. 2012, 51, 5556–5578. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Murphy, J.A. Recent advances in visible light-activated radical coupling reactions triggered by (i) ruthenium, (ii) iridium and (iii) organic photoredox agents. Chem. Soc. Rev. 2021, 50, 9540–9685. [Google Scholar] [CrossRef]
- Salamone, M.; Bietti, M. Tuning Reactivity and Selectivity in Hydrogen Atom Transfer from Aliphatic C–H Bonds to Alkoxyl Radicals: Role of Structural and Medium Effects. Acc. Chem. Res. 2015, 48, 2895–2903. [Google Scholar] [CrossRef]
- Zavitsas, A.A.; Pinto, J.A. Meaning of the polar effect in hydrogen abstractions by free radicals. Reactions of the tert-butoxy radical. J. Am. Chem. Soc. 1972, 94, 7390–7396. [Google Scholar] [CrossRef]
- Luo, Y.-R. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2002; pp. 18, 38, 41, 68, 69, 80, 146, 169, 170, 172, 209, 240–269, 291, 293, 298. [Google Scholar]
- Salamone, M.; Galeotti, M.; Romero-Montalvo, E.; van Santen, J.A.; Groff, B.D.; Mayer, J.M.; DiLabio, G.A.; Bietti, M. Bimodal Evans–Polanyi Relationships in Hydrogen Atom Transfer from C(sp3)–H Bonds to the Cumyloxyl Radical. A Combined Time-Resolved Kinetic and Computational Study. J. Am. Chem. Soc. 2021, 143, 11759–11776. [Google Scholar] [CrossRef]
- Dénès, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl Radicals in Organic Synthesis. Chem. Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef]
- Ide, T.; Barham, J.P.; Fujita, M.; Kawato, Y.; Egami, H.; Hamashima, Y. Regio- and chemoselective Csp3–H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis. Chem. Sci. 2018, 9, 8453–8460. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Zhu, Q.; Miller, D.C.; Gu, C.J.; Knowles, R.R. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 2016, 539, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: Concepts and applications in organic chemistry. Chem. Soc. Rev. 1999, 28, 25–35. [Google Scholar] [CrossRef]
- Giese, B. Formation of CC Bonds by Addition of Free Radicals to Alkenes. Angew. Chem. Int. Ed. 1983, 22, 753–764. [Google Scholar] [CrossRef]
- Tedder, J.M. Which Factors Determine the Reactivity and Regioselectivity of Free Radical Substitution and Addition Reactions? Angew. Chem. Int. Ed. 1982, 21, 401–410. [Google Scholar] [CrossRef]
- Ruffoni, A.; Mykura, R.C.; Bietti, M.; Leonori, D. The interplay of polar effects in controlling the selectivity of radical reactions. Nat. Synth. 2022, 1, 682–695. [Google Scholar] [CrossRef]
- Chan, B.; Easton, C.J.; Radom, L. Outcome-Changing Effect of Polarity Reversal in Hydrogen-Atom-Abstraction Reactions. J. Phys. Chem. A 2015, 119, 3843–3847. [Google Scholar] [CrossRef]
- Galeotti, M.; Trasatti, C.; Sisti, S.; Salamone, M.; Bietti, M. Factors Governing Reactivity and Selectivity in Hydrogen Atom Transfer from C(sp3)–H Bonds of Nitrogen-Containing Heterocycles to the Cumyloxyl Radical. J. Org. Chem. 2022, 87, 7456–7463. [Google Scholar] [CrossRef]
- Liu, F.; Ma, S.; Lu, Z.; Nangia, A.; Duan, M.; Yu, Y.; Xu, G.; Mei, Y.; Bietti, M.; Houk, K.N. Hydrogen Abstraction by Alkoxyl Radicals: Computational Studies of Thermodynamic and Polarity Effects on Reactivities and Selectivities. J. Am. Chem. Soc. 2022, 144, 6802–6812. [Google Scholar] [CrossRef]
- Martin, T.; Galeotti, M.; Salamone, M.; Liu, F.; Yu, Y.; Duan, M.; Houk, K.N.; Bietti, M. Deciphering Reactivity and Selectivity Patterns in Aliphatic C–H Bond Oxygenation of Cyclopentane and Cyclohexane Derivatives. J. Org. Chem. 2021, 86, 9925–9937. [Google Scholar] [CrossRef]
- Dantignana, V.; Milan, M.; Cussó, O.; Company, A.; Bietti, M.; Costas, M. Chemoselective Aliphatic C–H Bond Oxidation Enabled by Polarity Reversal. ACS Cent. Sci. 2017, 3, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Salamone, M.; Martin, T.; Milan, M.; Costas, M.; Bietti, M. Electronic and Torsional Effects on Hydrogen Atom Transfer from Aliphatic C–H Bonds: A Kinetic Evaluation via Reaction with the Cumyloxyl Radical. J. Org. Chem. 2017, 82, 13542–13549. [Google Scholar] [CrossRef] [PubMed]
- Bietti, M.; Forcina, V.; Lanzalunga, O.; Lapi, A.; Martin, T.; Mazzonna, M.; Salamone, M. Kinetic Study of the Reaction of the Phthalimide-N-oxyl Radical with Amides: Structural and Medium Effects on the Hydrogen Atom Transfer Reactivity and Selectivity. J. Org. Chem. 2016, 81, 11924–11931. [Google Scholar] [CrossRef] [PubMed]
- Salamone, M.; Mangiacapra, L.; Carboni, G.; Bietti, M. Hydrogen atom transfer from tertiary alkanamides to the cumyloxyl radical. The role of substrate structure on alkali and alkaline earth metal ion induced C–H bond deactivation. Tetrahedron 2016, 72, 7757–7763. [Google Scholar] [CrossRef]
- Salamone, M.; Carboni, G.; Bietti, M. Fine Control over Site and Substrate Selectivity in Hydrogen Atom Transfer-Based Functionalisation of Aliphatic C–H Bonds. J. Org. Chem. 2016, 81, 9269–9278. [Google Scholar] [CrossRef]
- Salamone, M.; DiLabio, G.A.; Bietti, M. Hydrogen Atom Abstraction Reactions from Tertiary Amines by Benzyloxyl and Cumyloxyl Radicals: Influence of Structure on the Rate-Determining Formation of a Hydrogen-Bonded Prereaction Complex. J. Org. Chem. 2011, 76, 6264–6270. [Google Scholar] [CrossRef]
- Salamone, M.; Mangiacapra, L.; Bietti, M. Kinetic Solvent Effects on the Reactions of the Cumyloxyl Radical with Tertiary Amides. Control over the Hydrogen Atom Transfer Reactivity and Selectivity through Solvent Polarity and Hydrogen Bonding. J. Org. Chem. 2015, 80, 1149–1154. [Google Scholar] [CrossRef]
- Salamone, M.; Carboni, G.; Mangiacapra, L.; Bietti, M. Binding to Redox-Inactive Alkali and Alkaline Earth Metal Ions Strongly Deactivates the C–H Bonds of Tertiary Amides toward Hydrogen Atom Transfer to Reactive Oxygen Centered Radicals. J. Org. Chem. 2015, 80, 9214–9223. [Google Scholar] [CrossRef]
- Salamone, M.; Ortega, V.B.; Bietti, M. Enhanced Reactivity in Hydrogen Atom Transfer from Tertiary Sites of Cyclohexanes and Decalins via Strain Release: Equatorial C–H Activation vs Axial C–H Deactivation. J. Org. Chem. 2015, 80, 4710–4715. [Google Scholar] [CrossRef]
- Salamone, M.; Basili, F.; Mele, R.; Cianfanelli, M.; Bietti, M. Reactions of the Cumyloxyl Radical with Secondary Amides. The Influence of Steric and Stereoelectronic Effects on the Hydrogen Atom Transfer Reactivity and Selectivity. Org. Lett. 2014, 16, 6444–6447. [Google Scholar] [CrossRef]
- Milan, M.; Salamone, M.; Bietti, M. Hydrogen Atom Transfer from 1,n-Alkanediamines to the Cumyloxyl Radical. Modulating C–H Deactivation Through Acid–Base Interactions and Solvent Effects. J. Org. Chem. 2014, 79, 5710–5716. [Google Scholar] [CrossRef] [PubMed]
- Kushch, O.V.; Hordieieva, I.O.; Kompanets, M.O.; Zosenko, O.O.; Opeida, I.A.; Shendrik, A.N. Hydrogen Atom Transfer from Benzyl Alcohols to N-Oxyl Radicals. Reactivity Parameters. J. Org. Chem. 2021, 86, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Ruffoni, A.; Hampton, C.; Simonetti, M.; Leonori, D. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature. 2022, 610, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hampton, C.; Simonetti, M.; Leonori, D. Olefin Dihydroxylation Using Nitroarenes as Photoresponsive Oxidants. Angew. Chem. Int. Ed. 2023, 62, e202214508. [Google Scholar] [CrossRef]
- Gaster, E.; Kozuch, S.; Pappo, D. Selective Aerobic Oxidation of Methylarenes to Benzaldehydes Catalyzed by N-Hydroxyphthalimide and Cobalt(II) Acetate in Hexafluoropropan-2-ol. Angew. Chem. Int. Ed. 2017, 56, 5912–5915. [Google Scholar] [CrossRef] [PubMed]
- Bietti, M.; Salamone, M. Kinetic Solvent Effects on Hydrogen Abstraction Reactions from Carbon by the Cumyloxyl Radical. The Role of Hydrogen Bonding. Org. Lett. 2010, 12, 3654–3657. [Google Scholar] [CrossRef] [PubMed]
- Bietti, M.; Martella, R.; Salamone, M. Understanding Kinetic Solvent Effects on Hydrogen Abstraction Reactions from Carbon by the Cumyloxyl Radical. Org. Lett. 2011, 13, 6110–6113. [Google Scholar] [CrossRef]
- Salamone, M.; Giammarioli, I.; Bietti, M. Kinetic Solvent Effects on Hydrogen Abstraction Reactions from Carbon by the Cumyloxyl Radical. The Importance of Solvent Hydrogen-Bond Interactions with the Substrate and the Abstracting Radical. J. Org. Chem. 2011, 76, 4645–4651. [Google Scholar] [CrossRef]
- Paul, V.; Roberts, B.P. Homolytic reactions of ligated boranes. Part 8. Electron spin resonance studies of radicals derived from ligated alkylboranes. J. Chem. Soc. Perkin Trans. II 1988, 1183–1193. [Google Scholar] [CrossRef]
- Paul, V.; Roberts, B.P.; Willis, C.R. Homolytic reactions of ligated boranes. Part 12. Amine–alkylboranes as polarity reversal catalysts for hydrogen-atom abstraction by t-butoxyl radicals. J. Chem. Soc. Perkin Trans. II 1989, 1953–1961. [Google Scholar] [CrossRef]
- Sheldon, R.A. Synthesis and uses of alkyl hydroperoxides and dialkyl peroxides. In Peroxides; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1983; p. 169. [Google Scholar]
- Okada, K.; Okamoto, K.; Oda, M. A new and practical method of decarboxylation: Photosensitised decarboxylation of N-acyloxyphthalimides via electron-transfer mechanism. J. Am. Chem. Soc. 1988, 110, 8736–8738. [Google Scholar] [CrossRef]
- Toriyama, F.; Cornella, J.; Wimmer, L.; Chen, T.G.; Dixon, D.D.; Creech, G.; Baran, P.S. Redox-Active Esters in Fe-Catalyzed C-C Coupling. J. Am. Chem. Soc. 2016, 138, 11132–11135. [Google Scholar] [CrossRef] [PubMed]
- Cornella, J.; Edwards, J.T.; Qin, T.; Kawamura, S.; Wang, J.; Pan, C.-M.; Gianatassio, R.; Schmidt, M.; Eastgate, M.D.; Baran, P.S. Practical Ni-Catalyzed Aryl–Alkyl Cross-Coupling of Secondary Redox-Active Esters. J. Am. Chem. Soc. 2016, 138, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Huihui, K.M.M.; Caputo, J.A.; Melchor, Z.; Olivares, A.M.; Spiewak, A.M.; Johnson, K.A.; DiBenedetto, T.A.; Kim, S.; Ackerman, L.K.G.; Weix, D.J. Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides. J. Am. Chem. Soc. 2016, 138, 5016–5019. [Google Scholar] [CrossRef] [PubMed]
- Leibler, I.N.-M.; Tekle-Smith, M.A.; Doyle, A.G. A general strategy for C(sp3)–H functionalisation with nucleophiles using methyl radical as a hydrogen atom abstractor. Nat. Commun. 2021, 12, 6950. [Google Scholar] [CrossRef]
- Kawamata, Y.; Yan, M.; Liu, Z.; Bao, D.-H.; Chen, J.; Starr, J.T.; Baran, P.S. Scalable, Electrochemical Oxidation of Unactivated C–H Bonds. J. Am. Chem. Soc. 2017, 139, 7448–7451. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, J.D.; MacMillan, D.W.C. The direct arylation of allylic sp3 C–H bonds via organic and photoredox catalysis. Nature. 2015, 519, 74–77. [Google Scholar] [CrossRef]
- Madani, A.; Anghileri, L.; Heydenreich, M.; Möller, H.M.; Pieber, B. Benzylic Fluorination Induced by a Charge-Transfer Complex with a Solvent-Dependent Selectivity Switch. Org. Lett. 2022, 24, 5376–5380. [Google Scholar] [CrossRef]
- Cismesia, M.A.; Yoon, T.P. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 2015, 6, 5426–5434. [Google Scholar] [CrossRef]
- Ye, T.; Zhang, F.-L.; Xia, H.-M.; Zhou, X.; Yu, Z.-X.; Wang, Y.-F. Stereoselective hydrogen atom transfer to acyclic radicals: A switch enabling diastereodivergent borylative radical cascades. Nat. Commun 2022, 13, 426. [Google Scholar] [CrossRef]
- Katz, R.B.; Mistry, J.; Mitchell, M.B. An Improved Method for the Mono-Hydroxymethylation of Pyridines. A Modification of the Minisci Procedure. Synth. Commun. 1989, 19, 317–325. [Google Scholar] [CrossRef]
- Chan, W.C.; Vinod, J.K.; Koide, K. Acetal Addition to Electron-Deficient Alkenes with Hydrogen Atom Transfer as a Radical Chain Propagation Step. J. Org. Chem. 2021, 86, 3674–3682. [Google Scholar] [CrossRef]
- Curran, D.P.; Kim, D.; Liu, H.T.; Shen, W. Translocation of radical sites by intramolecular 1,5-hydrogen atom transfer. J. Am. Chem. Soc. 1988, 110, 5900–5902. [Google Scholar] [CrossRef]
- Takahira, Y.; Chen, M.; Kawamata, Y.; Mykhailiuk, P.; Nakamura, H.; Peters, B.K.; Reisberg, S.H.; Li, C.; Chen, L.; Hoshikawa, T.; et al. Electrochemical C(sp3)–H Fluorination. Synlett 2019, 30, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.S.J.; Phipps, R.J. Recent Advances in Minisci-Type Reactions. Angew. Chem. Int. Ed. 2019, 58, 13666–13699. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; MacMillan, D.W.C. Photoredox α-Vinylation of α-Amino Acids and N-Aryl Amines. J. Am. Chem. Soc. 2014, 136, 11602–11605. [Google Scholar] [CrossRef]
- Jeffrey, J.L.; Petronijević, F.R.; MacMillan, D.W.C. Selective Radical–Radical Cross-Couplings: Design of a Formal β-Mannich Reaction. J. Am. Chem. Soc. 2015, 137, 8404–8407. [Google Scholar] [CrossRef]
- McManus, J.B.; Onuska, N.P.R.; Nicewicz, D.A. Generation and Alkylation of α-Carbamyl Radicals via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2018, 140, 9056–9060. [Google Scholar] [CrossRef]
- Liu, K.; Studer, A. Formal β-C–H Arylation of Aldehydes and Ketones by Cooperative Nickel and Photoredox Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202206533. [Google Scholar] [CrossRef]
- Landwehr, E.M.; Baker, M.A.; Oguma, T.; Burdge, H.E.; Kawajiri, T.; Shenvi, R.A. Concise syntheses of GB22, GB13, and himgaline by cross-coupling and complete reduction. Science. 2022, 375, 1270–1274. [Google Scholar] [CrossRef]
- Shen, Y.; Funez-Ardoiz, I.; Schoenebeck, F.; Rovis, T. Site-Selective α-C–H Functionalisation of Trialkylamines via Reversible Hydrogen Atom Transfer Catalysis. J. Am. Chem. Soc. 2021, 143, 18952–18959. [Google Scholar] [CrossRef] [PubMed]
- Pratley, C.; Fenner, S.; Murphy, J.A. Nitrogen-Centered Radicals in Functionalisation of sp2 Systems: Generation, Reactivity, and Applications in Synthesis. Chem. Rev. 2022, 122, 8181–8260. [Google Scholar] [CrossRef] [PubMed]
- Speckmeier, E.; Fischer, T.G.; Zeitler, K. A Toolbox Approach to Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor–Acceptor Cyanoarenes. J. Am. Chem. Soc. 2018, 140, 15353–15365. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Z.; Bordwell, F.G. Gas-Phase and Solution-Phase Homolytic Bond Dissociation Energies of H–N+ Bonds in the Conjugate Acids of Nitrogen Bases. J. Org. Chem. 1996, 61, 4778–4783. [Google Scholar] [CrossRef]
- Twilton, J.; Christensen, M.; DiRocco, D.A.; Ruck, R.T.; Davies, I.W.; MacMillan, D.W.C. Selective Hydrogen Atom Abstraction through Induced Bond Polarisation: Direct α-Arylation of Alcohols through Photoredox, HAT, and Nickel Catalysis. Angew. Chem. Int. Ed. 2018, 57, 5369–5373. [Google Scholar] [CrossRef]
- Jeffrey, J.L.; Terrett, J.A.; MacMillan, D.W. O-H hydrogen bonding promotes H-atom transfer from α C-H bonds for C-alkylation of alcohols. Science 2015, 349, 1532–1536. [Google Scholar] [CrossRef]
- Le, C.; Liang, Y.; Evans, R.W.; Li, X.; MacMillan, D.W.C. Selective sp3 C-H alkylation via polarity-match-based cross-coupling. Nature 2017, 547, 79–83. [Google Scholar] [CrossRef]
- Shaw, M.H.; Shurtleff, V.W.; Terrett, J.A.; Cuthbertson, J.D.; MacMillan, D.W.C. Native functionality in triple catalytic cross-coupling: sp3 C-H bonds as latent nucleophiles. Science 2016, 352, 1304–1308. [Google Scholar] [CrossRef]
- Yang, H.-B.; Feceu, A.; Martin, D.B.C. Catalyst-Controlled C–H Functionalisation of Adamantanes Using Selective H-Atom Transfer. ACS Catal. 2019, 9, 5708–5715. [Google Scholar] [CrossRef]
- Romano, C.; Talavera, L.; Gómez-Bengoa, E.; Martin, R. Conformational Flexibility as a Tool for Enabling Site-Selective Functionalisation of Unactivated sp3 C–O Bonds in Cyclic Acetals. J. Am. Chem. Soc. 2022, 144, 11558–11563. [Google Scholar] [CrossRef]
- Juliá, F.; Constantin, T.; Leonori, D. Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rueping, M. Direct Cross-Coupling of Allylic C(sp3)–H Bonds with Aryl- and Vinylbromides by Combined Nickel and Visible-Light Catalysis. Angew. Chem. Int. Ed. 2018, 57, 10333–10337. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.I.; Gygi, D.; Qin, Y.; Zhu, Q.; Johnson, E.J.; Chen, Y.-S.; Nocera, D.G. Taming the Chlorine Radical: Enforcing Steric Control over Chlorine-Radical-Mediated C–H Activation. J. Am. Chem. Soc. 2022, 144, 1464–1472. [Google Scholar] [CrossRef]
- Gygi, D.; Gonzalez, M.I.; Hwang, S.J.; Xia, K.T.; Qin, Y.; Johnson, E.J.; Gygi, F.; Chen, Y.-S.; Nocera, D.G. Capturing the Complete Reaction Profile of a C–H Bond Activation. J. Am. Chem. Soc. 2021, 143, 6060–6064. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Q.; Wu, Y.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Photoredox/Hydrogen Atom Transfer Cocatalyzed C–H Difluoroallylation of Amides, Ethers, and Alkyl Aldehydes. Org. Lett. 2021, 23, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; MacMillan, D.W.C. Direct Aldehyde C–H Arylation and Alkylation via the Combination of Nickel, Hydrogen Atom Transfer, and Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 11353–11356. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I.; Sonoda, N. Free-Radical Carbonylations: Then and Now. Angew. Chem. Int. Ed. 1996, 35, 1050–1066. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, Y.; Chen, H.; Wang, T.; Zhang, Z.-B.; Han, P.; Jing, L.-H. Photoredox Metal-Free Allylic Defluorinative Silylation of α-Trifluoromethylstyrenes with Hydrosilanes. Org. Lett. 2022, 24, 4286–4291. [Google Scholar] [CrossRef]
- Zhou, R.; Goh, Y.Y.; Liu, H.; Tao, H.; Li, L.; Wu, J. Visible-Light-Mediated Metal-Free Hydrosilylation of Alkenes through Selective Hydrogen Atom Transfer for Si–H Activation. Angew. Chem. Int. Ed. 2017, 56, 16621–16625. [Google Scholar] [CrossRef]
- Milligan, J.A.; Phelan, J.P.; Polites, V.C.; Kelly, C.B.; Molander, G.A. Radical/Polar Annulation Reactions (RPARs) Enable the Modular Construction of Cyclopropanes. Org. Lett. 2018, 20, 6840–6844. [Google Scholar] [CrossRef]
- Askey, H.E.; Grayson, J.D.; Tibbetts, J.D.; Turner-Dore, J.C.; Holmes, J.M.; Kociok-Kohn, G.; Wrigley, G.L.; Cresswell, A.J. Photocatalytic Hydroaminoalkylation of Styrenes with Unprotected Primary Alkylamines. J. Am. Chem. Soc. 2021, 143, 15936–15945. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guo, L.; Shi, C.; Zhu, Y.; Yang, C.; Xia, W. Transition Metal-Free Radical α-Oxy C–H Cyclobutylation via Photoinduced Hydrogen Atom Transfer. Adv. Synth. Catal. 2022, 364, 2140–2145. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, X.; Liu, R.; Wu, J. Quinuclidine and its derivatives as hydrogen-atom-transfer catalysts in photoinduced reactions. Chin. Chem. Lett. 2021, 32, 1847–1856. [Google Scholar] [CrossRef]
- Sun, T.; Jin, R.; Yang, Y.; Jia, Y.; Hu, S.; Jin, Y.; Wang, Q.; Li, Z.; Zhang, Y.; Wu, J.; et al. Direct α-C–H Alkylation of Structurally Diverse Alcohols via Combined Tavaborole and Photoredox Catalysis. Org. Lett. 2022, 24, 7637–7642. [Google Scholar] [CrossRef]
- Sakai, K.; Oisaki, K.; Kanai, M. Identification of Bond-Weakening Spirosilane Catalyst for Photoredox α-C–H Alkylation of Alcohols. Adv. Synth. Catal. 2020, 362, 337–343. [Google Scholar] [CrossRef]
- Sakai, K.; Oisaki, K.; Kanai, M. A Bond-Weakening Borinate Catalyst that Improves the Scope of the Photoredox α-C–H Alkylation of Alcohols. Synthesis 2020, 52, 2171–2189. [Google Scholar] [CrossRef]
- Dimakos, V.; Su, H.Y.; Garrett, G.E.; Taylor, M.S. Site-Selective and Stereoselective C–H Alkylations of Carbohydrates via Combined Diarylborinic Acid and Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 5149–5153. [Google Scholar] [CrossRef]
- Dimakos, V.; Gorelik, D.; Su, H.Y.; Garrett, G.E.; Hughes, G.; Shibayama, H.; Taylor, M.S. Site-selective redox isomerisations of furanosides using a combined arylboronic acid/photoredox catalyst system. Chem. Sci. 2020, 11, 1531–1537. [Google Scholar] [CrossRef]
- Merkens, K.; Sanosa, N.; Funes-Ardoiz, I.; Gómez-Suárez, A. Accessing α-Amino Ketyl Radicals from β-Amino Alcohols via Chemoselective Hydrogen Atom Transfer Catalysis. ACS Catal. 2022, 12, 13186–13192. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Ye, J.; Kalvet, I.; Schoenebeck, F.; Rovis, T. Direct α-alkylation of primary aliphatic amines enabled by CO2 and electrostatics. Nat. Chem. 2018, 10, 1037–1041. [Google Scholar] [CrossRef]
- Sakai, K.; Oisaki, K.; Kanai, M. A Germanium Catalyst Accelerates the Photoredox α-C(sp3)–H Alkylation of Primary Amines. Org. Lett. 2022, 24, 3325–3330. [Google Scholar] [CrossRef]
- Alder, R.W.; Arrowsmith, R.J.; Casson, A.; Sessions, R.B.; Heilbronner, E.; Kovac, B.; Huber, H.; Taagepera, M. Proton affinities and ionisation energies of bicyclic amines and diamines. Effects of ring strain and of 3-electron .sigma. bonding. J. Am. Chem. Soc. 1981, 103, 6137–6142. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yamamoto, M.; Maruoka, K. Cationic DABCO-Based Catalyst for Site-Selective C–H Alkylation via Photoinduced Hydrogen-Atom Transfer. ACS Catal. 2022, 12, 2045–2051. [Google Scholar] [CrossRef]
- Aguilar Troyano, F.J.; Merkens, K.; Gómez-Suárez, A. Selectfluor® Radical Dication (TEDA2+.)—A Versatile Species in Modern Synthetic Organic Chemistry. Asian J. Org. Chem. 2020, 9, 992–1007. [Google Scholar] [CrossRef]
- Xiang, M.; Xin, Z.-K.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Exploring the Reducing Ability of Organic Dye (Acr+-Mes) for Fluorination and Oxidation of Benzylic C(sp3)–H Bonds under Visible Light Irradiation. Org. Lett. 2017, 19, 3009–3012. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Liu, J.; Liang, X.-A.; Wang, S.; Lei, A. Visible light-induced direct α C–H functionalisation of alcohols. Nat. Commun. 2019, 10, 467. [Google Scholar] [CrossRef]
- Liang, X.-A.; Niu, L.; Wang, S.; Liu, J.; Lei, A. Visible-Light-Induced C(sp3)–H Oxidative Arylation with Heteroarenes. Org. Lett. 2019, 21, 2441–2444. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, J. Visible Light-Promoted Aliphatic C–H Arylation Using Selectfluor as a Hydrogen Atom Transfer Reagent. Org. Lett. 2019, 21, 6179–6184. [Google Scholar] [CrossRef]
- Danahy, K.E.; Cooper, J.C.; Van Humbeck, J.F. Benzylic Fluorination of Aza-Heterocycles Induced by Single-Electron Transfer to Selectfluor. Angew. Chem. Int. Ed. 2018, 57, 5134–5138. [Google Scholar] [CrossRef]
- Ventre, S.; Petronijevic, F.R.; MacMillan, D.W.C. Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis. J. Am. Chem. Soc. 2015, 137, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-D.; Wang, Y.; Xue, X.-S.; Cheng, J.-P. A Systematic Evaluation of the N–F Bond Strength of Electrophilic N–F Reagents: Hints for Atomic Fluorine Donating Ability. J. Org. Chem. 2017, 82, 4129–4135. [Google Scholar] [CrossRef]
- Capilato, J.N.; Pitts, C.R.; Rowshanpour, R.; Dudding, T.; Lectka, T. Site-Selective Photochemical Fluorination of Ketals: Unanticipated Outcomes in Selectivity and Stability. J. Org. Chem. 2020, 85, 2855–2864. [Google Scholar] [CrossRef]
- Ghorbani, F.; Harry, S.A.; Capilato, J.N.; Pitts, C.R.; Joram, J.; Peters, G.N.; Tovar, J.D.; Smajlagic, I.; Siegler, M.A.; Dudding, T.; et al. Carbonyl-Directed Aliphatic Fluorination: A Special Type of Hydrogen Atom Transfer Beats Out Norrish II. J. Am. Chem. Soc. 2020, 142, 14710–14724. [Google Scholar] [CrossRef] [PubMed]
- Harry, S.A.; Xiang, M.R.; Holt, E.; Zhu, A.; Ghorbani, F.; Patel, D.; Lectka, T. Hydroxy-directed fluorination of remote unactivated C(sp3)–H bonds: A new age of diastereoselective radical fluorination. Chem. Sci. 2022, 13, 7007–7013. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Wang, L.; Nicewicz, D.A. Synthesis and Characterisation of Acridinium Dyes for Photoredox Catalysis. Synlett 2019, 30, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Pangu, A.; Lévesque, F.; Roth, H.G.; Oliver, S.F.; Campeau, L.-C.; Nicewicz, D.; DiRocco, D.A. Acridinium-Based Photocatalysts: A Sustainable Option in Photoredox Catalysis. J. Org. Chem. 2016, 81, 7244–7249. [Google Scholar] [CrossRef]
- Bortolamei, N.; Isse, A.A.; Gennaro, A. Estimation of standard reduction potentials of alkyl radicals involved in atom transfer radical polymerisation. Electrochim. Acta 2010, 55, 8312–8318. [Google Scholar] [CrossRef]
- Davies, J.; Lyonnet, J.R.; Zimin, D.P.; Martin, R. The road to industrialisation of fine chemical carboxylation reactions. Chem 2021, 7, 2927–2942. [Google Scholar] [CrossRef]
- Lamy, E.; Nadjo, L.; Saveant, J.M. Standard potential and kinetic parameters of the electrochemical reduction of carbon dioxide in dimethylformamide. J. Electroanal. Chem. Interf. Electrochem. 1977, 78, 403–407. [Google Scholar] [CrossRef]
- Chmiel, A.F.; Williams, O.P.; Chernowsky, C.P.; Yeung, C.S.; Wickens, Z.K. Non-innocent Radical Ion Intermediates in Photoredox Catalysis: Parallel Reduction Modes Enable Coupling of Diverse Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 10882–10889. [Google Scholar] [CrossRef] [PubMed]
- Hendy, C.M.; Smith, G.C.; Xu, Z.; Lian, T.; Jui, N.T. Radical Chain Reduction via Carbon Dioxide Radical Anion (CO2•−). J. Am. Chem. Soc. 2021, 143, 8987–8992. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, Y.; Zhou, C.; Li, G. Visible-Light-Driven Reductive Carboarylation of Styrenes with CO2 and Aryl Halides. J. Am. Chem. Soc. 2020, 142, 8122–8129. [Google Scholar] [CrossRef] [PubMed]
- Grills, D.C.; Lymar, S.V. Radiolytic formation of the carbon dioxide radical anion in acetonitrile revealed by transient IR spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 10011–10017. [Google Scholar] [CrossRef]
- Till, N.A.; Tian, L.; Dong, Z.; Scholes, G.D.; MacMillan, D.W.C. Mechanistic Analysis of Metallaphotoredox C–N Coupling: Photocatalysis Initiates and Perpetuates Ni(I)/Ni(III) Coupling Activity. J. Am. Chem. Soc. 2020, 142, 15830–15841. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Xu, H.; Liu, Y.-Q.; Li, R.-B.; Liu, W.-W.; Wang, X.-Y.; Zou, M.-L.; Zhou, Y.; Guo, D.; et al. Dicarboxylation of Alkenes with CO2 and Formate via Photoredox Catalysis. ACS Catal. 2023, 13, 2149–2155. [Google Scholar] [CrossRef]
- Mangaonkar, S.R.; Hayashi, H.; Takano, H.; Kanna, W.; Maeda, S.; Mita, T. Photoredox/HAT-Catalyzed Dearomative Nucleophilic Addition of the CO2 Radical Anion to (Hetero)Aromatics. ACS Catal. 2023, 13, 2482–2488. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Q.; Zhu, J. Visible light photoredox-catalysed remote C–H functionalisation enabled by 1,5-hydrogen atom transfer (1,5-HAT). Chem. Soc. Rev. 2021, 50, 7359–7377. [Google Scholar] [CrossRef]
- Mao, R.; Bera, S.; Turla, A.C.; Hu, X. Copper-Catalyzed Intermolecular Functionalisation of Unactivated C(sp3)–H Bonds and Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2021, 143, 14667–14675. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Stable copper acetylacetonate-based oxidizing agents in redox (NIR photoactivated) polymerisation: An opportunity for the one pot grafting from approach and an example on a 3D printed object. Polym. Chem. 2018, 9, 2173–2182. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. A New Highly Efficient Amine-Free and Peroxide-Free Redox System for Free Radical Polymerisation under Air with Possible Light Activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
- Vasilopoulos, A.; Golden, D.L.; Buss, J.A.; Stahl, S.S. Copper-Catalyzed C–H Fluorination/Functionalisation Sequence Enabling Benzylic C–H Cross Coupling with Diverse Nucleophiles. Org. Lett. 2020, 22, 5753–5757. [Google Scholar] [CrossRef] [PubMed]
- Pitts, C.R.; Bloom, S.; Woltornist, R.; Auvenshine, D.J.; Ryzhkov, L.R.; Siegler, M.A.; Lectka, T. Direct, Catalytic Monofluorination of sp3 C–H Bonds: A Radical-Based Mechanism with Ionic Selectivity. J. Am. Chem. Soc. 2014, 136, 9780–9791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, H.; Lv, Y.; Tan, X.; Shen, H.; Yu, H.-Z.; Li, C. Radical Carbofluorination of Unactivated Alkenes with Fluoride Ions. J. Am. Chem. Soc. 2018, 140, 6169–6175. [Google Scholar] [CrossRef]
- Na, C.G.; Ravelli, D.; Alexanian, E.J. Direct Decarboxylative Functionalisation of Carboxylic Acids via O–H Hydrogen Atom Transfer. J. Am. Chem. Soc. 2020, 142, 44–49. [Google Scholar] [CrossRef]
- Quinn, R.K.; Könst, Z.A.; Michalak, S.E.; Schmidt, Y.; Szklarski, A.R.; Flores, A.R.; Nam, S.; Horne, D.A.; Vanderwal, C.D.; Alexanian, E.J. Site-Selective Aliphatic C–H Chlorination Using N-Chloroamides Enables a Synthesis of Chlorolissoclimide. J. Am. Chem. Soc. 2016, 138, 696–702. [Google Scholar] [CrossRef]
- Tierney, M.M.; Crespi, S.; Ravelli, D.; Alexanian, E.J. Identifying Amidyl Radicals for Intermolecular C–H Functionalisations. J. Org. Chem. 2019, 84, 12983–12991. [Google Scholar] [CrossRef]
- Williamson, J.B.; Na, C.G.; Johnson, R.R.; Daniel, W.F.M.; Alexanian, E.J.; Leibfarth, F.A. Chemo- and Regioselective Functionalisation of Isotactic Polypropylene: A Mechanistic and Structure–Property Study. J. Am. Chem. Soc. 2019, 141, 12815–12823. [Google Scholar] [CrossRef]
- Schmidt, V.A.; Quinn, R.K.; Brusoe, A.T.; Alexanian, E.J. Site-Selective Aliphatic C–H Bromination Using N-Bromoamides and Visible Light. J. Am. Chem. Soc. 2014, 136, 14389–14392. [Google Scholar] [CrossRef]
- Liang, L.; Guo, G.; Li, C.; Wang, S.-L.; Wang, Y.-H.; Guo, H.-M.; Niu, H.-Y. Copper-Catalyzed Intermolecular Alkynylation and Allylation of Unactivated C(sp3)–H Bonds via Hydrogen Atom Transfer. Org. Lett. 2021, 23, 8575–8579. [Google Scholar] [CrossRef]
- Fazekas, T.J.; Alty, J.W.; Neidhart, E.K.; Miller, A.S.; Leibfarth, F.A.; Alexanian, E.J. Diversification of aliphatic C-H bonds in small molecules and polyolefins through radical chain transfer. Science 2022, 375, 545–550. [Google Scholar] [CrossRef]
- Carestia, A.M.; Ravelli, D.; Alexanian, E.J. Reagent-dictated site selectivity in intermolecular aliphatic C–H functionalisations using nitrogen-centered radicals. Chem. Sci. 2018, 9, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.S.; Alexanian, E.J. Heteroarylation of unactivated C–H bonds suitable for late-stage functionalisation. Chem. Sci. 2022, 13, 11878–11882. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, P.; Xu, J.; Ma, W.; Tu, D.; Lu, C.-S.; Yan, H. Direct B–H Functionalisation of Icosahedral Carboranes via Hydrogen Atom Transfer. J. Am. Chem. Soc. 2023, 145, 7638–7647. [Google Scholar] [CrossRef] [PubMed]
- Ohmatsu, K.; Suzuki, R.; Furukawa, Y.; Sato, M.; Ooi, T. Zwitterionic 1,2,3-Triazolium Amidate as a Catalyst for Photoinduced Hydrogen-Atom Transfer Radical Alkylation. ACS Catal. 2020, 10, 2627–2632. [Google Scholar] [CrossRef]
- Czaplyski, W.L.; Na, C.G.; Alexanian, E.J. C–H Xanthylation: A Synthetic Platform for Alkane Functionalisation. J. Am. Chem. Soc. 2016, 138, 13854–13857. [Google Scholar] [CrossRef]
- Minami, K.; Ohmatsu, K.; Ooi, T. Hydrogen-Atom-Transfer-Mediated Acceptorless Dehydrogenative Cross-Coupling Enabled by Multiple Catalytic Functions of Zwitterionic Triazolium Amidate. ACS Catal. 2022, 12, 1971–1976. [Google Scholar] [CrossRef]
- Ohmatsu, K.; Fujita, H.; Suzuki, R.; Ooi, T. Hydrogen-Atom Transfer Catalysis for C–H Alkylation of Benzylic Fluorides. Org. Lett. 2022, 24, 3134–3137. [Google Scholar] [CrossRef]
- Ohmatsu, K.; Suzuki, R.; Fujita, H.; Ooi, T. Zwitterionic Diphenylphosphinyl Amidate as a Powerful Photoinduced Hydrogen-Atom-Transfer Catalyst for C–H Alkylation of Simple Alkanes. J. Org. Chem. 2023, 88, 6553–6556. [Google Scholar] [CrossRef]
- Shee, M.; Singh, N.D.P. Chemical versatility of azide radical: Journey from a transient species to synthetic accessibility in organic transformations. Chem. Soc. Rev. 2022, 51, 2255–2312. [Google Scholar] [CrossRef]
- Constantin, T.; Górski, B.; Tilby, M.J.; Chelli, S.; Juliá, F.; Llaveria, J.; Gillen, K.J.; Zipse, H.; Lakhdar, S.; Leonori, D. Halogen-atom and group transfer reactivity enabled by hydrogen tunneling. Science 2022, 377, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Groves, J.T. Taming Azide Radicals for Catalytic C–H Azidation. ACS Catal. 2016, 6, 751–759. [Google Scholar] [CrossRef]
- Ryder, A.S.H.; Cunningham, W.B.; Ballantyne, G.; Mules, T.; Kinsella, A.G.; Turner-Dore, J.; Alder, C.M.; Edwards, L.J.; McKay, B.S.J.; Grayson, M.N.; et al. Photocatalytic α-Tertiary Amine Synthesis via C–H Alkylation of Unmasked Primary Amines. Angew. Chem. Int. Ed. 2020, 59, 14986–14991. [Google Scholar] [CrossRef]
- Grayson, J.D.; Cresswell, A.J. γ-Amino phosphonates via the photocatalytic α-C–H alkylation of primary amines. Tetrahedron 2021, 81, 131896. [Google Scholar] [CrossRef]
- Sim, J.; Ryou, B.; Choi, M.; Lee, C.; Park, C.-M. Electrochemical C(sp3)–H Functionalisation of γ-Lactams Based on Hydrogen Atom Transfer. Org. Lett. 2022, 24, 4264–4269. [Google Scholar] [CrossRef]
- Wiss, J.; Fleury, C.; Onken, U. Safety Improvement of Chemical Processes Involving Azides by Online Monitoring of the Hydrazoic Acid Concentration. Org. Process Res. Dev. 2006, 10, 349–353. [Google Scholar] [CrossRef]
- Sneha, M.; Thornton, G.L.; Lewis-Borrell, L.; Ryder, A.S.H.; Espley, S.G.; Clark, I.P.; Cresswell, A.J.; Grayson, M.N.; Orr-Ewing, A.J. Photoredox-HAT Catalysis for Primary Amine α-C–H Alkylation: Mechanistic Insight with Transient Absorption Spectroscopy. ACS Catal. 2023, 13, 8004–8013. [Google Scholar] [CrossRef]
- Chinn, A.J.; Sedillo, K.; Doyle, A.G. Phosphine/Photoredox Catalyzed Anti-Markovnikov Hydroamination of Olefins with Primary Sulfonamides via α-Scission from Phosphoranyl Radicals. J. Am. Chem. Soc. 2021, 143, 18331–18338. [Google Scholar] [CrossRef]
- Guo, X.; Wenger, O.S. Reductive Amination by Photoredox Catalysis and Polarity-Matched Hydrogen Atom Transfer. Angew. Chem. Int. Ed. 2018, 57, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.-W.; Yang, L.; Wang, D.-Y.; Pu, C.-D.; Shen, Y.-M.; Wu, C.-D.; Peng, X.-G. Visible-Light Photocatalytic Synthesis of Amines from Imines via Transfer Hydrogenation Using Quantum Dots as Catalysts. J. Org. Chem. 2018, 83, 11886–11895. [Google Scholar] [CrossRef]
- Yue, W.-J.; Day, C.S.; Brenes Rucinski, A.J.; Martin, R. Catalytic Hydrodifluoroalkylation of Unactivated Olefins. Org. Lett. 2022, 24, 5109–5114. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, L.; Ji, Y.; Zou, G.; Shen, H.; Nicewicz, D.A.; Chen, J.; Huang, Y. Direct Synthesis of Bicyclic Acetals via Visible Light Catalysis. iScience 2020, 23, 101395. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Mendoza, A. Modular Enantioselective Synthesis of cis-Cyclopropanes through Self-Sensitised Stereoselective Photodecarboxylation with Benzothiazolines. ACS Catal. 2021, 11, 13312–13319. [Google Scholar] [CrossRef] [PubMed]
- Qvortrup, K.; Rankic, D.A.; MacMillan, D.W.C. A General Strategy for Organocatalytic Activation of C–H Bonds via Photoredox Catalysis: Direct Arylation of Benzylic Ethers. J. Am. Chem. Soc. 2014, 136, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Jencks, W.P.; Salvesen, K. Equilibrium deuterium isotope effects on the ionisation of thiol acids. J. Am. Chem. Soc. 1971, 93, 4433–4436. [Google Scholar] [CrossRef]
- Studer, A. The Persistent Radical Effect in Organic Synthesis. Chem. Eur. J. 2001, 7, 1159–1164. [Google Scholar] [CrossRef]
- Kobayashi, F.; Fujita, M.; Ide, T.; Ito, Y.; Yamashita, K.; Egami, H.; Hamashima, Y. Dual-Role Catalysis by Thiobenzoic Acid in Cα–H Arylation under Photoirradiation. ACS Catal. 2021, 11, 82–87. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Cao, K.; Zhang, X.; Jiang, H.; Li, J. Synthetic reactions driven by electron-donor-acceptor (EDA) complexes. Beilstein J. Org. Chem. 2021, 17, 771–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lim, C.-H.; Miyake, G.M. Visible-Light-Promoted C–S Cross-Coupling via Intermolecular Charge Transfer. J. Am. Chem. Soc. 2017, 139, 13616–13619. [Google Scholar] [CrossRef]
- Vu, M.D.; Das, M.; Guo, A.; Ang, Z.-E.; D̵okić, M.; Soo, H.S.; Liu, X.-W. Visible-Light Photoredox Enables Ketone Carbonyl Alkylation for Easy Access to Tertiary Alcohols. ACS Catal. 2019, 9, 9009–9014. [Google Scholar] [CrossRef]
- Walczyk, K.R.; Popkirov, G.S.; Schindler, R.N. Investigation of the Redox Couple Benzophenone/Benzophenone Anion Radical in Acetonitrile and N,N-Dimethylformamide by Electrochemical and Spectroelectrochemical Methods. Bunsenges. Phys. Chem. 1995, 99, 1028–1036. [Google Scholar] [CrossRef]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Photochemistry and Photophysics of Coordination Compounds: Iridium. In Photochemistry and Photophysics of Coordination Compounds II; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; p. 193. [Google Scholar]
- Zhou, R.; Li, J.; Cheo, H.W.; Chua, R.; Zhan, G.; Hou, Z.; Wu, J. Visible-light-mediated deuteration of silanes with deuterium oxide. Chem. Sci. 2019, 10, 7340–7344. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.; Luo, H.; Feng, Y.; Zhang, J.; Lin, L. Light-Promoted Arylsilylation of Alkenes with Hydrosilanes. Org. Lett. 2022, 24, 7145–7150. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Chiba, S. Leveraging of Sulfur Anions in Photoinduced Molecular Transformations. JACS Au 2021, 1, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, C.; Wang, Y.; Huang, X.; Zhao, X.; Qiao, B.; Jiang, Z. Catalytic Asymmetric Reductive Azaarylation of Olefins via Enantioselective Radical Coupling. J. Am. Chem. Soc. 2022, 144, 7805–7814. [Google Scholar] [CrossRef] [PubMed]
- Queen, A.E.; Selmani, A.; Schoenebeck, F. Hydrogermylation of Alkenes via Organophotoredox-Initiated HAT Catalysis. Org. Lett. 2022, 24, 406–409. [Google Scholar] [CrossRef]
- Fricke, C.; Schoenebeck, F. Organogermanes as Orthogonal Coupling Partners in Synthesis and Catalysis. Acc. Chem. Res. 2020, 53, 2715–2725. [Google Scholar] [CrossRef]
- Jia, J.; Kancherla, R.; Rueping, M.; Huang, L. Allylic C(sp3)–H alkylation via synergistic organo- and photoredox catalyzed radical addition to imines. Chem. Sci. 2020, 11, 4954–4959. [Google Scholar] [CrossRef]
- Nakashima, T.; Ohmatsu, K.; Ooi, T. Mannich-type allylic C–H functionalisation of enol silyl ethers under photoredox–thiol hybrid catalysis. Org. Biomol. Chem. 2021, 19, 141–145. [Google Scholar] [CrossRef]
- Shen, Y.; Rovis, T. Late-Stage N-Me Selective Arylation of Trialkylamines Enabled by Ni/Photoredox Dual Catalysis. J. Am. Chem. Soc. 2021, 143, 16364–16369. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Barham, J.P. Site-Selective C(sp3)–H Functionalisations Mediated by Hydrogen Atom Transfer Reactions via α-Amino/α-Amido Radicals. Synthesis 2022, 54, 1461–1477. [Google Scholar]
- Zhang, Y.-A.; Gu, X.; Wendlandt, A.E. A Change from Kinetic to Thermodynamic Control Enables trans-Selective Stereochemical Editing of Vicinal Diols. J. Am. Chem. Soc. 2022, 144, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Escoubet, S.; Gastaldi, S.; Vanthuyne, N.; Gil, G.; Siri, D.; Bertrand, M.P. Thiyl Radical Mediated Racemisation of Benzylic Amines. Eur. J. Org. Chem. 2006, 2006, 3242–3250. [Google Scholar] [CrossRef]
- Panferova, L.I.; Zubkov, M.O.; Kokorekin, V.A.; Levin, V.V.; Dilman, A.D. Using the Thiyl Radical for Aliphatic Hydrogen-Atom Transfer: Thiolation of Unactivated C–H Bonds. Angew. Chem. Int. Ed. 2021, 60, 2849–2854. [Google Scholar] [CrossRef]
- Wang, H.; Jui, N.T. Catalytic Defluoroalkylation of Trifluoromethylaromatics with Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 163–166. [Google Scholar] [CrossRef]
- Vogt, D.B.; Seath, C.P.; Wang, H.; Jui, N.T. Selective C–F Functionalisation of Unactivated Trifluoromethylarenes. J. Am. Chem. Soc. 2019, 141, 13203–13211. [Google Scholar] [CrossRef]
- Jacobsen, E.; Roberts, J.L.; Sawyer, D.T. Electrochemical oxidation of formate in dimethylsulfoxide at gold and platinum electrodes. J. Electroanal. Chem. Interf. Electrochem. 1968, 16, 351–360. [Google Scholar] [CrossRef]
- Alektiar, S.N.; Wickens, Z.K. Photoinduced Hydrocarboxylation via Thiol-Catalyzed Delivery of Formate Across Activated Alkenes. J. Am. Chem. Soc. 2021, 143, 13022–13028. [Google Scholar] [CrossRef]
- Xu, P.; Wang, X.-Y.; Wang, Z.; Zhao, J.; Cao, X.-D.; Xiong, X.-C.; Yuan, Y.-C.; Zhu, S.; Guo, D.; Zhu, X. Defluorinative Alkylation of Trifluoromethylbenzimidazoles Enabled by Spin-Center Shift: A Synergistic Photocatalysis/Thiol Catalysis Process with CO2•−. Org. Lett. 2022, 24, 4075–4080. [Google Scholar] [CrossRef]
- Alektiar, S.N.; Han, J.; Dang, Y.; Rubel, C.Z.; Wickens, Z.K. Radical Hydrocarboxylation of Unactivated Alkenes via Photocatalytic Formate Activation. J. Am. Chem. Soc. 2023, 145, 10991–10997. [Google Scholar] [CrossRef]
- Williams, O.P.; Chmiel, A.F.; Mikhael, M.; Bates, D.M.; Yeung, C.S.; Wickens, Z.K. Practical and General Alcohol Deoxygenation Protocol. Angew. Chem. Int. Ed. 2023, 62, e202300178. [Google Scholar] [CrossRef]
- Mikhael, M.; Alektiar, S.N.; Yeung, C.S.; Wickens, Z.K. Translating planar heterocycles into three-dimensional analogs via photoinduced hydrocarboxylation. Angew. Chem. Int. Ed. 2023, 62, e202303264. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.W.; Polites, V.C.; Patel, S.; Lipson, J.E.; Majhi, J.; Molander, G.A. Photochemical C–F Activation Enables Defluorinative Alkylation of Trifluoroacetates and -Acetamides. J. Am. Chem. Soc. 2021, 143, 19648–19654. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, B.; Majhi, J.; Granados, A.; Sharique, M.; Martin, R.T.; Gutierrez, O.; Molander, G.A. Transition metal-free photochemical C–F activation for the preparation of difluorinated-oxindole derivatives. Chem. Sci. 2023, 14, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-H.; Bellotti, P.; Heusel, C.; Glorius, F. Photoredox-Catalyzed Defluorinative Functionalisations of Polyfluorinated Aliphatic Amides and Esters. Angew. Chem. Int. Ed. 2022, 61, e202115456. [Google Scholar] [CrossRef] [PubMed]
- Fuse, H.; Mitsunuma, H.; Kanai, M. Catalytic Acceptorless Dehydrogenation of Aliphatic Alcohols. J. Am. Chem. Soc. 2020, 142, 4493–4499. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Mitsunuma, H.; Kanai, M. Catalytic Allylation of Aldehydes Using Unactivated Alkenes. J. Am. Chem. Soc. 2020, 142, 12374–12381. [Google Scholar] [CrossRef]
- Margrey, K.A.; Nicewicz, D.A. A General Approach to Catalytic Alkene Anti-Markovnikov Hydrofunctionalisation Reactions via Acridinium Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1997–2006. [Google Scholar] [CrossRef]
- Fuse, H.; Nakao, H.; Saga, Y.; Fukatsu, A.; Kondo, M.; Masaoka, S.; Mitsunuma, H.; Kanai, M. Photocatalytic redox-neutral hydroxyalkylation of N-heteroaromatics with aldehydes. Chem. Sci. 2020, 11, 12206–12211. [Google Scholar] [CrossRef]
- Jin, J.; MacMillan, D.W. Alcohols as alkylating agents in heteroarene C-H functionalisation. Nature. 2015, 525, 87–90. [Google Scholar] [CrossRef]
- Ji, X.; Liu, Q.; Wang, Z.; Wang, P.; Deng, G.-J.; Huang, H. LiBr-promoted photoredox neutral Minisci hydroxyalkylations of quinolines with aldehydes. Green Chem. 2020, 22, 8233–8237. [Google Scholar] [CrossRef]
- Bieszczad, B.; Perego, L.A.; Melchiorre, P. Photochemical C–H Hydroxyalkylation of Quinolines and Isoquinolines. Angew. Chem. Int. Ed. 2019, 58, 16878–16883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-L.; Li, B.; Houk, K.N.; Wang, Y.-F. Application of the Spin-Center Shift in Organic Synthesis. JACS Au 2022, 2, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Bellotti, P.; Chen, P.-P.; Houk, K.N.; Glorius, F. Allylic C(sp3)–H arylation of olefins via ternary catalysis. Nat. Synth. 2022, 1, 59–68. [Google Scholar] [CrossRef]
- Berman, R.S.; Kochi, J.K. Kinetics and mechanism of oxygen atom transfer from nitro compounds mediated by nickel(0) complexes. Inorg. Chem. 1980, 19, 248–254. [Google Scholar] [CrossRef]
- Percec, V.; Bae, J.-Y.; Zhao, M.; Hill, D.H. Aryl Mesylates in Metal-Catalyzed Homocoupling and Cross-Coupling Reactions. 1. Functional Symmetrical Biaryls from Phenols via Nickel-Catalyzed Homocoupling of Their Mesylates. J. Org. Chem. 1995, 60, 176–185. [Google Scholar] [CrossRef]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.-M.; Garg, N.K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon–Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef]
- Ting, S.I.; Williams, W.L.; Doyle, A.G. Oxidative Addition of Aryl Halides to a Ni(I)-Bipyridine Complex. J. Am. Chem. Soc. 2022, 144, 5575–5582. [Google Scholar] [CrossRef]
- McManus, J.B.; Griffin, J.D.; White, A.R.; Nicewicz, D.A. Homobenzylic Oxygenation Enabled by Dual Organic Photoredox and Cobalt Catalysis. J. Am. Chem. Soc. 2020, 142, 10325–10330. [Google Scholar] [CrossRef]
- Fischer, C.; Kerzig, C.; Zilate, B.; Wenger, O.S.; Sparr, C. Modulation of Acridinium Organophotoredox Catalysts Guided by Photophysical Studies. ACS Catal. 2020, 10, 210–215. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, E.; Zanini, M.; Melchiorre, P. Photochemical Organocatalytic Benzylation of Allylic C–H Bonds. J. Am. Chem. Soc. 2022, 144, 1113–1118. [Google Scholar] [CrossRef]
- Correia, J.T.M.; Fernandes, V.A.; Matsuo, B.T.; Delgado, J.A.C.; de Souza, W.C.; Paixão, M.W. Photoinduced deaminative strategies: Katritzky salts as alkyl radical precursors. Chem. Commun. 2020, 56, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Fuse, H.; Irie, Y.; Fuki, M.; Kobori, Y.; Kato, K.; Yamakata, A.; Higashi, M.; Mitsunuma, H.; Kanai, M. Identification of a Self-Photosensitizing Hydrogen Atom Transfer Organocatalyst System. J. Am. Chem. Soc. 2022, 144, 6566–6574. [Google Scholar] [CrossRef]
- Le Saux, E.; Georgiou, E.; Dmitriev, I.A.; Hartley, W.C.; Melchiorre, P. Photochemical Organocatalytic Functionalisation of Pyridines via Pyridinyl Radicals. J. Am. Chem. Soc. 2023, 145, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Li, J.; Liu, W.; Li, C.-J. Diacetyl as a “traceless” visible light photosensitiser in metal-free cross-dehydrogenative coupling reactions. Chem. Sci. 2019, 10, 5018–5024. [Google Scholar] [CrossRef]
- Proctor, R.S.J.; Chuentragool, P.; Colgan, A.C.; Phipps, R.J. Hydrogen Atom Transfer-Driven Enantioselective Minisci Reaction of Amides. J. Am. Chem. Soc. 2021, 143, 4928–4934. [Google Scholar] [CrossRef]
- Wang, B.; Ascenzi Pettenuzzo, C.; Singh, J.; McCabe, G.E.; Clark, L.; Young, R.; Pu, J.; Deng, Y. Photoinduced Site-Selective Functionalisation of Aliphatic C–H Bonds by Pyridine N-oxide Based HAT Catalysts. ACS Catal. 2022, 12, 10441–10448. [Google Scholar] [CrossRef]
- Schlegel, M.; Qian, S.; Nicewicz, D.A. Aliphatic C–H Functionalisation Using Pyridine N-Oxides as H-Atom Abstraction Agents. ACS Catal. 2022, 12, 10499–10505. [Google Scholar] [CrossRef]
- Markham, J.P.; Wang, B.; Stevens, E.D.; Burris, S.C.; Deng, Y. ortho-Alkylation of Pyridine N-Oxides with Alkynes by Photocatalysis: Pyridine N-Oxide as a Redox Auxiliary. Chem. Eur. J. 2019, 25, 6638–6644. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.; Bankhead, B.; Markham, J.P.; Zeller, M. Photoinduced oxidative cyclopropanation of ene-ynamides: Synthesis of 3-aza[n.1.0]bicycles via vinyl radicals. Chem. Commun. 2021, 57, 5254–5257. [Google Scholar] [CrossRef]
- Xu, J.-h.; Wu, W.-b.; Wu, J. Photoinduced Divergent Alkylation/Acylation of Pyridine N-Oxides with Alkynes under Anaerobic and Aerobic Conditions. Org. Lett. 2019, 21, 5321–5325. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, F.; Zhang, M.-T. Metal-Free Electrocatalyst for Water Oxidation Initiated by Hydrogen Atom Transfer. ACS Catal. 2021, 11, 68–73. [Google Scholar] [CrossRef]
- Chmurzynski, L.; Warnke, Z. Acid-Base Equilibria of Substituted Pyridine N-Oxides in N,N-Dimethylformamide and Dimethyl Sulfoxide. Aust. J. Chem. 1993, 46, 185–194. [Google Scholar] [CrossRef]
- Ciszewski, Ł.W.; Gryko, D. Pyridine N-oxides as HAT reagents for photochemical C–H functionalisation of electron-deficient heteroarenes. Chem. Commun. 2022, 58, 10576–10579. [Google Scholar] [CrossRef]
- Katsuki, T.; Martin, V. Asymmetric Epoxidation of Allylic Alcohols: The Katsuki–Sharpless Epoxidation Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 4–5. [Google Scholar]
- Martin, V.S.; Woodard, S.S.; Katsuki, T.; Yamada, Y.; Ikeda, M.; Sharpless, K.B. Kinetic resolution of racemic allylic alcohols by enantioselective epoxidation. A route to substances of absolute enantiomeric purity? J. Am. Chem. Soc. 1981, 103, 6237–6240. [Google Scholar] [CrossRef]
- Rossiter, B.E.; Katsuki, T.; Sharpless, K.B. Asymmetric epoxidation provides shortest routes to four chiral epoxy alcohols which are key intermediates in syntheses of methymycin, erythromycin, leukotriene C-1, and disparlure. J. Am. Chem. Soc. 1981, 103, 464–465. [Google Scholar] [CrossRef]
- Goor, G.; Glenneberg, J.; Jacobi, S.; Dadabhoy, J.; Candido, E. Hydrogen Peroxide. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 397–411. [Google Scholar]
- Vasilopoulos, A.; Krska, S.W.; Stahl, S.S. C(sp(3))–H methylation enabled by peroxide photosensitisation and Ni-mediated radical coupling. Science 2021, 372, 398–403. [Google Scholar] [CrossRef]
- Murakami, M.; Ishida, N. β-Scission of Alkoxy Radicals in Synthetic Transformations. Chem. Lett. 2017, 46, 1692–1700. [Google Scholar] [CrossRef]
- Avila, D.V.; Brown, C.E.; Ingold, K.U.; Lusztyk, J. Solvent effects on the competitive .beta.-scission and hydrogen atom abstraction reactions of the cumyloxyl radical. Resolution of a long-standing problem. J. Am. Chem. Soc. 1993, 115, 466–470. [Google Scholar] [CrossRef]
- Baciocchi, E.; Bietti, M.; Salamone, M.; Steenken, S. Spectral Properties and Absolute Rate Constants for β-Scission of Ring-Substituted Cumyloxyl Radicals. A Laser Flash Photolysis Study. J. Org. Chem. 2002, 67, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Kümmerle, A.E.; Fraga, C.A. The methylation effect in medicinal chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fu, J. Methyl-containing pharmaceuticals: Methylation in drug design. Bioorg. Med. Chem. Lett. 2018, 28, 3283–3289. [Google Scholar] [CrossRef]
- Colgan, A.C.; Proctor, R.S.J.; Gibson, D.C.; Chuentragool, P.; Lahdenperä, A.S.K.; Ermanis, K.; Phipps, R.J. Hydrogen Atom Transfer Driven Enantioselective Minisci Reaction of Alcohols. Angew. Chem. Int. Ed. 2022, 61, e202200266. [Google Scholar] [CrossRef]
- Proctor, R.S.J.; Davis, H.J.; Phipps, R.J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 2018, 360, 419–422. [Google Scholar] [CrossRef]
- Chen, X.; Lian, Z.; Kramer, S. Enantioselective Intermolecular Radical Amidation and Amination of Benzylic C–H Bonds via Dual Copper and Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202217638. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Y.-Y.; Xiao, L.-J.; Zhou, Q.-L. Intermolecular Enantioselective Benzylic C(sp3)–H Amination by Cationic Copper Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202304427. [Google Scholar] [CrossRef]
- Golden, D.L.; Zhang, C.; Chen, S.-J.; Vasilopoulos, A.; Guzei, I.A.; Stahl, S.S. Benzylic C–H Esterification with Limiting C–H Substrate Enabled by Photochemical Redox Buffering of the Cu Catalyst. J. Am. Chem. Soc. 2023, 145, 9434–9440. [Google Scholar] [CrossRef]
- Su, Y.-L.; Dong, K.; Zheng, H.; Doyle, M.P. Generation of Diazomethyl Radicals by Hydrogen Atom Abstraction and Their Cycloaddition with Alkenes. Angew. Chem. Int. Ed. 2021, 60, 18484–18488. [Google Scholar] [CrossRef]
- Das, M.; Zamani, L.; Bratcher, C.; Musacchio, P.Z. Azolation of Benzylic C–H Bonds via Photoredox-Catalyzed Carbocation Generation. J. Am. Chem. Soc. 2023, 145, 3861–3868. [Google Scholar] [CrossRef]
- Barthelemy, A.-L.; Tuccio, B.; Magnier, E.; Dagousset, G. Alkoxyl Radicals Generated under Photoredox Catalysis: A Strategy for anti-Markovnikov Alkoxylation Reactions. Angew. Chem. Int. Ed. 2018, 57, 13790–13794. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Q.; Zhu, J. Remote C(sp3)–H Arylation and Vinylation of N-Alkoxypyridinium Salts to δ-Aryl and δ-Vinyl Alcohols. Chem. Eur. J. 2019, 25, 11630–11634. [Google Scholar] [CrossRef] [PubMed]
- Rammal, F.; Gao, D.; Boujnah, S.; Hussein, A.A.; Lalevée, J.; Gaumont, A.-C.; Morlet-Savary, F.; Lakhdar, S. Photochemical C–H Silylation and Hydroxymethylation of Pyridines and Related Structures: Synthetic Scope and Mechanisms. ACS Catal. 2020, 10, 13710–13717. [Google Scholar] [CrossRef]
- Kim, I.; Min, M.; Kang, D.; Kim, K.; Hong, S. Direct Phosphonation of Quinolinones and Coumarins Driven by the Photochemical Activity of Substrates and Products. Org. Lett. 2017, 19, 1394–1397. [Google Scholar] [CrossRef]
- Zheng, M.; Hou, J.; Zhan, L.-W.; Huang, Y.; Chen, L.; Hua, L.-L.; Li, Y.; Tang, W.-Y.; Li, B.-D. Visible-Light-Driven, Metal-Free Divergent Difunctionalisation of Alkenes Using Alkyl Formates. ACS Catal. 2021, 11, 542–553. [Google Scholar] [CrossRef]
- Kim, I.; Park, B.; Kang, G.; Kim, J.; Jung, H.; Lee, H.; Baik, M.-H.; Hong, S. Visible-Light-Induced Pyridylation of Remote C(sp3)–H Bonds by Radical Translocation of N-Alkoxypyridinium Salts. Angew. Chem. Int. Ed. 2018, 57, 15517–15522. [Google Scholar] [CrossRef]
- Kim, I.; Kang, G.; Lee, K.; Park, B.; Kang, D.; Jung, H.; He, Y.-T.; Baik, M.-H.; Hong, S. Site-Selective Functionalisation of Pyridinium Derivatives via Visible-Light-Driven Photocatalysis with Quinolinone. J. Am. Chem. Soc. 2019, 141, 9239–9248. [Google Scholar] [CrossRef]
- Fitzpatrick, N.A.; Zamani, L.; Das, M.; Yayla, H.G.; Lall, M.S.; Musacchio, P.Z. A SN1 mechanistic approach to the Williamson ether reaction via photoredox catalysis applied to benzylic C(sp3)–H bonds. Tetrahedron 2022, 125, 132986. [Google Scholar] [CrossRef]
- Zhang, Y.; Fitzpatrick, N.A.; Das, M.; Bedre, I.P.; Yayla, H.G.; Lall, M.S.; Musacchio, P.Z. A photoredox-catalyzed approach for formal hydride abstraction to enable Csp3–H functionalisation with nucleophilic partners (F, C, O, N, and Br/Cl). Chem. Catal. 2022, 2, 292–308. [Google Scholar] [CrossRef]
- Fan, L.-F.; Liu, R.; Ruan, X.-Y.; Wang, P.-S.; Gong, L.-Z. Asymmetric 1,2-oxidative alkylation of conjugated dienes via aliphatic C–H bond activation. Nat. Synth. 2022, 1, 946–955. [Google Scholar] [CrossRef]
- Citterio, A.; Arnoldi, A.; Minisci, F. Nucleophilic character of alkyl radicals. 18. Absolute rate constants for the addition of primary alkyl radicals to conjugated olefins and 1,4-benzoquinone. J. Org. Chem. 1979, 44, 2674–2682. [Google Scholar] [CrossRef]
- Jin, J.; MacMillan, D.W.C. Direct α-Arylation of Ethers through the Combination of Photoredox-Mediated C–H Functionalisation and the Minisci Reaction. Angew. Chem. Int. Ed. 2015, 54, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- McCallum, T.; Jouanno, L.-A.; Cannillo, A.; Barriault, L. Persulfate-Enabled Direct C–H Alkylation of Heteroarenes with Unactivated Ethers. Synlett 2016, 27, 1282–1286. [Google Scholar]
- Ueda, M.; Kamikawa, K.; Fukuyama, T.; Wang, Y.-T.; Wu, Y.-K.; Ryu, I. Site-Selective Alkenylation of Unactivated C(sp3)–H Bonds Mediated by Compact Sulfate Radical. Angew. Chem. Int. Ed. 2021, 60, 3545–3550. [Google Scholar] [CrossRef]
- Kim, J.H.; Constantin, T.; Simonetti, M.; Llaveria, J.; Sheikh, N.S.; Leonori, D. A radical approach for the selective C–H borylation of azines. Nature 2021, 595, 677–683. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Hu, G.-W.; Luan, Y.-X. Development of N-Hydroxy Catalysts for C–H Functionalisation via Hydrogen Atom Transfer: Challenges and Opportunities. ACS Catal. 2022, 12, 11716–11733. [Google Scholar] [CrossRef]
- Weidmann, V.; Maison, W. Allylic Oxidations of Olefins to Enones. Synthesis 2013, 45, 2201–2221. [Google Scholar]
- Recupero, F.; Punta, C. Free Radical Functionalisation of Organic Compounds Catalyzed by N-Hydroxyphthalimide. Chem. Rev. 2007, 107, 3800–3842. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F.; Minisci, F.; Recupero, F.; Fontana, F.; Astolfi, P.; Greci, L. Hydroxylamines as Oxidation Catalysts: Thermochemical and Kinetic Studies. J. Org. Chem. 2003, 68, 1747–1754. [Google Scholar] [CrossRef]
- Nutting, J.E.; Rafiee, M.; Stahl, S.S. Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions. Chem. Rev. 2018, 118, 4834–4885. [Google Scholar] [CrossRef]
- Ueda, C.; Noyama, M.; Ohmori, H.; Masui, M. Reactivity of Phthalimide-N--oxyl: A Kinetic Study. Chem. Pharm. Bull. 1987, 35, 1372–1377. [Google Scholar] [CrossRef]
- Hoque, M.A.; Twilton, J.; Zhu, J.; Graaf, M.D.; Harper, K.C.; Tuca, E.; DiLabio, G.A.; Stahl, S.S. Electrochemical PINOylation of Methylarenes: Improving the Scope and Utility of Benzylic Oxidation through Mediated Electrolysis. J. Am. Chem. Soc. 2022, 144, 15295–15302. [Google Scholar] [CrossRef] [PubMed]
- Sanyo, H. Effects of Cyclodextrins on the Fluorescence of Diphenyl Phosphate in Aqueous Solution. Bull. Chem. Soc. Jpn. 1986, 59, 2979–2982. [Google Scholar] [CrossRef]
- Wakaki, T.; Sakai, K.; Enomoto, T.; Kondo, M.; Masaoka, S.; Oisaki, K.; Kanai, M. C(sp3)–H Cyanation Promoted by Visible-Light Photoredox/Phosphate Hybrid Catalysis. Chem. Eur. J. 2018, 24, 8051–8055. [Google Scholar] [CrossRef]
- Li, D.-S.; Liu, T.; Hong, Y.; Cao, C.-L.; Wu, J.; Deng, H.-P. Stop-Flow Microtubing Reactor-Assisted Visible Light-Induced Hydrogen-Evolution Cross Coupling of Heteroarenes with C(sp3)–H Bonds. ACS Catal. 2022, 12, 4473–4480. [Google Scholar] [CrossRef]
- Hamilton, D.S.; Nicewicz, D.A. Direct Catalytic Anti-Markovnikov Hydroetherification of Alkenols. J. Am. Chem. Soc. 2012, 134, 18577–18580. [Google Scholar] [CrossRef]
- Sarkar, S.; Cheung, K.P.S.; Gevorgyan, V. C–H functionalisation reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci. 2020, 11, 12974–12993. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Wang, C.-S.; Dixneuf, P.H.; Soulé, J.-F. Photoredox Catalysis for Building C–C Bonds from C(sp2)–H Bonds. Chem. Rev. 2018, 118, 7532–7585. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Reckenthäler, M.; Griesbeck, A.G. Photoredox Catalysis for Organic Syntheses. Adv. Synth. Catal. 2013, 355, 2727–2744. [Google Scholar] [CrossRef]
- De Vleeschouwer, F.; Van Speybroeck, V.; Waroquier, M.; Geerlings, P.; De Proft, F. Electrophilicity and Nucleophilicity Index for Radicals. Org. Lett. 2007, 9, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Kubisiak, M.; Zelga, K.; Bury, W.; Justyniak, I.; Budny-Godlewski, K.; Ochal, Z.; Lewiński, J. Development of zinc alkyl/air systems as radical initiators for organic reactions. Chem. Sci. 2015, 6, 3102–3108. [Google Scholar] [CrossRef]
- Akindele, T.; Yamada, K.-i.; Tomioka, K. Dimethylzinc-Initiated Radical Reactions. Acc. Chem. Res. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- Clerici, A.; Cannella, R.; Panzeri, W.; Pastori, N.; Regolini, E.; Porta, O. TiCl3/PhN2+-mediated radical addition of ethers to aldimines generated in situ under aqueous conditions. Tetrahedron Lett. 2005, 46, 8351–8354. [Google Scholar] [CrossRef]
- Ye, B.; Zhao, J.; Zhao, K.; McKenna, J.M.; Toste, F.D. Chiral Diaryliodonium Phosphate Enables Light Driven Diastereoselective α-C(sp3)–H Acetalisation. J. Am. Chem. Soc. 2018, 140, 8350–8356. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Deng, G.; Wei, W.; Feng, P.; Zi, Q.; Li, T.; Zhang, H.; Yang, X.; Walsh, P.J. Transition-metal-free C(sp3)–H/C(sp3)–H dehydrogenative coupling of saturated heterocycles with N-benzyl imines. Chem. Sci. 2020, 11, 7619–7625. [Google Scholar] [CrossRef]
- Uchikura, T.; Hara, Y.; Tsubono, K.; Akiyama, T. Visible-Light-Driven C–S Bond Formation Based on Electron Donor–Acceptor Excitation and Hydrogen Atom Transfer Combined System. ACS Org. Inorg. Au 2021, 1, 23–28. [Google Scholar] [CrossRef]
- Li, T.; Liang, K.; Tang, J.; Ding, Y.; Tong, X.; Xia, C. A photoexcited halogen-bonded EDA complex of the thiophenolate anion with iodobenzene for C(sp3)–H activation and thiolation. Chem. Sci. 2021, 12, 15655–15661. [Google Scholar] [CrossRef] [PubMed]
- Uchikura, T.; Tsubono, K.; Hara, Y.; Akiyama, T. Dual-Role Halogen-Bonding-Assisted EDA-SET/HAT Photoreaction System with Phenol Catalyst and Aryl Iodide: Visible-Light-Driven Carbon–Carbon Bond Formation. J. Org. Chem. 2022, 87, 15499–15510. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Poole, D.L.; Murphy, J.A. The role of organic electron donors in the initiation of BHAS base-induced coupling reactions between haloarenes and arenes. Sci. China Chem. 2019, 62, 1425–1438. [Google Scholar] [CrossRef]
- Sun, C.-L.; Shi, Z.-J. Transition-Metal-Free Coupling Reactions. Chem. Rev. 2014, 114, 9219–9280. [Google Scholar] [CrossRef]
- Gurry, M.; Aldabbagh, F. A new era for homolytic aromatic substitution: Replacing Bu3SnH with efficient light-induced chain reactions. Org. Biomol. Chem. 2016, 14, 3849–3862. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Pak Shing Cheung, K.; Fang, J.; Mukherjee, K.; Mihranyan, A.; Gevorgyan, V. Asymmetric intermolecular allylic C-H amination of alkenes with aliphatic amines. Science 2022, 378, 1207–1213. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Rovis, T. Photoinduced Nickel-Catalyzed Selective N-Demethylation of Trialkylamines Using C(sp2)–Bromides as HAT Reagents. J. Am. Chem. Soc. 2023, 145, 3294–3300. [Google Scholar] [CrossRef]
- Zuo, Z.; MacMillan, D.W.C. Decarboxylative Arylation of α-Amino Acids via Photoredox Catalysis: A One-Step Conversion of Biomass to Drug Pharmacophore. J. Am. Chem. Soc. 2014, 136, 5257–5260. [Google Scholar] [CrossRef]
- Qin, T.; Cornella, J.; Li, C.; Malins, L.R.; Edwards, J.T.; Kawamura, S.; Maxwell, B.D.; Eastgate, M.D.; Baran, P.S. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 2016, 352, 801–805. [Google Scholar] [CrossRef]
- Capaldo, L.; Noël, T.; Ravelli, D. Photocatalytic generation of ligated boryl radicals from tertiary amine-borane complexes: An emerging tool in organic synthesis. Chem Catal. 2022, 2, 957–966. [Google Scholar] [CrossRef]
- Lei, G.; Xu, M.; Chang, R.; Funes-Ardoiz, I.; Ye, J. Hydroalkylation of Unactivated Olefins via Visible-Light-Driven Dual Hydrogen Atom Transfer Catalysis. J. Am. Chem. Soc. 2021, 143, 11251–11261. [Google Scholar] [CrossRef]
- Gonçalves, C.R.; Lemmerer, M.; Teskey, C.J.; Adler, P.; Kaiser, D.; Maryasin, B.; González, L.; Maulide, N. Unified Approach to the Chemoselective α-Functionalisation of Amides with Heteroatom Nucleophiles. J. Am. Chem. Soc. 2019, 141, 18437–18443. [Google Scholar] [CrossRef]
- Hamada, T.; Chieffi, A.; Åhman, J.; Buchwald, S.L. An Improved Catalyst for the Asymmetric Arylation of Ketone Enolates. J. Am. Chem. Soc. 2002, 124, 1261–1268. [Google Scholar] [CrossRef]
- He, Z.-T.; Hartwig, J.F. Palladium-catalyzed α-arylation for the addition of small rings to aromatic compounds. Nat. Commun. 2019, 10, 4083. [Google Scholar] [CrossRef]
- Baran, P.S.; Maimone, T.J.; Richter, J.M. Total synthesis of marine natural products without using protecting groups. Nature. 2007, 446, 404–408. [Google Scholar] [CrossRef]
- Baran, P.S.; Richter, J.M. Enantioselective Total Syntheses of Welwitindolinone A and Fischerindoles I and G. J. Am. Chem. Soc. 2005, 127, 15394–15396. [Google Scholar] [CrossRef]
- Baran, P.S.; DeMartino, M.P. Intermolecular Oxidative Enolate Heterocoupling. Angew. Chem. Int. Ed. 2006, 45, 7083–7086. [Google Scholar] [CrossRef]
- Guo, F.; Clift, M.D.; Thomson, R.J. Oxidative Coupling of Enolates, Enol Silanes, and Enamines: Methods and Natural Product Synthesis. Eur. J. Org. Chem. 2012, 2012, 4881–4896. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Sang, Y.-Q.; Wang, C.-Q.; Dai, P.; Xue, X.-S.; Piper, J.L.; Peng, Z.-H.; Ma, J.-A.; Zhang, F.-G.; Wu, J. Difluoromethylation of Unactivated Alkenes Using Freon-22 through Tertiary Amine-Borane-Triggered Halogen Atom Transfer. J. Am. Chem. Soc. 2022, 144, 14288–14296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).