Untargeted Metabolomic Analysis Combined with Chemometrics Revealed the Effects of Different Cooking Methods on Lentinus edodes
Abstract
1. Introduction
2. Results and Discussions
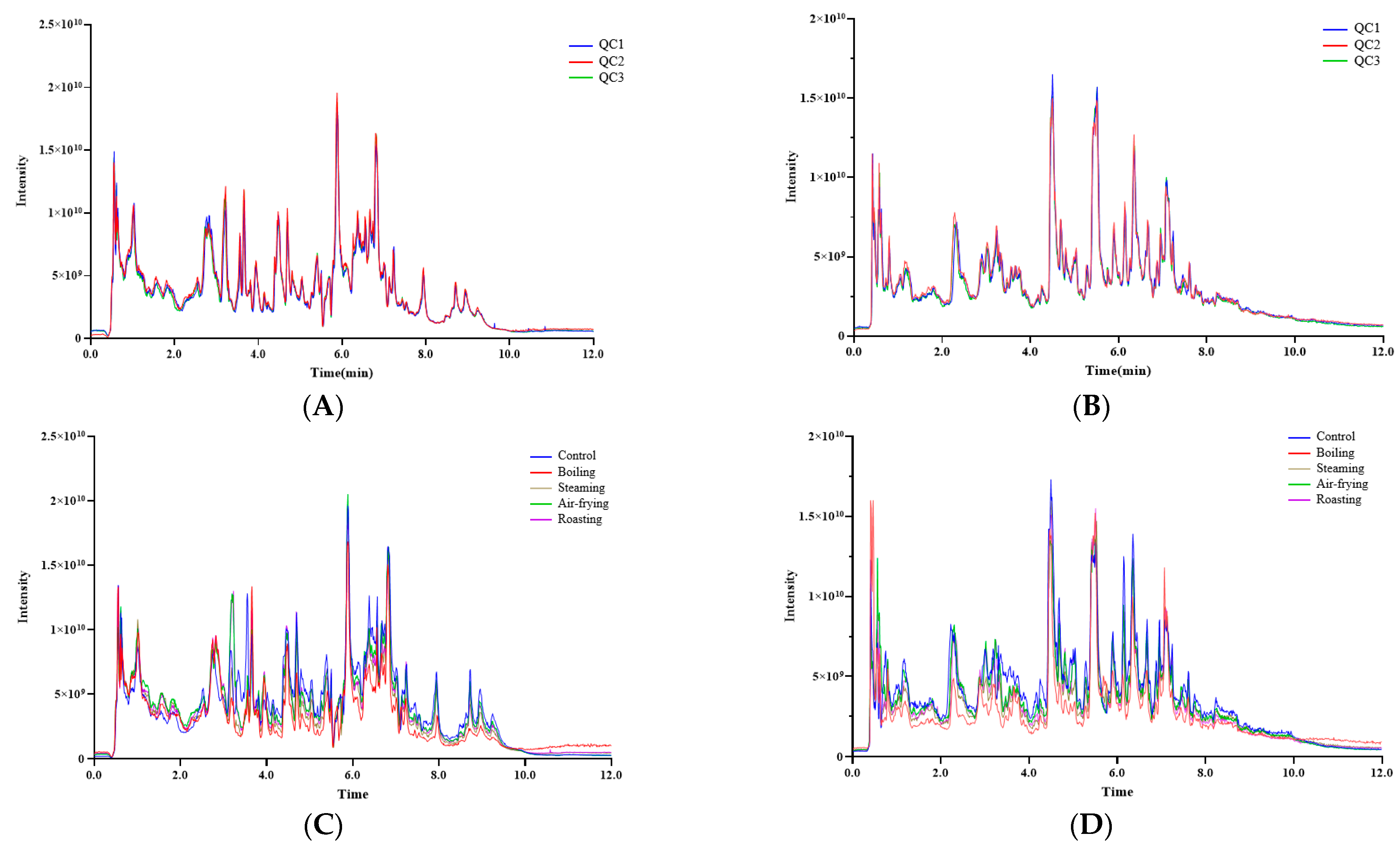
2.1. Overview of the Metabolites in Lentinus edodes with Different Cooking Methods
2.2. Heatmap for All Identified Metabolites in Lentinus edodes
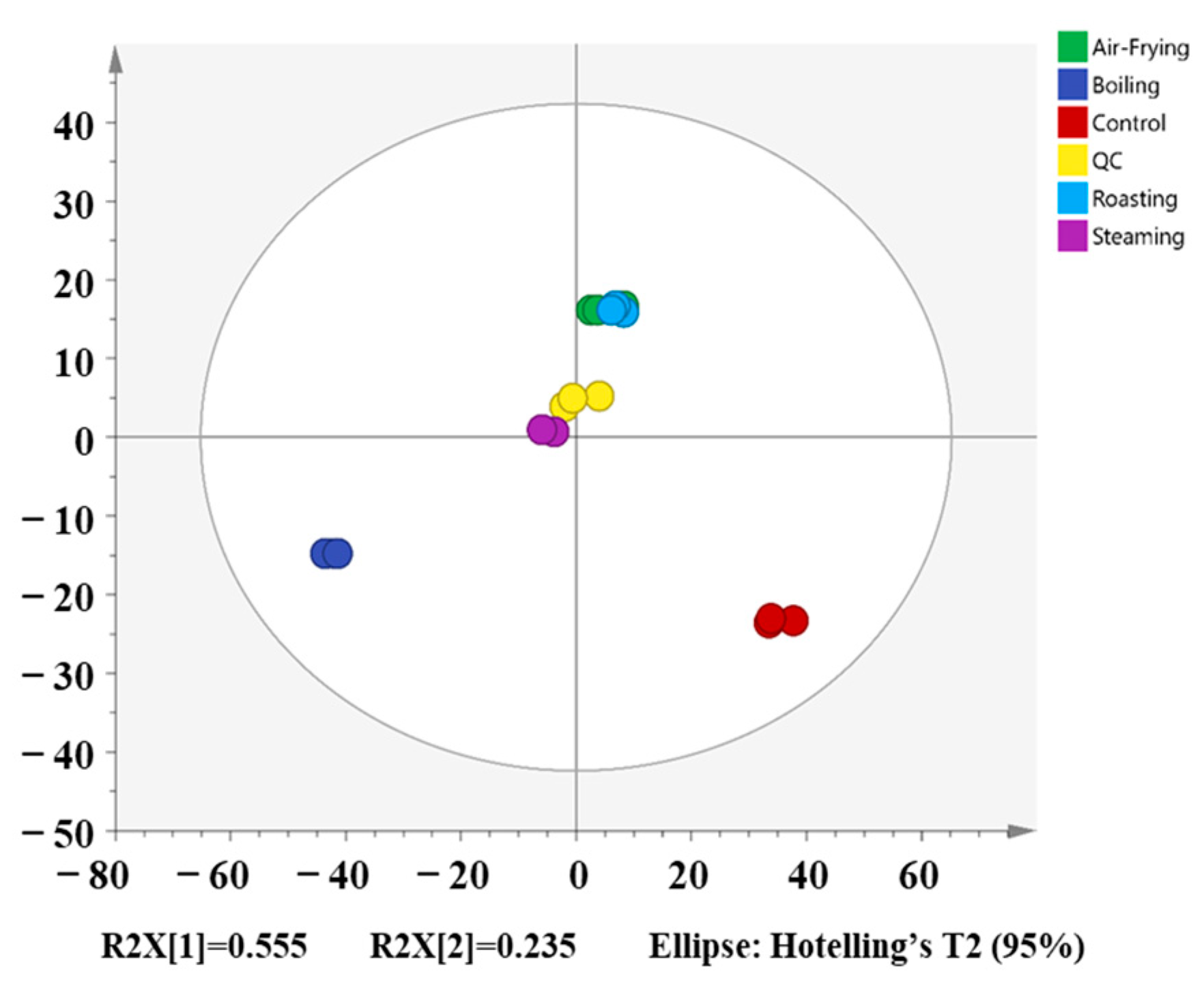
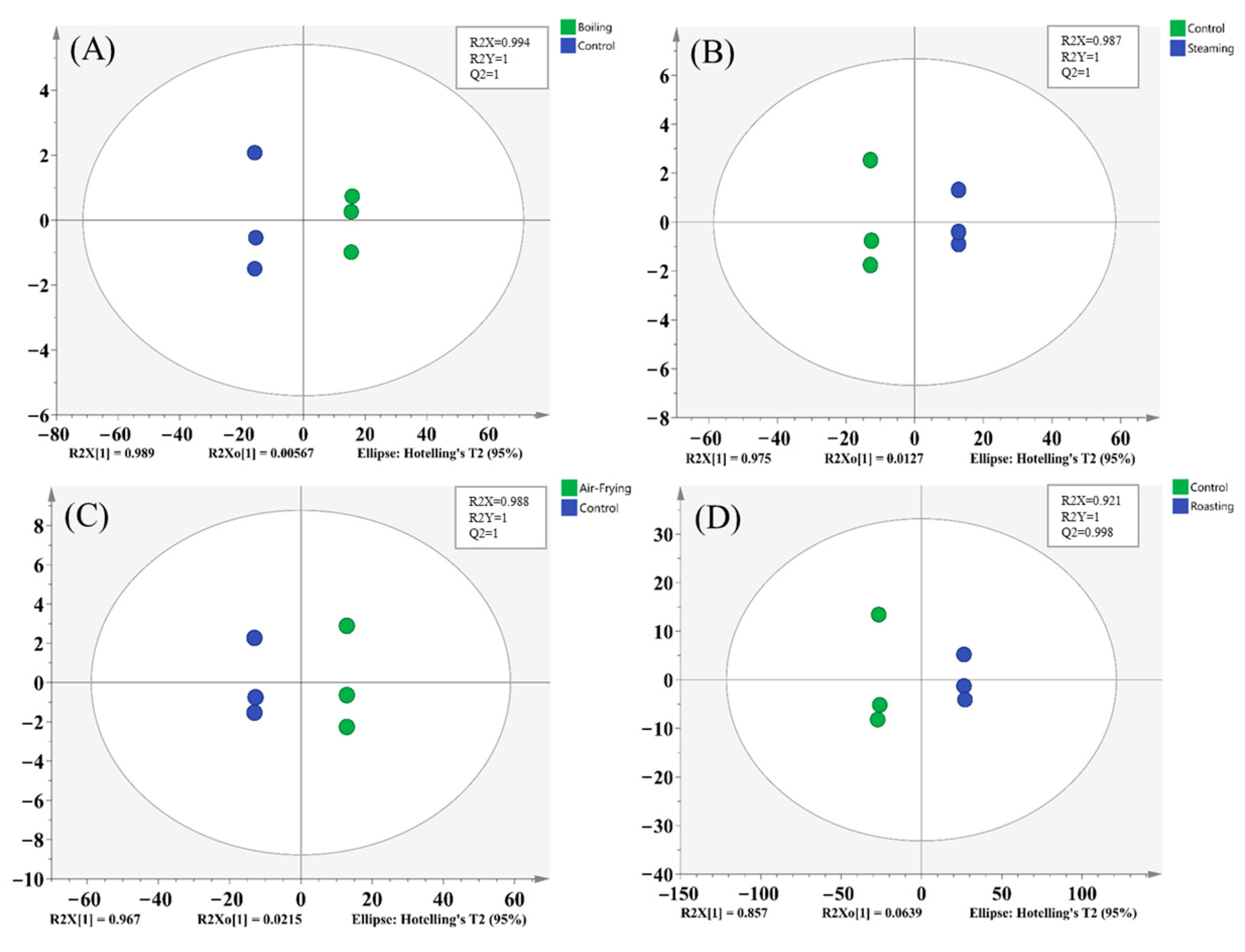
2.3. Multivariate Statistical Analysis of the Metabolites
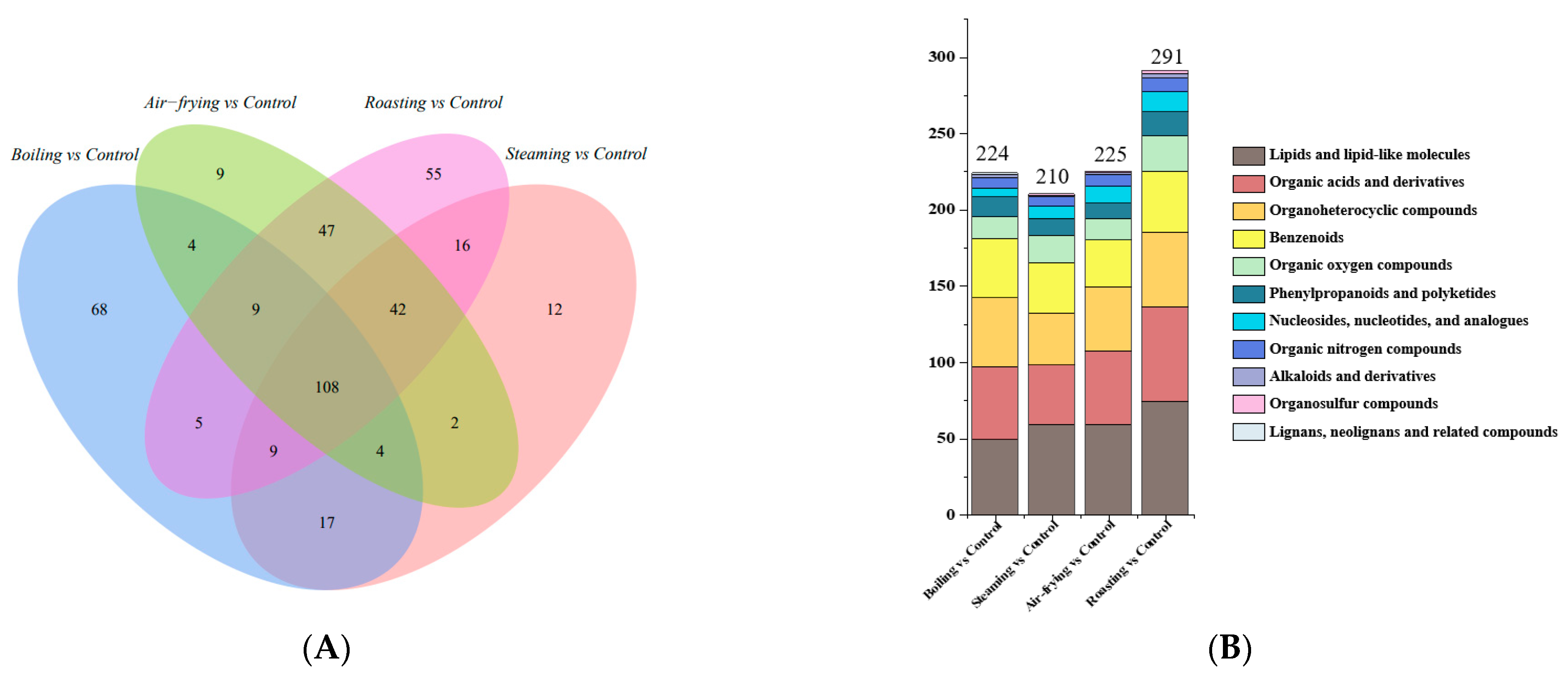
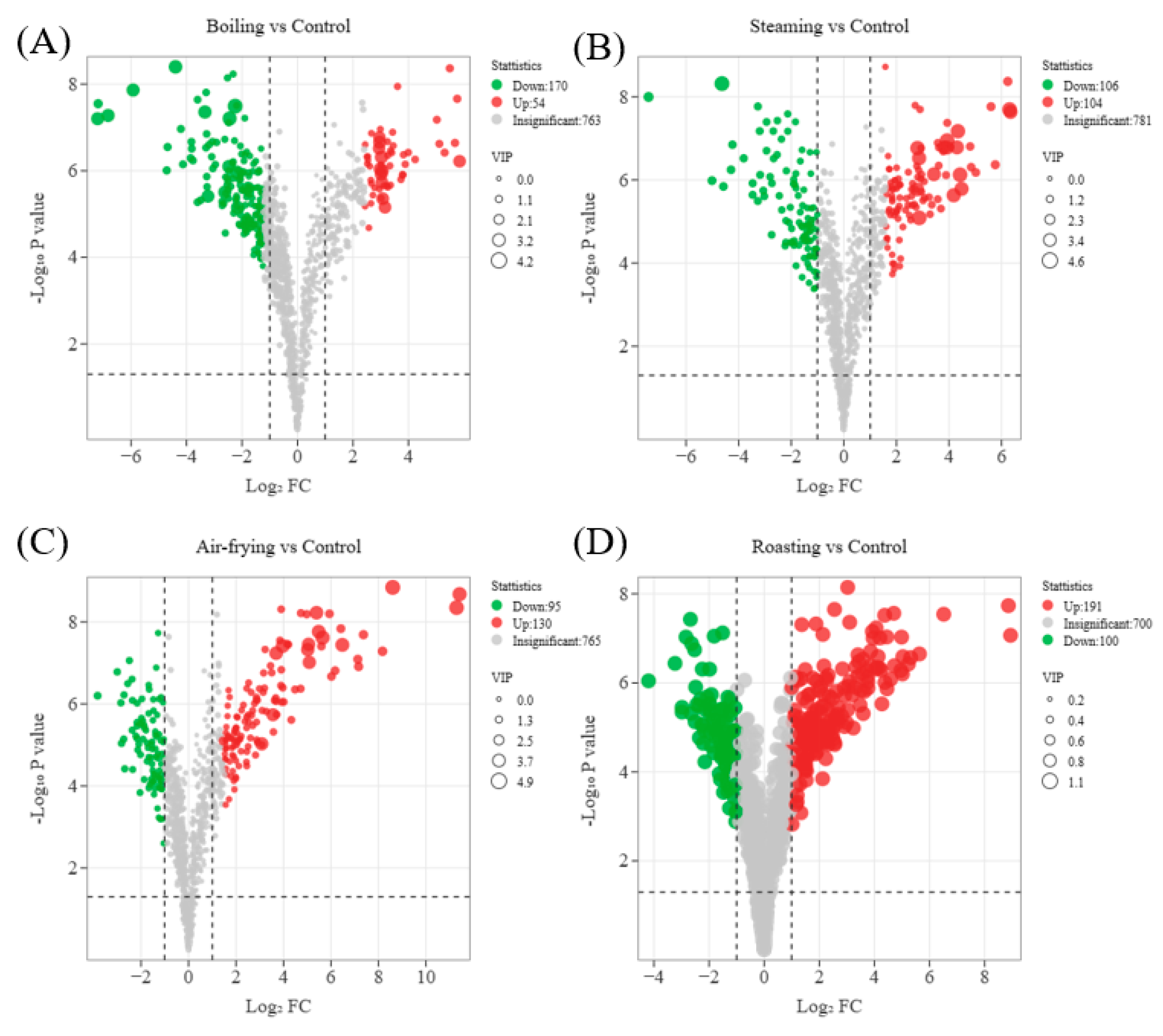
2.4. Screening of Significantly Different Metabolites in Lentinus edodes with Distinct Cooking Methods
2.5. Changes in Different Metabolites in Lentinus edodes with Distinct Cooking Methods
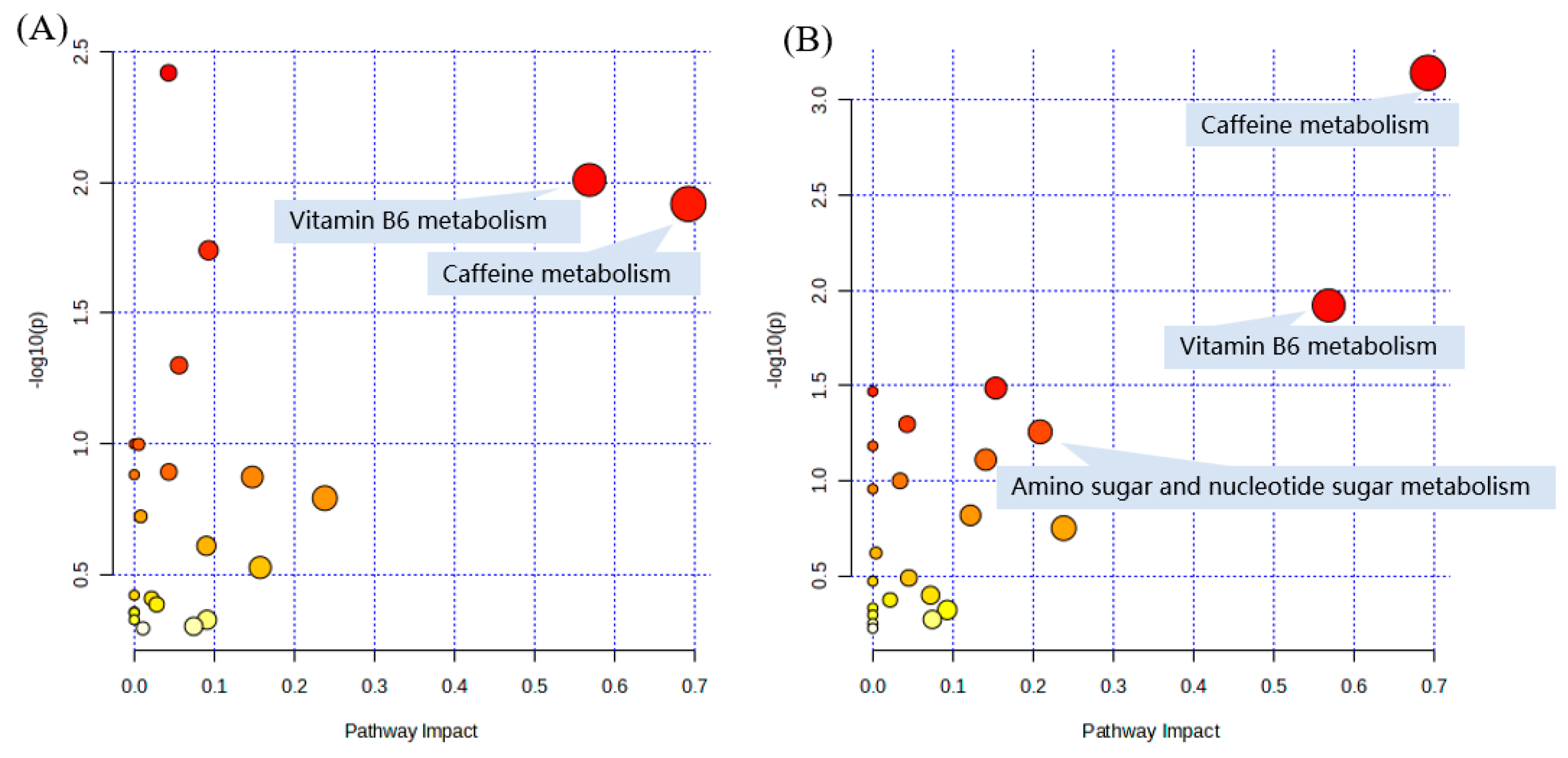
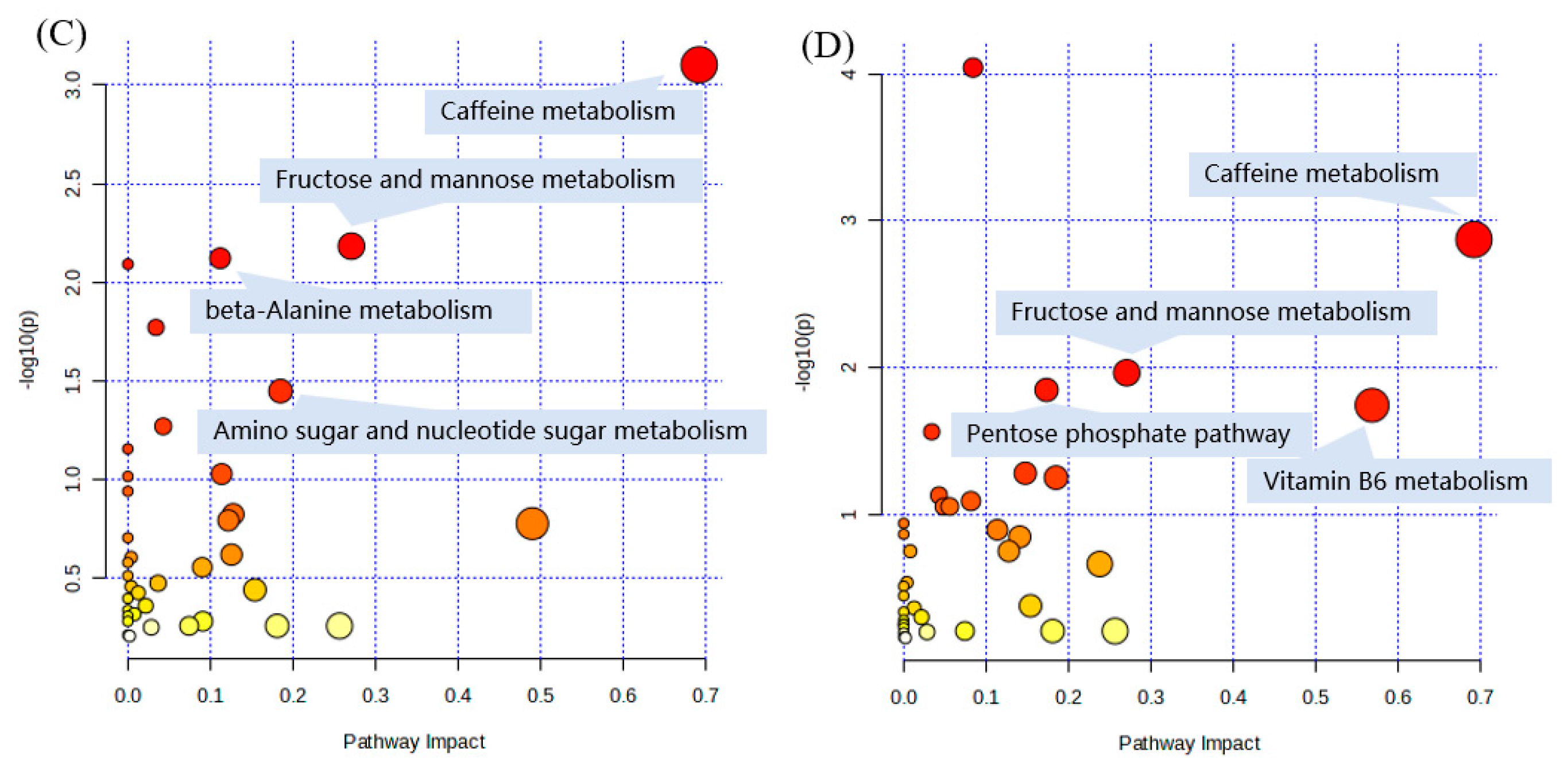
2.6. Pathway and Enrichment Analysis Based on KEGG Annotation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Extraction of Metabolites
3.4. Metabolomic Analysis
3.5. Data Processing
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, L.B.; Zhang, Z.Y.; Xin, G.; Sun, B.X.; Bao, X.J.; Wei, Y.Y.; Zhao, X.M.; Xu, H.R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Muszynska, B.; Grzywacz-Kisielewska, A.; Kala, K.; Gdula-Argasinska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, R.; Jiao, Z.; Chen, W.; Peng, D.; Wang, L.; Yu, N.; Peng, C.; Cai, B.; Song, H.; et al. Current Advancements in Antitumor Properties and Mechanisms of Medicinal Components in Edible Mushrooms. Nutrients 2022, 14, 2622. [Google Scholar] [CrossRef] [PubMed]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Fayez, S.; Xiao, H.; Xu, B. New insights into antimicrobial and antibiofilm effects of edible mushrooms. Food Res. Int. 2022, 162, 111982. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Li, C.; Xu, S. Edible mushroom industry in China: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef]
- Hwang, I.S.; Chon, S.Y.; Bang, W.S.; Kim, M.K. Influence of Roasting Temperatures on the Antioxidant Properties, beta-Glucan Content, and Volatile Flavor Profiles of Shiitake Mushroom. Foods 2020, 10, 54. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Avinash, J.; Vinay, S.; Jha, K.; Das, D.; Goutham, B.S.; Kumar, G. The Unexplored Anticaries Potential of Shiitake Mushroom. Pharmacogn. Rev. 2016, 10, 100–104. [Google Scholar]
- Chiocchetti, G.M.; Latorre, T.; Clemente, M.J.; Jadan-Piedra, C.; Devesa, V.; Velez, D. Toxic trace elements in dried mushrooms: Effects of cooking and gastrointestinal digestion on food safety. Food Chem. 2020, 306, 125478. [Google Scholar] [CrossRef]
- Rotola-Pukkila, M.; Yang, B.; Hopia, A. The effect of cooking on umami compounds in wild and cultivated mushrooms. Food Chem. 2019, 278, 56–66. [Google Scholar] [CrossRef]
- Davila, M.; Muniz, A.; Du, X. The impact of roasting and steaming on savory flavors contributed by amino acids, 5′-nucleotides, and volatiles in Agaricus bisporus mushrooms. Int. J. Gastron. Food Sci. 2022, 30, 100590. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; Xie, Y.; Li, C.; Fang, Z.; Hu, B.; Wang, C.; Chen, S.; Wu, W.; Li, X.; et al. Effect of different cooking methods on the nutrient, and subsequent bioaccessibility and biological activities in Boletus auripes. Food Chem. 2023, 405, 134358. [Google Scholar] [CrossRef]
- Sun, L.; Bai, X.; Zhuang, Y. Effect of different cooking methods on total phenolic contents and antioxidant activities of four Boletus mushrooms. J. Food Sci. Technol. 2014, 51, 3362–3368. [Google Scholar] [CrossRef]
- Zhou, X.; Guan, Q.; Wang, Y.; Lin, D.; Du, B. Effect of Different Cooking Methods on Nutrients, Antioxidant Activities and Flavors of Three Varieties of Lentinus edodes. Foods 2022, 11, 2713. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Choi, Y.; Kim, Y.; Jeong, H.S.; Lee, J. Effect of Different Cooking Methods on the True Retention of Vitamins, Minerals, and Bioactive Compounds in Shiitake Mushrooms (Lentinula edodes). Food Sci. Technol. Res. 2019, 25, 115–122. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Q.; Miao, R.; Lin, J.; Feng, R.; Ni, Y.; Li, W.; Yang, D.; Zhao, X. Application of omics technology in the research on edible fungi. Curr. Res. Food Sci. 2023, 6, 100430. [Google Scholar] [CrossRef]
- Ibáñez, C.; García-Cañas, V.; Valdés, A.; Simó, C. Novel MS-based approaches and applications in food metabolomics. Trends Anal. Chem. 2013, 52, 100–111. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Wang, L.; Huang, Y.; Wang, L. Key taste components in two wild edible Boletus mushrooms using widely targeted metabolomics. Biochem. Syst. Ecol. 2021, 96, 104268. [Google Scholar] [CrossRef]
- de Oliveira Gorgulho Silva, C.; Raisa Barbosa Cunha, J.; Almeida Conceicao, A.; Gonzaga Almeida, E.; Cunha Zied, D.; Goncalves Vieira Junior, W.; Souza Dias, E.; Isikhuemhen, O.S.; Verardi Abdelnur, P.; Goncalves de Siqueira, F. Outdoor versus indoor cultivation: Effects on the metabolite profile of Agaricus subrufescens strains analyzed by untargeted metabolomics. Food Chem. 2022, 374, 131740. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, Y.; Tan, H.; Wang, B.; Peng, W.; Sun, Q. Metabolomics reveals dopa melanin involved in the enzymatic browning of the yellow cultivars of East Asian golden needle mushroom (Flammulina filiformis). Food Chem. 2022, 370, 131295. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, L.; Zhou, J. Analytical Strategies for LC-MS-Based Untargeted and Targeted Metabolomics Approaches Reveal the Entomological Origins of Honey. J. Agric. Food Chem. 2022, 70, 1358–1366. [Google Scholar] [CrossRef]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.T.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y.; et al. UPLC-QQQ-MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef]
- Cui, H.-N.; Gu, H.-W.; Li, Z.-Q.; Sun, W.; Ding, B.; Li, Z.; Chen, Y.; Long, W.; Yin, X.-L.; Fu, H. Integration of lipidomics and metabolomics approaches for the discrimination of harvest time of green tea in spring season by using UPLC-Triple-TOF/MS coupled with chemometrics. Front. Sustain. Food Syst. 2023, 7, 1119314. [Google Scholar] [CrossRef]
- Sissons, J.; Davila, M.; Du, X. Sautéing and roasting effect on free amino acid profiles in portobello and shiitake mushrooms, and the effect of mushroom- and cooking-related volatile aroma compounds on meaty flavor enhancement. Int. J. Gastron. Food Sci. 2022, 28, 100550. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, S.; Song, Z.; Cui, X.; Kong, W.; Song, K.; Zhang, Y. Relationship between flavor and energy status in shiitake mushroom (Lentinula edodes) harvested at different developmental stages. J. Food Sci. 2021, 86, 4288–4302. [Google Scholar] [CrossRef]
- Maria Pellegrino, R.; Ianni, F.; Blasi, F.; Angelini, P.; Emiliani, C.; Venanzoni, R.; Cossignani, L. Lipidomic profiling of Pleurotus ostreatus by LC/MS Q-TOF analysis. Food Res. Int. 2022, 156, 111335. [Google Scholar] [CrossRef]
- Chen, H.; Wei, F.; Dong, X.-y.; Xiang, J.-q.; Quek, S.-y.; Wang, X. Lipidomics in food science. Curr. Opin. Food Sci. 2017, 16, 80–87. [Google Scholar] [CrossRef]
- Reid, T.; Munyanyi, M.; Mduluza, T. Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Sci. Nutr. 2017, 5, 538–544. [Google Scholar] [CrossRef]
- Sun, R.; Wu, T.; Guo, H.; Xu, J.; Chen, J.; Tao, N.; Wang, X.; Zhong, J. Lipid profile migration during the tilapia muscle steaming process revealed by a transactional analysis between MS data and lipidomics data. NPJ Sci. Food 2021, 5, 30. [Google Scholar] [CrossRef]
- Ngan, H.L.; Ip, S.Y.; Wang, M.; Zhou, Q. Comparative Study of Sensory and Physicochemical Characteristics of Green-Tea-Fortified Cupcakes upon Air Frying and Oven Baking. Foods 2023, 12, 1266. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Lu, Q.; Li, H.; Liu, Q.; Li, S.; Liu, H.; Varshney, R.K.; Liang, X.; Hong, Y.; et al. Lipid profile variations in high olecic acid peanuts by following different cooking processes. Food Res. Int. 2022, 155, 110993. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xue, J.; Wang, J.; Song, G.; Zhang, Y.; Shen, Q. Effect of air-frying conditions on the quality attributes and lipidomic characteristics of surimi during processing. Innov. Food Sci. Emerg. Technol. 2020, 60, 102305. [Google Scholar] [CrossRef]
- Manninen, H.; Rotola-Pukkila, M.; Aisala, H.; Hopia, A.; Laaksonen, T. Free amino acids and 5’-nucleotides in Finnish forest mushrooms. Food Chem. 2018, 247, 23–28. [Google Scholar] [CrossRef]
- Feng, Y.; Xin, G.; Wei, Y.; Xu, H.; Sun, L.; Hou, Z.; Sun, B. Comparison of the umami taste and aroma of dried Suillus granulatus packed using four different packaging methods. Food Chem. 2022, 366, 130570. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.; Du, X. Primary exploration of mushroom protein hydrolysis and cooking impact on the protein amino acid profiles of Agaricus bisporus and Lentinula edodes mushrooms. Int. J. Gastron. Food Sci. 2023, 32, 100710. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; Hu, L.T.; Ni, Z.J.; Thakur, K.; Wei, Z.J. Maillard conjugates and their potential in food and nutritional industries: A review. Food Front. 2020, 1, 382–397. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- Chiang, P.; Yen, C.; Mau, J. Non-volatile taste components of canned mushrooms. Food Chem. 2006, 97, 431–437. [Google Scholar] [CrossRef]
- Misharina, T.A.; Mukhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I.; Medvedeva, I.B. The composition of volatile components of dry cepe and oyster mushroom. Appl. Biochem. Microbiol. 2009, 45, 544–549. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.-H.; Claver, I.P.; Zhu, K.-X.; Peng, W.; Zhou, H.-M. Effect of different cooking methods on the flavour constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]
- Ochieng, B.O.; Anyango, J.O.; Nduko, J.M.; Cheseto, X.; Mudalungu, C.M.; Khamis, F.M.; Ghemoh, C.J.; Egonyu, P.J.; Subramanian, S.; Nakimbugwe, D.; et al. Dynamics in nutrients, sterols and total flavonoid content during processing of the edible Long-Horned grasshopper (Ruspolia differens Serville) for food. Food Chem. 2022, 383, 132397. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, R.; Wu, X.; Liu, S.; Shi, L. UHPLC-Q-Orbitrap HRMS-based quantitative lipidomics reveals the chemical changes of phospholipids during thermal processing methods of Tan sheep meat. Food Chem. 2021, 360, 130153. [Google Scholar] [CrossRef]
- Li, S.F.; Guo, X.M.; Hao, X.F.; Feng, S.H.; Hu, Y.J.; Yang, Y.Q.; Wang, H.F.; Yu, Y.J. Untargeted metabolomics study of Lonicerae japonicae flos processed with different drying methods via GC-MS and UHPLC-HRMS in combination with chemometrics. Ind. Crop. Prod. 2022, 186, 115179. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhou, L.; Yao, J.; Hu, Y.; Li, Z.; Liu, J.; Marchioni, E. Untargeted Metabolomic Analysis Combined with Chemometrics Revealed the Effects of Different Cooking Methods on Lentinus edodes. Molecules 2023, 28, 6009. https://doi.org/10.3390/molecules28166009
Zhu J, Zhou L, Yao J, Hu Y, Li Z, Liu J, Marchioni E. Untargeted Metabolomic Analysis Combined with Chemometrics Revealed the Effects of Different Cooking Methods on Lentinus edodes. Molecules. 2023; 28(16):6009. https://doi.org/10.3390/molecules28166009
Chicago/Turabian StyleZhu, Jinrui, Li Zhou, Jiaxu Yao, Yueqi Hu, Zhenghui Li, Jikai Liu, and Eric Marchioni. 2023. "Untargeted Metabolomic Analysis Combined with Chemometrics Revealed the Effects of Different Cooking Methods on Lentinus edodes" Molecules 28, no. 16: 6009. https://doi.org/10.3390/molecules28166009
APA StyleZhu, J., Zhou, L., Yao, J., Hu, Y., Li, Z., Liu, J., & Marchioni, E. (2023). Untargeted Metabolomic Analysis Combined with Chemometrics Revealed the Effects of Different Cooking Methods on Lentinus edodes. Molecules, 28(16), 6009. https://doi.org/10.3390/molecules28166009





