Optimum Reaction Conditions for the Synthesis of Selenized Ornithogalum caudatum Ait. (Liliaceae) Polysaccharides and Measurement of Their Antioxidant Activity In Vivo
Abstract
1. Introduction
2. Results and Discussion
2.1. Establishment and Analysis of RSM
2.2. Optimization and Analysis of the Synthesis of Se-OCAPIIA
2.3. Physicochemical Properties of Se-OCAPIIA
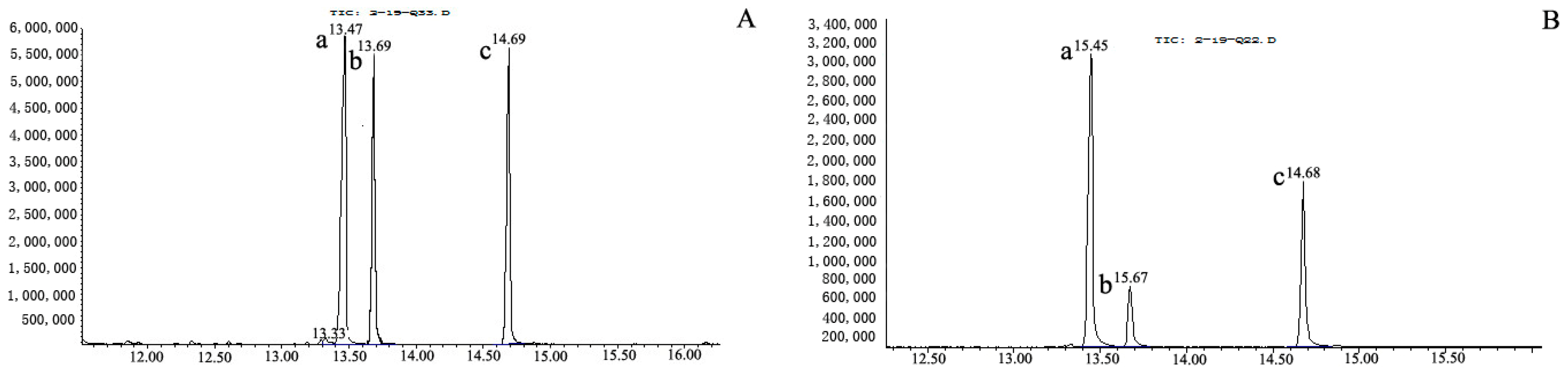
2.4. Monosaccharide Composition of Se-OCAPIIA
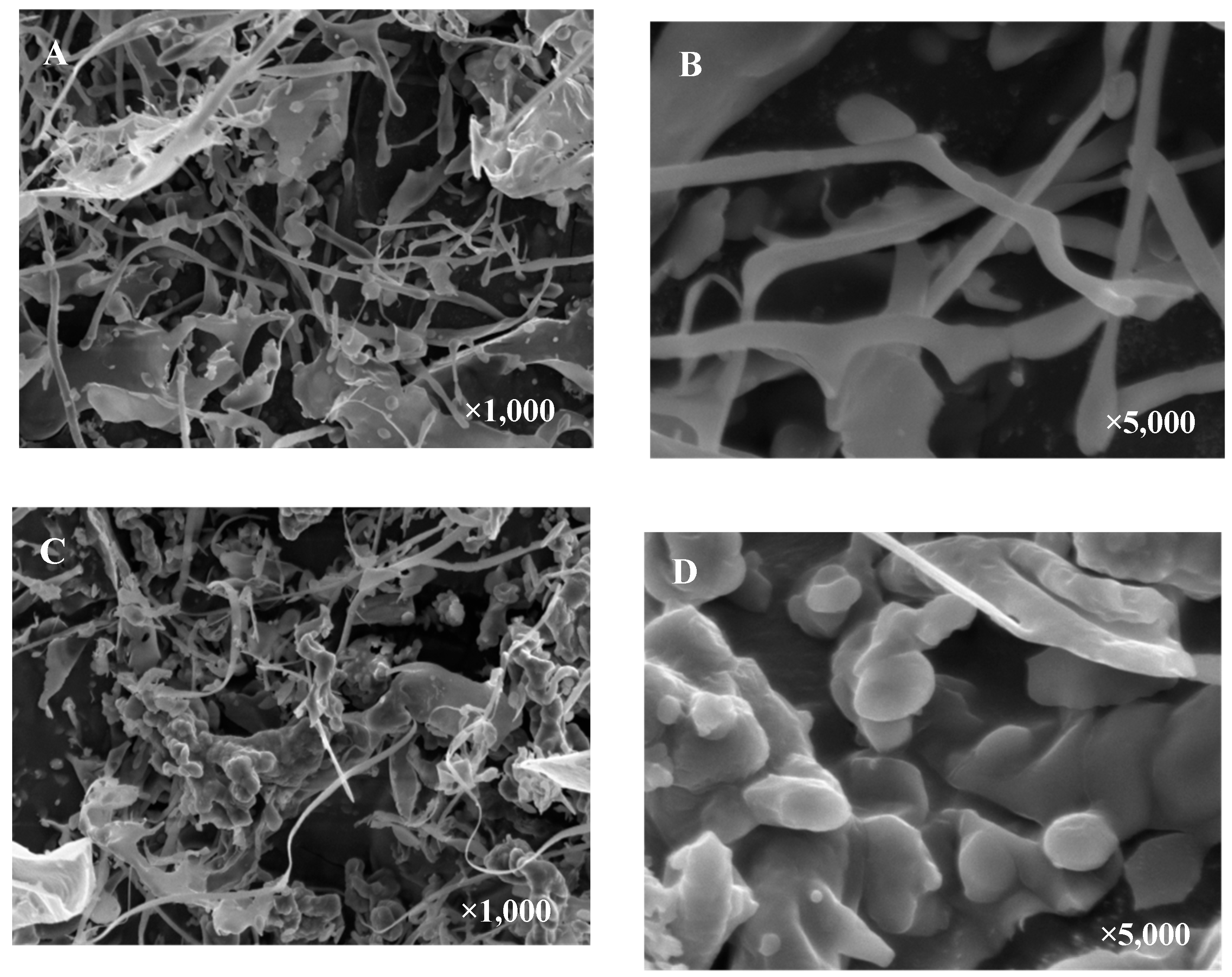
2.5. Morphologies of OCAPIIA and Se-OCAPIIA
2.6. FT-IR Analysis of OCAPIIA before and after Selenization
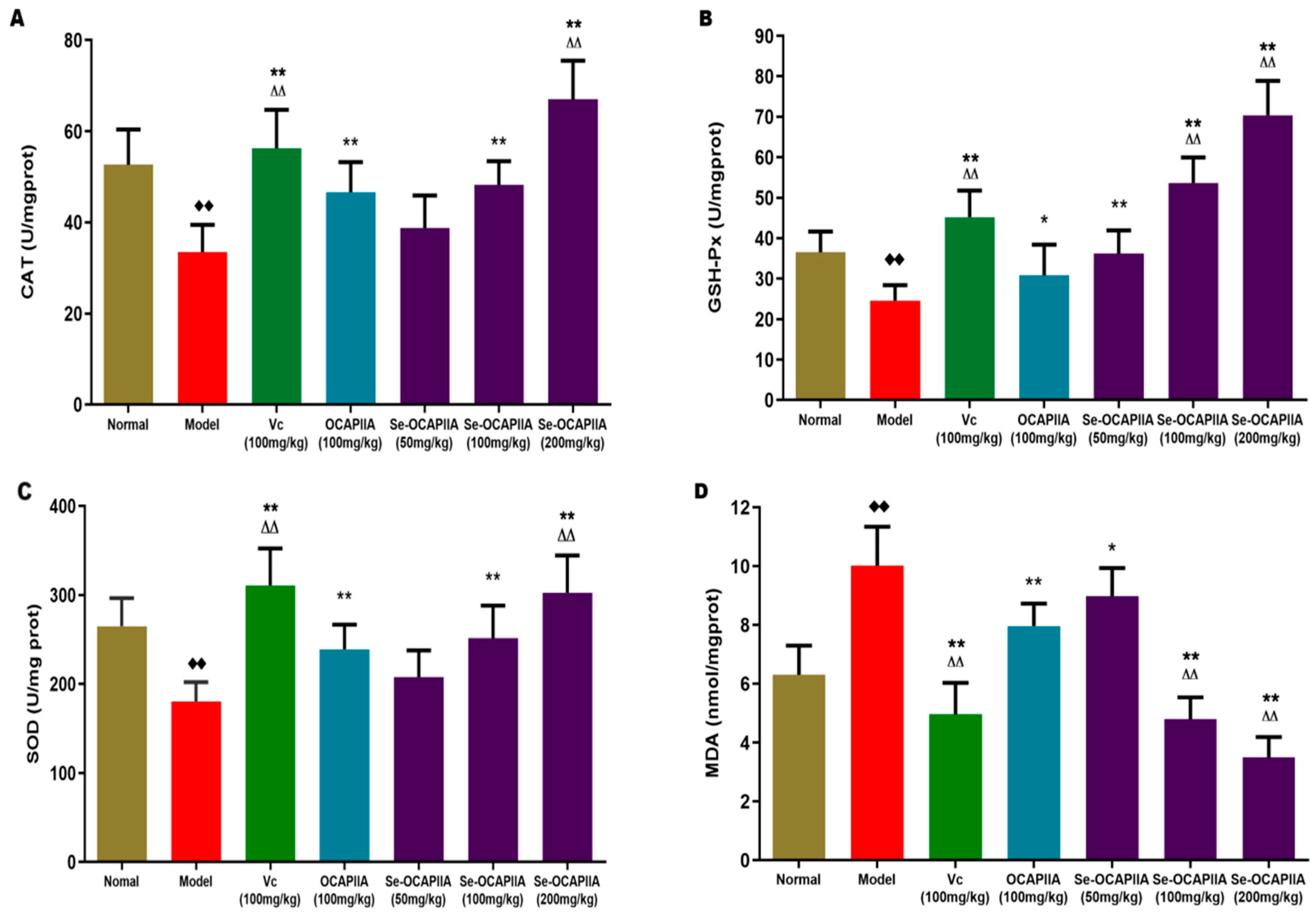
2.7. Antioxidant Effect of Se-OCAPIIA In Vivo
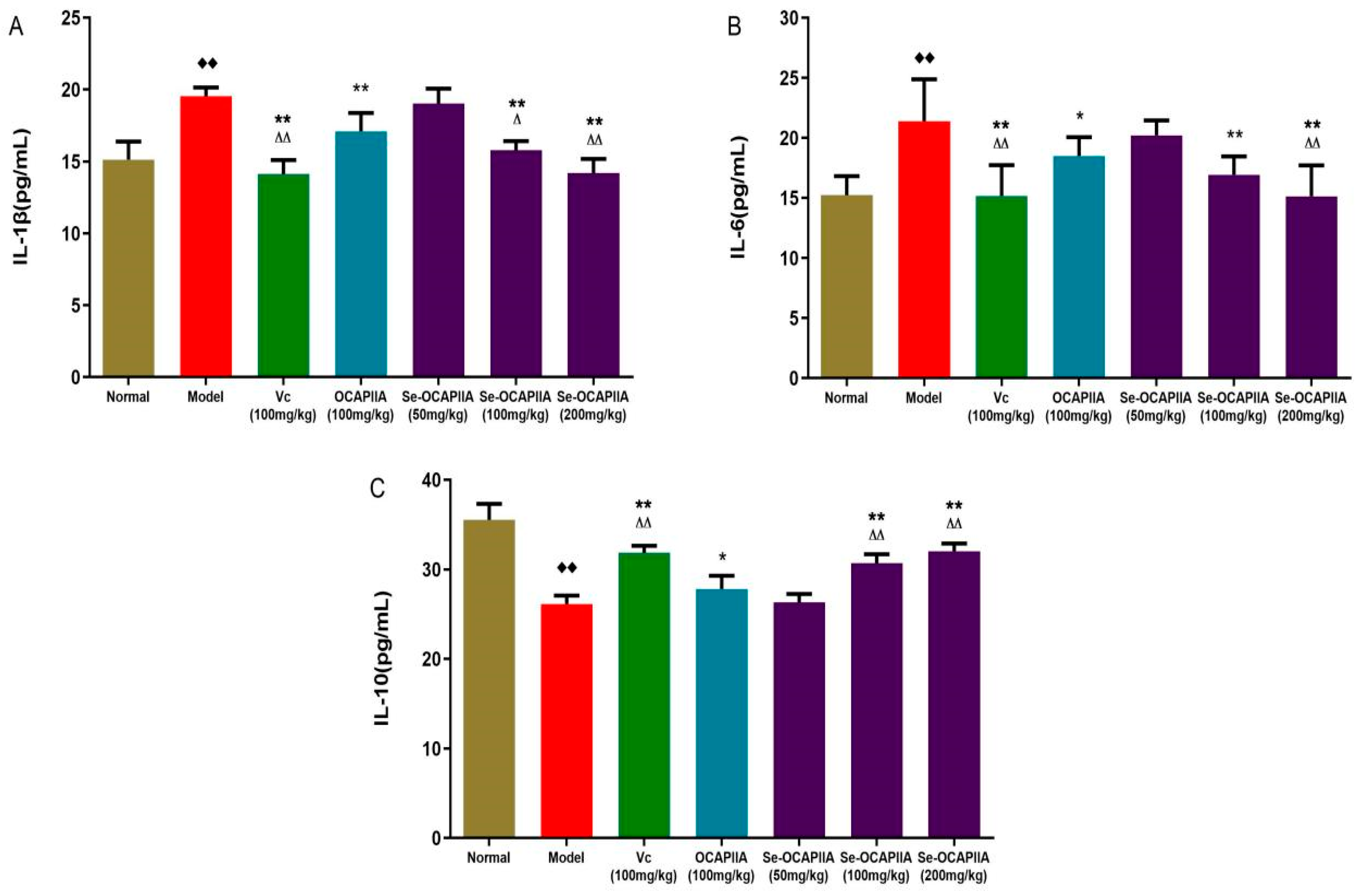
2.8. Effect of Se-OCAPIIA on Serum Cytokine Levels
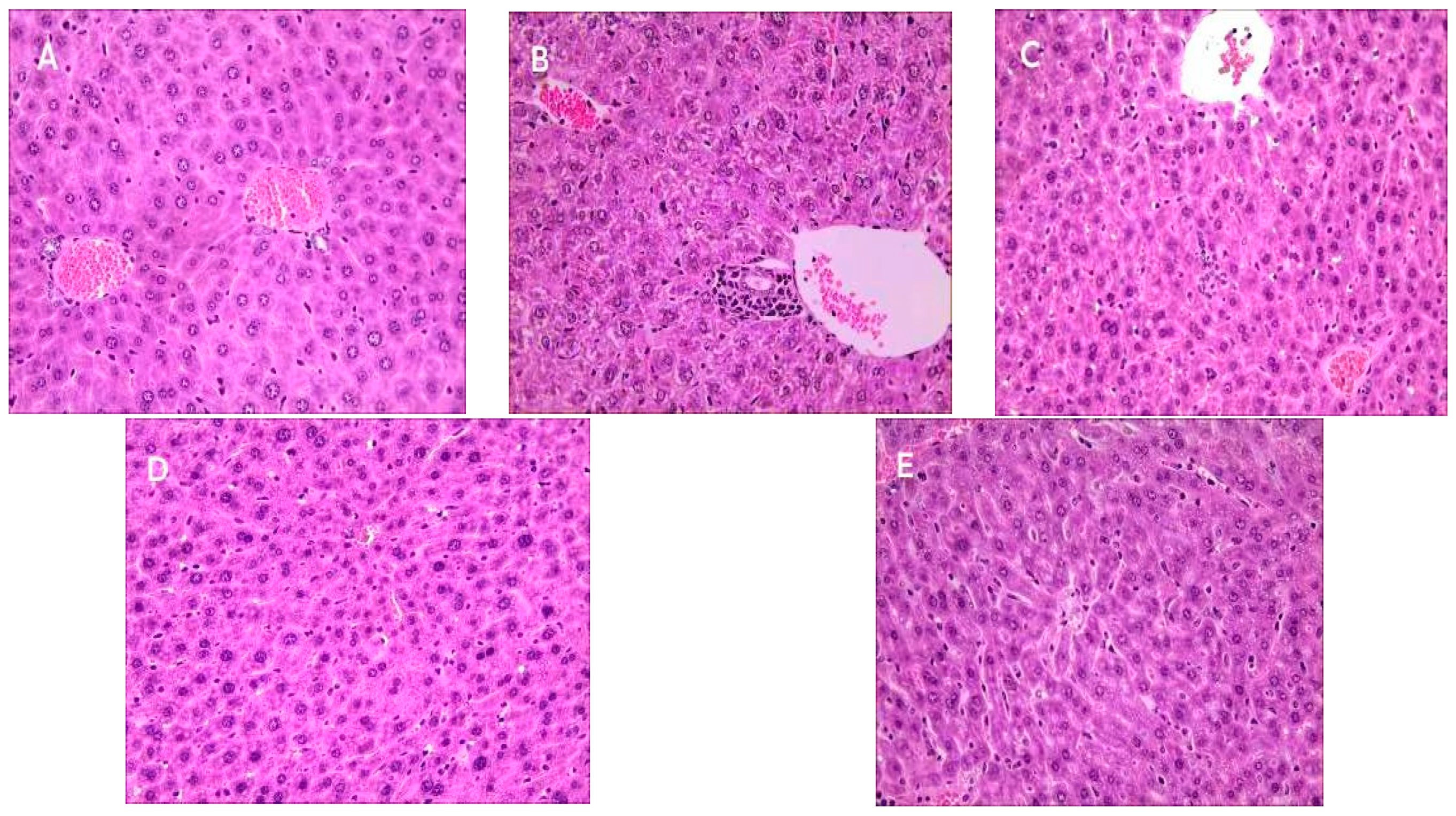
2.9. Effect of Se-OCAPIIA on the Morphology of Liver Tissue
3. Materials and Methods
3.1. Samples
3.2. Reagents
3.3. Instruments
3.4. Experimental Animals
3.5. Extraction and Purification of OCAPIIA
3.6. Selenization of OCAPIIA
3.7. Analysis of the Physical and Chemical Properties of Se-OCAPIIA
3.8. Analysis of the Monosaccharide Composition of Se-OCAPIIA by Gas Chromatography (GC)
3.9. Scanning Electron Microscopy (SEM) Analysis of Se-OCAPIIA
3.10. FT-IR Analysis
3.11. Antioxidant Activity of Se-OCAPIIA In Vivo
3.12. Histopathological Analysis of Liver Tissues
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.Z.; Li, Y.; Dong, H.; Liu, Z.Q.; Li, S.Z.; Yang, S.M.; Li, X.L. Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum caudatum Ait and evaluation of its biological activities. Ultrason. Sonochem. 2012, 19, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, L.; Gao, L. Recent advances in studies on chemical constituents and pharmacological action of Ornithogalum caudatum Ait. Asia-Pac. Tradit. Med. 2016, 12, 54–56. [Google Scholar]
- Zou, X.; Zhou, L.; Zhang, Y.; Gong, T.; Feng, J.; Qu, Z.; Xue, L.; Li, W. Study on the apoptotic mechanisms of human breast cancer MCF-7 cells induced by total saponins of Ornithogalum caudatum ait. Nat. Prod. Res. Dev. 2020, 32, 32–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Fan, K.; Zhang, Y.; Zhang, J.; Guo, H.; Yu, P.; Ma, J. Effective cytotoxic activity of OSW-1 on colon cancer by inducing apoptosis in vitro and in vivo. Oncol. Rep. 2017, 37, 3509–3519. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y. Effects of Ornithogalum caudatum Ait Saponins on Proliferation and Apoptosis of Breast Cancer Cell Line MDA-MB-231. Tradit Chin. Drug. Res. Clin. Pharmacol. 2012, 23, 121–124. [Google Scholar]
- Zou, X.; Liu, Y.; Qu, Z.; Shi, X.; Liu, X.; Wang, B.; Chen, M. Research progress on chemical constituents of Ornithogalum plants. J. Harbin Univ. Commer. (Nat. Sci. Ed.) 2016, 32, 136–141. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, M.; Su, T.; Qu, J.L.; Jiang, W.Y. Research ontherapeutics effect of extract of Ornithogalum caudatum on liver fibrosis. China J. Chin. Mater. Medica 2016, 41, 2303–2308. [Google Scholar] [CrossRef]
- Qu, Z.Y.; Shi, X.; Zou, X.; Ji, Y.B. Study on the Apoptotic mechanisms of human liver cancer HepG-2 cells induced by total saponins of Ornithogalum caudatum. Chin. Med. Mater. 2016, 39, 867–871. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, Y.; Zou, X.; Shi, X.; Fang, Y.; Liu, X.; Ji, Y. Purification of Total Saponins from Ornithogalum caudatum and its Antioxidant Activity. Nat. Prod. Res. Dev. 2016, 28, 404–408. [Google Scholar] [CrossRef]
- Shi, L.; Li, J.; Liu, Z.; Liu, S. Immunopotentiating effects of polysaccharides of the Ornithogalum caudatum Ait on non-specific and humoral immunity. Jilin. Univ. Med. Ed. 2002, 28, 232–234. [Google Scholar] [CrossRef]
- Chen, R.; Li, S.; Liu, Z.; Dong, H.; Li, Y.; Yang, S. Purification and Antitumor Activities of Polysaccharide from Ornithogalum caudatum Ait. Chin. Pharm. J. 2011, 46, 1630–1634. [Google Scholar] [CrossRef]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, Z.Y.; Sun, X.; Gao, H.; Zhang, Y.M. The preparation of three selenium-containing Cordyceps militaris polysaccharides: Characterization and anti-tumor activities. Int. J. Biol. Macromol. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, S.; Duan, X.; Sun, Q.; Qiu, L.; Bian, X.; Wu, Y.; Liu, A.; Li, C. A Method for Simulating Digestion of Polysaccharides and Selenide Derivatives of Edible Fungi In Vitro. CN112710822A, 27 April 2021. [Google Scholar]
- Li, Q.; Chen, G.; Chen, H.; Zhang, W.; Ding, Y.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice via MAPKs signal transduction pathway. Carbohydr. Polym. 2018, 196, 445–456. [Google Scholar] [CrossRef]
- Huang, S.; Yang, W.; Huang, G. Preparation and activities of selenium polysaccharide from plant such as Grifola frondosa. Carbohydr. Polym. 2020, 242, 116409. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and immunomodulatory activity of selenium exopolysaccharide produced by Lactococcus lactis subsp. lactis. Food Chem. 2013, 138, 84–89. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, B.; Xiang, X.; Wen, Z.; Zhou, Y.; Ma, J.; Qu, Y. Immunomodulatory effect of low-molecular-mass seleno-aminopolysaccharide in RAW264.7 macrophages. Food Sci. 2018, 39, 198–204. [Google Scholar] [CrossRef]
- Qin, T.; Chen, J.; Wang, D.; Hu, Y.; Wang, M.; Zhang, J.; Nguyen, T.L.; Liu, C.; Liu, X. Optimization of selenylation conditions for Chinese angelica polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 2013, 92, 645–650. [Google Scholar] [CrossRef]
- Shang, L.; Wu, S.; Zhang, C.; Liao, H.; Liu, X. Preparation, characterization and activity analysis of selenium-containing pumpkin polysaccharide. Food Sci. 2016, 37, 48–53. [Google Scholar] [CrossRef]
- Mu, X.; Shi, J.; Wang, Y.; Zhao, L.; Zhao, H.; Zhang, J. Preparation, Characterization and in Vitro Antioxidant Activity of Selenium Potentilla anserina L. Polysaccharide with Different Substitution Degrees. Sci. Technol. Food Ind. 2019, 40, 191–198. [Google Scholar] [CrossRef]
- Gao, P.; Bian, J.; Xu, S.; Liu, C.; Sun, Y.; Zhang, G.; Li, D.; Liu, X. Structural features, selenization modification, antioxidant and anti-tumor effects of polysaccharides from alfalfa roots. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Li, D.; Liu, C.; Jin, M.; Bian, J.; Lv, M.; Sun, Y.; Zhang, L.; Gao, P. The neuroprotective and antioxidant profiles of selenium-containing polysaccharides from the fruit of Rosa laevigata. Food Funct. 2018, 9, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, C.; Jing, L.; Feng, M.; Li, R.; Yang, Y. The structural characterization and immune modulation activitives comparison of Codonopsis pilosula polysaccharide (CPPS) and selenizing CPPS (sCPPS) on mouse in vitro and vivo. Int. J. Biol. Macromol. 2020, 160, 814–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, H.; Wang, D.; Wang, J.; Deng, Z.; Li, T.; He, Y.; Yang, Y.; Zhong, S. Physicochemical characterization and antitumor activity in vitro of a selenium polysaccharide from Pleurotus ostreatus. Int. J. Biol. Macromol. 2020, 165, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of selenium-containing polysaccharides isolated from selenium-enriched tea and its bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef]
- He, J.; Wu, Z.; Pan, D.; Guo, Y.; Zeng, X. Effect of selenylation modification on antitumor activity of peptidoglycan from Lactobacillus acidophilus. Carbohydr. Polym. 2017, 165, 344–350. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Selvaratnam, J.; Robaire, B. Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production. Exp. Gerontol. 2016, 84, 12–20. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.M.; Qi, Z.M.; Wang, S.Y.; Liu, S.X.; Li, X.; Wang, H.J.; Xia, X.C. An overview on natural polysaccharides with antioxidant properties. Curr. Med. Chem. 2013, 20, 2899–2913. [Google Scholar] [CrossRef]
- Zhao, F.F.; Zhou, Y.Z.; Gao, L.; Qin, X.M.; Du, G.H. Advances in the study of the rat model of aging induced by D-galactose. Acta Pharm. Sin. 2017, 52, 347–354. [Google Scholar] [CrossRef]
- Csiszar, A.; Yabluchanskiy, A.; Ungvari, A.; Ungvari, Z.; Tarantini, S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience 2019, 41, 609–617. [Google Scholar] [CrossRef]
- Flores-Mateo, G.; Elosua, R.; Rodriguez-Blanco, T.; Basora-Gallisà, J.; Bulló, M.; Salas-Salvadó, J.; Martínez-González, M.; Estruch, R.; Corella, D.; Fitó, M.; et al. Oxidative stress is associated with an increased antioxidant defense in elderly subjects: A multilevel approach. PLoS ONE 2014, 9, e105881. [Google Scholar] [CrossRef][Green Version]
- Kaur, N.; Sharma, A.K.; Shakeel, A.; Kumar, V.; Singh, A.; Gupta, A.; Suhag, D.; Rajput, S.K.; Mukherjee, M. Therapeutic Implications of Superoxide Dismutase And Its Importance in Kinase Drug Discovery. Curr. Top. Med. Chem. 2017, 17, 2495–2508. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q.; Xiao, Y.; Liu, X.; Li, Y.; Zhang, J.; Pan, J.; Zhang, Z. Trehalose targets Nrf2 signal to alleviate d-galactose induced aging and improve behavioral ability. Biochem. Biophys. Res. Commun. 2020, 521, 113–119. [Google Scholar] [CrossRef]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free. Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Singh, S.; Garg, G.; Singh, A.K.; Bissoyi, A.; Rizvi, S.I. Fisetin, a potential caloric restriction mimetic, attenuates senescence biomarkers in rat erythrocytes. Biochem. Cell Biol. 2019, 97, 480–487. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Yin, M.C. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by D-galactose. Food Chem. Toxicol. 2012, 50, 3198–3205. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Hao, C.; Xu, G.; Yang, J.; Sun, R. Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J. Food Drug Anal. 2017, 25, 766–775. [Google Scholar] [CrossRef]
- Qin, H.; Yang, C.; Fan, Y.; Zhu, Q. Establishment and evaluation of aging mouse model induced by D-galactose. Chin. J. Tissue Eng. Res. 2009, 13, 1275–1278. [Google Scholar]
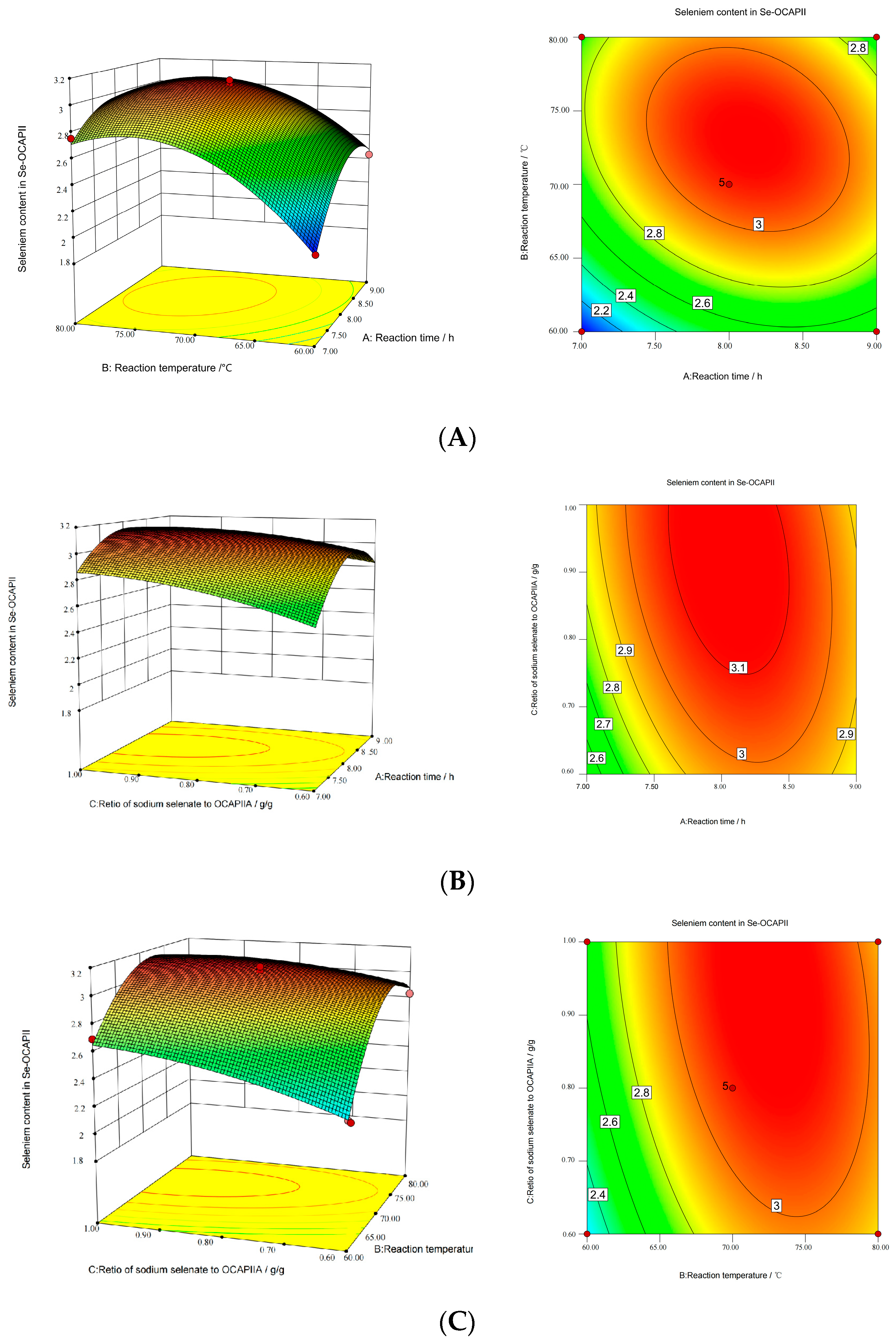

| Run Number | Coded Levels | Selenium Concentration (mg/g) | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| 1 | 8 | 60 | 1 | 2.693 |
| 2 | 8 | 70 | 0.8 | 3.076 |
| 3 | 9 | 70 | 1 | 2.889 |
| 4 | 9 | 80 | 0.8 | 2.663 |
| 5 | 7 | 70 | 0.6 | 2.438 |
| 6 | 8 | 60 | 0.6 | 2.253 |
| 7 | 7 | 80 | 0.8 | 2.754 |
| 8 | 7 | 70 | 1 | 2.766 |
| 9 | 8 | 70 | 0.8 | 3.053 |
| 10 | 9 | 60 | 0.8 | 2.44 |
| 11 | 8 | 70 | 0.8 | 3.081 |
| 12 | 8 | 70 | 0.8 | 3.091 |
| 13 | 8 | 70 | 0.8 | 3.125 |
| 14 | 8 | 80 | 0.6 | 2.833 |
| 15 | 8 | 80 | 1 | 2.936 |
| 16 | 7 | 60 | 0.8 | 2.006 |
| 17 | 9 | 70 | 0.6 | 2.862 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.63 | 9 | 0.18 | 83.63 | <0.0001 b |
| X1 | 0.099 | 1 | 0.099 | 45.85 | 0.0003 b |
| X2 | 0.40 | 1 | 0.40 | 186.32 | <0.0001 b |
| X3 | 0.10 | 1 | 0.10 | 46.68 | 0.0002 b |
| X1X2 | 0.069 | 1 | 0.069 | 31.91 | 0.0008 b |
| X1X3 | 0.023 | 1 | 0.023 | 10.49 | 0.0143 a |
| X2X3 | 0.028 | 1 | 0.028 | 13.15 | 0.0084 a |
| X12 | 0.33 | 1 | 0.33 | 152.58 | <0.0001 b |
| X22 | 0.49 | 1 | 0.49 | 225.05 | <0.0001 b |
| X32 | 0.019 | 1 | 0.019 | 8.68 | 0.0215 a |
| Residual | 0.015 | 7 | 0.0022 | ||
| Lack of fit | 0.012 | 3 | 0.0041 | 5.98 | 0.0584 c |
| Pure error | 0.0028 | 4 | 0.0007 | ||
| Total correlation | 1.64 | 16 | |||
| R2 | 0.9908 | ||||
| Adjusted R2 | 0.9789 | ||||
| Adeq Precision (signal-to-noise ratio) | 30.478 | ||||
| Coefficient of variation | 1.68 |
| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: reaction time (h) | 7 | 8 | 9 |
| X2: reaction temperature (°C) | 60 | 70 | 80 |
| X3: Na2SeO3-to-OCAPIIA mass ratio (g/g) | 0.6 | 0.8 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Qu, Z.; Ji, C.; Yang, X.; Zhang, Y.; Zou, X. Optimum Reaction Conditions for the Synthesis of Selenized Ornithogalum caudatum Ait. (Liliaceae) Polysaccharides and Measurement of Their Antioxidant Activity In Vivo. Molecules 2023, 28, 5929. https://doi.org/10.3390/molecules28155929
Sun R, Qu Z, Ji C, Yang X, Zhang Y, Zou X. Optimum Reaction Conditions for the Synthesis of Selenized Ornithogalum caudatum Ait. (Liliaceae) Polysaccharides and Measurement of Their Antioxidant Activity In Vivo. Molecules. 2023; 28(15):5929. https://doi.org/10.3390/molecules28155929
Chicago/Turabian StyleSun, Renshuang, Zhongyuan Qu, Chenfeng Ji, Xiaolong Yang, Yiqiao Zhang, and Xiang Zou. 2023. "Optimum Reaction Conditions for the Synthesis of Selenized Ornithogalum caudatum Ait. (Liliaceae) Polysaccharides and Measurement of Their Antioxidant Activity In Vivo" Molecules 28, no. 15: 5929. https://doi.org/10.3390/molecules28155929
APA StyleSun, R., Qu, Z., Ji, C., Yang, X., Zhang, Y., & Zou, X. (2023). Optimum Reaction Conditions for the Synthesis of Selenized Ornithogalum caudatum Ait. (Liliaceae) Polysaccharides and Measurement of Their Antioxidant Activity In Vivo. Molecules, 28(15), 5929. https://doi.org/10.3390/molecules28155929