Plant-Waste-Derived Sorbents for Nitazoxanide Adsorption
Abstract
1. Introduction
2. Results and Discussion
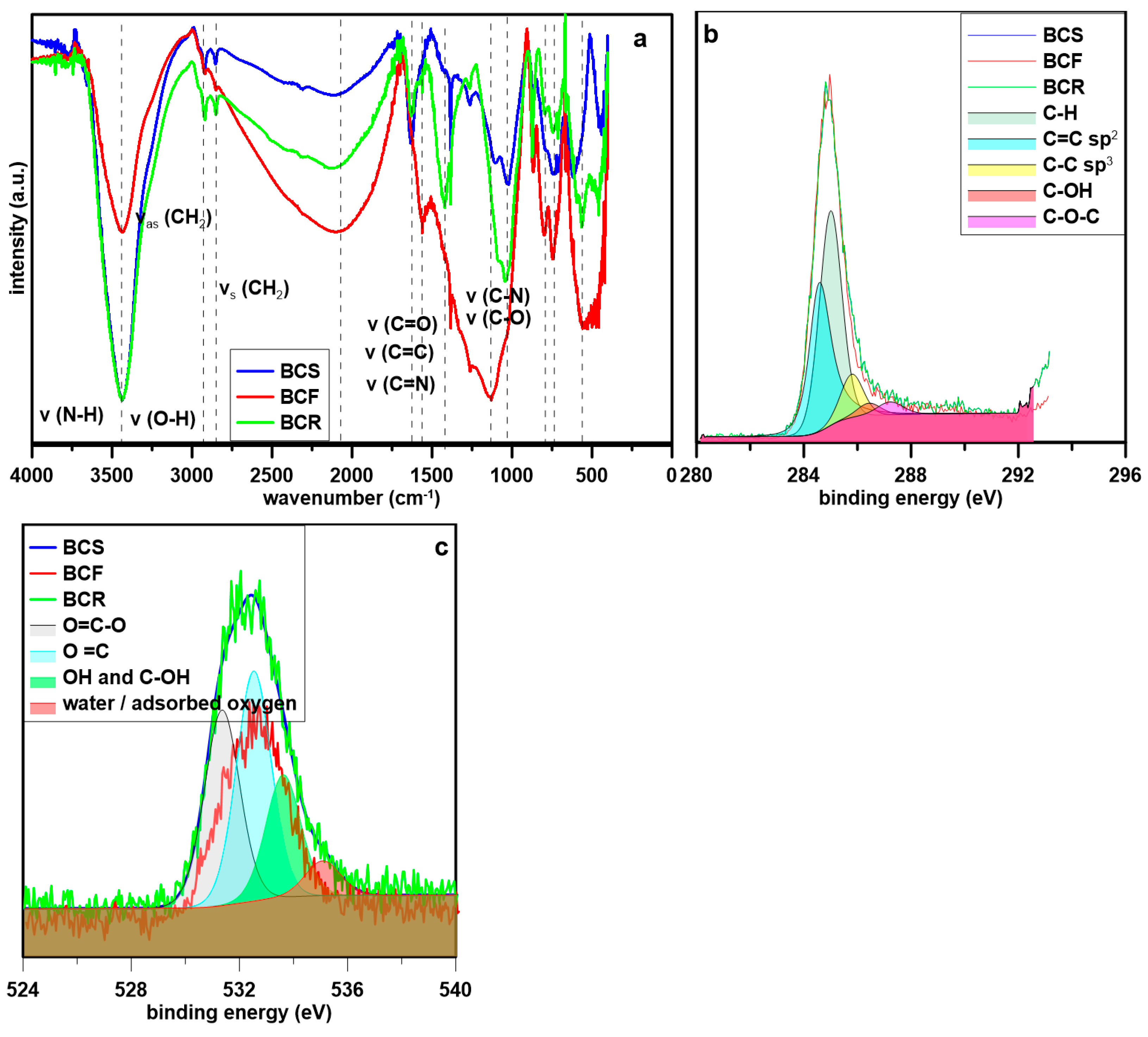
2.1. Biochar Physicochemical Properties
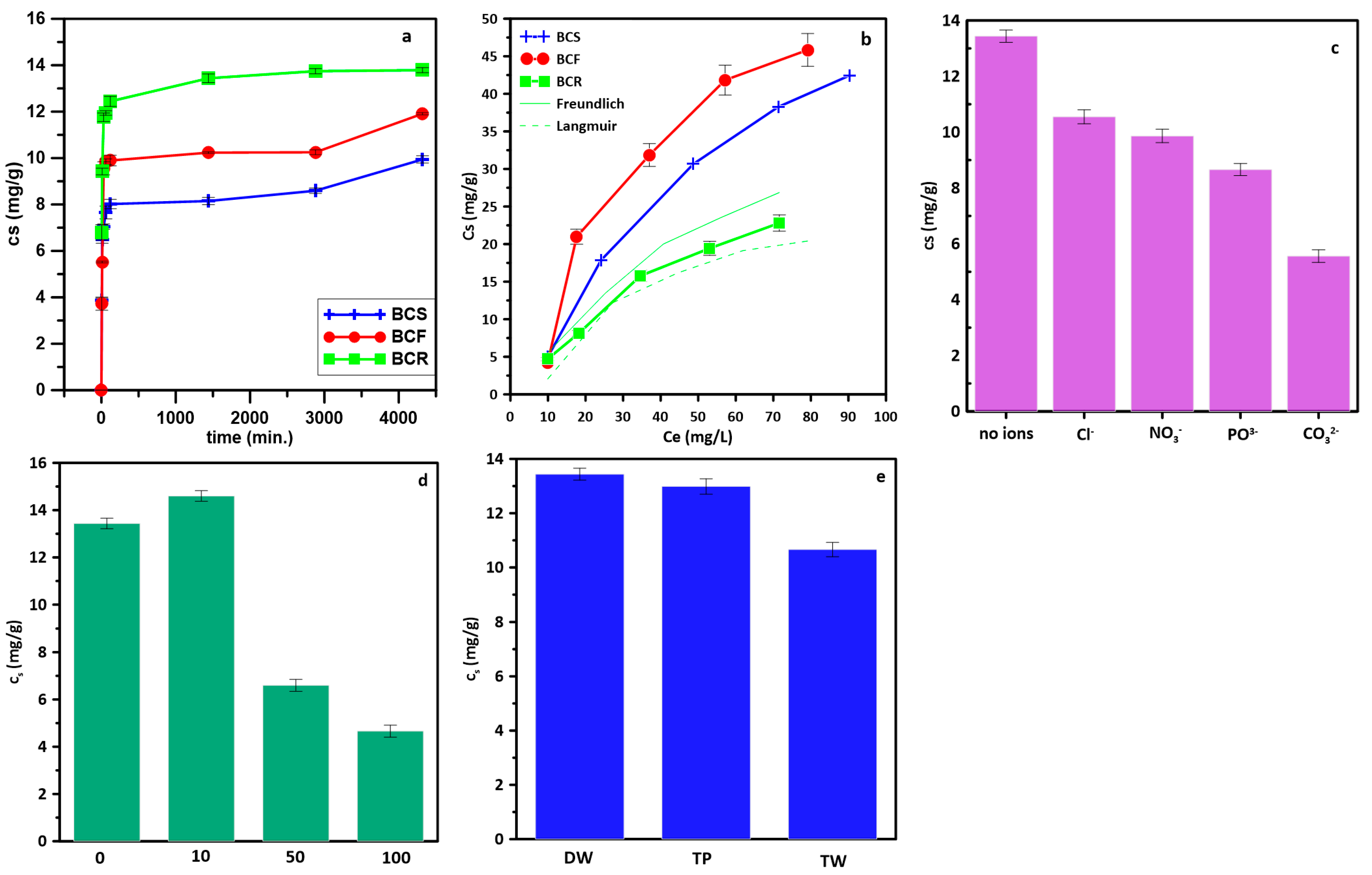
2.2. Adsorption Studies
3. Materials and Methods
3.1. Biochar Production and Characterization
3.2. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, S.; Yamamoto, N. Anti-Infective Nitazoxanide Disrupts Transcription of Ribosome Biogenesis-Related Genes in Yeast. Genes Genom. 2020, 42, 915–926. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Batiha, G.E.-S. Nitazoxanide and COVID-19: A Review. Mol. Biol. Rep. 2022, 49, 11169–11176. [Google Scholar] [CrossRef]
- Liu, L.X.; Weller, P.F. Antiparasitic Drugs. N. Engl. J. Med. 1996, 334, 1178–1184. [Google Scholar] [CrossRef]
- Musher, D.M.; Logan, N.; Hamill, R.J.; DuPont, H.L.; Lentnek, A.; Gupta, A.; Rossignol, J.-F. Nitazoxanide for the Treatment of Clostridium difficile Colitis. Clin. Infect. Dis. 2006, 43, 421–427. [Google Scholar] [CrossRef]
- Arnold, M.; Lang, E.; Modicano, P.; Bissinger, R.; Faggio, C.; Abed, M.; Lang, F. Effect of Nitazoxanide on Erythrocytes. Basic Clin. Pharmacol. Toxicol. 2014, 114, 421–426. [Google Scholar] [CrossRef]
- Sukanya, R.; Mohandoss, S.; Lee, Y.R. Synthesis of Active-Site Rich Molybdenum-Doped Manganese Tungstate Nanocubes for Effective Electrochemical Sensing of the Antiviral Drug (COVID-19) Nitazoxanide. Chemosphere 2023, 311, 137005. [Google Scholar] [CrossRef]
- Blum, V.F.; Cimerman, S.; Hunter, J.R.; Tierno, P.; Lacerda, A.; Soeiro, A.; Cardoso, F.; Bellei, N.C.; Maricato, J.; Mantovani, N.; et al. Nitazoxanide Superiority to Placebo to Treat Moderate COVID-19—A Pilot Prove of Concept Randomized Double-Blind Clinical Trial. eClinicalMedicine 2021, 37, 100981. [Google Scholar] [CrossRef]
- Fox, L.M.; Saravolatz, L.D. Nitazoxanide: A New Thiazolide Antiparasitic Agent. Clin. Infect. Dis. 2005, 40, 1173–1180. [Google Scholar] [CrossRef]
- AlGabbani, Q. Nanotechnology: A Promising Strategy for the Control of Parasitic Infections. Exp. Parasitol. 2023, 250, 108548. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Xiong, C.; Liu, F.; Gao, J.; Jiang, X. A N-Doped Porous Carbon Derived from Deep Eutectic Solvent for Adsorption of Organic Contaminants from Aqueous or Oil Solution. RSC Adv. 2020, 10, 34953–34958. [Google Scholar] [CrossRef]
- Adeola, A.O.; de Lange, J.; Forbes, P.B.C. Adsorption of Antiretroviral Drugs, Efavirenz and Nevirapine from Aqueous Solution by Graphene Wool: Kinetic, Equilibrium, Thermodynamic and Computational Studies. Appl. Surf. Sci. Adv. 2021, 6, 100157. [Google Scholar] [CrossRef]
- Leininger, A.; Ren, Z.J. Circular Utilization of Food Waste to Biochar Enhances Thermophilic Co-Digestion Performance. Bioresour. Technol. 2021, 332, 125130. [Google Scholar] [CrossRef]
- Zhang, X.; Gang, D.D.; Sun, P.; Lian, Q.; Yao, H. Goethite Dispersed Corn Straw-Derived Biochar for Phosphate Recovery from Synthetic Urine and Its Potential as a Slow-Release Fertilizer. Chemosphere 2021, 262, 127861. [Google Scholar] [CrossRef]
- Jeong, N.; Han, S.O.; Kim, H.; Kim, C.; Hong, S.; Park, S.; Jang, M. Synthesis of Calcium Aluminate Nanoflakes and Nanochains from Woody Biomass. Mater. Charact. 2015, 110, 68–76. [Google Scholar] [CrossRef]
- Alves, B.S.Q.; Zelaya, K.P.S.; Colen, F.; Frazão, L.A.; Napoli, A.; Parikh, S.J.; Fernandes, L.A. Effect of Sewage Sludge and Sugarcane Bagasse Biochar on Soil Properties and Sugar Beet Production. Pedosphere 2021, 31, 572–582. [Google Scholar] [CrossRef]
- Gui, X.; Liu, C.; Li, F.; Wang, J. Effect of Pyrolysis Temperature on the Composition of DOM in Manure-Derived Biochar. Ecotoxicol. Environ. Saf. 2020, 197, 110597. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P.; Różyło, K. Application of Different Carrying Gases and Ratio between Sewage Sludge and Willow for Engineered (Smart) Biochar Production. J. CO2 Util. 2019, 29, 20–28. [Google Scholar] [CrossRef]
- Monisha, R.S.; Mani, R.L.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Green Remediation of Pharmaceutical Wastes Using Biochar: A Review. Environ. Chem. Lett. 2022, 20, 681–704. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar Production and Applications in Agro and Forestry Systems: A Review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.-P.; Gerber, H. Biochar Organic Fertilizers from Natural Resources as Substitute for Mineral Fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar Production by Sewage Sludge Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of Humic and Fulvic Acids onto a Range of Adsorbents in Aqueous Systems, and Their Effect on the Adsorption of Other Species: A Review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Sadowska, U.; Zaleski, T.; Kuboń, M.; Latawiec, A.; Klimek-Kopyra, A.; Sikora, J.; Gliniak, M.; Kobyłecki, R.; Zarzycki, R. Effect of the Application of Sunflower Biochar and Leafy Trees Biochar on Soil Hydrological Properties of Fallow Soils and under Soybean Cultivation. Materials 2023, 16, 1737. [Google Scholar] [CrossRef]
- Bhanvase, B.; Sonawane, S.; Pawade, V.; Pandit, A. (Eds.) Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-821496-1. [Google Scholar]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the Intraparticle Diffusion Adsorption Kinetics Model: Interpretation, Solving Methods and Applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Matsui, Y.; Ando, N.; Yoshida, T.; Kurotobi, R.; Matsushita, T.; Ohno, K. Modeling High Adsorption Capacity and Kinetics of Organic Macromolecules on Super-Powdered Activated Carbon. Water Res. 2011, 45, 1720–1728. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Carbon Nanostructures for Hydrogen Storage. In Solid-State Hydrogen Storage; Elsevier: Amsterdam, The Netherlands, 2008; pp. 261–287. ISBN 978-1-84569-270-4. [Google Scholar]
- Singh, A.K. Engineered Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-801406-6. [Google Scholar]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, Isotherms and Thermodynamic Modeling of Liquid Phase Adsorption of Rhodamine B Dye onto Raphia Hookerie Fruit Epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Nam, S.-W.; Choi, D.-J.; Kim, S.-K.; Her, N.; Zoh, K.-D. Adsorption Characteristics of Selected Hydrophilic and Hydrophobic Micropollutants in Water Using Activated Carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Różyło, K.; Jędruchniewicz, K.; Krasucka, P.; Biszczak, W.; Oleszczuk, P. Physicochemical Characteristics of Biochar from Waste Cricket Chitin (Acheta Domesticus). Molecules 2022, 27, 8071. [Google Scholar] [CrossRef] [PubMed]
- Lazar, G.; Ureche, D.; Ifrim, I.L.; Stamate, M.; Ureche, C.; Nedeff, V.; Nistor, I.D.; Finaru, A.L.; Lazar, I.M. Effects of the Environmental Stress on Two Fish Populations Revealed by Statistical and Spectral Analysis. Environ. Eng. Manag. J. 2012, 11, 109–124. [Google Scholar] [CrossRef]
- Mecozzi, M.; Pietroletti, M.; Scarpiniti, M.; Acquistucci, R.; Conti, M.E. Monitoring of Marine Mucilage Formation in Italian Seas Investigated by Infrared Spectroscopy and Independent Component Analysis. Environ. Monit. Assess. 2012, 184, 6025–6036. [Google Scholar] [CrossRef]
- Tomczyk, B.; Siatecka, A.; Jędruchniewicz, K.; Sochacka, A.; Bogusz, A.; Oleszczuk, P. Polycyclic Aromatic Hydrocarbons (PAHs) Persistence, Bioavailability and Toxicity in Sewage Sludge- or Sewage Sludge-Derived Biochar-Amended Soil. Sci. Total Environ. 2020, 747, 141123. [Google Scholar] [CrossRef] [PubMed]
- Koinuma, M.; Tateishi, H.; Hatakeyama, K.; Miyamoto, S.; Ogata, C.; Funatsu, A.; Taniguchi, T.; Matsumoto, Y. Analysis of Reduced Graphene Oxides by X-Ray Photoelectron Spectroscopy and Electrochemical Capacitance. Chem. Lett. 2013, 42, 924–926. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Dideikin, A.T.; Kirilenko, D.A.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile Reduction of Graphene Oxide Suspensions and Films Using Glass Wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, G.; Heredia-Guerrero, J.A.; Masood, M.T.; Ceseracciu, L.; Davis, A.; Carzino, R.; Prato, M.; Bayer, I.S.; Athanassiou, A. Highly Effective Antiadhesive Coatings from PH-Modified Water-Dispersed Perfluorinated Acrylic Copolymers: The Case of Vulcanizing Rubber. Adv. Mater. Interfaces 2016, 3, 1600069. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.-H.; Park, S.B. Oxygen Functional Groups and Electrochemical Capacitive Behavior of Incompletely Reduced Graphene Oxides as a Thin-Film Electrode of Supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, B.; Wiącek, A.E.; Ćwikła-Bundyra, W.; Naebe, M. Sustainable Carbon Microtube Derived from Cotton Waste for Environmental Applications. Chem. Eng. J. 2018, 361, 1605–1616. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; Zhou, B.; Yuan, R.; Chen, Z.; Chen, H. Removal of PFASs from Water by Carbon-Based Composite Photocatalysis with Adsorption and Catalytic Properties: A Review. Sci. Total Environ. 2022, 836, 155652. [Google Scholar] [CrossRef]
- Diniz, V.; Gasparini Fernandes Cunha, D.; Rath, S. Adsorption of Recalcitrant Contaminants of Emerging Concern onto Activated Carbon: A Laboratory and Pilot-Scale Study. J. Environ. Manag. 2023, 325, 116489. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and Adsorption Capacities of Biochar for the Removal of Organic and Inorganic Pollutants from Industrial Wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Saleh, T.A. Isotherm Models of Adsorption Processes on Adsorbents and Nanoadsorbents. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 34, pp. 99–126. ISBN 978-0-12-849876-7. [Google Scholar]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis Tef) Agricultural Waste. J. Thermodyn. 2013, 2013, 375830. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, H.; Pan, W.; Wang, C.; Liang, Y. The Role of Dissolved Organic Matter during Per- and Polyfluorinated Substance (PFAS) Adsorption, Degradation, and Plant Uptake: A Review. J. Hazard. Mater. 2022, 436, 129139. [Google Scholar] [CrossRef]
- Keerthanan, S.; Bhatnagar, A.; Mahatantila, K.; Jayasinghe, C.; Ok, Y.S.; Vithanage, M. Engineered Tea-Waste Biochar for the Removal of Caffeine, a Model Compound in Pharmaceuticals and Personal Care Products (PPCPs), from Aqueous Media. Environ. Technol. Innov. 2020, 19, 100847. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]

| Compound | Groundwater | River Water | WWTP on River Doubs (Eastern France) | WWTP Effluents (Greece Volos) | WWTP Influents (Portugal Coimbra) | WWTP Effluents (Portugal Coimbra) | Hospital Effluents (Portugal Coimbra) |
|---|---|---|---|---|---|---|---|
| Metronidazole | 4.9–35.6 Taiwan | 0.05–13.51 Bangladesh | 35.8 | 35.2 | 0–113 | 19.4–83.5 | 1559–12,315 |
| Thiabendazole | (detection limit −1.6 China Jiulong River) | 0–15.3 | 0.493–12.1 | 9.17–1746 | |||
| Albendazole | 1.9 Serbia | 0–1.79 | 28.3 |
| SBET 1 (m2/g) | Vp 2 (cm3/g) | D 3 (nm) | Ash 4 (%) | N 5 (%) | C 5 (%) | H 5 (%) | O 6 (%) | H/C (-) | (O + N)/C (-) | O/C (-) | TC 7 (mg/g) | IC 8 (mg/g) | TOC 9 (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCS | 0.093 | 0.001424 | 95.41 | 14.99 | 1.03 | 77.84 | 1.48 | 4.65 | 0.019 | 0.073 | 0.060 | 768.02 | 0.55 | 767.46 |
| BCF | 0.941 | 0.000798 | 14.04 | 6.70 | 0.96 | 86.71 | 1.67 | 3.96 | 0.019 | 0.057 | 0.046 | 878.52 | 0 | 878.52 |
| BCR | 26.05 | 0.004706 | 10.63 | 25.93 | 3.66 | 23.49 | 0.57 | 46.35 | 0.024 | 2.129 | 1.973 | 633.00 | 1.97 | 631.00 |
| PFO | PSO | Elovich | IPD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | k1 | q1 | R2 | k2 | q2 | R2 | α | β | R2 | KIPD | β | R2 | |
| BCS | 9.94 | 3.852 | 0.974 | 0.5269 | 0.0156 | 9.53 | 0.9914 | 1055.2 | 1.667 | 0.7665 | 0.05 | 6.21 | 0.5876 |
| BCF | 10.25 | 3.828 | 1.052 | 0.3022 | 0.0323 | 11.41 | 0.9922 | 147.0 | 1.139 | 0.6889 | 0.07 | 7.17 | 0.4461 |
| BCR | 13.44 | 13.996 | 1.066 | 0.9601 | 0.2371 | 13.80 | 0.9999 | 1720.6 | 0.985 | 0.7871 | 0.07 | 10.05 | 0.5205 |
| L | F | T | DR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QL | KL ×10−3 | R2 | RL | KF | n | R2 | QT | B | R2 | QD | E | B | R2 | |
| BCS | 133.62 | 3.73 | 0.9762 | 0.67 | 0.653 | 0.961 | 0.9576 | 0.1272 | 144.53 | 0.9961 | 35.485 | 118.11 | 220.03 | 0.9461 |
| BCF | 34.93 | 12.17 | 0.8652 | 0.70 | 0.594 | 1.053 | 0.8432 | 0.1405 | 126.32 | 0.9838 | 43.898 | 110.08 | 253.27 | 0.9925 |
| BCR | 418.55 | 1.18 | 0.9996 | 0.67 | 0.464 | 1.033 | 0.9975 | 0.1152 | 143.77 | 0.9524 | 30.480 | 125.09 | 196.15 | 0.8294 |
| Material | pH | Temp. [°C] | Surface Area [m2/g] | Langmuir Sorption Capacity [mg/g] | Recovery/ Regeneration |
|---|---|---|---|---|---|
| Carbon materials F400 | 7 | 25 | 919 | 248.6 | Yes |
| Carbon materials ACF | 7 | 25 | 1441 | 249.2 | Yes |
| Carbon materials MWCNT | 7 | 25 | 144 | 49.8 | Yes |
| Carbon materials CMK-3 | 7 | 25 | 917 | 219.3 | Yes |
| Carbon materials MWCNT-HNO3 | 7 | 25 | 31 | 54.4 | Yes |
| Carbon materials F400-HNO3 | 7 | 25 | 823 | 234.4 | Yes |
| Commercial activated carbon (S) | 6–7 | 25 | 1225 | 328.61 | No |
| Commercial activated carbon (M) | 6–7 | 25 | 1301 | 213.94 | No |
| Petroleum coke activated carbon © | 6–7 | 25 | 848 | 287.53 | No |
| Siris Seedpod activated carbon | 7 | 30 | 1676.61 | 180.77 | No |
| Siris Seedpod KOH activated carbon | 7 | 30 | 1824.88 | 191.68 | No |
| BCR—this study | 6–7 | 25 | 26.05 | 418.55 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowski, A.; Jędruchniewicz, K.; Kobyłecki, R.; Zarzycki, R.; Różyło, K.; Wang, H.; Czech, B. Plant-Waste-Derived Sorbents for Nitazoxanide Adsorption. Molecules 2023, 28, 5919. https://doi.org/10.3390/molecules28155919
Sokołowski A, Jędruchniewicz K, Kobyłecki R, Zarzycki R, Różyło K, Wang H, Czech B. Plant-Waste-Derived Sorbents for Nitazoxanide Adsorption. Molecules. 2023; 28(15):5919. https://doi.org/10.3390/molecules28155919
Chicago/Turabian StyleSokołowski, Artur, Katarzyna Jędruchniewicz, Rafał Kobyłecki, Robert Zarzycki, Krzysztof Różyło, Haitao Wang, and Bożena Czech. 2023. "Plant-Waste-Derived Sorbents for Nitazoxanide Adsorption" Molecules 28, no. 15: 5919. https://doi.org/10.3390/molecules28155919
APA StyleSokołowski, A., Jędruchniewicz, K., Kobyłecki, R., Zarzycki, R., Różyło, K., Wang, H., & Czech, B. (2023). Plant-Waste-Derived Sorbents for Nitazoxanide Adsorption. Molecules, 28(15), 5919. https://doi.org/10.3390/molecules28155919