Oxide Materials for Thermoelectric Conversion
Abstract
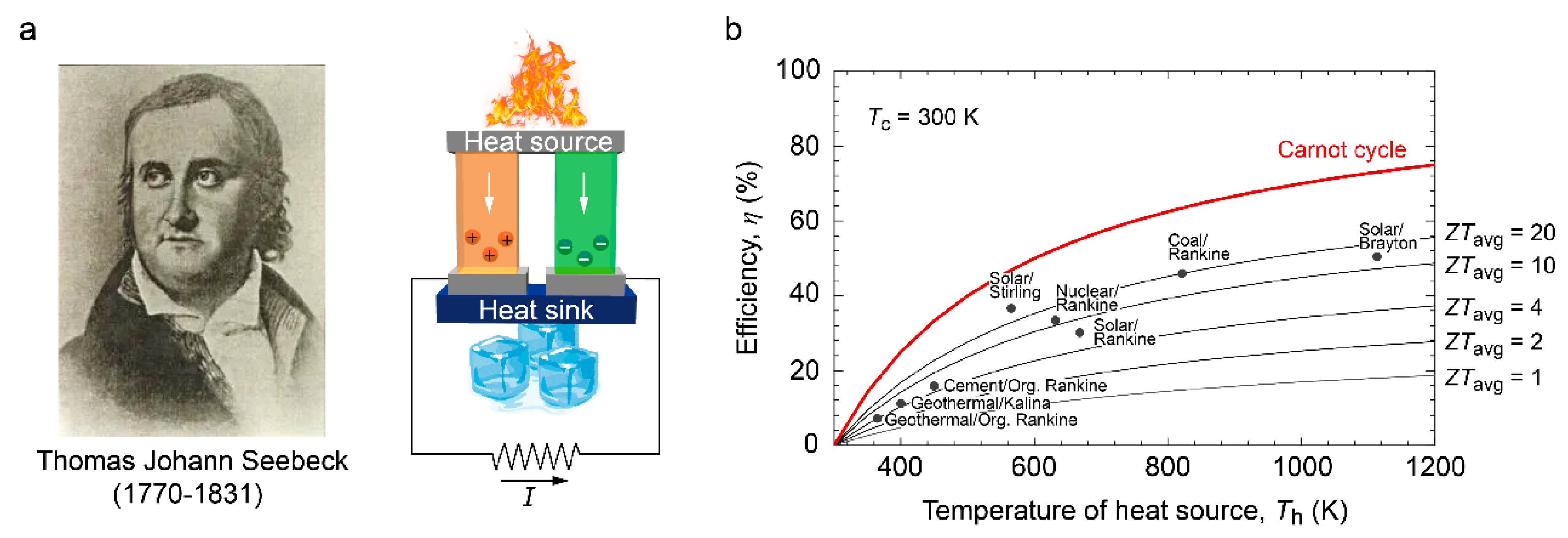
1. Introduction
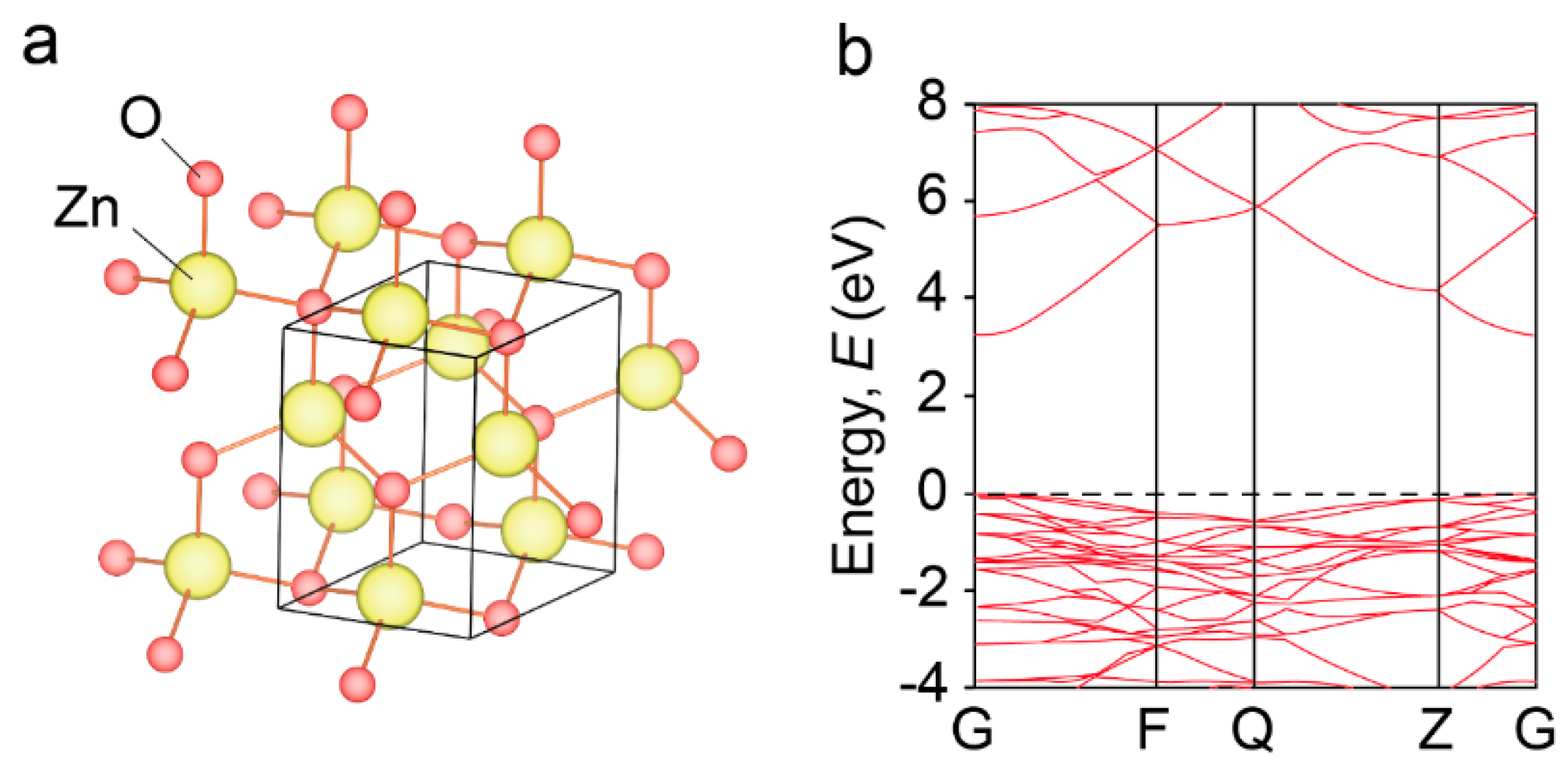
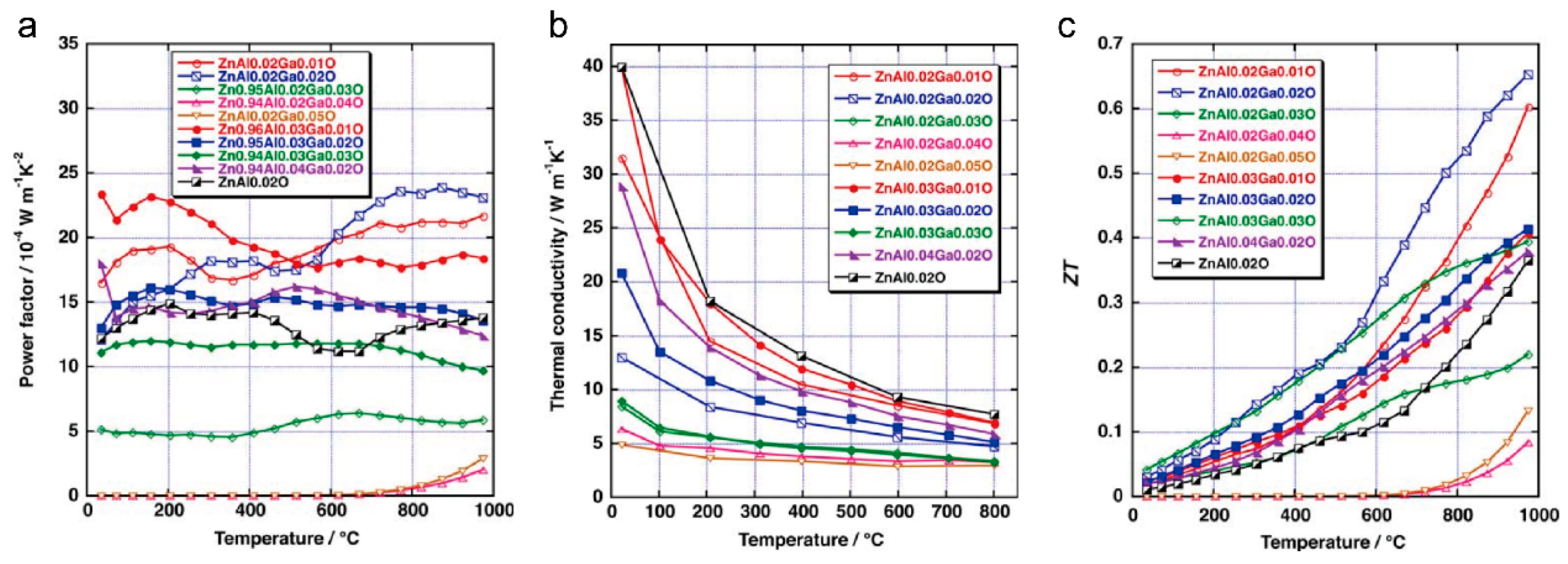
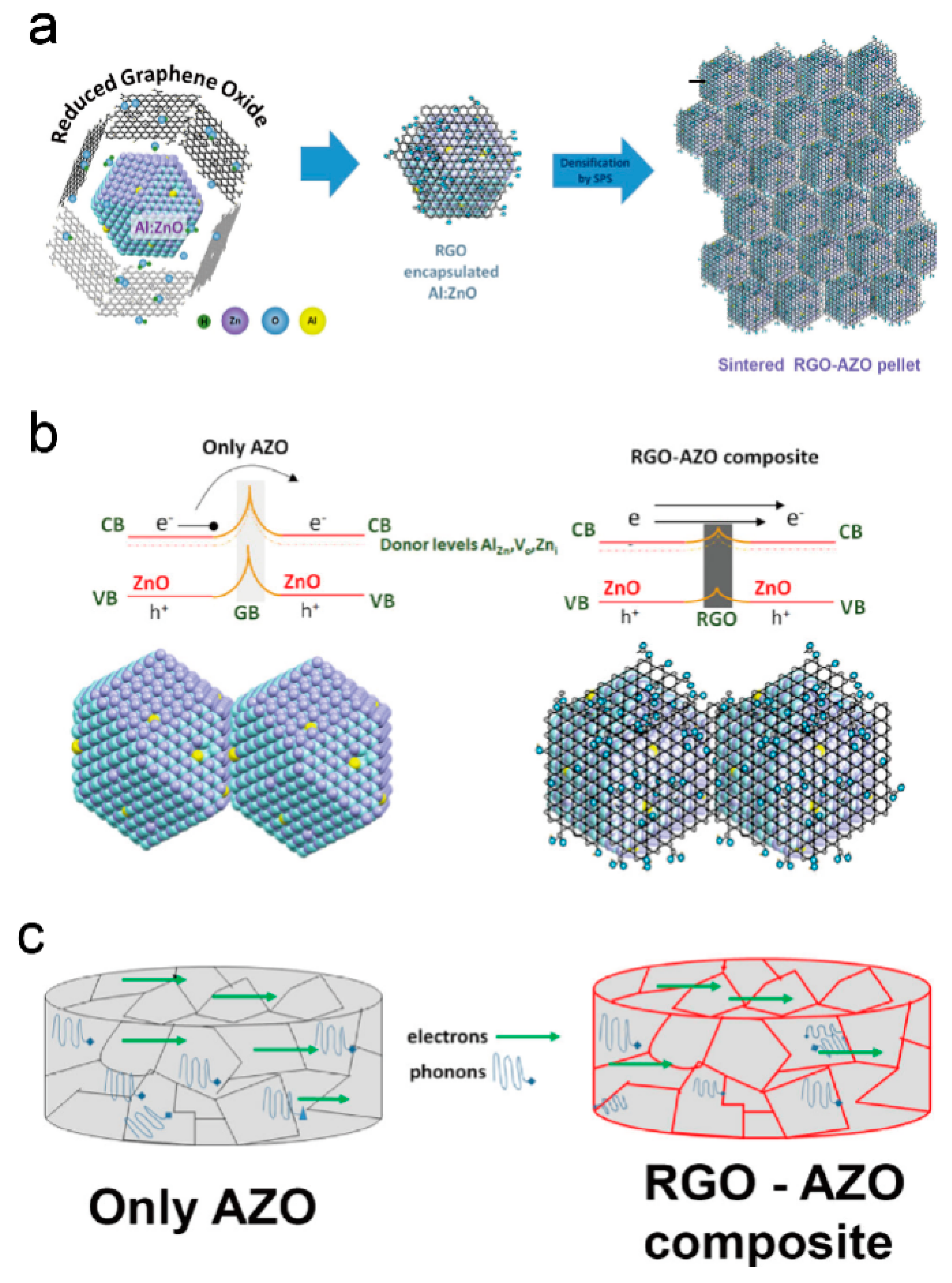
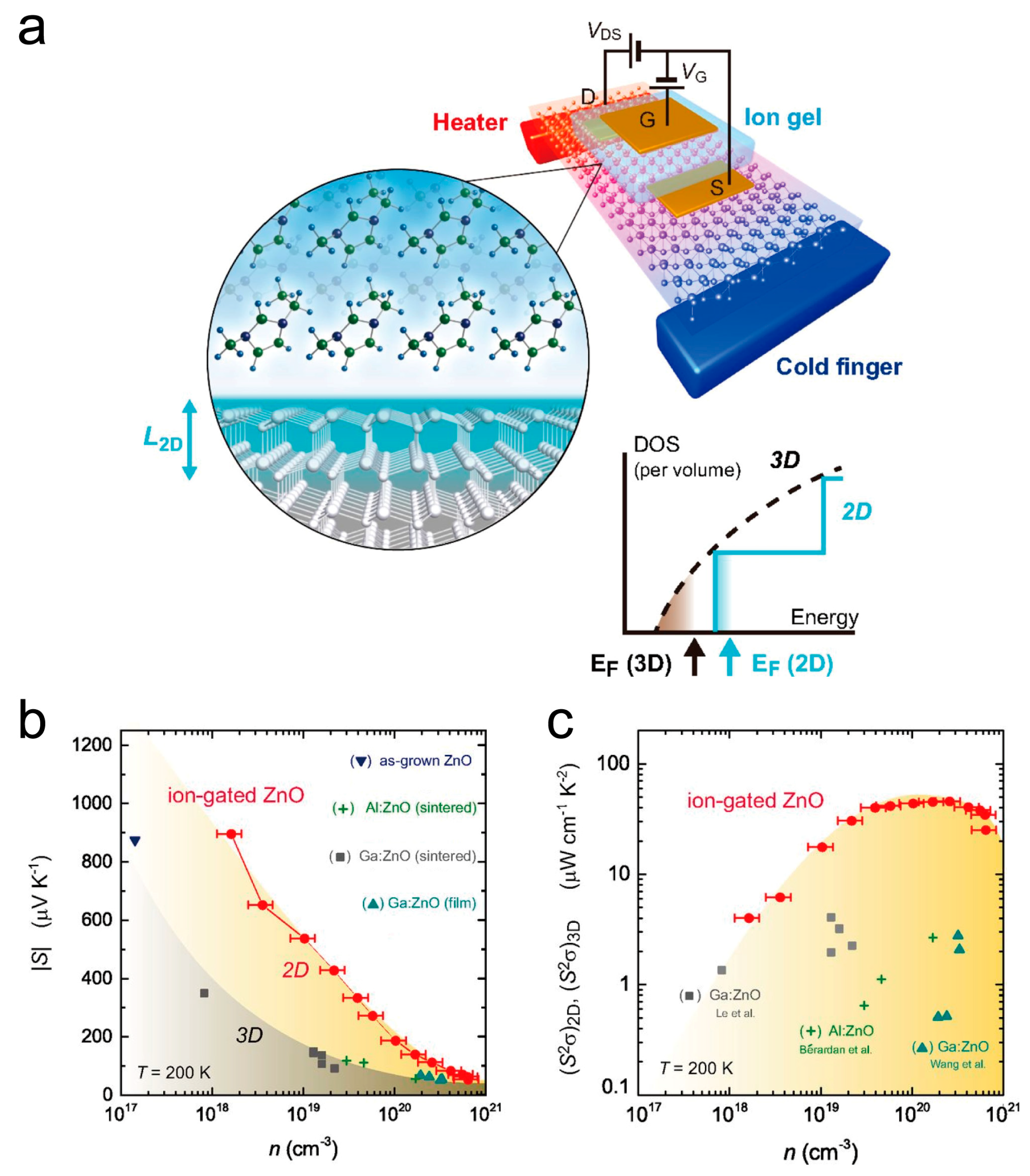
2. ZnO
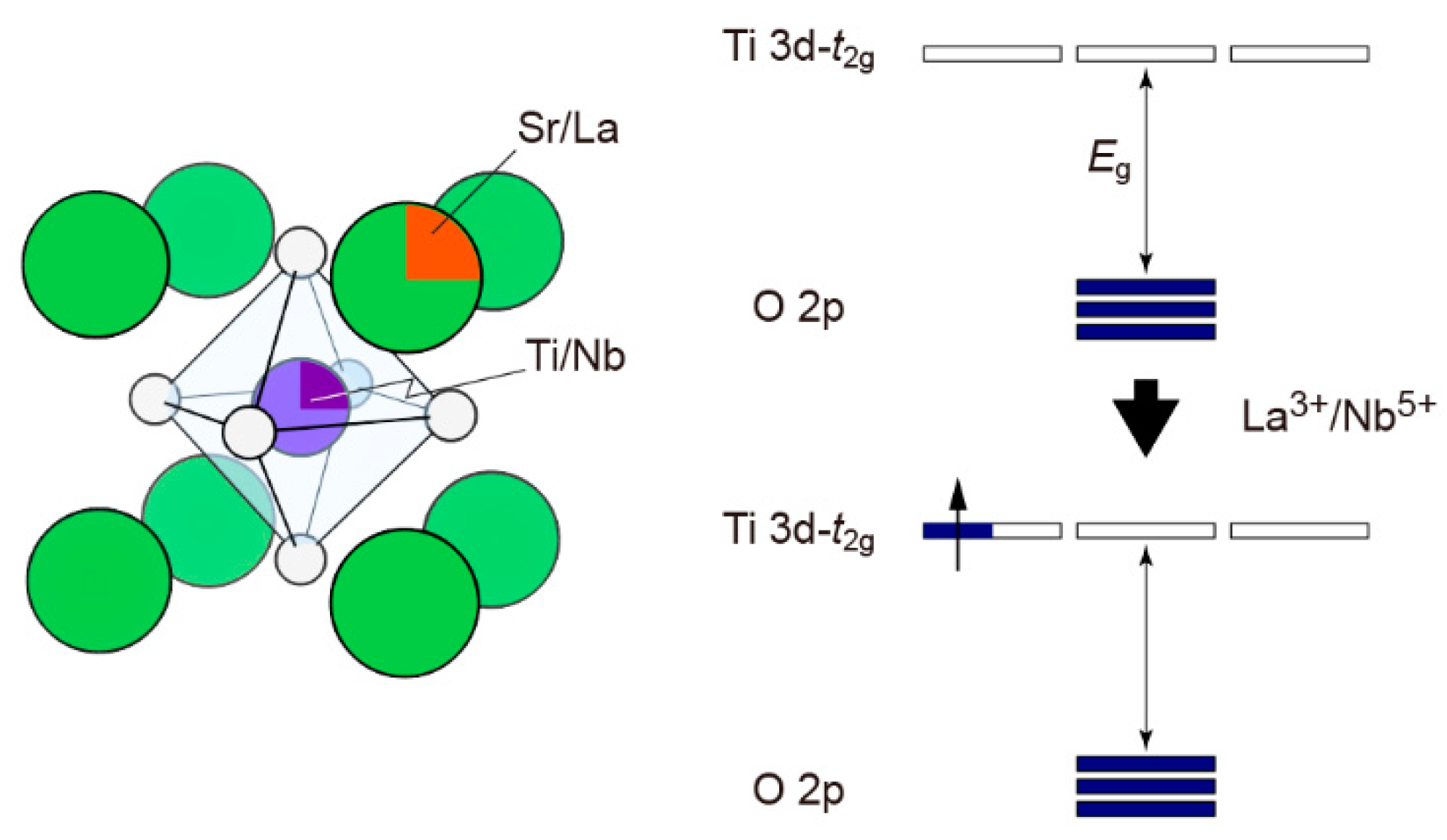
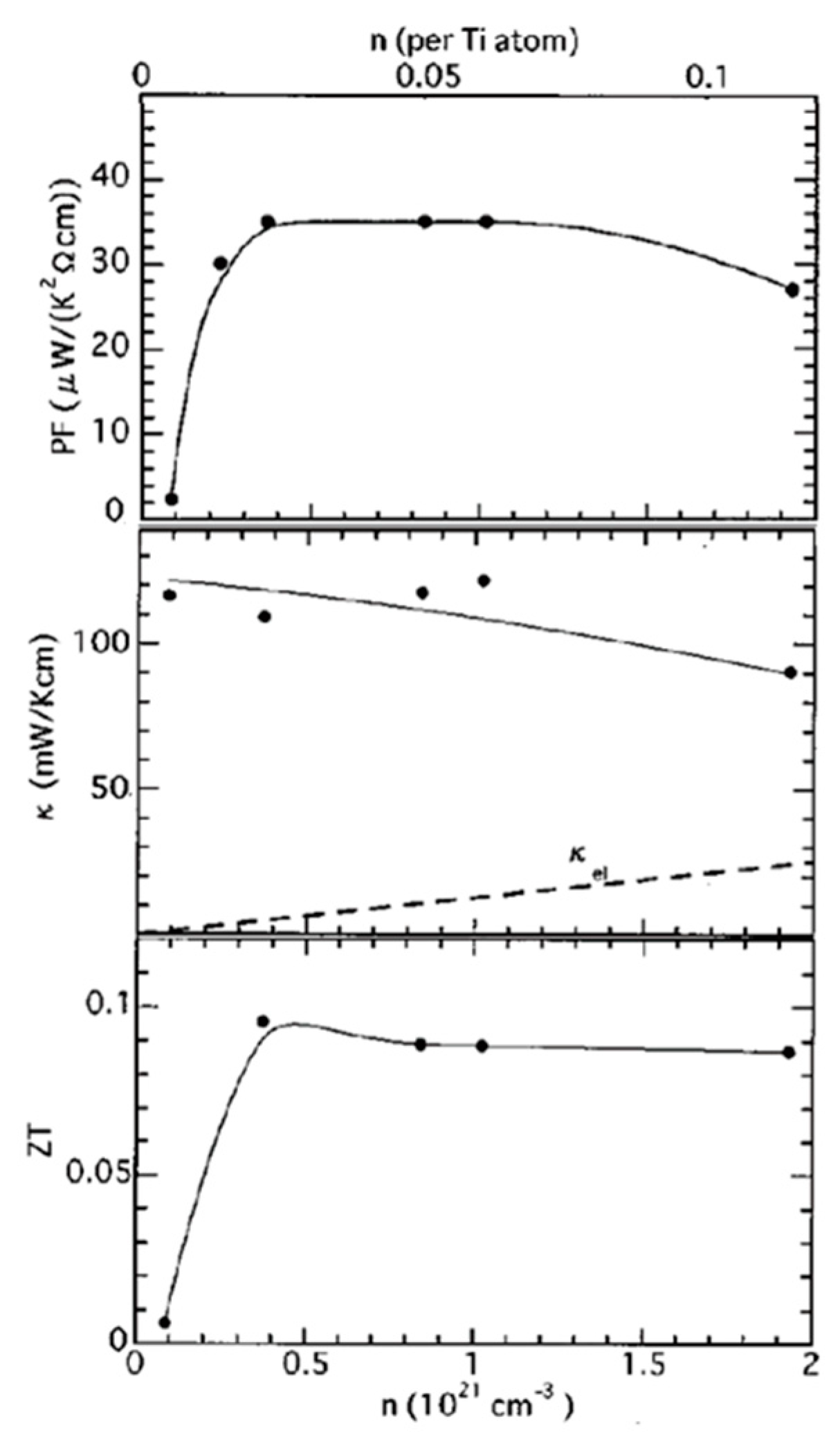
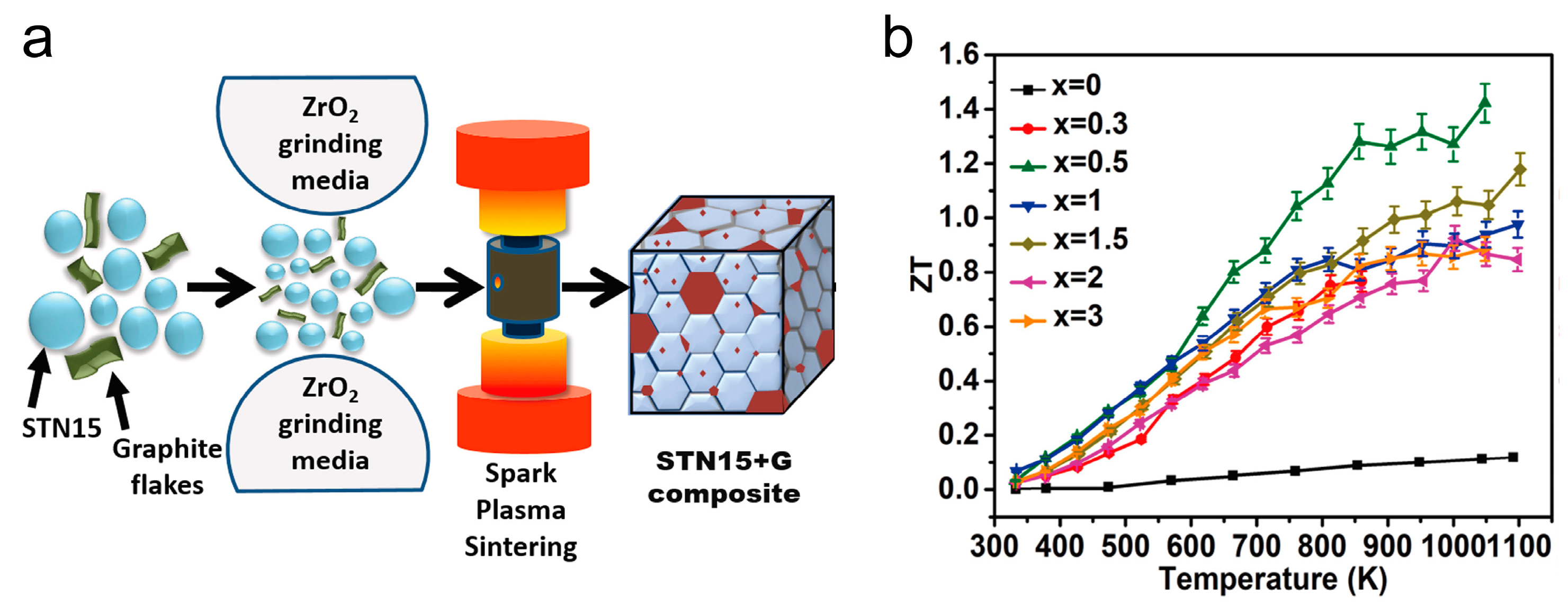
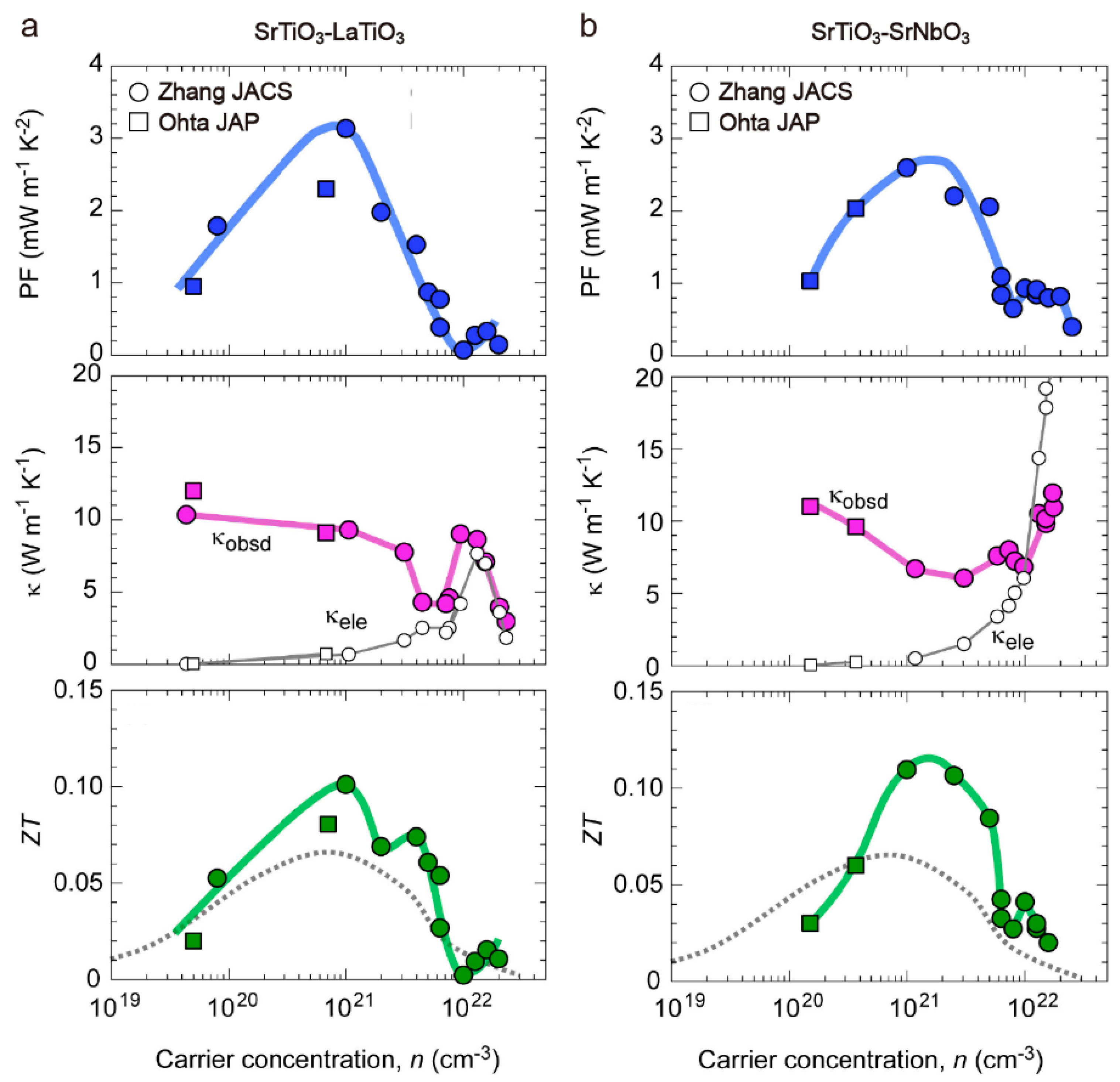
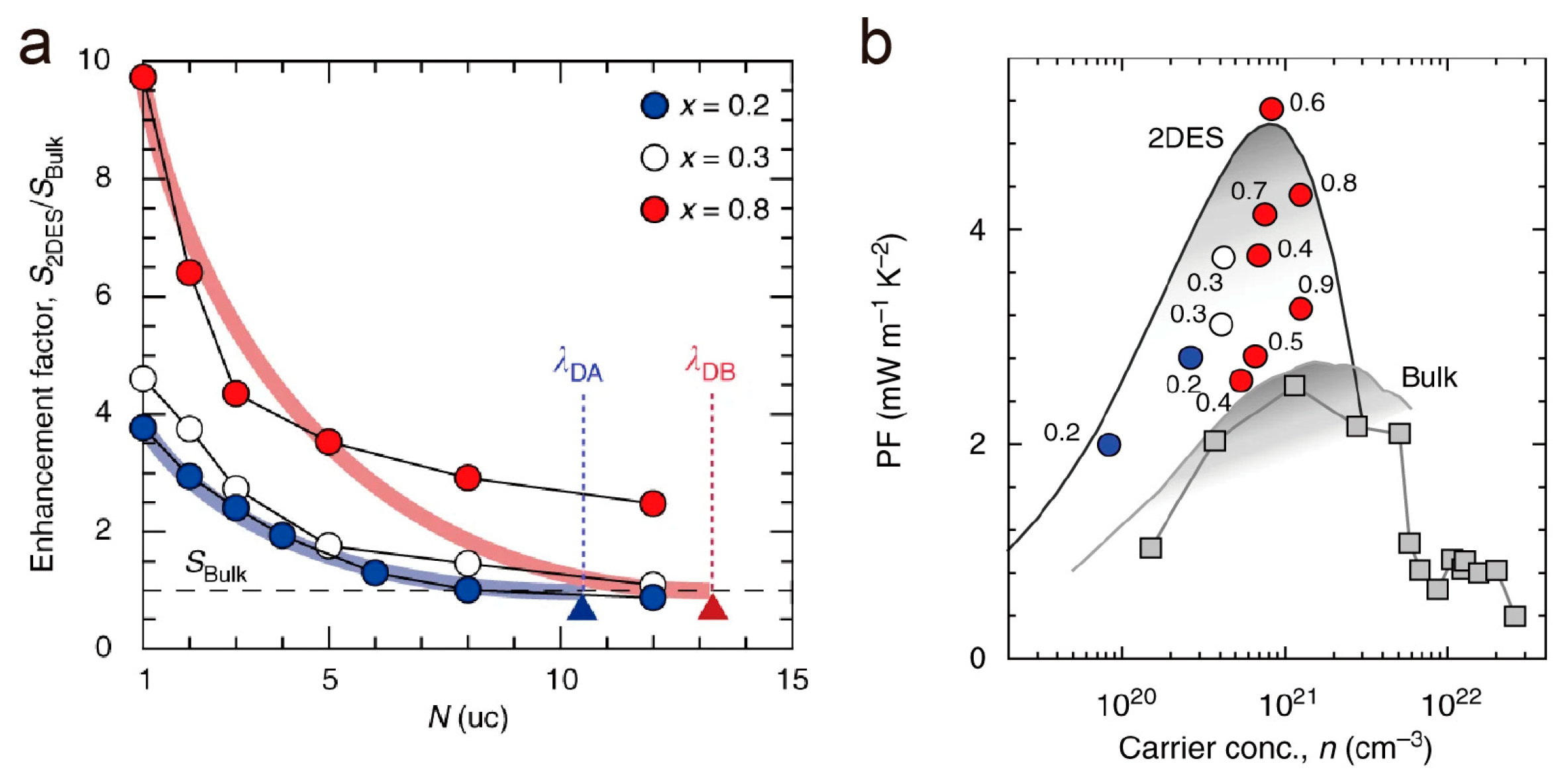
3. SrTiO3
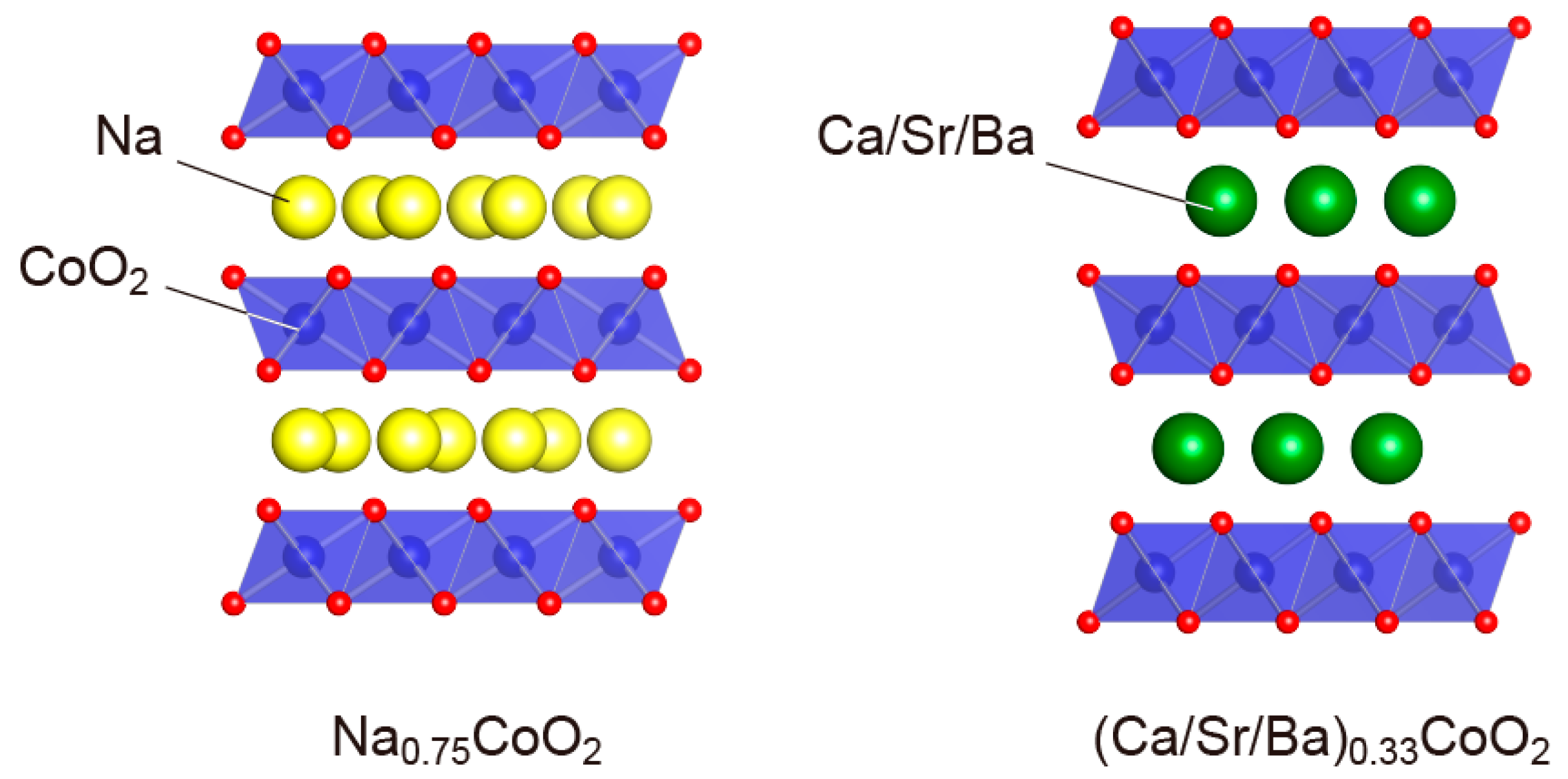
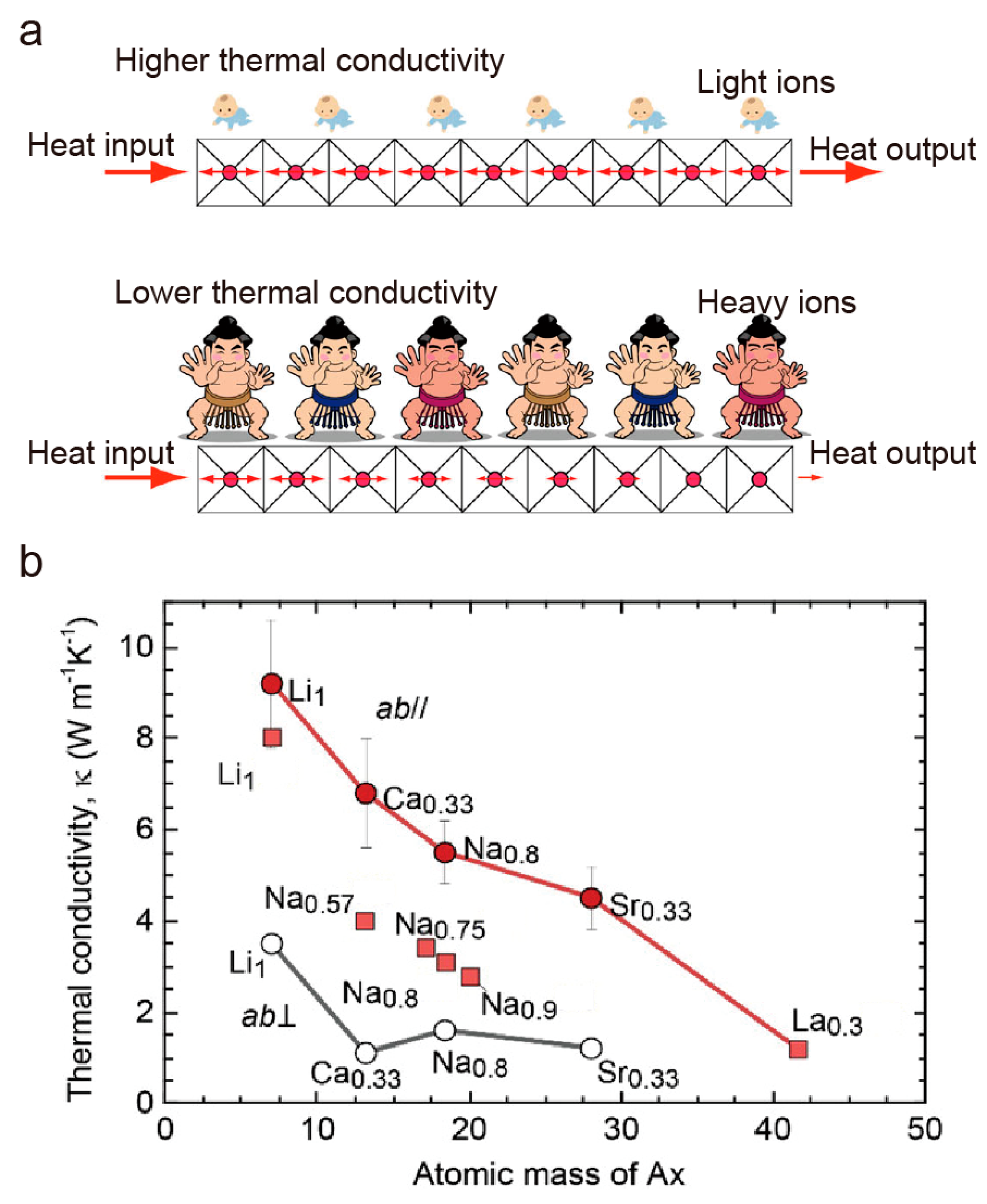
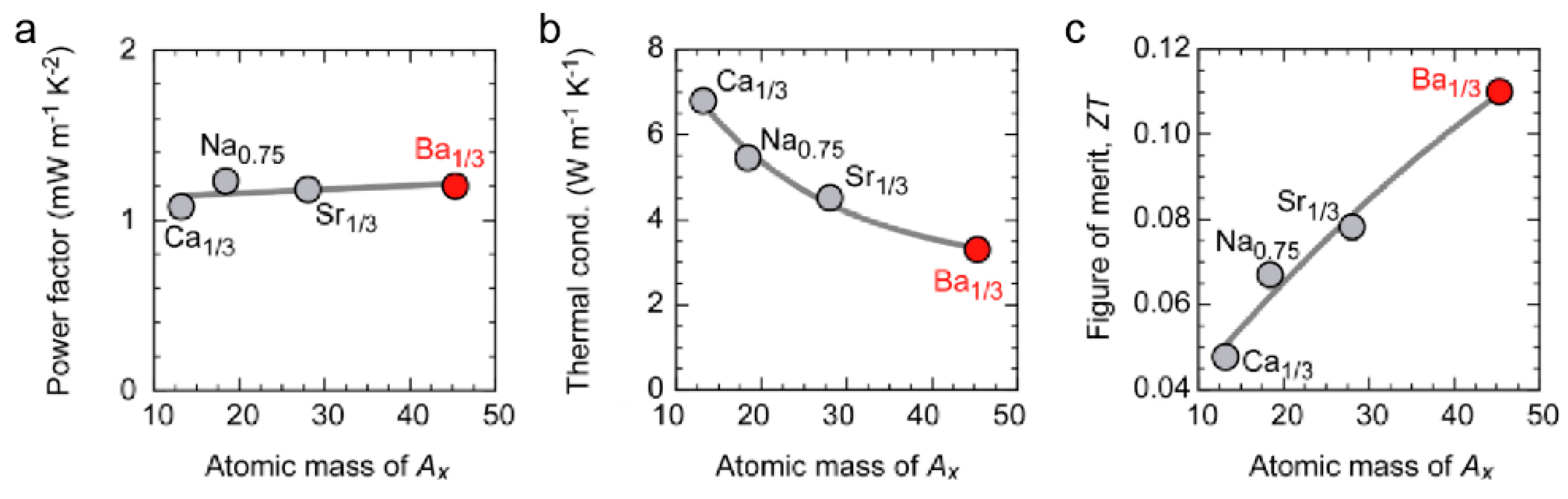
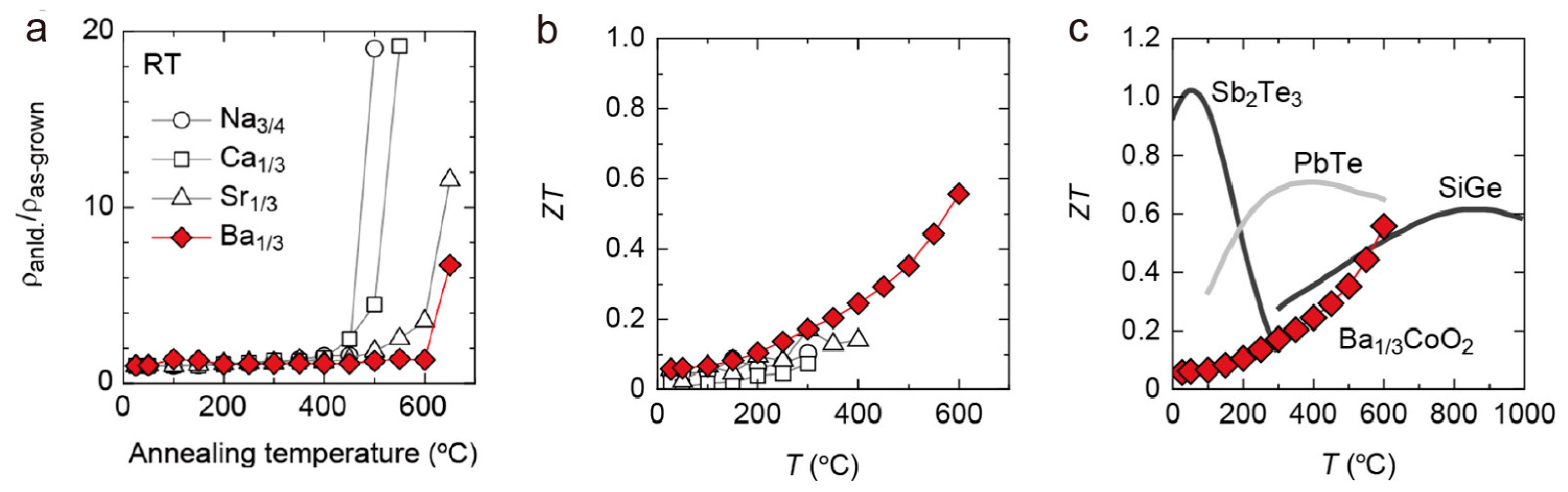
4. Layered Cobalt Oxides (AxCoO2 (Ax = Na0.75, Ca0.33, Sr0.33, Ba0.33))
5. Summary and Perspective
Funding
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Liu, Y.; Wang, B.; Fang, L.; Qiu, J.; Zhang, K.; Wang, S. Exceptional thermoelectric properties of flexible organic−inorganic hybrids with monodispersed and periodic nanophase. Nat. Commun. 2018, 9, 3817. [Google Scholar] [CrossRef]
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Sustainable development goals for people and planet. Nature 2013, 495, 305–307. [Google Scholar] [CrossRef]
- Rowe, D.M. CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Goldsmid, H.J. Introduction to Thermoelectricity; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Seebeck, T. Abh. K. Akad. Wiss 1823, 265, 1822. [Google Scholar]
- Vining, C.B. An inconvenient truth about thermoelectrics. Nat. Mater. 2009, 8, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Rull-Bravo, M.; Moure, A.; Fernández, J.F.; Martín-González, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2015, 5, 41653–41667. [Google Scholar] [CrossRef]
- Quinn, R.J.; Bos, J.-W.G. Advances in half-Heusler alloys for thermoelectric power generation. Mater. Adv. 2021, 2, 6246–6266. [Google Scholar] [CrossRef]
- Scheele, M.; Oeschler, N.; Veremchuk, I.; Peters, S.-O.; Littig, A.; Kornowski, A.; Klinke, C.; Weller, H. Thermoelectric Properties of Lead Chalcogenide Core-Shell Nanostructures. Acs Nano 2011, 5, 8541–8551. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Pedersen, S.H.; Yin, H.; Hung, L.T.; Iversen, B.B. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat. Commun. 2017, 8, 13901. [Google Scholar] [CrossRef]
- Liu, W.D.; Wang, D.Z.; Liu, Q.; Zhou, W.; Shao, Z.; Chen, Z.G. High-Performance GeTe-Based Thermoelectrics: From Materials to Devices. Adv. Energy Mater. 2020, 10, 2000367. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Day, T.; Zhang, T.; Liu, H.; Shi, X.; Chen, L.; Snyder, G.J. High Thermoelectric Performance in Non-Toxic Earth-Abundant Copper Sulfide. Adv. Mater. 2014, 26, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef]
- Chang, C.; Wu, M.; He, D.; Pei, Y.; Wu, C.-F.; Wu, X.; Yu, H.; Zhu, F.; Wang, K.; Chen, Y.; et al. 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 2018, 360, 778–783. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, Y.K.; Yu, Y.; Byun, S.; Luo, Z.-Z.; Lee, H.; Ge, B.; Lee, Y.-L.; Chen, X.; Lee, J.Y.; et al. Polycrystalline SnSe with a thermoelectric figure of merit greater than the single crystal. Nat. Mater. 2021, 20, 1378–1384. [Google Scholar] [CrossRef]
- Su, L.; Wang, D.; Wang, S.; Qin, B.; Wang, Y.; Qin, Y.; Jin, Y.; Chang, C.; Zhao, L.-D. High thermoelectric performance realized through manipulating layered phonon-electron decoupling. Science 2022, 375, 1385–1389. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Andrews, J.P. XXV. Variation with oxygen pressure of the thermoelectric power of cadmium oxide. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1949, 40, 273–282. [Google Scholar] [CrossRef]
- Parravano, G. Thermoelectric behavior of nickel oxide. J. Chem. Phys. 1955, 23, 5–10. [Google Scholar] [CrossRef]
- Hutson, A. Electronic properties of ZnO. J. Phys. Chem. Solids 1959, 8, 467–472. [Google Scholar] [CrossRef]
- Arvin, M. Electrical conductivity and thermoelectric power of indium oxide. Phys. Chem. Solids 1962, 23, 1681–1683. [Google Scholar] [CrossRef]
- Thurber, W.; Mante, A. Thermal Conductivity and Thermoelectric Power of Rutile (TiO2). Phys. Rev. 1965, 139, A1655. [Google Scholar] [CrossRef]
- Marley, J.; Dockerty, R. Electrical properties of stannic oxide single crystals. Phys. Rev. 1965, 140, A304. [Google Scholar] [CrossRef]
- Young, A.; Schwartz, C. Electrical conductivity and thermoelectric power of Cu2O. J. Phys. Chem. Solids 1969, 30, 249–252. [Google Scholar] [CrossRef]
- Griffiths, B.; Elwell, D.; Parker, R. The thermoelectric power of the system NiFe2O4-Fe3O4. Philos. Mag. 1970, 22, 163–174. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Possible high Tc superconductivity in the Ba−La−Cu−O system. Z. Für Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Cooper, J.; Alavi, B.; Zhou, L.; Beyermann, W.; Grüner, G. Thermoelectric power of some high-Tc oxides. Phys. Rev. B 1987, 35, 8794. [Google Scholar] [CrossRef]
- Chen, J.; McEwan, C.; Wenger, L.; Logothetis, E. Determination of charge carriers in superconducting La-Ba-Cu-O by thermoelectric measurements. Phys. Rev. B 1987, 35, 7124. [Google Scholar] [CrossRef]
- Mitra, N.; Trefny, J.; Yarar, B.; Pine, G.; Sheng, Z.; Hermann, A. Thermoelectric power of the Tl-Ca-Ba-Cu-O superconductor. Phys. Rev. B 1988, 38, 7064. [Google Scholar] [CrossRef]
- Ohtaki, M.; Koga, H.; Tokunaga, T.; Eguchi, K.; Arai, H. Electrical transport properties and high-temperature thermoelectric performance of (Ca0.9M0.1)MnO3 (M = Y, La, Ce, Sm, In, Sn, Sb, Pb, Bi). J. Solid State Chem. 1995, 120, 105–111. [Google Scholar] [CrossRef]
- Ohtaki, M.; Tsubota, T.; Eguchi, K.; Arai, H. High-temperature thermoelectric properties of (Zn1−xAlx)O. J. Appl. Phys. 1996, 79, 1816–1818. [Google Scholar] [CrossRef]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 1997, 56, R12685–R12687. [Google Scholar] [CrossRef]
- Masset, A.; Michel, C.; Maignan, A.; Hervieu, M.; Toulemonde, O.; Studer, F.; Raveau, B.; Hejtmanek, J. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys. Rev. B 2000, 62, 166. [Google Scholar] [CrossRef]
- Funahashi, R.; Matsubara, I.; Ikuta, H.; Takeuchi, T.; Mizutani, U.; Sodeoka, S. An oxide single crystal with high thermoelectric performance in air. Jpn. J. Appl. Phys. 2000, 39, L1127. [Google Scholar] [CrossRef]
- Ishikawa, R.; Ono, Y.; Miyazaki, Y.; Kajitani, T. Low-Temperature Synthesis and Electric Properties of New Layered Cobaltite, SrxCoO2. Jpn. J. Appl. Phys. 2002, 41, L337–L339. [Google Scholar] [CrossRef]
- Kanno, T.; Yotsuhashi, S.; Adachi, H. Anisotropic thermoelectric properties in layered cobaltite AxCoO2 (A = Sr and Ca) thin films. Appl. Phys. Lett. 2004, 85, 739–741. [Google Scholar] [CrossRef]
- Takashima, Y.; Zhang, Y.-Q.; Wei, J.; Feng, B.; Ikuhara, Y.; Cho, H.J.; Ohta, H. Layered cobalt oxide epitaxial films exhibiting thermoelectric ZT = 0.11 at room temperature. J. Mater. Chem. A 2021, 9, 274–280. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wu, L.; Tsuruta, A.; Mikami, M.; Cho, H.J.; Ohta, H. Ba1/3CoO2: A Thermoelectric Oxide Showing a Reliable ZT of ~0.55 at 600 °C in Air. ACS Appl. Mater. Interfaces 2022, 14, 33355–33360. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Nakanishi, K.; Miyasaka, S.; Tokura, Y. Large thermoelectric response of metallic perovskites: Sr1−xLaxTiO3 (0 < ~x < ~0.1). Phys. Rev. B 2001, 63, 113104. [Google Scholar] [CrossRef]
- Ohta, S.; Nomura, T.; Ohta, H.; Koumoto, K. High-temperature carrier transport and thermoelectric properties of heavily La-or Nb-doped SrTiO3 single crystals. J. Appl. Phys. 2005, 97, 034106. [Google Scholar] [CrossRef]
- Ohta, S.; Nomura, T.; Ohta, H.; Hirano, M.; Hosono, H.; Koumoto, K. Large thermoelectric performance of heavily Nb-doped SrTiO3 epitaxial film at high temperature. Appl. Phys. Lett. 2005, 87, 092108. [Google Scholar] [CrossRef]
- Lu, Z.L.; Zhang, H.R.; Lei, W.; Sinclair, D.C.; Reaney, I.M. High-Figure-of-Merit Thermoelectric La-Doped A-Site-Deficient SrTiO3 Ceramics. Chem Mater 2016, 28, 925–935. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.-Y.; Kang, H.-J.; Li, Y.; Yaer, X.; Li, J.-F.; Tan, Q.; Zhang, S.; Fan, G.-H.; Liu, C.-Y.; et al. Record high thermoelectric performance in bulk SrTiO3 via nano-scale modulation doping. Nano Energy 2017, 35, 387–395. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, J.; Ghorpade, U.; Shin, H.H.; Gang, M.G.; Park, S.D.; Kim, H.-J.; Lee, D.S.; Kim, J.H. Comparison study of ZnO-based quaternary TCO materials for photovoltaic application. J. Alloys Compd. 2019, 793, 499–504. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Le, A.T.; Ahmadipour, M.; Pung, S.-Y. A review on ZnO-based piezoelectric nanogenerators: Synthesis, characterization techniques, performance enhancement and applications. J. Alloys Compd. 2020, 844, 156172. [Google Scholar] [CrossRef]
- Tsubota, T.; Ohtaki, M.; Eguchi, K.; Arai, H. Thermoelectric properties of Al-doped ZnO as a promising oxide material for high-temperature thermoelectric conversion. J. Mater. Chem. 1997, 7, 85–90. [Google Scholar] [CrossRef]
- Wu, X.; Lee, J.; Varshney, V.; Wohlwend, J.L.; Roy, A.K.; Luo, T. Thermal Conductivity of Wurtzite Zinc-Oxide from First-Principles Lattice Dynamics—A Comparative Study with Gallium Nitride. Sci. Rep. 2016, 6, 22504. [Google Scholar] [CrossRef]
- Wu, M.; Sun, D.; Tan, C.; Tian, X.; Huang, Y. Al-Doped ZnO Monolayer as a Promising Transparent Electrode Material: A First-Principles Study. Materials 2017, 10, 359. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Peleckis, G.; Wang, X.; Siegel, R.W.; Borca-Tasciuc, T.; Dou, S.X.; Ramanath, G. Al-Doped Zinc Oxide Nanocomposites with Enhanced Thermoelectric Properties. Nano Lett. 2011, 11, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Seong, J.K.; Nahm, S. Improvement of thermoelectric properties with the addition of Sb to ZnO. J. Alloys Compd. 2008, 455, 331–335. [Google Scholar] [CrossRef]
- Colder, H.; Guilmeau, E.; Harnois, C.; Marinel, S.; Retoux, R.; Savary, E. Preparation of Ni-doped ZnO ceramics for thermoelectric applications. J. Eur. Ceram. Soc. 2011, 31, 2957–2963. [Google Scholar] [CrossRef]
- Liang, X. Thermoelectric Transport Properties of Fe-Enriched ZnO with High-Temperature Nanostructure Refinement. ACS Appl. Mater. Interfaces 2015, 7, 7927–7937. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, M.; Araki, K.; Yamamoto, K. High Thermoelectric Performance of Dually Doped ZnO Ceramics. J. Electron. Mater. 2009, 38, 1234–1238. [Google Scholar] [CrossRef]
- Acharya, S.; Yu, B.-K.; Hwang, J.; Kim, J.; Kim, W. High Thermoelectric Performance of ZnO by Coherent Phonon Scattering and Optimized Charge Transport. Adv. Funct. Mater. 2021, 31, 2105008. [Google Scholar] [CrossRef]
- Biswas, S.; Singh, S.; Singh, S.; Chattopadhyay, S.; De Silva, K.K.H.; Yoshimura, M.; Mitra, J.; Kamble, V.B. Selective Enhancement in Phonon Scattering Leads to a High Thermoelectric Figure-of-Merit in Graphene Oxide-Encapsulated ZnO Nanocomposites. ACS Appl. Mater. Interfaces 2021, 13, 23771–23786. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Ren, G.-K.; Tan, X.; Liu, R.; Liu, C.; Lin, Y.-H.; Nan, C.-W. Enhancing the thermoelectric performance of ZnO epitaxial films by Ga doping and thermal tuning. J. Mater. Chem. A 2018, 6, 24128–24135. [Google Scholar] [CrossRef]
- Shimizu, S.; Bahramy, M.S.; Iizuka, T.; Ono, S.; Miwa, K.; Tokura, Y.; Iwasa, Y. Enhanced thermopower in ZnO two-dimensional electron gas. Proc. Natl. Acad. Sci. USA 2016, 113, 6438–6443. [Google Scholar] [CrossRef]
- Hutson, A.R. Piezoelectric Scattering and Phonon Drag in ZnO and CdS. J. Appl. Phys. 1961, 32, 2287–2292. [Google Scholar] [CrossRef]
- Bérardan, D.; Byl, C.; Dragoe, N. Influence of the Preparation Conditions on the Thermoelectric Properties of Al-Doped ZnO. J. Am. Ceram. Soc. 2010, 93, 2352–2358. [Google Scholar] [CrossRef]
- Le, T.N.H.; Hou, F.; Vo, T.T.X.; Pham, Q.N.; Berardan, D.; Dragoe, N. Preparation and study of thermoelectric properties of fine grains GdxZn1−xO. Phys. Status Solidi A 2013, 210, 2693–2698. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Tang, W. Extracting the effective mass of electrons in transparent conductive oxide thin films using Seebeck coefficient. Appl. Phys. Lett. 2014, 104, 212103. [Google Scholar] [CrossRef]
- Okhay, O.; Zlotnik, S.; Xie, W.; Orlinski, K.; Hortiguela Gallo, M.J.; Otero-Irurueta, G.; Fernandes, A.J.S.; Pawlak, D.A.; Weidenkaff, A.; Tkach, A. Thermoelectric performance of Nb-doped SrTiO3 enhanced by reduced graphene oxide and Sr deficiency cooperation. Carbon 2019, 143, 215–222. [Google Scholar] [CrossRef]
- Shi, X.-L.; Wu, H.; Liu, Q.; Zhou, W.; Lu, S.; Shao, Z.; Dargusch, M.; Chen, Z.-G. SrTiO3-based thermoelectrics: Progress and challenges. Nano Energy 2020, 78, 105195. [Google Scholar] [CrossRef]
- Acharya, M.; Jana, S.S.; Ranjan, M.; Maiti, T. High performance (ZT > 1) n-type oxide thermoelectric composites from earth abundant materials. Nano Energy 2021, 84, 105905. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, B.; Hayashi, H.; Tohei, T.; Tanaka, I.; Ikuhara, Y.; Ohta, H. Thermoelectric phase diagram of the SrTiO3–SrNbO3 solid solution system. J. Appl. Phys. 2017, 121, 185102. [Google Scholar] [CrossRef]
- Zhang, Y.; Sugo, K.; Cho, H.J.; Ohta, H. Thermoelectric phase diagram of the SrTiO3-LaTiO3 solid-solution system through a metal to Mott insulator transition. J. Appl. Phys. 2019, 126, 075104. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, H.J.; Sugo, K.; Mikami, M.; Woo, S.; Jung, M.C.; Zhuang, Y.H.; Feng, B.; Sheu, Y.M.; Shin, W.; et al. Low thermal conductivity of SrTiO3−LaTiO3 and SrTiO3−SrNbO3 thermoelectric oxide solid solutions. J. Am. Ceram. Soc. 2021, 104, 4075–4085. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B 1993, 47, 12727–12731. [Google Scholar] [CrossRef]
- Ohta, H.; Kim, S.; Mune, Y.; Mizoguchi, T.; Nomura, K.; Ohta, S.; Nomura, T.; Nakanishi, Y.; Ikuhara, Y.; Hirano, M.; et al. Giant thermoelectric Seebeck coefficient of a two-dimensional electron gas in SrTiO3. Nat. Mater. 2007, 6, 129–134. [Google Scholar] [CrossRef]
- Hung, N.T.; Hasdeo, E.H.; Nugraha, A.R.T.; Dresselhaus, M.S.; Saito, R. Quantum effects in the thermoelectric power factor of low-dimensional semiconductors. Phys. Rev. Lett. 2016, 117, 036602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, B.; Hayashi, H.; Chang, C.-P.; Sheu, Y.-M.; Tanaka, I.; Ikuhara, Y.; Ohta, H. Double thermoelectric power factor of a 2D electron system. Nat. Commun. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Banerjee, R.; Chatterjee, S.; Ranjan, M.; Bhattacharya, T.; Mukherjee, S.; Jana, S.S.; Dwivedi, A.; Maiti, T. High-Entropy Perovskites: An Emergent Class of Oxide Thermoelectrics with Ultralow Thermal Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 17022–17032. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, M.; Zhang, W.; Yi, D.; Lan, J.; Nan, C.-W.; Lin, Y.-H. Electrical and thermal transport behaviours of high-entropy perovskite thermoelectric oxides. J. Adv. Ceram. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Koshibae, W.; Tsutsui, K.; Maekawa, S. Thermopower in cobalt oxides. Phys. Rev. B 2000, 62, 6869–6872. [Google Scholar] [CrossRef]
- Tang, G.D.; Xu, F.; Zhang, D.W.; Wang, Z.H. Improving the spin entropy by suppressing Co4+ concentration in thermoelectric Ca3Co4O9+δ. Ceram. Int. 2013, 39, 1341–1344. [Google Scholar] [CrossRef]
- Koshibae, W.; Maekawa, S. Effects of spin and orbital degeneracy on the thermopower of strongly correlated systems. Phys. Rev. Lett. 2001, 87, 236603. [Google Scholar] [CrossRef]
- Wang, Y.; Rogado, N.S.; Cava, R.J.; Ong, N.P. Spin entropy as the likely source of enhanced thermopower in NaxCo2O4. Nature 2003, 423, 425–428. [Google Scholar] [CrossRef]
- Roca, A.G.; Mele, P.; Kijima-Aoki, H.; Fantechi, E.; Vejpravova, J.K.; Kalbac, M.; Kaneko, S.; Endo, T. Surfaces and Interfaces of Metal Oxide Thin Films, Multilayers, Nanoparticles and Nano-composites: In Memory of Prof. Dr. Hanns-Ulrich Habermeier; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Sales, B.C.; Jin, R.; Affholter, K.A.; Khalifah, P.; Veith, G.M.; Mandrus, D. Magnetic, thermodynamic, and transport characterization of Na0.75CoO2 single crystals. Phys. Rev. B 2004, 70, 174419. [Google Scholar] [CrossRef]
- Cho, H.J.; Takashima, Y.; Nezu, Y.; Onozato, T.; Ohta, H. Anisotropic Heat Conduction in Ion-Substituted Layered Cobalt Oxides. Adv. Mater. Interfaces 2020, 7, 1901816. [Google Scholar] [CrossRef]
- Huang, R.; Mizoguchi, T.; Sugiura, K.; Ohta, H.; Koumoto, K.; Hirayama, T.; Ikuhara, Y. Direct observations of Ca ordering in Ca0.33CoO2 thin films with different superstructures. Appl. Phys. Lett. 2008, 93, 181907. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhi, J.; Li, W.; Yang, Q.; Zhang, L.; Zhang, Y. Oxide Materials for Thermoelectric Conversion. Molecules 2023, 28, 5894. https://doi.org/10.3390/molecules28155894
Liu Y, Zhi J, Li W, Yang Q, Zhang L, Zhang Y. Oxide Materials for Thermoelectric Conversion. Molecules. 2023; 28(15):5894. https://doi.org/10.3390/molecules28155894
Chicago/Turabian StyleLiu, Yucen, Jun Zhi, Wannuo Li, Qian Yang, Long Zhang, and Yuqiao Zhang. 2023. "Oxide Materials for Thermoelectric Conversion" Molecules 28, no. 15: 5894. https://doi.org/10.3390/molecules28155894
APA StyleLiu, Y., Zhi, J., Li, W., Yang, Q., Zhang, L., & Zhang, Y. (2023). Oxide Materials for Thermoelectric Conversion. Molecules, 28(15), 5894. https://doi.org/10.3390/molecules28155894






