Identification of Salivary Metabolic Signatures Associated with Primary Sjögren’s Disease
Abstract
1. Introduction
2. Results
2.1. Study Population Characteristics
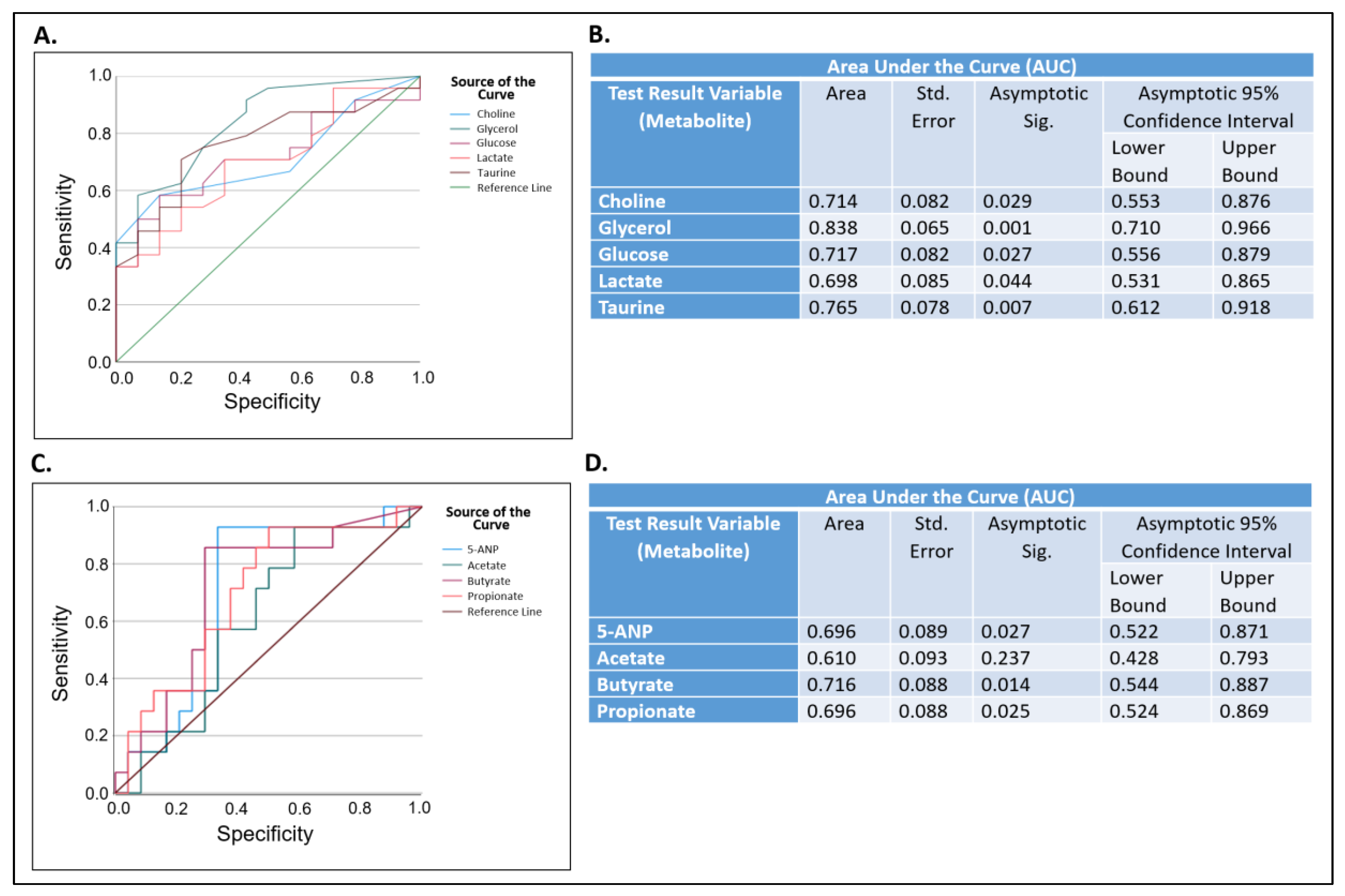
2.2. Metabolomics and Statistical Analysis of All Saliva Samples
2.3. The Effect of Age on Salivary Metabolic Profiles
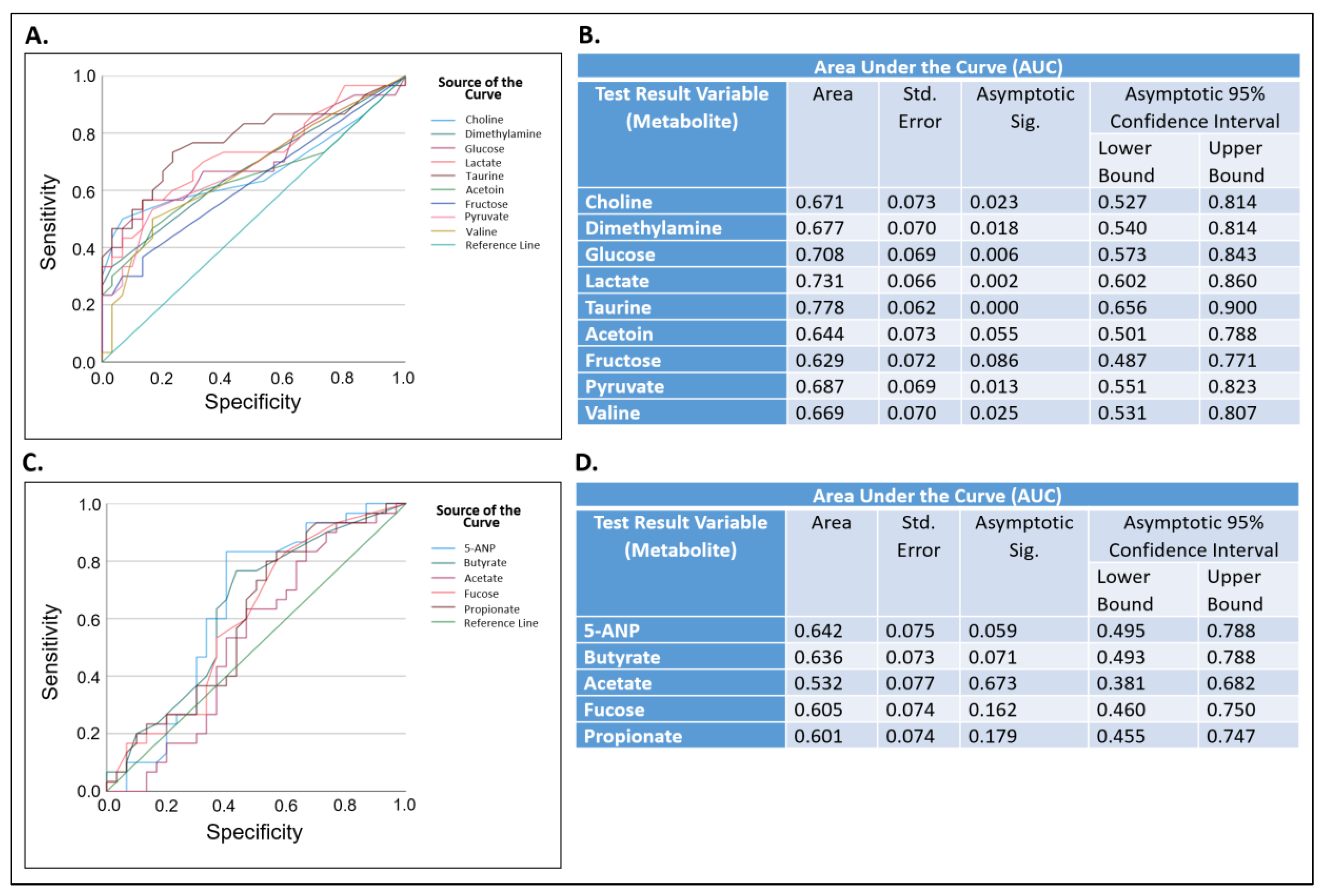
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Data Collection
4.3. Collection and Processing of Saliva Samples
4.4. Nuclear Magnetic Resonance Spectroscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, R.; Shahane, A. The epidemiology of Sjögren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [PubMed]
- Chevet, B.; Chiche, L.Y.; Devauchelle-Pensec, V.; Cornec, D.Y.K. How rare is primary Sjogren’s syndrome? Jt. Bone Spine 2023, 90, 105480. [Google Scholar] [CrossRef]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Maciel, G.; Crowson, C.S.; Matteson, E.L.; Cornec, D. Prevalence of primary Sjögren’s syndrome in a population-based cohort in the United States. Arthritis Care Res. 2017, 69, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.M.; Talal, N. Sjögren’s syndrome comes of age. Semin. Arthritis Rheum. 1999, 28, 35–39. [Google Scholar] [CrossRef]
- Peri, Y.; Agmon-Levin, N.; Theodor, E.; Shoenfeld, Y. Sjögren’s syndrome, the old and the new. Best Pract. Res. Clin. Rheumatol. 2012, 26, 105–117. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Tzioufas, A.G.; Font, J. Primary Sjoegren’s syndrome: New clinical and therapeutic concepts. Ann. Rheum. Dis. 2005, 64, 347–354. [Google Scholar] [CrossRef]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef]
- Gomes, P.D.; Juodzbalys, G.; Fernandes, M.H.; Guobis, Z. Diagnostic approaches to Sjögren’s syndrome: A literature review and own clinical experience. J. Oral Maxillofac. Res. 2012, 3, e3. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Baldini, C.; Talarico, R.; Tzioufas, A.G.; Bombardieri, S. Classification criteria for Sjogren’s syndrome: A critical review. J. Autoimmun. 2012, 39, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Routsias, J.G.; Tzioufas, A.G. Autoimmune response and target autoantigens in Sjögren’s syndrome. Eur. J. Clin. Investig. 2010, 40, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Giovelli, R.A.; Santos, M.C.S.; Serrano, É.V.; Valim, V. Clinical characteristics and biopsy accuracy in suspected cases of Sjögren’s syndrome referred to labial salivary gland biopsy. BMC Musculoskelet. Disord. 2015, 16, 30. [Google Scholar] [CrossRef]
- Guellec, D.; Cornec, D.; Jousse-Joulin, S.; Marhadour, T.; Marcorelles, P.; Pers, J.-O.; Saraux, A.; Devauchelle-Pensec, V. Diagnostic value of labial minor salivary gland biopsy for Sjogren’s syndrome: A systematic review. Autoimmun. Rev. 2013, 12, 416–420. [Google Scholar] [CrossRef]
- Fisher, B.A.; Jonsson, R.; Daniels, T.; Bombardieri, M.; Brown, R.M.; Morgan, P.; Bombardieri, S.; Ng, W.-F.; Tzioufas, A.G.; Vitali, C.; et al. Standardization of labial salivary gland histopathology in clinical trials in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.F.; Farag, A.; Papas, A.; Ganguly, R.; Campos, H.; Ramesh, A. Salivary glands ultrasonography as a diagnostic aid in Sjögren syndrome: A prospective pilot investigation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 172–181. [Google Scholar] [CrossRef]
- Baldini, C.; Luciano, N.; Tarantini, G.; Pascale, R.; Sernissi, F.; Mosca, M.; Caramella, D.; Bombardieri, S. Salivary gland ultrasonography: A highly specific tool for the early diagnosis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2015, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, R.; Brokstad, K.A.; Jonsson, M.V.; Delaleu, N.; Skarstein, K. Current concepts on Sjögren’s syndrome—Classification criteria and biomarkers. Eur. J. Oral Sci. 2018, 126 (Suppl. S1), 37–48. [Google Scholar] [CrossRef]
- Tong, L.; Koh, V.; Thong, B.Y. Review of autoantigens in Sjögren’s syndrome: An update. J. Inflamm. Res. 2017, 10, 97–105. [Google Scholar] [CrossRef]
- Fayyaz, A.; Kurien, B.T.; Scofield, R.H. Autoantibodies in Sjögren’s syndrome. Rheum. Dis. Clin. N. Am. 2016, 42, 419–434. [Google Scholar] [CrossRef]
- Delaleu, N.; Mydel, P.; Kwee, I.; Brun, G.B.; Jonsson, M.V.; Jonsson, R. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjögren’s syndrome. Arthritis Rheum. 2015, 67, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Meijer, J.; Ieong, S.; Xie, Y.; Yu, T.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary proteomic and genomic biomarkers for primary Sjogren’s syndrome. Arthritis Rheum. 2007, 56, 3588–3600. [Google Scholar] [CrossRef] [PubMed]
- Cecchettini, A.; Finamore, F.; Puxeddu, I.; Ferro, F.; Baldini, C. Salivary extracellular vesicles versus whole saliva: New perspectives for the identification of proteomic biomarkers in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2019, 118, 240–248. [Google Scholar]
- Das, N.; Menon, N.G.; de Almeida, L.G.N.; Woods, P.S.; Heynen, M.L.; Jay, G.D.; Caffery, B.; Jones, L.; Krawetz, R.; Schmidt, T.A.; et al. Proteomics analysis of tears and saliva from Sjogren’s syndrome patients. Front. Pharmacol. 2021, 12, 787193. [Google Scholar] [CrossRef]
- Cuevas-Cordoba, B.; Santiago-Garcia, J. Saliva: A fluid of study for OMICS. Omics 2014, 18, 87–97. [Google Scholar] [CrossRef]
- Shah, S. Salivaomics: The current scenario. J. Oral Maxillofac. Pathol. 2018, 22, 375–381. [Google Scholar] [CrossRef]
- Mougeot, J.L.; Noll, B.D.; Bahrani, M.F.K. Sjögren’s syndrome X-chromosome dose effect: An epigenetic perspective. Oral Dis. 2019, 2, 372–384. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, P. Identification of differentially expressed genes in primary Sjögren’s syndrome. J. Cell Biochem. 2019, 120, 17368–17377. [Google Scholar] [CrossRef]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic phenotyping in health and disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-throughput metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2018, 58, 968–994. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Carpenter, G.; So, P.-W. Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef]
- Bartsch, T.; Alfke, K.; Wolff, S.; Rohr, A.; Jansen, O.; Deuschl, G. Focal MR spectroscopy of hippocampal CA-1 lesions in transient global amnesia. Neurology 2008, 70, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Mal, M. Noninvasive metabolic profiling for painless diagnosis of human diseases and disorders. Future Sci. 2016, 2, FSO106. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, J.J.W.; Singh, S.P.; Herrala, M.; Lappalainen, R.; Myllymaa, S.; Kulla, A.M. Salivary metabolomics in the diagnosis of oral cancer and periodontal diseases. J. Periodontal Res. 2016, 51, 431–437. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2011, 129, 2207–2217. [Google Scholar] [CrossRef]
- Aimetti, M.; Cacciatore, S.; Graziano, A.; Tenori, L. Metabonomic analysis of saliva reveals generalized chronic periodontitis signature. Metabolomics 2012, 8, 465–474. [Google Scholar] [CrossRef]
- De Oliveira, L.R.; Martins, C.; Fidalgo, T.K.; Freitas-Fernandes, L.B.; Torres, R.O.; Soares, A.L.; Almeida, F.C.; Valente, A.P.; de Souza, I.P. Salivary metabolite fingerprint of type 1 diabetes in young children. J. Proteome Res. 2016, 15, 2491–2499. [Google Scholar] [CrossRef]
- Yilmaz, A.; Geddes, T.; Han, B.; Bahado-Singh, R.O.; Wilson, G.D.; Imam, K.; Maddens, M.; Graham, S.F. Diagnostic biomarkers of Alzheimer’s disease as identified in saliva using 1H NMR-based metabolomics. J. Alzheimer’s Dis. 2017, 58, 355–359. [Google Scholar] [CrossRef]
- Pereira, J.L.S.; Duarte, D.; Carneiro, T.J.; Ferreira, S.; Cunha, B.; Soares, D.; Costa, A.L.; Gil, A.M. Saliva NMR metabolomics: Analytical issues in pediatric oral health research. Oral Dis. 2019, 25, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Zoukhri, D.; Rawe, I.; Singh, M.; Brown, A.; Kublin, C.L.; Dawson, K.; Haddon, W.; White, E.L.; Hanley, K.M.; Tusé, D.; et al. Discovery of putative salivary biomarkers for Sjögren’s syndrome using high resolution mass spectrometry and bioinformatics. J. Oral Sci. 2012, 54, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, J.J.W.; Herrala, M.; Soininen, P.; Lappalainen, R.; Tjäderhane, L.; Seitsalo, H.; Niemelä, R.; Salo, T.; Kullaa, A.M.; Myllymaa, S. Metabolic profiling of saliva in patients with primary Sjögren’s syndrome. Metabolomics 2013, 3, 128. [Google Scholar]
- Herrala, M.; Mikkonen, J.J.W.; Pesonen, P.; Lappalainen, R.; Tjaderhane, L.; Niemela, R.K.; Seitsalo, H.; Salo, T.; Myllymaa, S.; Kullaa, A.M. Variability of salivary metabolite levels in patients with Sjögren’s syndrome. J. Oral Sci. 2020, 63, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, G.; Saegusa, J.; Irino, Y.; Tanaka, S.; Tsuda, K.; Takahashi, S.; Sendo, S.; Morinobu, A. Metabolomics analysis of saliva from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2015, 182, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mu, Y.; Guo, C.; You, X.; Liu, X.; Li, Q.; Sun, W. Analysis of the saliva metabolic signature in patients with primary Sjogren’s syndrome. PLoS ONE 2022, 17, e0269275. [Google Scholar] [CrossRef]
- Hynne, H.; Sandås, E.M.; Elgstøen, K.B.P.; Rootwelt, H.; Utheim, T.P.; Galtung, H.K.; Jensen, J.L. Saliva metabolomics in dry mouth patients with head and neck cancer or Sjögren’s syndrome. Cells 2022, 11, 323. [Google Scholar] [CrossRef]
- Setti, G.; Righi, V.; Mucci, A.; Panari, L.; Bernardelli, G.; Tarentini, E.; Gambini, A.; Consolo, U.; Generali, L.; Magnoni, C.; et al. Metabolic profile of whole unstimulated saliva in patients with Sjögren’s syndrome. Metabolites 2023, 13, 348. [Google Scholar] [CrossRef]
- Ismail, I.T.; Showalter, M.R.; Fiehn, O. Inborn errors of metabolism in the era of untargeted metabolomics and lipidomics. Metabolites 2019, 9, 242. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Johnson, M.; Mandal, R.; Wishart, D.S. Comprehensive targeted metabolomic assay for urine analysis. Anal. Chem. 2020, 92, 10627–10634. [Google Scholar] [CrossRef]
- Craig, A.; Cloarec, O.; Holmes, E.; Nicholson, J.K.; Lindon, J.C. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal. Chem. 2006, 78, 2262–2267. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2000; pp. 160–164. [Google Scholar]
- Izmirly, P.M.; Buyon, J.P.; Wan, I.; Belmont, M.H.; Sahl, S.; Salmon, J.E.; Askanase, A.; Bathon, J.M.; Geraldino-Pardilla, L.; Ali, Y.; et al. The Incidence and prevalence of adult primary Sjögren’s syndrome in New York county. Arthritis Care Res. 2019, 71, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Shin, Y.-J. Role of Choline in Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 4733. [Google Scholar] [CrossRef]
- Oshima, M.; Sugahara, K.; Kasahara, K.; Katakura, A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017, 37, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, G. Saliva between normal and pathological: Important factors in determining systemic and oral health. J. Med. Life 2009, 2, 303–307. [Google Scholar]
- Gomar-Vercher, S.; SimoÂn-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef]
- Rutherfurd-Markwick, K.; Starck, C.; Dulson, D.K.; Ali, A. Comparison of three saliva collection methods to assess physiological markers. J. Food Nutr Metabol. 2020, 3, 3–11. [Google Scholar]
- Maruyama, Y.; Nishimoto, Y.; Umezawa, K.; Kawamata, R.; Ichiba, Y.; Tsutsumi, K.; Kimura, M.; Murakami, S.; Kakizawa, Y.; Kumagai, T.; et al. Comparison of oral metabolome profiles of stimulated saliva, unstimulated saliva, and mouth-rinsed water. Sci. Rep. 2022, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.; Gouveia-Figueira, S.; Öhman, C.; Holgerson, P.L.; Nording, M.L.; Öhman, A. Metabolite quantification by NMR and LC-MS/MS reveals differences between unstimulated, stimulated, and pure parotid saliva. J. Pharm. Biomed. Anal. 2017, 140, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Takeda, I.; Stretch, C.; Barnaby, P.; Bhatnager, K.; Rankin, K.; Fu, H.; Weljie, A.; Jha, N.; Slupsky, C. Understanding the human salivary metabolome. NMR Biomed. 2009, 22, 577–584. [Google Scholar] [CrossRef]
- Okuma, N.; Saita, M.; Hoshi, N.; Soga, T.; Tomita, M.; Sugimoto, M.; Kimoto, K. Effect of masticatory stimulation on the quantity and quality of saliva and the salivary metabolomic profile. PLoS ONE 2017, 12, e0183109. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wu, K.-L.; Yao, C.-C.; Tsai, C.-E.; Tai, W.-C.; Liang, C.-M.; Chou, Y.-P.; Chuah, S.-K. Clinical characteristics and esophageal motility in Sjögren’s syndrome: A single-center study in Taiwan. Adv. Dig. Med. 2021, 8, 218–223. [Google Scholar] [CrossRef]
- Dawes, C. Circadian rhythms in human salivary flow rate and composition. J. Physiol. 1972, 220, 529–545. [Google Scholar] [CrossRef]
- Tahara, Y.; Aoyama, S.; Shibata, S. The mammalian circadian clock and its entrainment by stress and exercise. J. Physiol. Sci. 2017, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Hoshi, N.; Masahiro, S.; Enomoto, A.; Ota, S.; Kaneko, M.; Soga, T.; Tomita, M.; Kimoto, K. Effects of inter-day and intra-day variation on salivary metabolomic profiles. Clin. Chim. Acta 2019, 489, 41–48. [Google Scholar] [CrossRef] [PubMed]

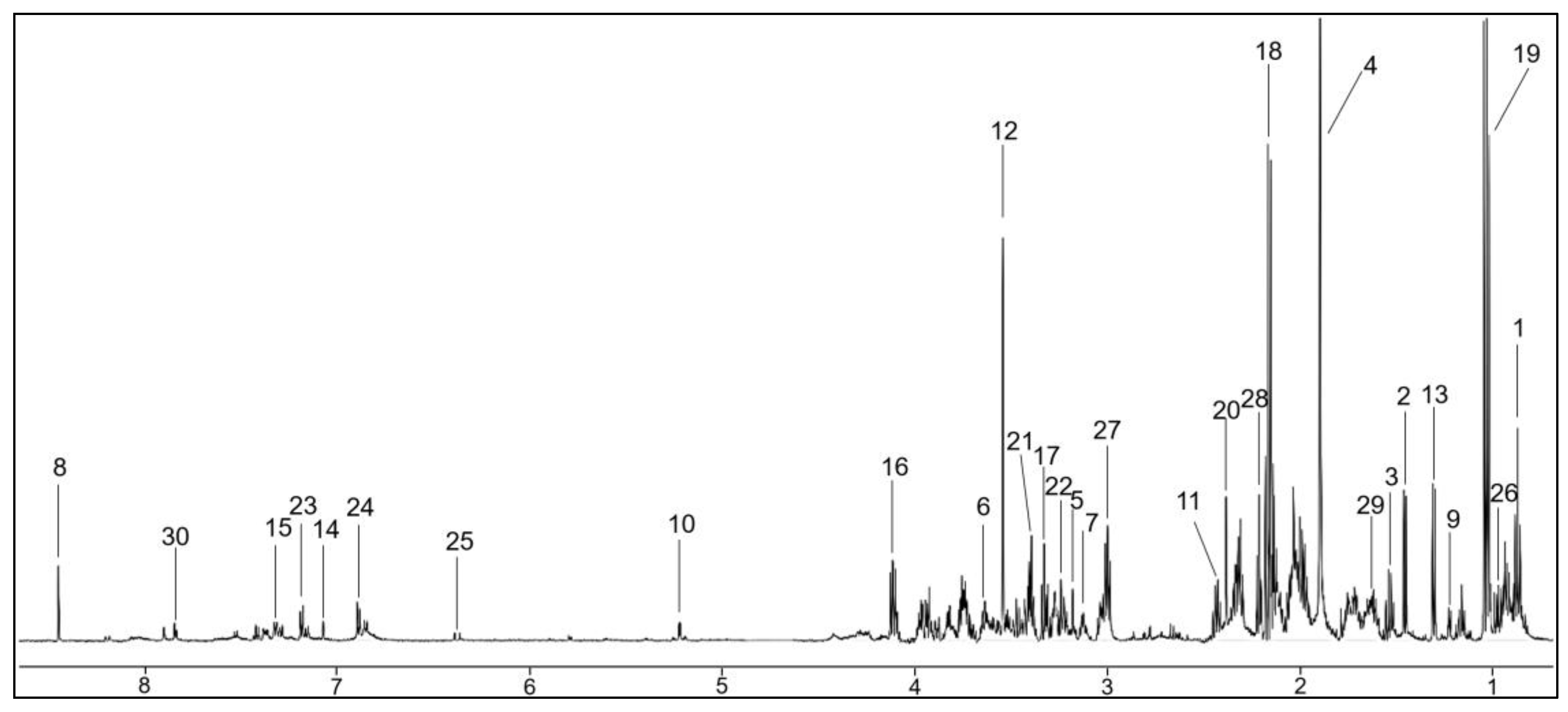

| Metabolite | Metabolite Concentration (mM) | p Value Corrected | FC | Normalized Data | p Value Corrected | FC | ||
|---|---|---|---|---|---|---|---|---|
| Metabolites significantly higher in pSjD relatively to NS-C in both Metabolite Concentration and Normalized Data | ||||||||
| p50 (iqr) | FC (%) | p50 (iqr) | FC (%) | |||||
| NS-C | pSjD | NS-C | pSjD | |||||
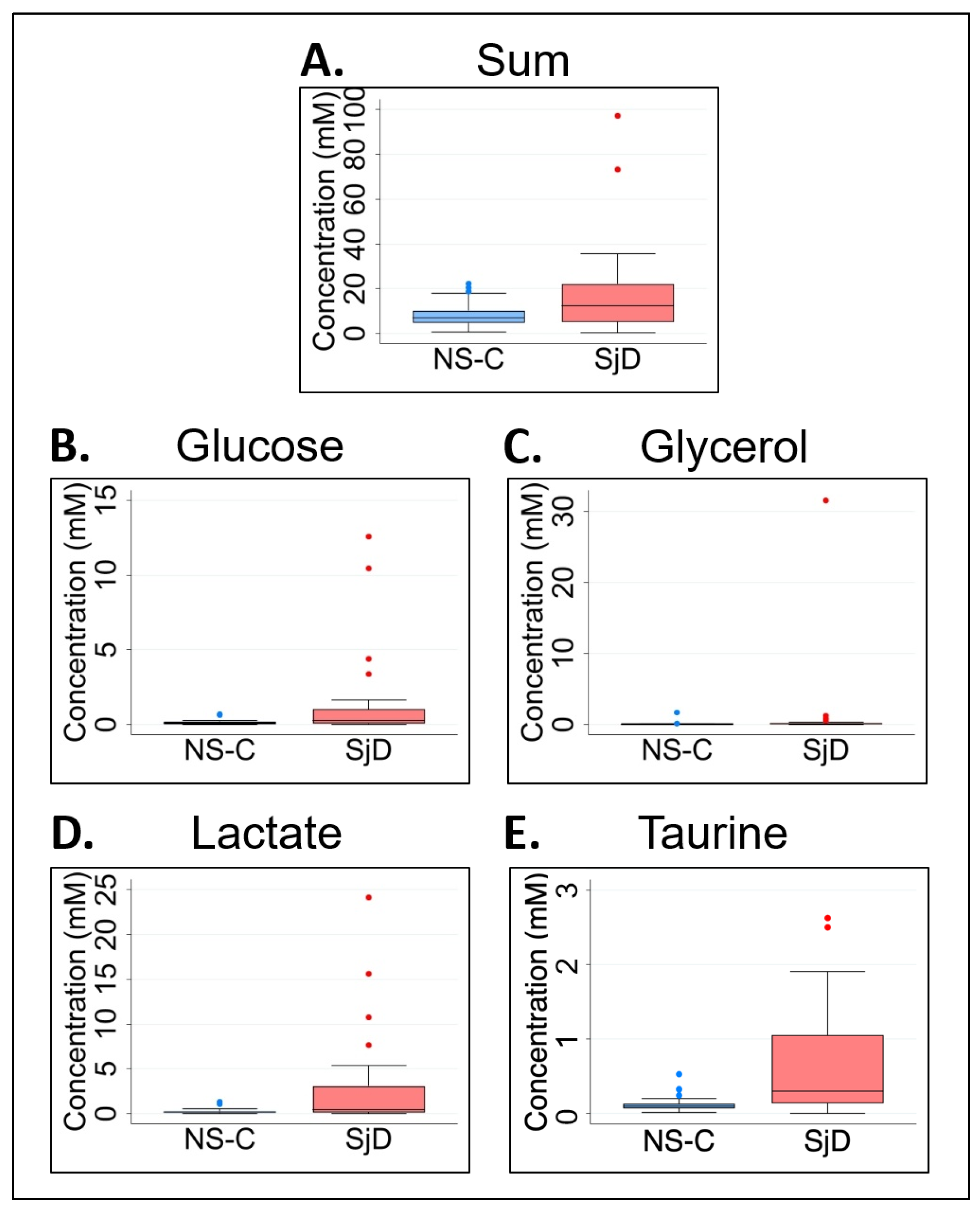
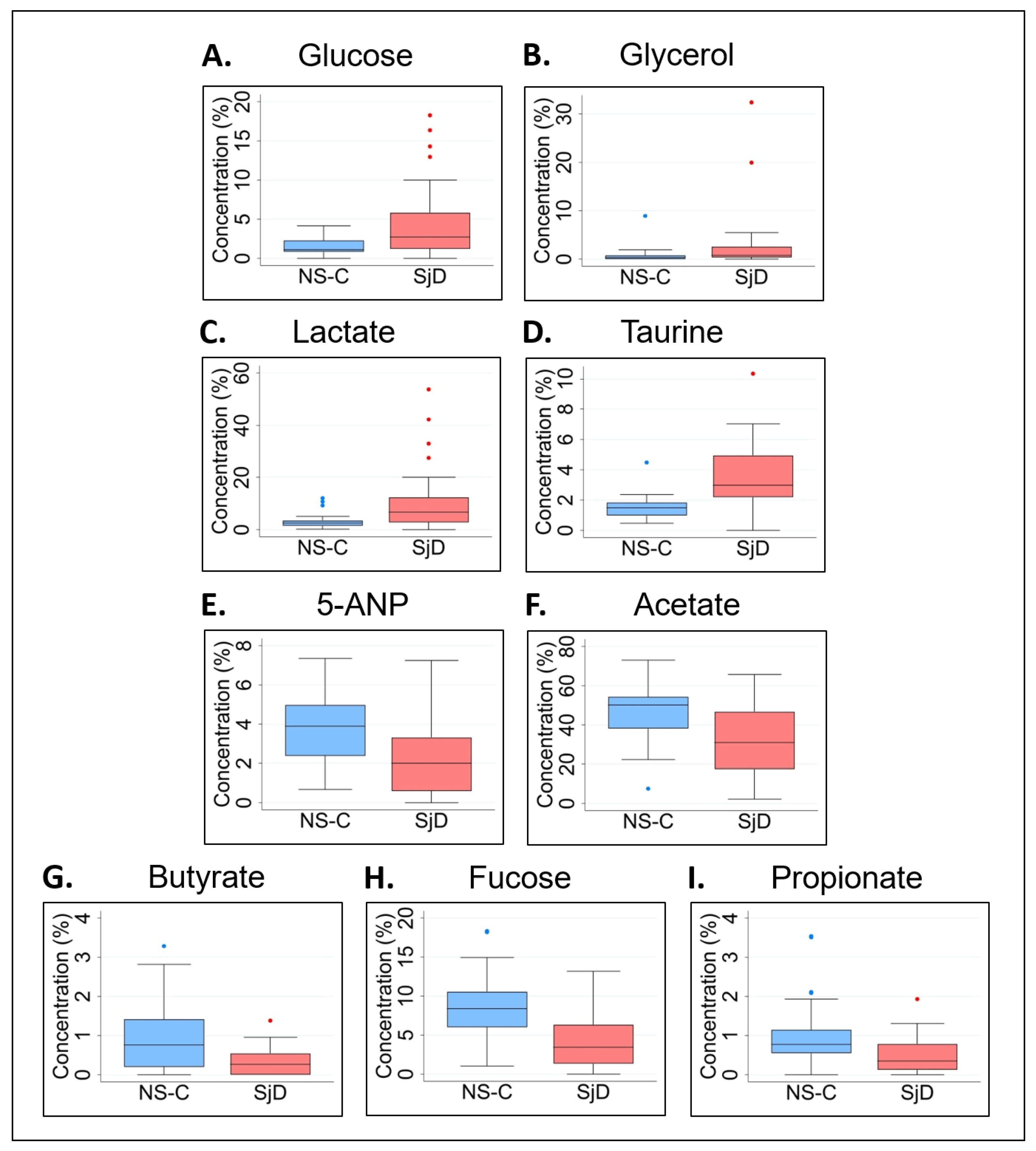
| Glucose | 0.100 (0.160) | 0.277 (0.947) | 0.0442 | 1.8 (177%) | 1.158 (1.447) | 2.760 (4.585) | 0.0289 | 1.4 (138%) |
| Glycerol | 0.027 (0.510) | 0.071 (0.177) | 0.0051 | 1.6 (163%) | 0.389 (0.803) | 0.770 (2.149) | 0.0302 | 1.0 (98%) |
| Lactate | 0.189 (0.162) | 0.432 (2.910) | 0.0272 | 1.3 (129%) | 2.484 (1.984) | 6.753 (9.361) | 0.0074 | 1.7 (172%) |
| Taurine | 0.100 (0.063) | 0.300 (0.917) | 0.0068 | 2.0 (200%) | 1.481 (0.837) | 2.971 (2.751) | 0.0001 | 1.0 (101%) |
| Metabolites significantly lower in pSjD relatively to NS-C in Normalized Data analysis only | ||||||||
| 5-ANP | 0.218 (0.291) | 0.123 (0.350) | 0.1580 | 0.8 (77%) | 3.893 (2.588) | 2.013 (2.706) | 0.0027 | 0.9 (93%) |
| Acetate | 3.489 (3.678) | 2.620 (6.843) | 0.7328 | 0.3 (33%) | 50.133 (16.004) | 30.961 (29.140) | 0.0043 | 0.6 (62%) |
| Butyrate | 0.061 (0.075) | 0.016 (0.094) | 0.1544 | 2.8 (281%) | 0.766 (1.210) | 0.270 (0.544) | 0.0048 | 1.8 (184%) |
| Fucose | 0.060 (0.108) | 0.039 (0.103) | 0.2905 | 0.5 (54%) | 0.782 (0.592) | 0.356 (0.657) | 0.0117 | 1.2 (120%) |
| Propionate | 0.535 (1.170) | 0.292 (0.940) | 0.2833 | 0.8 (83%) | 8.368 (4.524) | 3.488 (4.980) | 0.0027 | 1.4 (140%) |
| Metabolite | Metabolite Concentration (mM) | p Value Corrected | FC | Normalized Data | p Value Corrected | FC | ||
|---|---|---|---|---|---|---|---|---|
| Metabolites significantly higher in pSjD relatively to NS-C in Metabolite Normalized Data | ||||||||
| p50 (iqr) | FC (%) | p50 (iqr) | FC (%) | |||||
| NS-C | pSjD | NS-C | pSjD | |||||
| Choline | 0.016 (0.012) | 0.038 (0.083) | 0.1927 | 1.4 (138%) | 0.161 (0.121) | 0.330 (0.392) | 0.0191 | 1.0 (105%) |
| Glucose | 0.096 (0.146) | 0.277 (0.925) | 0.2244 | 1.9 (189%) | 1.158 (0.602) | 2.768 (4.088) | 0.0194 | 1.4 (139%) |
| Lactate | 0.189 (0.294) | 0.458 (2.508) | 0.2129 | 1.4 (142%) | 2.215 (1.965) | 5.943 (8.914) | 0.0559 | 1.7 (168%) |
| Taurine | 0.099 (0.081) | 0.299 (0.812) | 0.0969 | 2.0 (202%) | 1.578 (0.790) | 3.093 (2.767) | 0.0041 | 1.0 (96%) |
| *Fructose | 0 (0) | 0 (0.104) | 0.1813 | 0 (0) | 0 (1.119) | 0.0544 | ||
| Metabolites significantly higher in pSjD relatively to NS-C in both Metabolite Concentration and Normalized Data | ||||||||
| Glycerol | 0.006 (0.039) | 0.068 (0.127) | 0.0102 | 10.3 (1033%) | 0.093 (0.450) | 0.706 (1.694) | 0.0148 | 6.6 (659%) |
| Metabolites significantly lower in pSjD relatively to NS-C in Metabolite Concentration and Normalized Data | ||||||||
| 5-ANP | 0.302 (0.426) | 0.115 (0.365) | 0.2411 | 1.6 (163%) | 4.230 (1.694) | 2.013 (1.971) | 0.0034 | 1.1 (110%) |
| Acetate | 4.303 (3.636) | 2.309 (6.407) | 0.4423 | 0.9 (86%) | 50.335 (19.184) | 30.961 (28.922) | 0.0148 | 0.6 (63%) |
| Butyrate | 0.079 (0.064) | 0.013 (0.088) | 0.1836 | 5.1 (508%) | 0.789 (0.394) | 0.245 (0.453) | 0.0041 | 2.2 (222%) |
| Propionate | 0.776 (1.463) | 0.236 (0.917) | 0.2411 | 2.3 (229%) | 9.431 (3.452) | 3.488 (4.660) | 0.0003 | 1.7 (170%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alt-Holland, A.; Huang, X.; Mendez, T.; Singh, M.L.; Papas, A.S.; Cimmino, J.; Bairos, T.; Tzavaras, E.; Foley, E.; Pagni, S.E.; et al. Identification of Salivary Metabolic Signatures Associated with Primary Sjögren’s Disease. Molecules 2023, 28, 5891. https://doi.org/10.3390/molecules28155891
Alt-Holland A, Huang X, Mendez T, Singh ML, Papas AS, Cimmino J, Bairos T, Tzavaras E, Foley E, Pagni SE, et al. Identification of Salivary Metabolic Signatures Associated with Primary Sjögren’s Disease. Molecules. 2023; 28(15):5891. https://doi.org/10.3390/molecules28155891
Chicago/Turabian StyleAlt-Holland, Addy, Xuejian Huang, Tatiana Mendez, Mabi L. Singh, Athena S. Papas, Joseph Cimmino, Tiffany Bairos, Elizabeth Tzavaras, Elizabeth Foley, Sarah E. Pagni, and et al. 2023. "Identification of Salivary Metabolic Signatures Associated with Primary Sjögren’s Disease" Molecules 28, no. 15: 5891. https://doi.org/10.3390/molecules28155891
APA StyleAlt-Holland, A., Huang, X., Mendez, T., Singh, M. L., Papas, A. S., Cimmino, J., Bairos, T., Tzavaras, E., Foley, E., Pagni, S. E., & Baleja, J. D. (2023). Identification of Salivary Metabolic Signatures Associated with Primary Sjögren’s Disease. Molecules, 28(15), 5891. https://doi.org/10.3390/molecules28155891






