Comparative Molecular Characterization and Pharmacokinetics of IgG1-Fc and Engineered Fc Human Antibody Variants to Insulin-like Growth Factor 2 Receptor (IGF2R)
Abstract
1. Introduction
2. Results
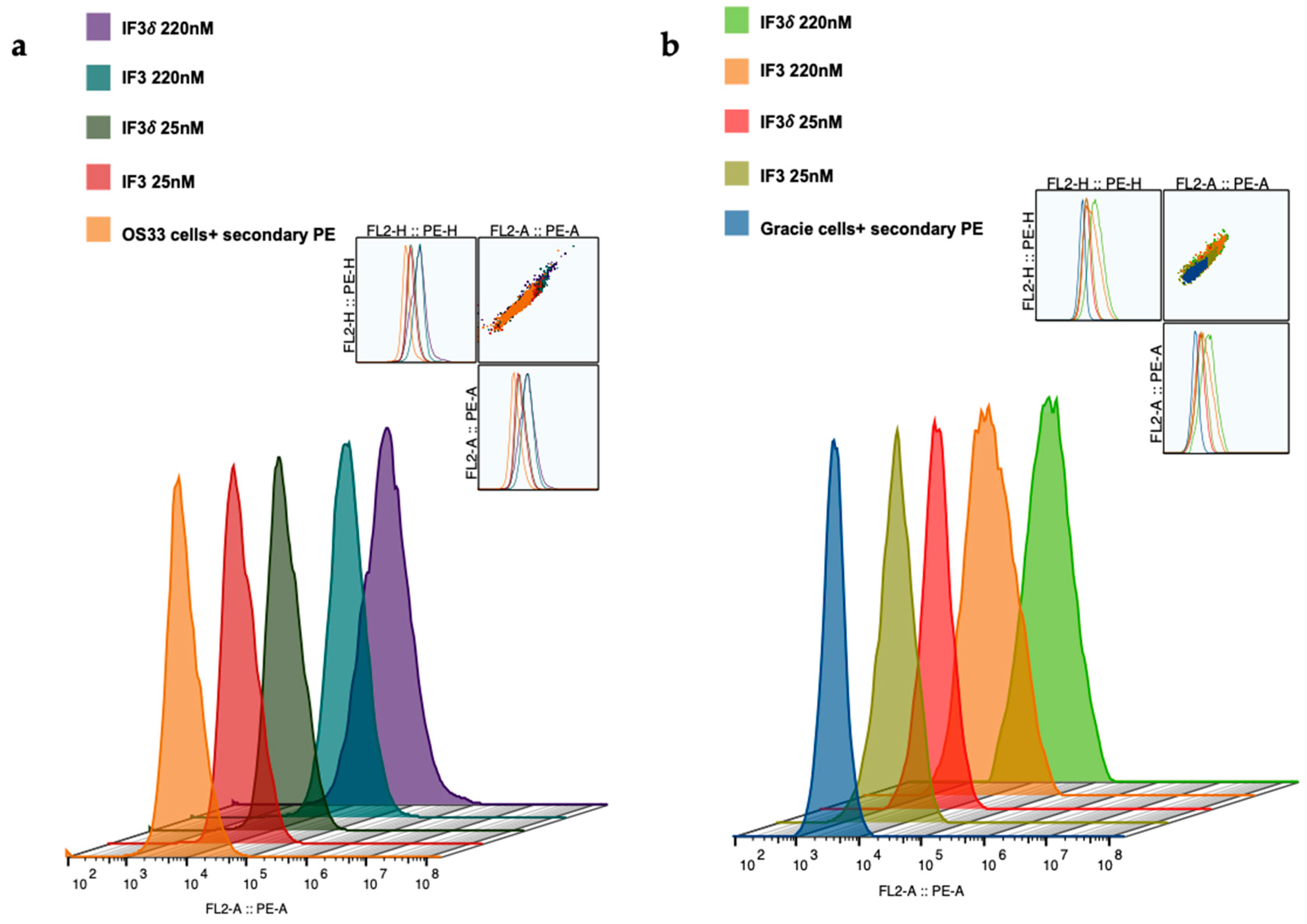
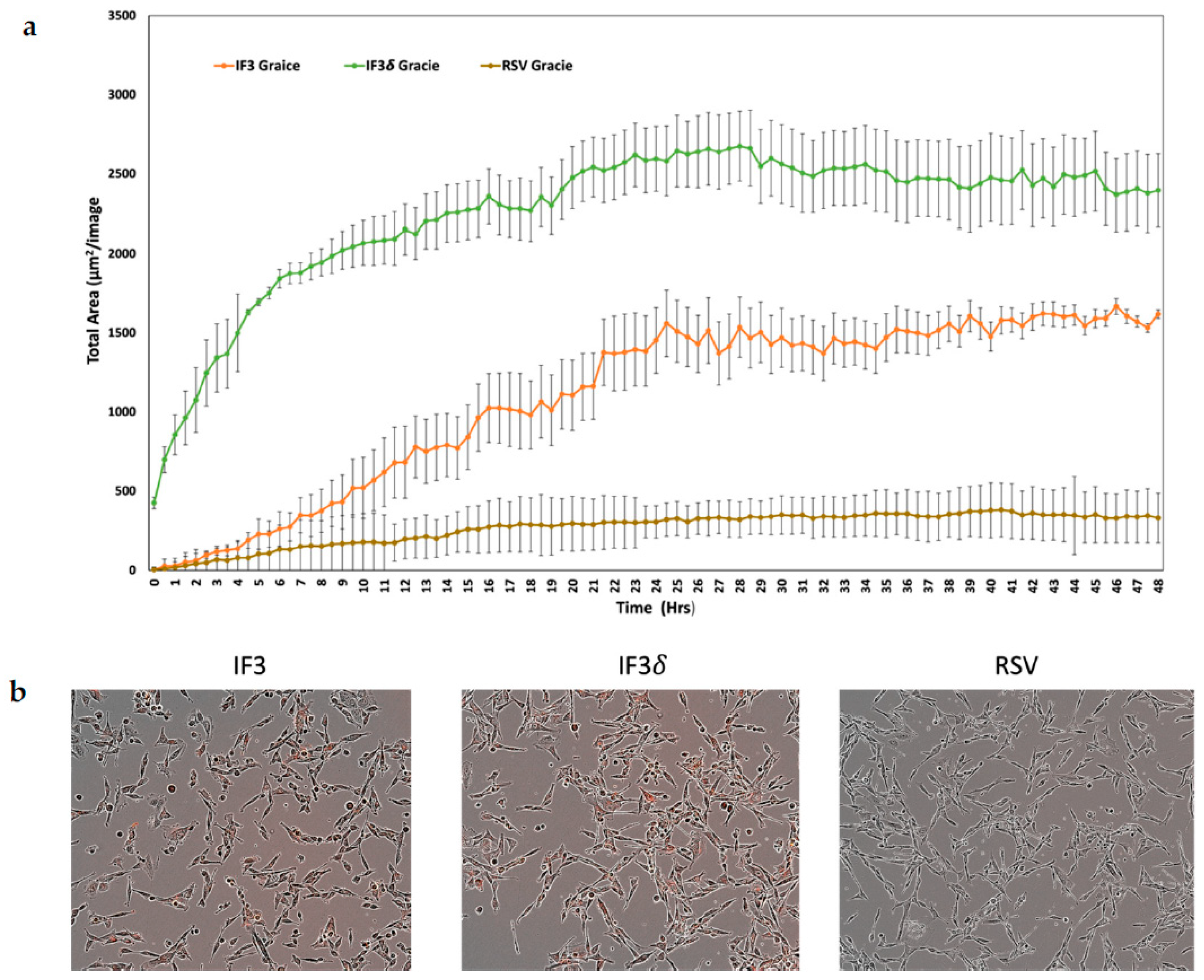
2.1. Expression, Purification, and Characterization of IF3 and IF3δ
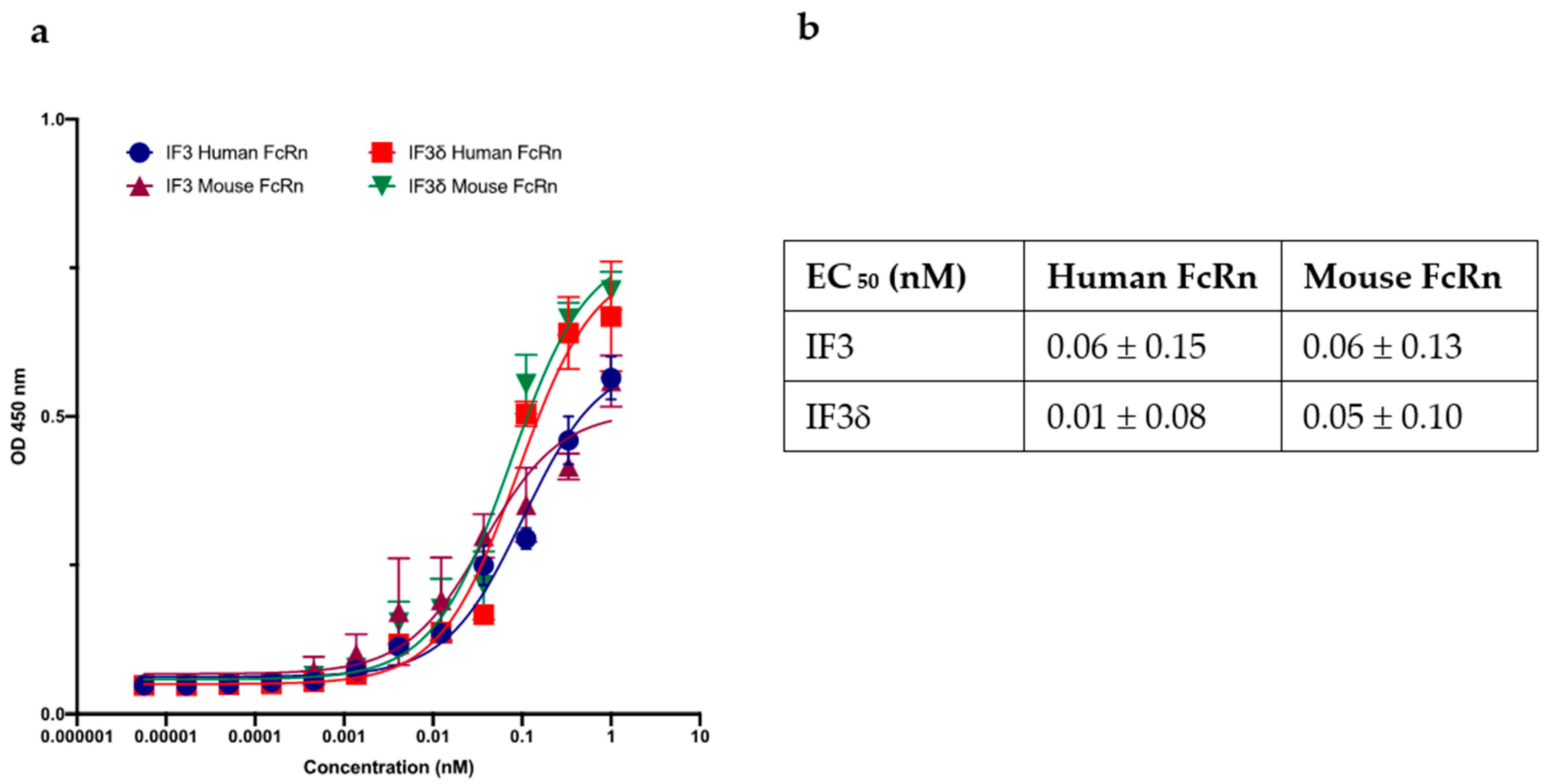
2.2. pH 5.8 Binding of IF3 and IF3δ Antibodies to Human and Mouse FcRn Receptors
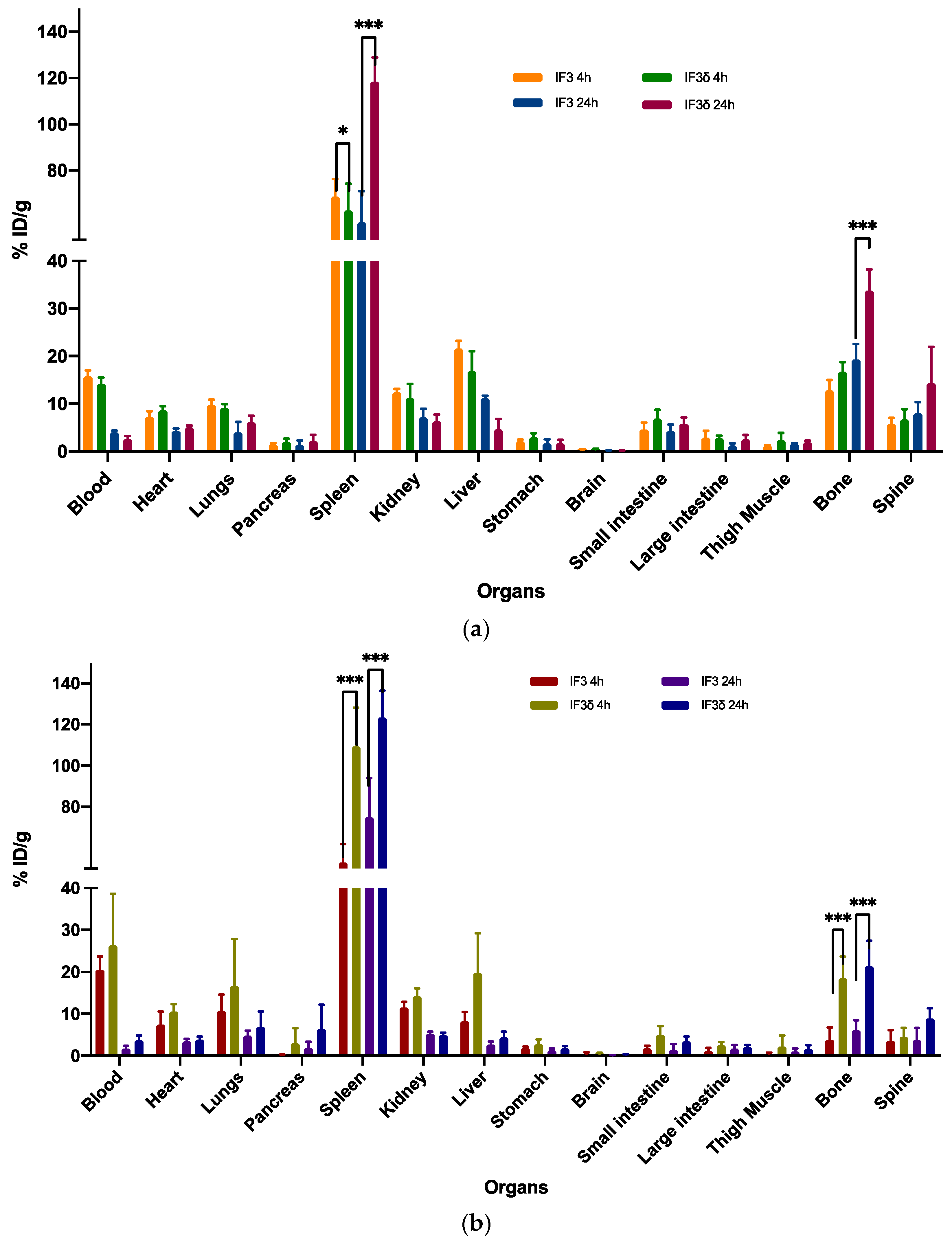
2.3. Biodistribution Comparison of 111In-Radiolabeled IF3 and IF3 Antibodies in SCID and C57BL/6 Mice Reveals Differences in Accumulation in Spleen and Bone
3. Discussion
4. Materials and Methods
4.1. Construction of Mammalian Expression Vector with DHS Mutation
4.2. Production of IgG1 Antibodies
4.3. Cell Lines
4.4. ELISA for Purified IgG1 Antibodies against IGF2R
4.5. ELISA for Purified IgG1 Antibodies against FcRn
4.6. Flow Cytometry
4.7. Antibody Internalization Assay
4.8. Animal Models
4.9. Conjugation and Radiolabeling of Antibodies
4.10. Biodistribution
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ehrhart, N.P.; Ryan, S.D.; Fan, T.M. 25—Tumors of the Skeletal System. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Liptak, J.M.; Dernell, W.S.; Withrow, S.J. Canine Appendicular Osteosarcoma: Diagnosis and Palliative Treatment. Compend. Contin. Educ. Pract. Vet. 2004, 26, 172–185. [Google Scholar]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Spodnick, G.J.; Berg, J.; Rand, W.M.; Schelling, S.H.; Couto, G.; Harvey, H.J.; Henderson, R.A.; MacEwen, G.; Mauldin, N.; McCaw, D.L.; et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J. Am. Vet. Med. Assoc. 1992, 200, 995–999. [Google Scholar] [PubMed]
- Bergman, P.J.; MacEwen, E.G.; Kurzman, I.D.; Henry, C.J.; Hammer, A.S.; Knapp, D.W.; Hale, A.; Kruth, S.A.; Klein, M.K.; Klausner, J.; et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J. Vet. Intern. Med. 1996, 10, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.; Powers, B.E.; Dernell, W.S.; Straw, R.C.; Khanna, C.; Hogge, G.S.; Vail, D.M. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 2009, 45, 33–38. [Google Scholar] [CrossRef]
- Saam, D.E.; Liptak, J.M.; Stalker, M.J.; Chun, R. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996–2006). J. Am. Vet. Med. Assoc. 2011, 238, 195–206. [Google Scholar] [CrossRef]
- Farese, J.P.; Milner, R.; Thompson, M.S.; Lester, N.; Cooke, K.; Fox, L.; Hester, J.; Bova, F.J. Stereotactic radiosurgery for treatment of osteosarcomas involving the distal portions of the limbs in dogs. J. Am. Vet. Med. Assoc. 2004, 225, 1567–1572, 1548. [Google Scholar] [CrossRef]
- Covey, J.L.; Farese, J.P.; Bacon, N.J.; Schallberger, S.P.; Amsellem, P.; Cavanaugh, R.P.; Milner, R.J. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet. Surg. 2014, 43, 174–181. [Google Scholar] [CrossRef]
- Boston, S.E.; Vinayak, A.; Lu, X.; Larue, S.; Bacon, N.J.; Bleedorn, J.A.; Souza, C.H.M.; Ehrhart, N.P. Outcome and complications in dogs with appendicular primary bone tumors treated with stereotactic radiotherapy and concurrent surgical stabilization. Vet. Surg. 2017, 46, 829–837. [Google Scholar] [CrossRef]
- Kim, C.; Matsuyama, A.; Mutsaers, A.J.; Woods, J.P. Retrospective evaluation of toceranib (Palladia) treatment for canine metastatic appendicular osteosarcoma. Can. Vet. J. 2017, 58, 1059–1064. [Google Scholar]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef]
- Rowell, J.L.; McCarthy, D.O.; Alvarez, C.E. Dog models of naturally occurring cancer. Trends Mol. Med. 2011, 17, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Brady, E.D.; Brechbiel, M.W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 488–499. [Google Scholar] [CrossRef]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Heinrich, D.; Mariados, N.; Mendez Vidal, M.J.; Keizman, D.; Thellenberg Karlsson, C.; Peer, A.; Procopio, G.; Frank, S.J.; Pulkkanen, K.; et al. Re-treatment with radium-223: 2-year follow-up from an international, open-label, phase 1/2 study in patients with castration-resistant prostate cancer and bone metastases. Prostate 2019, 79, 1683–1691. [Google Scholar] [CrossRef]
- Bayers Pharmaceuticals. Xofigo (Radium Ra223 Dichloride) Injection for Intravenous Use [Package Insert]. May 2013. Available online: http://hcp.xofigo-us.com/index.php (accessed on 17 October 2018).
- Eberle, A.N.; Beglinger, C. Does 177Lu-labeled octreotate improve the rate of remission of endocrine gastroenteropancreatic tumors? Nat. Clin. Pract. Endocrinol. Metab. 2005, 1, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; Goldenberg, D.M. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J. Nucl. Med. 2005, 46 (Suppl. 1), 115S–127S. [Google Scholar]
- Hassan, S.E.; Bekarev, M.; Kim, M.Y.; Lin, J.; Piperdi, S.; Gorlick, R.; Geller, D.S. Cell surface receptor expression patterns in osteosarcoma. Cancer 2012, 118, 740–749. [Google Scholar] [CrossRef]
- Geller, D.S.; Morris, J.; Revskaya, E.; Kahn, M.; Zhang, W.; Piperdi, S.; Park, A.; Koirala, P.; Guzik, H.; Hall, C.; et al. Targeted therapy of osteosarcoma with radiolabeled monoclonal antibody to an insulin-like growth factor-2 receptor (IGF2R). Nucl. Med. Biol. 2016, 43, 812–817. [Google Scholar] [CrossRef]
- Karkare, S.; Allen, K.J.H.; Jiao, R.; Malo, M.E.; Dawicki, W.; Helal, M.; Godson, D.L.; Dickinson, R.; MacDonald-Dickinson, V.; Yang, R.; et al. Detection and targeting insulin growth factor receptor type 2 (IGF2R) in osteosarcoma PDX in mouse models and in canine osteosarcoma tumors. Sci. Rep. 2019, 9, 11476. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, R.N.; Song, X.; Olson, L.J.; Kudo, M.; Gotschall, R.R.; Canfield, W.M.; Cummings, R.D.; Smith, D.F.; Dahms, N.M. Cation-independent mannose 6-phosphate receptor: A composite of distinct phosphomannosyl binding sites. J. Biol. Chem. 2009, 284, 35215–35226. [Google Scholar] [CrossRef]
- Takeda, T.; Komatsu, M.; Chiwaki, F.; Komatsuzaki, R.; Nakamura, K.; Tsuji, K.; Kobayashi, Y.; Tominaga, E.; Ono, M.; Banno, K.; et al. Upregulation of IGF2R evades lysosomal dysfunction-induced apoptosis of cervical cancer cells via transport of cathepsins. Cell Death Dis. 2019, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Martin-Kleiner, I.; Gall Troselj, K. Mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) in carcinogenesis. Cancer Lett. 2010, 289, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Broqueza, J.; Prabaharan, C.B.; Allen, K.J.H.; Jiao, R.; Fisher, D.R.; Dickinson, R.; MacDonald-Dickinson, V.; Uppalapati, M.; Dadachova, E. Radioimmunotherapy Targeting IGF2R on Canine-Patient-Derived Osteosarcoma Tumors in Mice and Radiation Dosimetry in Canine and Pediatric Models. Pharmaceuticals 2021, 15, 10. [Google Scholar] [CrossRef]
- Broqueza, J.; Prabaharan, C.B.; Andrahennadi, S.; Allen, K.J.H.; Dickinson, R.; MacDonald-Dickinson, V.; Dadachova, E.; Uppalapati, M. Novel Human Antibodies to Insulin Growth Factor 2 Receptor (IGF2R) for Radioimmunoimaging and Therapy of Canine and Human Osteosarcoma. Cancers 2021, 13, 2208. [Google Scholar] [CrossRef]
- Allen, K.J.H.; Kwon, O.; Hutcheson, M.R.; Grudzinski, J.J.; Cain, S.M.; Cruz, F.A.; Vinayakamoorthy, R.M.; Sun, Y.S.; Fairley, L.; Prabaharan, C.B.; et al. Image-Based Dosimetry in Dogs and Cross-Reactivity with Human Tissues of IGF2R-Targeting Human Antibody. Pharmaceuticals 2023, 16, 979. [Google Scholar] [CrossRef]
- Lee, C.H.; Kang, T.H.; Godon, O.; Watanabe, M.; Delidakis, G.; Gillis, C.M.; Sterlin, D.; Hardy, D.; Cogne, M.; Macdonald, L.E.; et al. An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat. Commun. 2019, 10, 5031. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Ward, E.S.; Devanaboyina, S.C.; Ober, R.J. Targeting FcRn for the modulation of antibody dynamics. Mol. Immunol. 2015, 67, 131–141. [Google Scholar] [CrossRef]
- Challa, D.K.; Velmurugan, R.; Ober, R.J.; Sally Ward, E. FcRn: From molecular interactions to regulation of IgG pharmacokinetics and functions. Curr. Top. Microbiol. Immunol. 2014, 382, 249–272. [Google Scholar] [CrossRef]
- Robbie, G.J.; Criste, R.; Dall’acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lombardi, R.; Nevoltris, D.; Luthra, A.; Schofield, P.; Zimmermann, C.; Christ, D. Transient expression of human antibodies in mammalian cells. Nat. Protoc. 2018, 13, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2018, 9, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; DiLillo, D.J.; Ravetch, J.V. humanized mice to study FcgammaR function. Curr. Top. Microbiol. Immunol. 2014, 382, 237–248. [Google Scholar] [CrossRef]
- Cataldi, M.; Vigliotti, C.; Mosca, T.; Cammarota, M.; Capone, D. Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int. J. Mol. Sci. 2017, 18, 1249. [Google Scholar] [CrossRef]
- Datta-Mannan, A. Mechanisms Influencing the Pharmacokinetics and Disposition of Monoclonal Antibodies and Peptides. Drug Metab. Dispos. 2019, 47, 1100–1110. [Google Scholar] [CrossRef]
- Li, F.; Ulrich, M.L.; Shih, V.F.; Cochran, J.H.; Hunter, J.H.; Westendorf, L.; Neale, J.; Benjamin, D.R. Mouse Strains Influence Clearance and Efficacy of Antibody and Antibody-Drug Conjugate via Fc-FcgammaR Interaction. Mol. Cancer Ther. 2019, 18, 780–787. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chow, A.; Monette, S.; Vivier, D.; Pourat, J.; Edwards, K.J.; Dilling, T.R.; Abdel-Atti, D.; Zeglis, B.M.; Poirier, J.T.; et al. Fc-Mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models. Cancer Res. 2018, 78, 1820–1832. [Google Scholar] [CrossRef]
- de Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V., Jr.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araujo, J.M.G.; Fernandes, J.V. Biology and pathogenesis of human osteosarcoma. Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef] [PubMed]

| EC 50 (nM) | Human IGF2R | Canine IGF2R | Mouse IGF2R |
|---|---|---|---|
| IF3 | 0.22 ± 0.45 | 0.41 ± 0.75 | 0.81 ± 1.77 |
| IF3δ | 0.17 ± 0.41 | 0.32 ± 0.75 | 0.79 ± 1.46 |
| CHXA″-DTPA-IF3 | 0.15 ± 0.32 | 0.73 ± 1.28 | 1.65 ± 2.63 |
| CHXA″-DTPA-IF3δ | 0.11 ± 0.24 | 0.79 ± 1.49 | 1.1 ± 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabaharan, C.B.; Giri, S.; Allen, K.J.H.; Bato, K.E.M.; Mercado, T.R.; Malo, M.E.; Carvalho, J.L.C.; Dadachova, E.; Uppalapati, M. Comparative Molecular Characterization and Pharmacokinetics of IgG1-Fc and Engineered Fc Human Antibody Variants to Insulin-like Growth Factor 2 Receptor (IGF2R). Molecules 2023, 28, 5839. https://doi.org/10.3390/molecules28155839
Prabaharan CB, Giri S, Allen KJH, Bato KEM, Mercado TR, Malo ME, Carvalho JLC, Dadachova E, Uppalapati M. Comparative Molecular Characterization and Pharmacokinetics of IgG1-Fc and Engineered Fc Human Antibody Variants to Insulin-like Growth Factor 2 Receptor (IGF2R). Molecules. 2023; 28(15):5839. https://doi.org/10.3390/molecules28155839
Chicago/Turabian StylePrabaharan, Chandra B., Sabeena Giri, Kevin J. H. Allen, Katrina E. M. Bato, Therese R. Mercado, Mackenzie E. Malo, Jorge L. C. Carvalho, Ekaterina Dadachova, and Maruti Uppalapati. 2023. "Comparative Molecular Characterization and Pharmacokinetics of IgG1-Fc and Engineered Fc Human Antibody Variants to Insulin-like Growth Factor 2 Receptor (IGF2R)" Molecules 28, no. 15: 5839. https://doi.org/10.3390/molecules28155839
APA StylePrabaharan, C. B., Giri, S., Allen, K. J. H., Bato, K. E. M., Mercado, T. R., Malo, M. E., Carvalho, J. L. C., Dadachova, E., & Uppalapati, M. (2023). Comparative Molecular Characterization and Pharmacokinetics of IgG1-Fc and Engineered Fc Human Antibody Variants to Insulin-like Growth Factor 2 Receptor (IGF2R). Molecules, 28(15), 5839. https://doi.org/10.3390/molecules28155839






