Abstract
Perovskite is the largest mineral on earth and has a variety of excellent physical and chemical properties. La1−xRxFeO3 (R = Co, Al, Nd, Sm) were synthesized using the sol-gel method and analyzed by XRD, TG-DTA, and VSM. With the increase in the Co2+ doping content, the diffraction peak drifted in the direction of a larger angle. The grain size of La1−xRxFeO3(R = Co) is mainly concentrated between 50.7 and 133.5 nm. As the concentration of Co2+ increased, the magnetic loop area and magnetization increased. La1−xRxFeO3(R = Al) is an orthorhombic perovskite structure, the grain size decreased with the increase in Al3+ doping concentration, and the minimum crystallite is 17.9 nm. The magnetic loop area and magnetization increased with the increase in Al3+ ion concentration. The enclosed area of the M-H curve of the sample decreased, and the ferromagnetic order gradually weakened and tended to be antiferromagnetic, which may be due to the increase in sintering temperature, decrease in the iron oxide composition, and changes in the magnetic properties. Proper doping can improve the magnetization of La1−xRxFeO3(R = Nd), refine the particles, and obtain better magnetic performance. As the Nd3+ ion concentration increased, the magnetic properties of the samples increased. Ms of La0.85Co0.15FeO3 prepared by different calcination time increases with the increase in calcination time. As the Sm3+ ion concentration increased, the magnetic properties of the samples increased. Proper doping can improve the magnetization of La1−xRxFeO3(R = Sm), refine the particles, and generate better magnetic performance.
1. Introduction
Perovskite is the largest mineral on earth and has a variety of excellent physical and chemical properties, such as magnetoelectric effect, magnetostriction, variable magnetic phase transition, catalytic activity, and piezoelectric effect. It is one of the new functional materials with great development prospects [1,2]. LaFeO3 oxides with perovskite structure have a unique crystal structure; a slight change in its structure, especially defects in the crystal structure or changes in the performance caused by doping, will lead to new properties [3,4]. In the perovskite structure oxide ABO3, the A-site is generally a rare earth element with large radius (i.e., La, Pr, and Gd). The interaction between A-site ions and oxygen ions plays a decisive role in the mutual evolution of perovskite structures [5,6]. Lili Liu et al. [7] studied synthesis and characterization of Al3+ doped LaFeO3 compounds. Sr-doped porous LaFeO3 samples were fabricated via the sol-gel method [8]. Nabasmita Saikia et al. [9] prepared (La0.75Gd0.25)FeO3 successfully via the typical solid casting route method. Chethana Aranthady et al. [10] studied the Ca substituted LaFeO3. Yongfang Rao et al. [11] prepared Cu-doped LaFeO3 samples and studied heterogeneous catalysts of PMS for the degradation of pharmaceuticals. Wankassama Haron et al. [12] studied the structural characteristics and dielectric properties of La1−xCoxFeO3 and LaFe1−xCoxO3 samples using the thermal decomposition method. Xiutao Ge et al. [3] studied the gas sensitivity of LaFe1−yCoyO3 via the co-precipitation method. Yutana Janbutrach et al. [5] synthesized La1−xAlxFeO3 nanocrystals and studied their magnetic and optical properties. The saturation magnetization, the coercivity, and the remanent magnetization of the samples increase with the increase in the concentration of Al3+ ion. There are few studies that investigate the Al3+-doped LaFeO3 nanoparticles synthesized at low temperature using the citric acid sol-gel method and their structure and magnetic properties. LI Fa-tang et al. [1] synthesized La1−xNdxFeO3 via the citric acid complexation method and studied its morphology. Enrico traversa et al. [2] synthesized La1−xSmxFeO3 sample via the thermal decomposition method and studied its structure and composition. In this paper, La1−xRxFeO3 (R = Co, Al, Nd, Sm) powder was synthesized using the citric acid sol-gel method [13,14,15,16,17,18,19,20], and the influences of different Co, Al, Nd, and Sm doping ratios and calcination temperatures on the structure, morphology, and magnetic properties of the samples were studied.
2. Results and Discussion
2.1. XRD Analysis of La1−xRxFeO3 (R = Co)
Figure 1 is the XRD diffraction pattern of La1−xCoxFeO3 (x = 0~0.25). The XRD diagram can analyze the structural changes caused by atomic doping. When the doping amount x = 0.20, the samples showed a CoFe2O4 mixed phase, indicating that with the increase in Co2+ doped, Co2+ does not fully enter the La3+ node, and part of Co2+ and Fe3+ form a CoFe2O4 compound, which will decrease the magnetic properties of the samples.
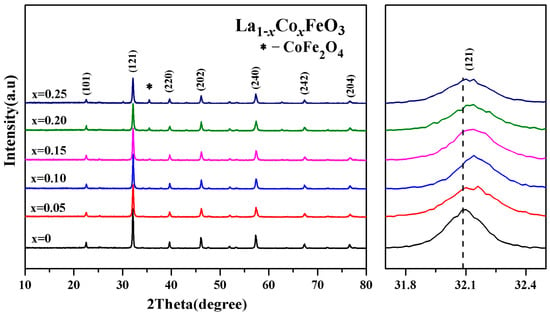
Figure 1.
XRD diffraction and (121) peak drift patterns of La1−xCoxFeO3 (x = 0~0.25) samples calcined at 700 °C for 6 h.
The diffraction peak (121) intensity is decreased with the increase in Co2+ doping concentration. The diffraction peak (121) is wider. The crystallinity of the sample is reduced [21,22,23,24]. The drift pattern shows that with the increase in the Co2+ doping amount, the diffraction peak (121) drifted in the direction of a larger angle. Wankassama Haron [12] came to a similar conclusion. Wankassama Haron et al. [12] explained that the substitution of small radius Co2+ (ionic radius of 0.074 nm) for larger radius La3+ (ionic radius 0.274 nm) will reduce the c/a. Therefore, the lattice spacing d decreased. According to the Bragg diffraction condition 2dsinθ = nλ, it is known that diffraction peak drifted in the direction of 2θ increased. This explains that according to the equation below, the smaller radius of Co3+ (ionic radius of 0.167 nm) replaced the larger radius of La3+ (ionic radius of 0.274 nm) [14,15,16]:
Figure 2 shows the change trend of the average grain size obtained via jade software. After doping Co3+, the diffraction peak was wide, and the grain size decreased. When the doping amount x > 0.05, the average grain size changed irregularly with the increase in x value. This may be related to the second phase CoFe2O4 [3]. Figure 3 is an XRD diffraction pattern of the La0.85Co0.15FeO3 sample. When the calcination temperature is 1000 °C, XRD detects impurities CoFe2O4. Rare earth transition metal composite oxide LaFeO3 material is a typical perovskite orthorhombic structure and a p-type semiconductor [5,6]. The rare earth ion La3+ with relatively large ion radius is more likely to occupy position A, which is located in the hole composed of FeO6 octahedron, whereas the transition metal ion Fe3+ with relatively small ion radius is more likely to occupy position B [7,12]. Fe3+ and the surrounding six O2™ form a FeO6 octahedron structure [17].
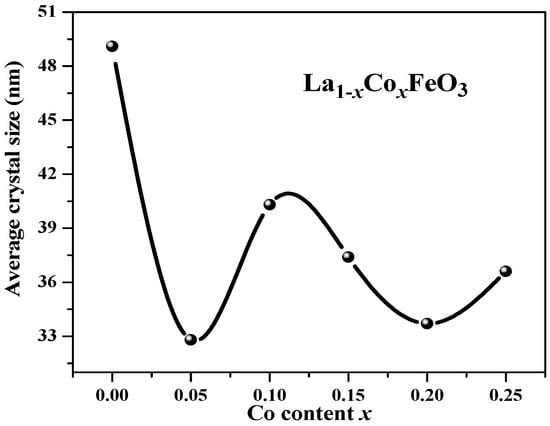
Figure 2.
Average grain size of La1−xCoxFeO3 (x = 0~0.25) calcined at 700 °C for 6 h.
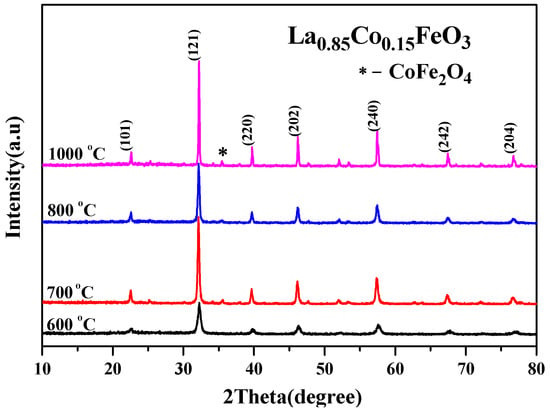
Figure 3.
XRD diffraction pattern of La0.85Co0.15FeO3 calcined at 600~1000 °C for 2 h.
2.2. XRD and TG-DTA Analysis of La1−xRxFeO3 (R = Al)
Figure 4 is the XRD pattern of the La1−xAlxFeO3 (x = 0~0.10) sample. This may be due to the fact that the Al3+ ionic radius (0.0535 nm) is similar to the B-site Fe3+ ionic radius (0.078 nm), resulting in the formation of the Fe2O3 impurity phase. Table 1 shows that the lattice parameters (a, b, c), crystal cell volume and particle size showed a trend of being smaller with the increase in Al3+ doping concentration because La3+ ion radiuses (0.274 nm) are replaced by a smaller Al3+ ion radiuses [25].
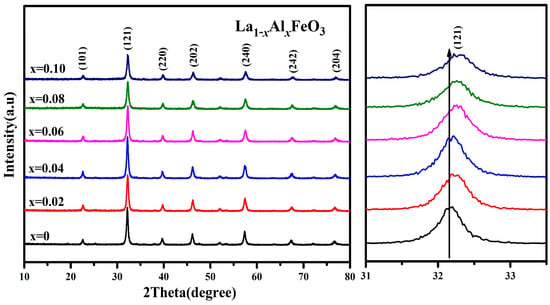
Figure 4.
XRD diffraction pattern of La1−xAlxFeO3 (x = 0~0.10) calcined at 600 °C for 2 h.

Table 1.
Lattice parameters of La1−xAlxFeO3 (x = 0~0.10) samples calcined at 600 °C for 2 h.
Table 1 shows that as the Al3+ ion doping amount increased, the cell volume decreased. It was estimated using Scherrer’s formula:
The XRD pattern clearly shows that the diffraction peak drifted in the direction of a larger angle, which can also indicate that the lattice parameters and the unit cell volume have a tendency to decrease.
Figure 5 is the XRD diffraction pattern of La0.9Al0.1FeO3 samples. When the calcination temperature was 800 °C, Fe2O3 peaks began to appear. Table 2 presents the lattice parameters of the La0.9Al0.1FeO3 sample calcined at 600 °C, 800 °C, and 1000 °C. This explains that according to the equation below, the smaller radius of Al3+ (ionic radius of 0.182 nm) replaced the larger radius of La3+ (ionic radius of 0.274 nm). At the same time, to maintain the charge balance, Fe3+ was oxidized to Fe4+ or oxygen vacancies appeared, resulting in lattice distortion.
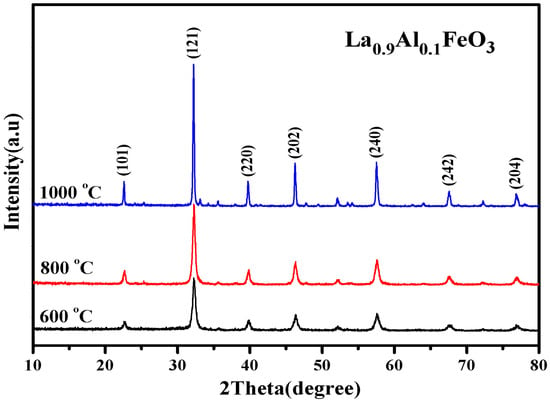
Figure 5.
XRD diffraction pattern of La0.9Al0.1FeO3 samples calcined at temperatures between 600 and 1000 °C for 2 h.

Table 2.
Lattice parameters of La0.9Al0.1FeO3 sample calcined at 600 °C, 800 °C, and 1000 °C for 2 h.
Figure 6 is the TG and DTA curves of La0.9Al0.1FeO3 xerogel. When the temperature rose from 30 °C to 140 °C, the TG curve showed that the weight loss rate was about 10%. Then, at 90 °C, the DTA curve shows a weak endothermic peak due to water evaporation from the wet gel and the expulsion of water inside the dry gel (adsorbed water, crystallization water, and water vapor generated during the reaction). When the temperature increases from 140 °C to 207 °C, there is a weight loss of about 70 percent and a sharp exothermic peak corresponding to the DTA curves at about 207 °C. When the temperature is higher than 207 °C, the weight loss rate was less than 5%, forming La1−xAlxFeO3 [7].
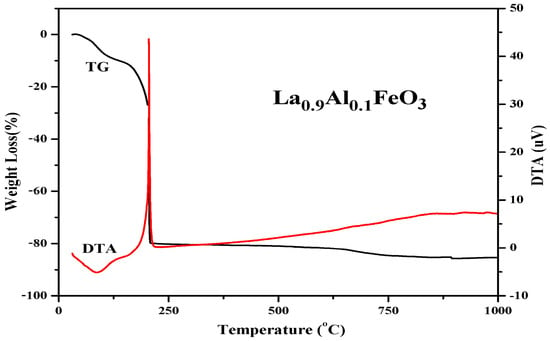
Figure 6.
TG and DTA curves of La0.9Al0.1FeO3 xerogel.
2.3. XRD and TG-DTA Analysis of La1−xRxFeO3 (R = Nd)
Figure 7 shows the XRD diffraction pattern of La1−xNdxFeO3 (x = 0~0.25) samples calcined at 600 °C for 2 h and (121) peak drift pattern. According to Figure 7b, the (121) diffraction peak slightly increased with the increase in Nd3+ doped concentration and drifted in the direction of 2θ [24]. The substitution of small radius Nd3+ (ionic radius of 0.127 nm) for larger radius La3+ (ionic radius of 0.274 nm) leads to lattice distortion [26]. The lattice parameters of corresponding samples are as shown in Table 3. As the Nd3+ content increased, the crystal lattice parameters (a, b, and c) changed accordingly [27], and the cell volume of the samples decreased. The reason is that the La ion was gradually replaced by Nd ion, which reduced the average ionic radius of A-bit and, in turn, caused cell shrinkage, resulting in a decrease in cell volume [28]. The grain size of the samples can be estimated according to Scherrer’s formula. When x ≤ 0.05, the average grain size decreased. When x > 0.05, as the doping amount increased, the grain size increased. It is possible that the ion size of A-bit changed with its nearest neighbor, and the size of the neighboring oxygen atoms depends on the grain size [29,30].
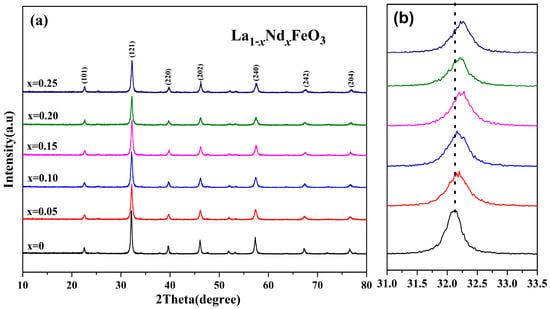
Figure 7.
(a) XRD of La1−xNdxFeO3 (x = 0~0.25) samples calcined at 600 °C and (b) (121) peak drift.

Table 3.
Lattice parameters of La1−xNdxFeO3 (x = 0~0.25) calcined at 600 °C for 2 h.
The XRD diffraction pattern in Figure 8 shows that the main diffraction peak is consistent with the standard sample LaFeO3 card (JCPDS No. 37-1493). No other phases were generated, and the space group is Pnma.
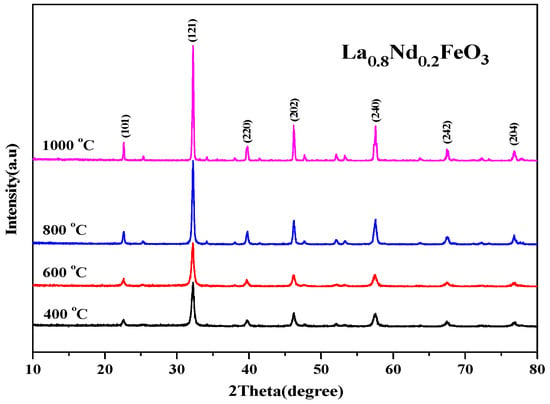
Figure 8.
XRD diffraction pattern of La0.8Nd0.2FeO3 samples calcined at between 400 and 1000 °C for 2 h.
The results show that under four calcination conditions, Nd3+ is better immersed in the crystal lattice of the perovskite, and all characteristic peaks have been indexed according to the orthorhombic structure [1]. As the calcination temperature rose, the diffraction peak became sharper, the half-height width decreased, and the average grain size estimated by Scherrer’s formula gradually increased. The results show that the calcination temperature had a direct effect on the grain size of the powder when the samples were synthesized using the sol-gel method. The higher the calcination temperature, the higher the energy, the larger the grain size, and the larger the size of the powder.
2.4. XRD and TG-DTA Analysis of La1−xRxFeO3 (R = Sm)
Figure 9 shows an XRD diffraction pattern of the La1−xSmxFeO3 (x = 0~0.5) samples. According to the tolerance factor t = rA + rO/1.414 (rB + rO), rA, rB, and rO are A-bit ionic radius, B-bit ionic radius, and O ionic radius, respectively. For part of the A-bit doped samples, rA = (A′ ionic radius) (1 − x) + (radius of doped ions) x; when t values were between 0.75 and 1.00, the perovskite structure was stable. According to the ionic radius records [31,32], La3+ ionic radius was 0.274 nm, Sm3+ ionic radius was 0.124 nm, Fe3+ ionic radius was 0.0645 nm, O ionic radius was 0.132 nm, and the t value of the series samples was between 0.75 and 1.00, which had a stable perovskite structure [33,34]. As the Sm3+ doping concentration increased, the intensity of diffraction peaks decreased. The drift pattern shows that as the Sm3+ doping content increased, the diffraction peak drifted in the direction of the larger angle. This explains that according to the equation below, the smaller radius of Sm3+ (ionic radius of 0.124 nm) replaced the larger radius of La3+ (ionic radius of 0.274 nm).
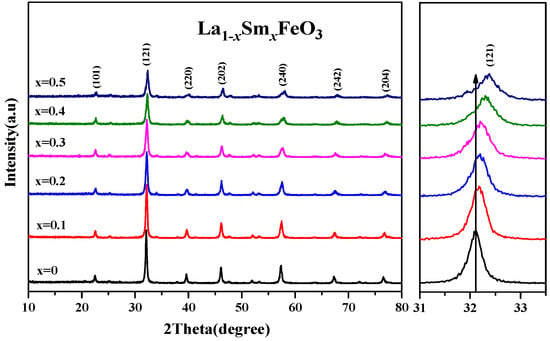
Figure 9.
XRD of La1−xSmxFeO3 (x = 0~0.5) and the peak drift (121).
Table 4 shows that as the Sm3+ ion doping amount increased, the cell volume decreased. Figure 10 shows the XRD diffraction pattern of uncalcined La0.8Sm0.2FeO3 samples, and then calcined at 700 °C and 800 °C for 2 h. The sample diffraction peaks were sharp, suggesting that the samples crystallized well after sintering at a certain temperature. The half-width of the diffraction peak of the samples was 0.371, 0.333, and 0.378, respectively, corresponding to the aforementioned calcination temperatures, with a first decreasing then increasing change trend [1,2]. The average grain size of the samples was 22.7, 25.9, and 21.1 nm, respectively, corresponding to the aforementioned calcination temperatures, with a first increasing then decreasing change trend [6,7].

Table 4.
Lattice parameters of uncalcined La1−xSmxFeO3 (x = 0~0.5).
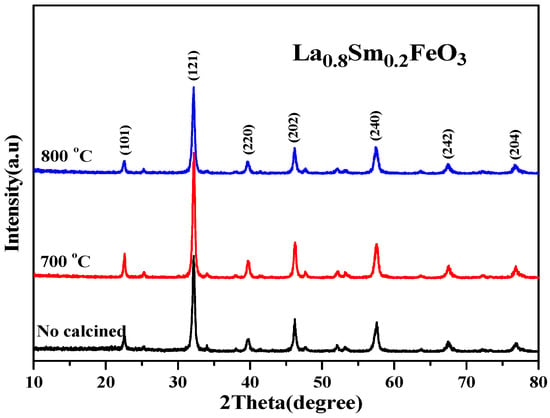
Figure 10.
XRD diffraction pattern of uncalcined La0.8Sm0.2FeO3 samples that were then calcined at 700 °C and 800 °C for 2 h.
Figure 11 shows the TG and DTA curves of La0.8Sm0.2FeO3 xerogel. When the temperature rose from 30 °C to 200 °C, the TG curve showed that the weight loss rate was about 13%. Then, the DTA curve showed a weak endothermic peak at 98.5 °C due to the water evaporation from the wet gel. The weak endothermic peaks at 133 °C and 171 °C were due to the expulsion of water from inside the dry gel. When the temperature rose from 200 °C to 226 °C, the weight loss was about 63%, showing a sharp exothermic peak corresponding to the DTA curves at about 226 °C, which may be due to emissions of nitrates and organic substances, including NOx, COx, and H2O. When the temperature was higher than 226 °C, the weight loss rate was less than 3%, indicating the formation of La1−xSmxFeO3.
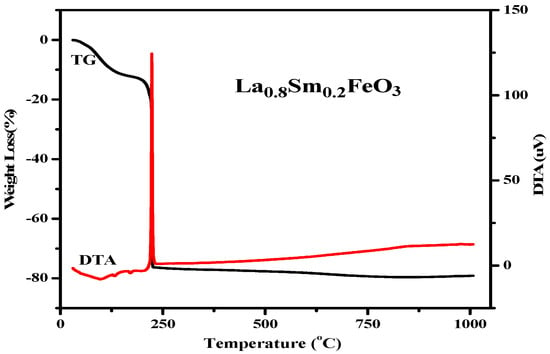
Figure 11.
TG and DTA curves of La0.8Sm0.2FeO3 xerogel.
2.5. Magnetic Analysis of La1−xRxFeO3 (R = Co)
Figure 12 shows the hysteresis loop of the La1−xCoxFeO3 (x = 0~0.25) sample. When the applied magnetic field is 8000 Oe, the magnetization reaches a saturation state [35]. As shown in Table 5, with the increase in Co2+ concentration, the magnetic loop area and magnetization increased. The change of valence state of the A-site ions will directly affect the state of the oxygen ions, which will eventually lead to the generation of oxygen vacancies.
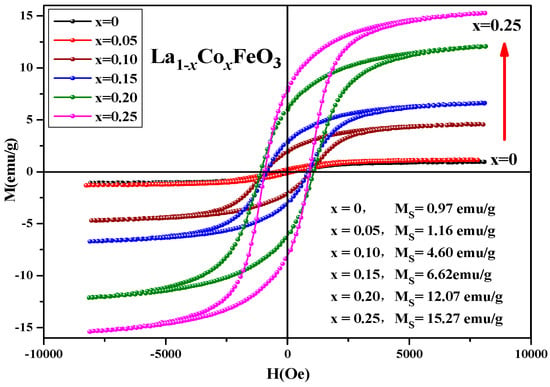
Figure 12.
Hysteresis loop of La1−xCoxFeO3 (x = 0~0.25) samples calcined at 700 °C for 6 h.

Table 5.
Magnetic parameters of La1−xCoxFeO3 sample calcined at 700 °C.
Figure 13 shows the hysteresis loop of La0.85Co0.15FeO3 sample calcined at temperatures between 600 and 1000 °C for 6 h. When the applied magnetic field is 8000 Oe, the magnetization reaches a saturation state. Figure 13 and Table 6 show that when the samples calcined temperature rose from 600 °C to 700 °C, the loop area of the M-H curve changed significantly. With the increase in calcination temperature, the saturation magnetization, remanent magnetization, and coercivity of the samples increased, which may be affected by the mixed-phase CoFe2O4 samples.
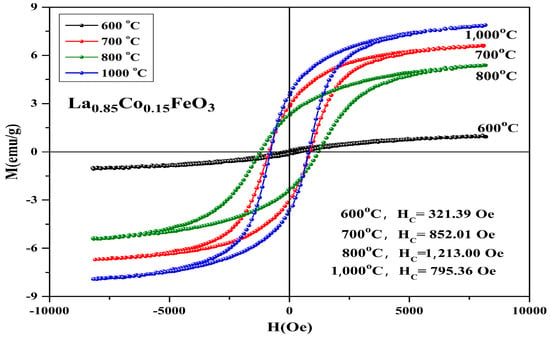
Figure 13.
Hysteresis loop of La0.85Co0.15FeO3 samples calcined at 600~1000 °C for 6 h.

Table 6.
Magnetic parameters of La0.85Co0.15FeO3 sample calcined between 600 and 1000 °C.
In Table 7, Co2+ doping improves the coercive force of a sample. It is caused by the relation of coercive force Hc and magnetocrystalline anisotropy [18,19]. The magnetic moments of the sublattice array composed of iron ions are oppositely aligned in the same straight line, so the samples show antiferromagnetic properties on a macroscopic scale. The magnetic ion Co has a 3d7 electronic configuration and stronger spin-orbit coupling. When Co2+ replaced A-bit non-magnetic ion La3+, the La1−xCoxFeO3 samples had a stronger magnetocrystalline anisotropy constant. When the doping amount x ≥ 0.10, the changes in the coercivity were irregular. There may be a relationship between coercivity and grain size [36]. When the calcination temperature was 600 °C, no CoFe2O4 mixed phase was detected. When it rose to 700 °C, we found the second phase CoFe2O4, and when it rose to 1000 °C, the magnetization of the sample was the largest and the coercive force of sample was 1213 Oe. Figure 14 shows the hysteresis loop of La0.85CO0.15FeO3 samples. The calcination time can regulate the magnetic properties of the sample, as shown in Table 7. From 2 h to 6 h, the magnetic properties reduced significantly.

Table 7.
Magnetic parameters of La0.85CO0.15FeO3 sample calcined at 700 °C.
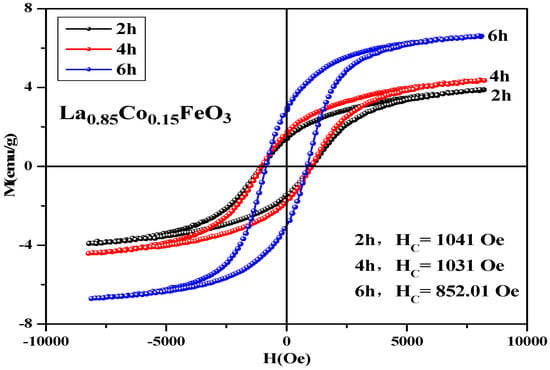
Figure 14.
Hysteresis loop of La0.85Co0.15FeO3 samples calcined at 700 °C for 2 h, 4 h, and 6 h.
2.6. Magnetic Analysis of La1−xRxFeO3 (R = Al)
Figure 15 is hysteresis loop of La1−xAlxFeO3 (x = 0~0.10) samples, the magnetic loop area and magnetization increased with the increase in Al3+ ion concentration and the weak ferromagnetic properties are shown in Table 8. In fact, in the perovskite structure, Fe3+ was in a distorted octahedral B-site location, and the octahedron distortion was inclined along the c-axis direction, and the inclination depended on the size of the adjacent A-site ions, which ultimately determined the Fe-O-Fe super-exchange angle. The reason this may enhance the magnetic properties is as follows: First, when Al3+ ions substituted La3+ ions, the effective size of the A-site of octahedral reduced, changing the Fe-O-Fe super-exchange angle and promoting super-exchange interaction. Second, when the sample was doped, only a small fraction of La3+ was substituted by Al3+ ions, which tend to occupy Fe3+ ion positions at octahedral B-bit. When Al3+ ion substituted the Fe3+ ion, Fe3+ was squeezed into the La3+ ion position of the regular tetrahedron A-bit. This time, the Fe3+ ion spin was not compensated, and the magnetic properties of the sample can be improved. In addition, Fe2O3 impurity phase was detected by XRD, which may also enhance the magnetic properties of the La1−xAlxFeO3 samples [21,22]. Figure 16 shows the hysteresis loop of La0.9Al0.1FeO3 samples. It can be seen from the figure that as the calcination temperature increased, the M-H curve of the sample enclosed area reduced, the ferromagnetic order gradually weakened and tended to be of the antiferromagnetic order, which may be due to with the increase in sintering temperature, decrease in iron oxide composition, and changes in the magnetic properties [14].
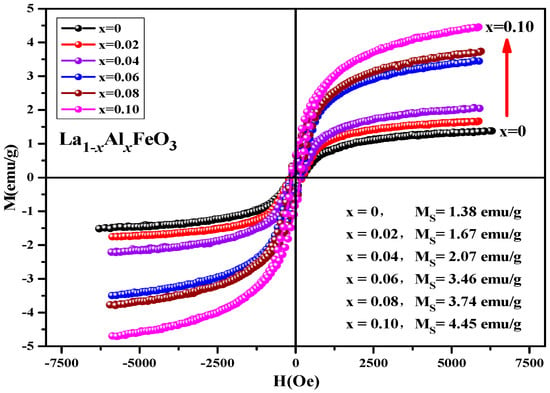
Figure 15.
Hysteresis loop of La1−xAlxFeO3 samples calcined at 600 °C for 2 h.

Table 8.
Magnetic parameters of La1−xAlxFeO3 (x = 0~0.10) samples calcined at 600 °C for 2 h.
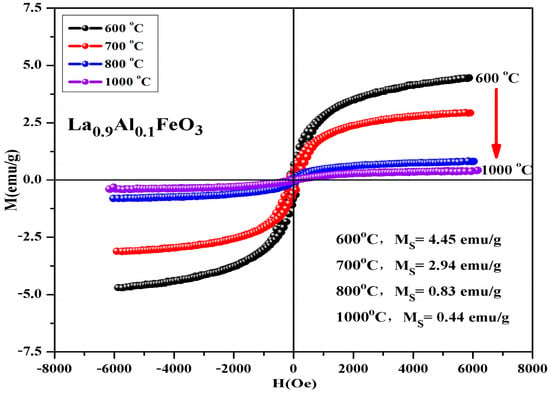
Figure 16.
Hysteresis loop of La0.9Al0.1FeO3 samples calcined at between 400 and 1000 °C for 2 h.
Table 9 shows that the calcination temperature has the same effect on Ms, Mr, and Hc; all of which showed a declining trend as T increased. Figure 17 shows the hysteresis loop of La0.9Al0.1FeO3 samples. The calcination time can regulate the magnetic properties of the sample, as shown in Table 10. From 2 h to 6 h, the magnetic properties reduced significantly. When the calcination time increased from 6 h to 10 h, the sintering time showed little impact on the changes in the magnetic properties.

Table 9.
Magnetic parameters of La0.9Al0.1FeO3 samples calcined at temperatures between 600 and 1000 °C for 2 h.
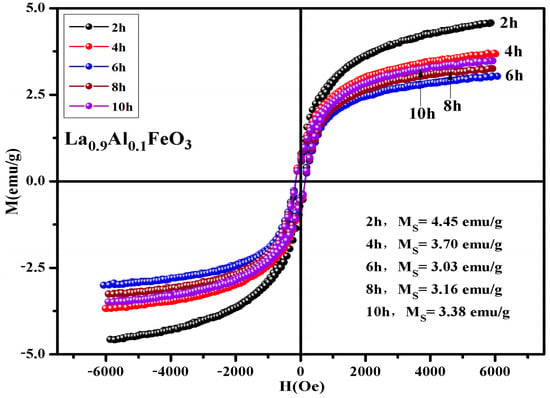
Figure 17.
Hysteresis loop of La0.9Al0.1FeO3 samples calcined at 600 °C between 2 and 10 h.

Table 10.
Magnetic parameters of La0.9Al0.1FeO3 samples calcined at 600 °C for between 2 and 10 h.
2.7. Magnetic Analysis of La1−xRxFeO3 (R = Nd)
Figure 18 is the hysteresis loop of the La1−xNdxFeO3 (x = 0~0.25) samples calcined at 600 °C for 2 h, which was measured at room temperature, and has an applied magnetic field of 0.6 T. It can be seen that the hysteresis loop had a narrow shape, and all samples did not reach a saturation state, showing weak ferromagnetic properties. When x ≤ 0.20, as the Nd3+ doped concentration increased, the magnetization of sample increased. This may be due to the fact that the Nd atomic magnetic moment is not zero. The introduction of Nd3+ leads to an unbalanced distribution of magnetic moment of the whole structure, thereby increasing the magnetization of the samples. When Nd3+ was doped, the magnetization of sample began to decrease.
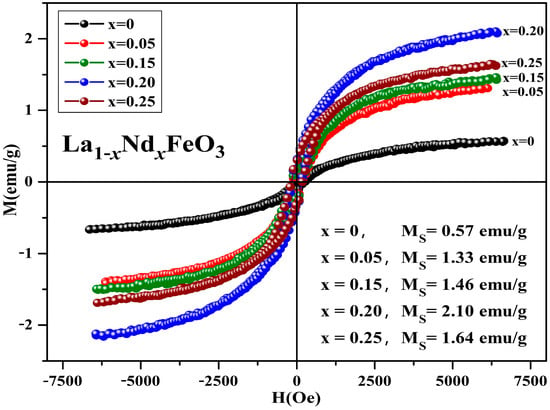
Figure 18.
Hysteresis loop of La1−xNdxFeO3 (x = 0~0.25) samples calcined at 600 °C for 2 h.
The Nd3+ ion concentration reached a certain amount, the Fe-O bond length decreased, and the inclination of the octahedron decreased, thereby strengthening antiferromagnetic exchange interaction and weakening the interaction between weak ferromagnetics. Eventually, the magnetization of the samples was weakened. Table 11 shows the magnetic parameters of the samples. As the Nd3+ content increased, the remanent magnetization and saturation magnetization of the samples first increased then decreased. The magnetic moment and ionic radius of rare earth elements ions are the main factors affecting the saturation magnetization of the doping samples [37]. The coercivity of the samples shows an upward trend, and the main factors affecting the coercivity of the samples include magnetic anisotropy, grain size, micro strain, stress, crystal symmetry and spin-orbit coupling effect, magnetic single domain size, and impurities [31].

Table 11.
Magnetic parameters of La1−xNdxFeO3 (x = 0~0.25) samples calcined at 600 °C for 2 h.
From Figure 19, it can be seen that the magnetization of the samples hardly changed when the calcination temperature rose from 400 °C to 600 °C. As the calcination temperature decreased, the saturation magnetization (Ms) of the samples decreased. The magnetization of the samples changed little when the temperature continued to rise to 1000 °C. As the calcination temperature increased, the magnetization of the samples decreased. It can be seen from Figure 20 that as the calcination time extended, the saturation magnetization of the samples first decreased then increased. When the calcination time was extended, the magnetization changed significantly, and the optimum calcination time was 2 h. The main factors affecting the coercivity of the samples include magnetic anisotropy, grain size, micro strain, stress, crystal symmetry and spin-orbit coupling effect, magnetic single domain size, impurities, and calcination temperature.
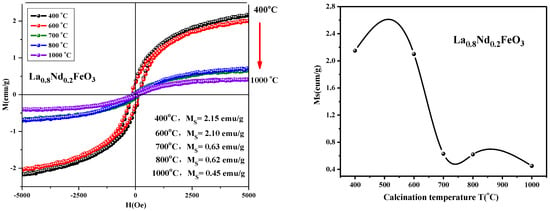
Figure 19.
Hysteresis loop and saturation magnetization of La0.8Nd0.2FeO3 samples calcined between 400 and 1000 °C for 2 h.
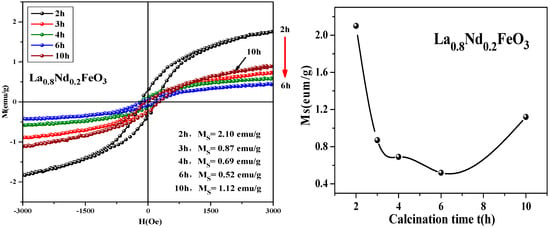
Figure 20.
Hysteresis loop and saturation magnetization of La0.8Nd0.2FeO3 samples calcined at 600 °C for different durations.
2.8. Magnetic Analysis of La1−xRxFeO3 (R = Sm)
Figure 21 shows the hysteresis loop of the uncalcined La1−xSmxFeO3 (x = 0~0.5) samples. It can be seen that the hysteresis loop had a narrow shape, and all samples did not reach a saturation state, showing weak ferromagnetic properties. Table 12 shows the distribution of magnetic parameters of the sample. This may be because the Sm3+ ions are magnetic in nature [37], and together with Fe3+ and O2−, generate a super-exchange interaction, which affected the net magnetic moment size of the crystals, ultimately affecting the magnetization of the samples.
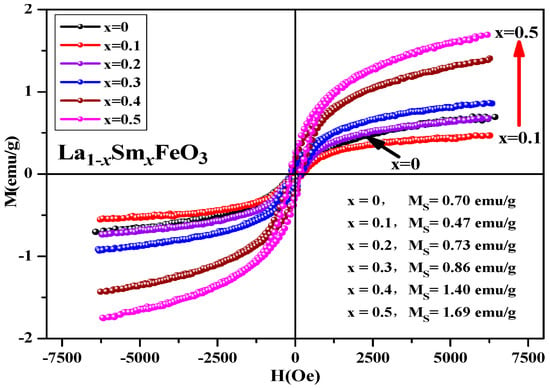
Figure 21.
Hysteresis loop of uncalcined La1−xSmxFeO3 (x = 0~0.5) samples.

Table 12.
Magnetic parameters of uncalcined La1−xSmxFeO3 (x = 0~0.5) samples.
3. Experimental
Compared with other preparation methods, the advantages of the sol-gel method are as follows [13,14]: (1) The uniformity of components in the reaction process is good, and the ratio of reactive ions is controllable [15,16]; (2) It is easy to obtain smaller particle size, better dispersion, and higher purity [17,18]; (3) The experimental operation is simple and the reaction temperature is low [19,20]. La1−xRxFeO3 (R = Co, Al, Nd, Sm) was synthesised by the sol-gel combustion method. The synthetic raw material of the sample is analytically pure nitrate La(NO3)3·nH2O, Fe(NO3)3·9H2O, Co(NO3)3·6H2O, Al(NO3)3·9H2O, Sm(NO3)3·6H2O, Nd(NO3)3·6H2O ammonia (NH3·H2O), and citric acid (C6H8O7·H2O). The crystalline structure was investigated using X-ray diffraction (D/max-2500V/PC, Rigaku). Magnetization measurements were carried out with vibrating sample magnetometer (VSM-100) at room temperature.
4. Conclusions
The effects of sol gel self propagating synthesis method, treatment temperature and doping amount of rare earth ions were studied in this paper. The doping relative sample shape, particle size, microstructure, and magnetic properties were also discussed. In this paper, the synthesis of LaFeO3 nanoparticles doped with Co2+, Al3+, Nd3+, and Sm3+ ion doping LaFeO3 on the structure and magnetic properties of La1−xRxFeO3 (R = Co, Al, Nd, Sm). The diffraction peak intensity is decreased with the increase in Co2+ doping concentration. The crystallinity of the sample is reduced. Fe3+ was oxidized to Fe4+, or oxygen vacancies appeared, resulting in lattice distortion. When Co2+ replaced A-bit non-magnetic ion La3+, the La1−xCoxFeO3 sample will obtain a larger magnetocrystalline anisotropy constant. TG and DTA analysis show that when the temperature is higher than 207 °C, La1−xRxFeO3 (R = Al) is formed. Magnetic properties show that La1−xAlxFeO3 has a weak ferromagnetism; with increasing Al3+ concentration, magnetic properties of the samples showed an increasing trend, and the sintering time that continues to extend with the change of the magnetic properties has a little influence. The results of XRD analysis show that Nd3+ substitutes the perovskite La to form a solid La1−xSmxFeO3 solution, which is a single orthogonal perovskite structure. Through calcination at different temperatures, La0.8Nd0.2FeO3 has a pure phase. The peak of Fe-O-Fe bond antisymmetric stretching vibration in the FeO6 regular octahedron was at about 569 cm−1. The hysteresis loop of La0.8Nd0.2FeO3 samples calcined at temperatures between 400 and 1000 °C for 2 h, respectively, show that as the temperature increased, the saturation magnetization decreased, and the maximum value and minimum value of the saturation magnetization was 2.15 emu/g and 0.45 emu/g, respectively. As the calcination time increased, the saturation magnetization first decreased then increased, and the minimum value of the saturation magnetization was 0.52 emu/g. The results of XRD analysis show that Nd3+ substitutes the perovskite La entering the lattice to form La1−xSmxFeO3 solid solution, which is a single orthogonal perovskite structure with a space group of Pnma, and no impurity peaks were observed. In the future, the reaction mechanism of the sol-gel process can be studied to improve the magnetic properties of LaFeO3 nanoparticles.
Author Contributions
Conceptualization, Q.L. and J.L.; validation, F.Y., X.Y. and K.S.; formal analysis, F.Y., X.Y., K.S. and Y.H.; investigation, F.Y., X.Y. and Q.L.; writing—original draft preparation, J.L., X.Y. and Q.L.; writing—review and editing, J.L., F.Y. and Q.L.; supervision, Q.L. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 12164006, 11364004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
F.Y. and X.Y. contributed equally to this work. All authors discussed the results and commented on the manuscript. Q.L. and J.L. are co-corresponding authors who contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Li, F.-T.; Liu, Y.; Li, Z.-J.; Liu, R.-H.; Yin, R. Preparation and visible-light photocatalytic activity of Nd-doped LaFeO3 nanopowders. Nanotechnol. Precis. Eng. 2010, 8, 231–234. [Google Scholar]
- Traversa, E.; Nunziante, P.; Sangaletti, L.; Allieri, B.; Depero, L.E.; Aono, H.; Sadaoka, Y. Synthesis and Structural Characterization of Trimetallic Perovskite-Type Rare-Earth Orthoferrites, LaxSm1−xFeO3. J. Am. Ceram. Soc. 2000, 83, 1087–1092. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Liu, X. Preparation and gas-sensitive properties of LaFe1−yCoyO3 semiconducting materials. Sens. Actuators B 2011, 79, 171–174. [Google Scholar] [CrossRef]
- Nejat, A.; Ece, A.; Erkan, Y. Solution combustion synthesis of LaMO3, (M=Fe, Co, Mn) perovskite nanoparticles and the measurement of their electrocatalytic properties for air cathode. Int. J. Hydrogen Energy 2013, 38, 13238–13248. [Google Scholar]
- Janbutrach, Y.; Hunpratub, S.; Swatsitang, E. Ferromagnetism and optical properties of La1−xAlxFeO3 nanopowders. Nanoscale Res. Lett. 2014, 9, 498. [Google Scholar] [CrossRef]
- Khine, M.S.S.; Chen, L.; Zhang, S.; Lin, J.; Jiang, S.P. Syngas production by catalytic partial oxidation of methane over (La0.7A0.3)BO3 (A = Ba, Ca, Mg, Sr, and B = Cr or Fe) perovskite oxides for portable fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 13300–13308. [Google Scholar] [CrossRef]
- Liu, L.; Han, A.; Ye, M.; Zhao, M. Synthesis and characterization of Al3+ doped LaFeO3 compounds: A novel inorganic pigments with high near-infrared reflectance. Sol. Energy Mater. Sol. Cells 2015, 132, 377–384. [Google Scholar] [CrossRef]
- Yin, X.-T.; Huang, H.; Xie, J.-L.; Dastan, D.; Li, J.; Liu, Y.; Tan, X.-M.; Gao, X.-C.; Shah, W.A.; Ma, X.-G. High-performance visible-light active Sr-doped porous LaFeO3 semiconductor prepared via sol–gel method. Green Chem. Lett. Rev. 2022, 15, 546–556. [Google Scholar] [CrossRef]
- Saikia, N.; Chakravarty, R.; Bhattacharjee, S.; Hota, R.; Parida, R.; Parida, B. Synthesis and characterization of Gd-doped LaFeO3 for device application. Mater. Sci. Semicond. Process. 2022, 151, 106969. [Google Scholar] [CrossRef]
- Aranthady, C.; Jangid, T.; Gupta, K.; Mishra, A.K.; Kaushik, S.; Siruguri, V.; Rao, G.M.; Shanbhag, G.V.; Sundaram, N.G. Selective SO2 detection at low concentration by Ca substituted LaFeO3 chemiresistive gas sensor: A comparative study of LaFeO3 pellet vs thin film. Sens. Actuators B Chem. 2021, 329, 129211. [Google Scholar] [CrossRef]
- Rao, Y.; Zhang, Y.; Fan, J.; Wei, G.; Wang, D.; Han, F.; Huang, Y.; Croué, J.-P. Enhanced peroxymonosulfate activation by Cu-doped LaFeO3 with rich oxygen vacancies: Compound-specific mechanisms. Chem. Eng. J. 2022, 435, 134882. [Google Scholar] [CrossRef]
- Haron, W.; Thaweechai, T.; Wattanathana, W.; Laobuthee, A.; Manaspiya, H.; Veranitisagul, C.; Koonsaeng, N. Structural Characteristics and Dielectric Properties of La1−xCoxFeO3 and LaFe1−xCoxO3 Synthesized via Metal Organic Complexes. Energy Procedia 2013, 34, 791–800. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent No. 3330697, 11 July 1967. [Google Scholar]
- Bhat, I.; Husain, S.; Khan, W.; Patil, S.I. Effect of Zn doping on structural, magnetic and dielectric properties of LaFeO3 synthesized through sol-gel auto-combustion process. Mater. Res. Bull. 2013, 48, 4506–4512. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Du, Z.; Yang, Z.; Zhao, H.; Skinner, S.J. LaxPr4−xNi3O10−δ: Mixed A-Site Cation Higher-Order Ruddlesden-Popper Phase Materials as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes. Crystals 2020, 10, 428. [Google Scholar] [CrossRef]
- Li, S.; Jing, L.; Fu, W.; Yang, L.; Xin, B.; Fu, H. Photoinduced charge property of nanosized perovskite-type LaFeO3 and its relationships with photocatalytic activity under visible irradiation. Mater. Res. Bull. 2007, 42, 203–212. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Seymour, I.D.; Skinner, S.J. Neutron diffraction and DFT studies of oxygen defect and transport in higher-order Ruddlesden–Popper phase materials. RSC Adv. 2023, 13, 13786–13797. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.M.; Acharya, S.A. Novel perovskite-spinel composite approach to enhance the magnetization of LaFeO3. RSC Adv. 2015, 5, 14366–14373. [Google Scholar] [CrossRef]
- Gou, L.; Shen, X.; Song, F.; Lin, L.; Zhu, Y. Structure and magnetic property of CoFe2−xSmxO4 (x = 0–0.2) nanofibers prepared by Sol-gel route. Mater. Chem. Phys. 2011, 129, 943–947. [Google Scholar]
- Abdel-Khalek, E.K.; Mohamed, H.M. Synthesis, structural and magnetic properties of La1−xCaxFeO3 prepared by the co-precipitation method. Hyperfine Interact. 2013, 222, S57–S67. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S. Perovskite LaFeO3 nanoparticles synthesized by the reverse microemulsion nanoreactors in the presence of aerosol-OT: Morphology, crystal structure, and their optical properties. Superlattices Microstruct. 2013, 64, 148–157. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Saghatforoush, L.A. A unique and facile preparation of lanthanum ferrite nanoparticles in emulsion nanoreactors Morphology, structure, and efficient photocatalysis. Mater. Sci. Semicond. Process. 2014, 25, 301–306. [Google Scholar] [CrossRef]
- Lebid, M.; Omari, M. Synthesis and Electrochemical Properties of LaFeO3 Oxides Prepared Via Sol-Gel Method. Arab. J. Sci. Eng. 2014, 39, 147–152. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Ganesh, V.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Novel synthesis of LaFeO3 nanostructure dendrites a systematic investigation of growth mechanism, properties, and biosensing for highly selective determination of neurotransmitter compounds. Cryst. Growth Des. 2013, 13, 291–302. [Google Scholar] [CrossRef]
- Li, M.; Feng, M.; Guo, C.; Qiu, S.; Zhang, L.; Zhao, D.; Guo, H.; Zhang, K.; Wang, F. Green and Efficient Al-Doped LaFexAl1−xO3 Perovskite Oxide for Enhanced Phosphate Adsorption with Creation of Oxygen Vacancies. ACS Appl. Mater. Interfaces 2023, 15, 16942–16952. [Google Scholar] [CrossRef]
- Berger, C.; Bucher, E.; Lammer, J.; Nader, C.; Sitte, W. Fundamental material property trends in the La0.8−xNdxCa0.2FeO3−δ series: Crystal structure and thermal expansion. J. Mater. Sci. 2021, 56, 10191–10203. [Google Scholar] [CrossRef]
- Ateia, E.E.; Ismail, H.; Elshimy, H.; Abdelmaksoud, M.K. Structural and Magnetic Tuning of LaFeO3 Orthoferrite Substituted Different Rare Earth Elements to Optimize Their Technological Applications. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1713–1725. [Google Scholar] [CrossRef]
- Nakhaei, M.; Khoshnoud, D.S. Study on structural, magnetic and electrical properties of ReFeO3 (Re = La, Pr, Nd, Sm & Gd) orthoferrites. Phys. B Condens. Matter 2021, 612, 412899. [Google Scholar]
- Pawar, G.S.; Tahir, A.A. Unbiased Spontaneous Solar Fuel Production using Stable LaFeO3 Photoelectrode. Sci. Rep. 2018, 8, 3501. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Yelon, W.B.; James, W.J.; Zhou, X.D.; Xie, Y.X.; Anderson, H.U.; Chu, Z. Magnetic and Mössbauer studies on oxygen deficient perovskite, La0.6Sr0.4FeO3−δ. J. Appl. Phys. 2002, 91, 7718–7720. [Google Scholar] [CrossRef]
- Sani, P.; Choudhary, V.; Singh, B.P.; Mathur, R.B.; Dhawan, S.K. Enhanced microwave absorption behavior of polyaniline CNT/polystyrene blend in 12.4–18.0 GHz range. Synth. Met. 2011, 161, 1522–1526. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, Y.-M. Effect of Gd substitution on structural and magnetic properties of Zn-Cu-Cr ferrites prepared by novel rheological technique. Mater. Sci. Technol. 2009, 25, 415. [Google Scholar] [CrossRef]
- Rahman, S.; Nadeem, K.; Anis-ur-Rehman, M.; Mumtaz, M.; Naeem, S.; Letofsky-Papst, I. Structural and magnetic properties of ZnMg-ferrite nanoparticles prepared using theco-precipitation method. Ceram. Int. 2013, 39, 5235–5239. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Ganesh, I.; Mangalaraj, D.; Viswanathan, C.; Balamurugan, A.; Ponpandian, N. Controlled synthesis of perovskite LaFeO3 microsphere composed of nanoparticles via self-assembly process and their associated photocatalytic activity. Chem. Eng. J. 2012, 209, 420–428. [Google Scholar] [CrossRef]
- Kuppan, M.; Yamamoto, D.; Egawa, G.; Kalainathan, S.; Yoshimura, S. Magnetic properties of (Bi1−xLax)(Fe,Co)O3 films fabricated by a pulsed DC reactive sputtering and demonstration of magnetization reversal by electric field. Sci. Rep. 2021, 11, 11118. [Google Scholar] [CrossRef]
- Sanpo, N.; Berndt, C.C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef]
- Jia, L.; Lloyd, M.D.; Lees, M.R.; Huang, L.; Walton, R.I. Limits of Solid Solution and Evolution of Crystal Morphology in (La1−xREx)FeO3 Perovskites by Low Temperature Hydrothermal Crystallization. Inorg. Chem. 2023, 62, 4503–4513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).