Novel Cytotoxic Sesquiterpene Coumarin Ethers and Sulfur-Containing Compounds from the Roots of Ferula turcica
Abstract
1. Introduction
2. Results
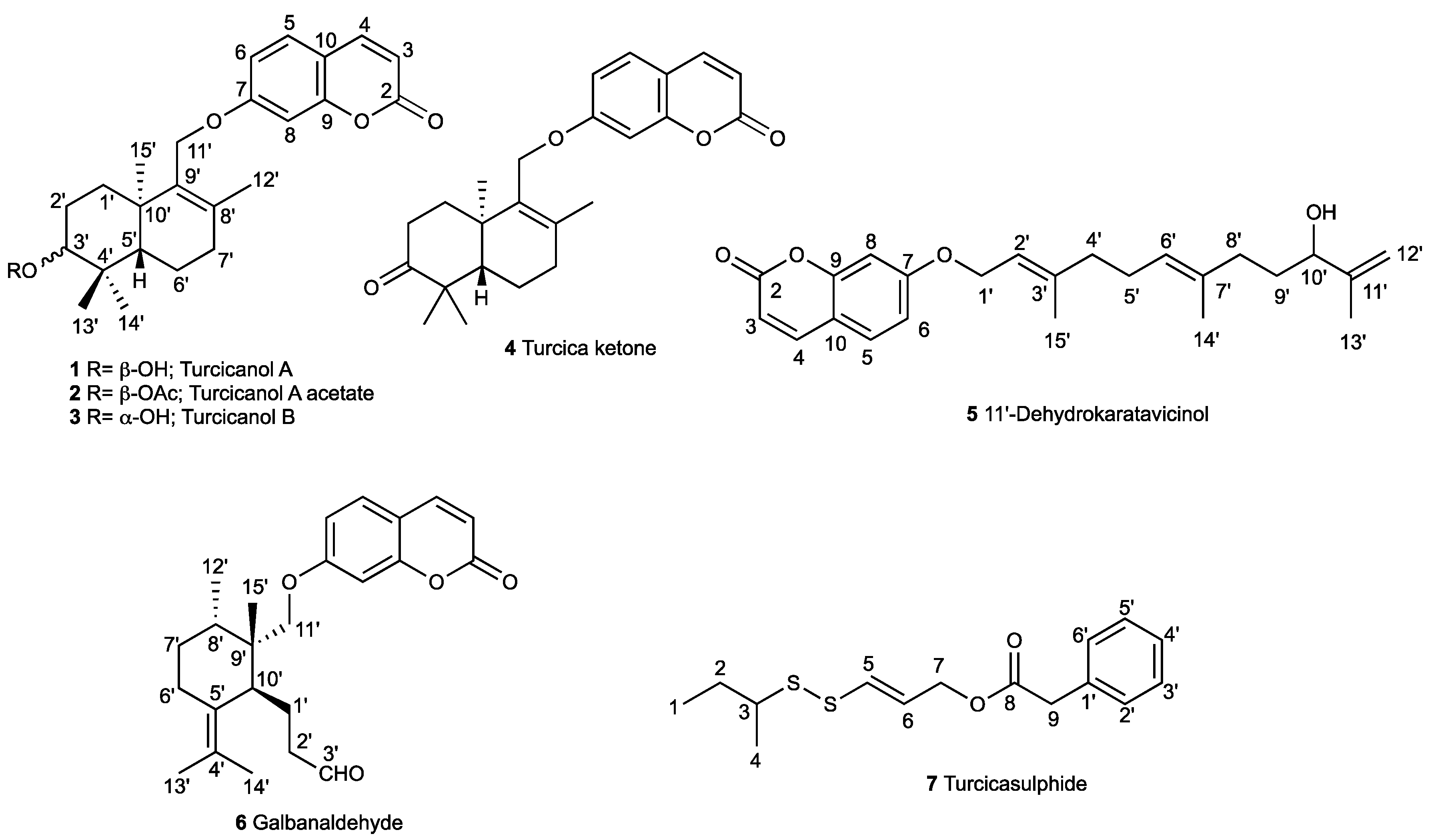
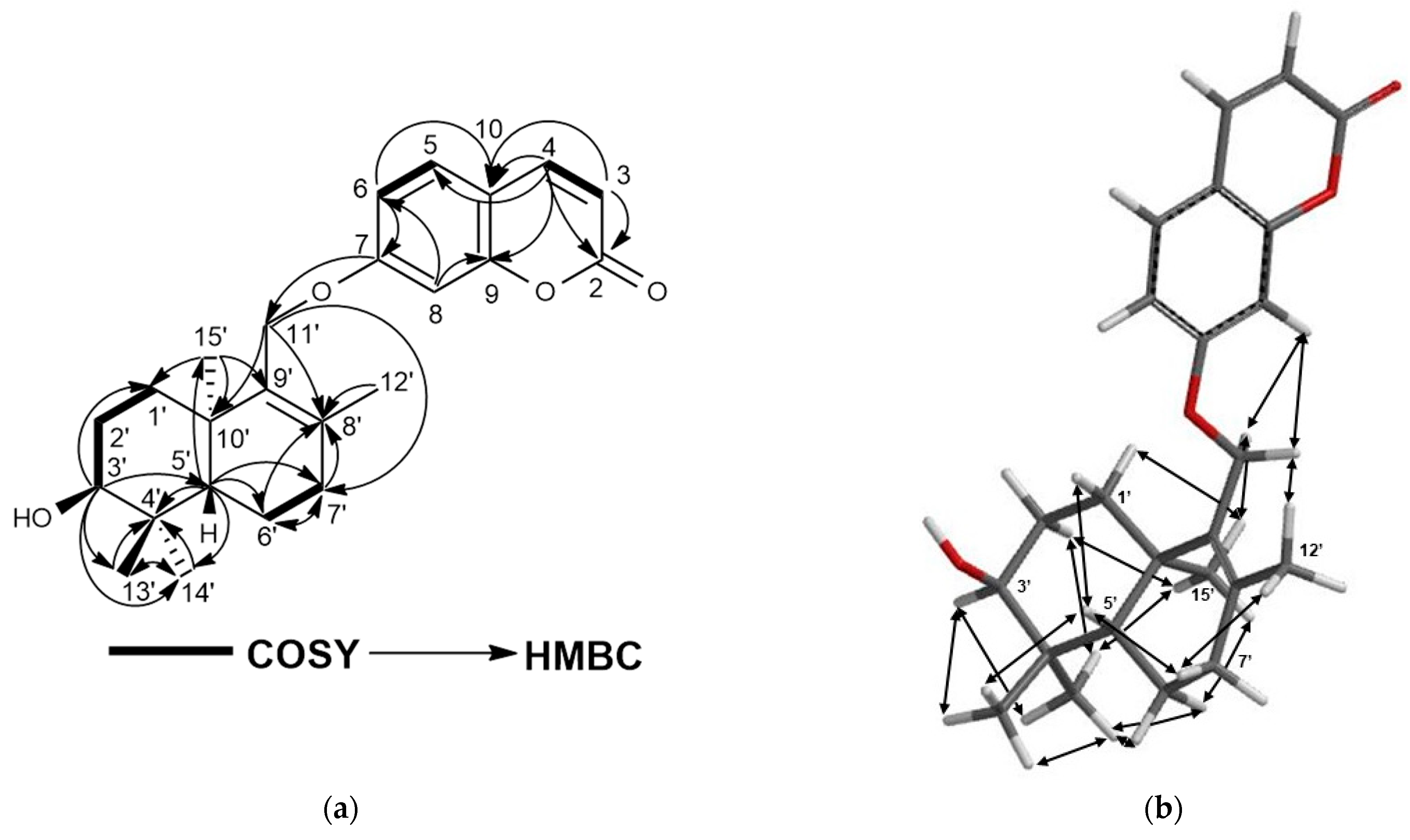
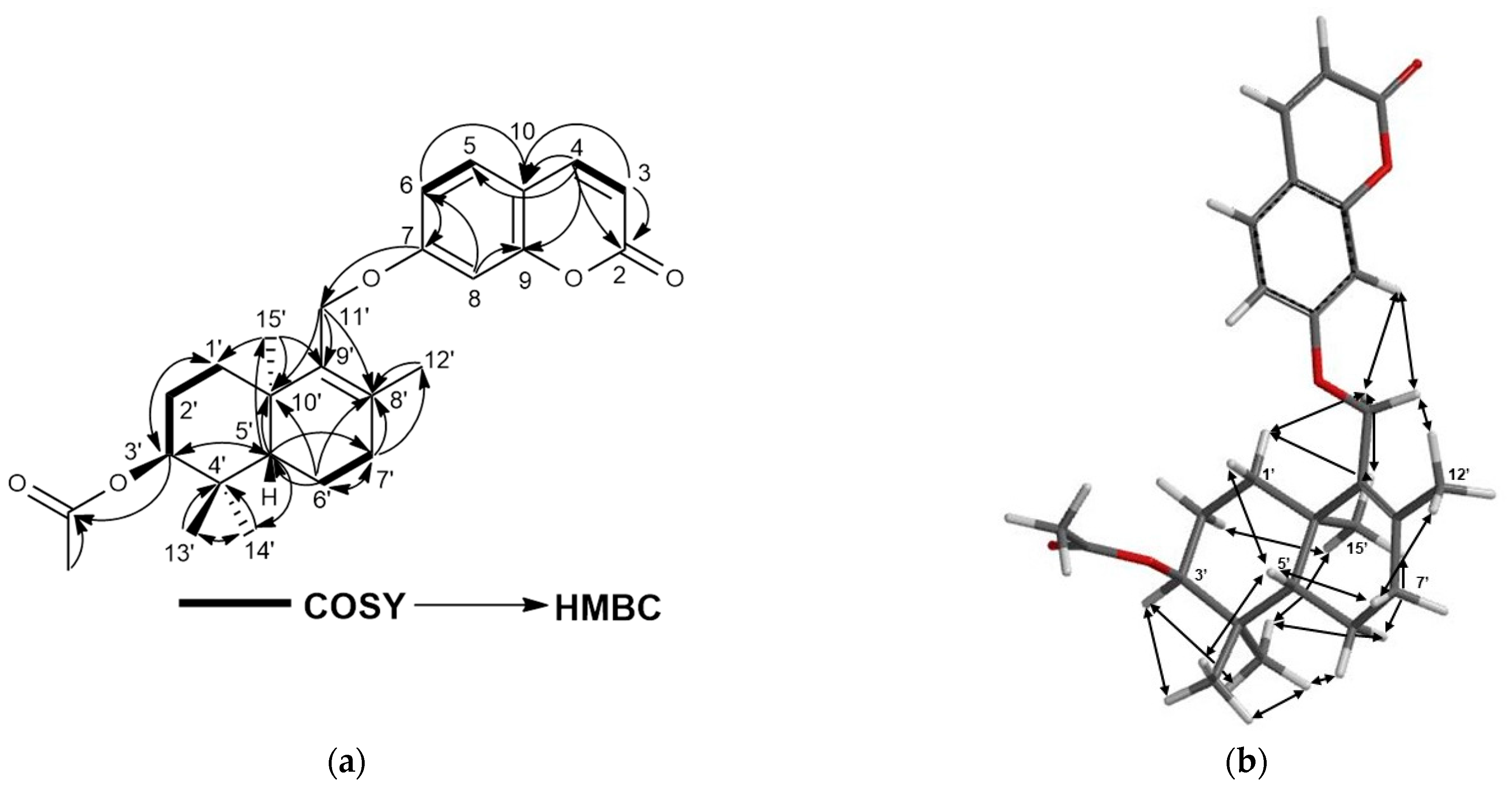
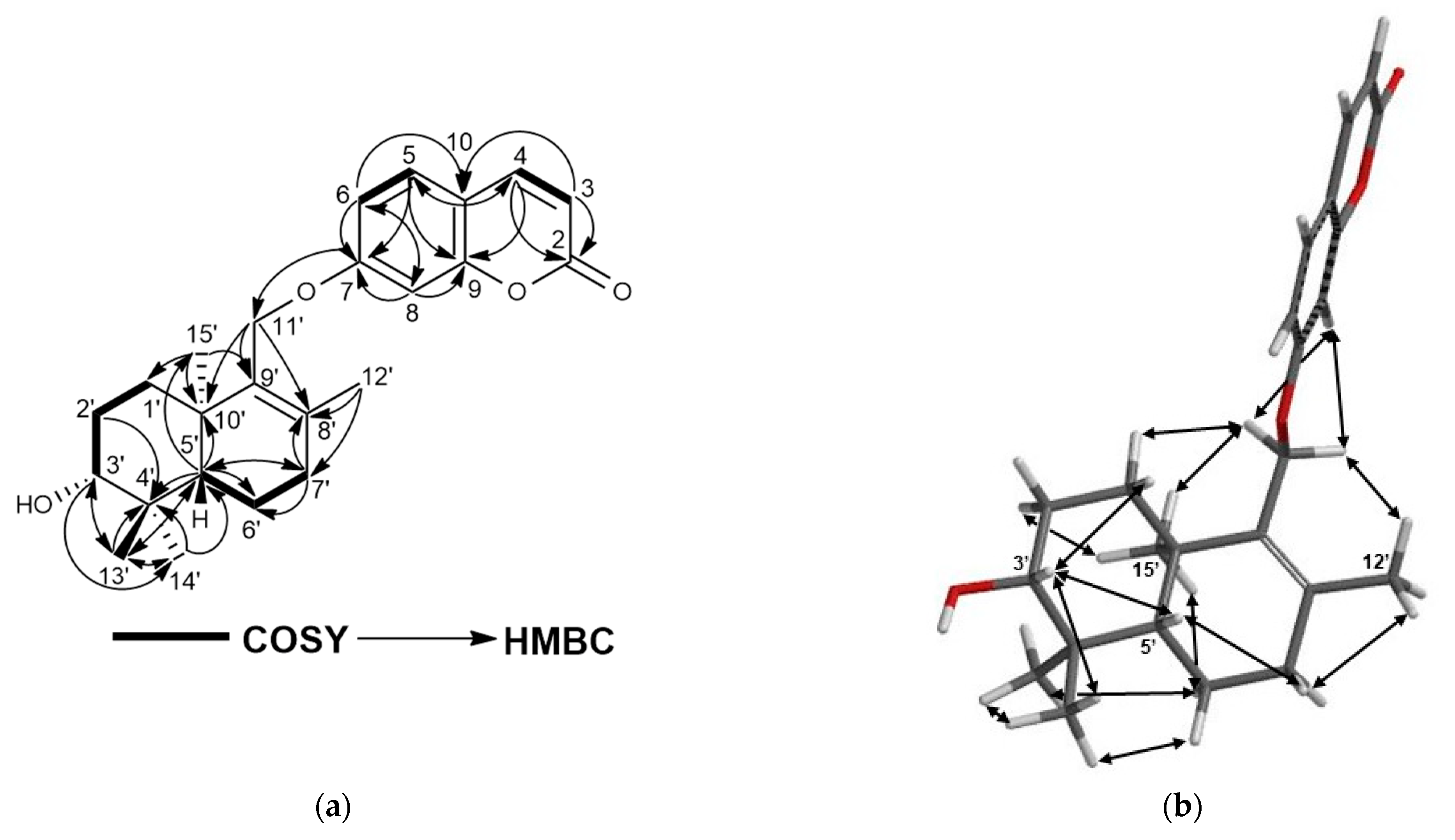
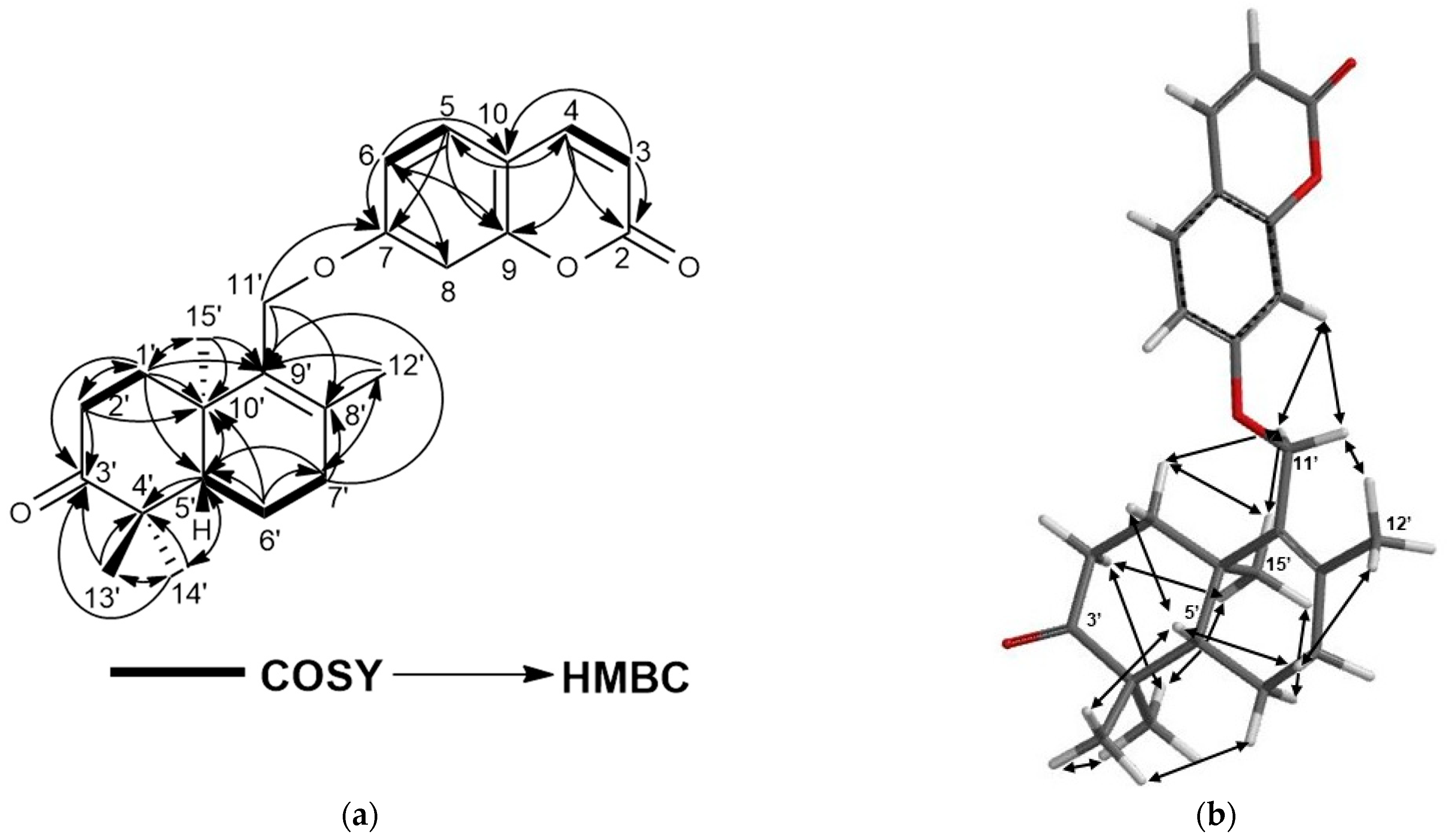
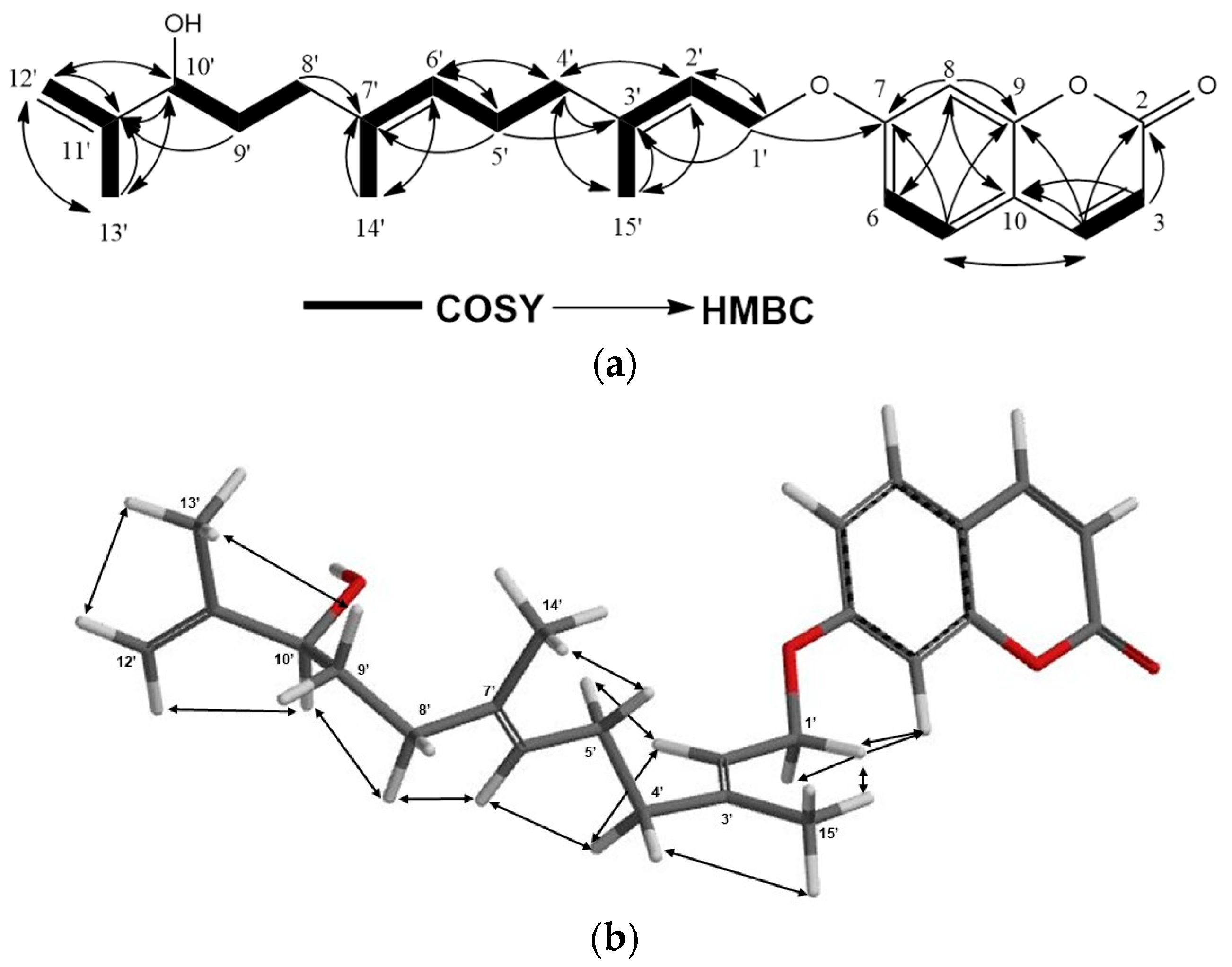
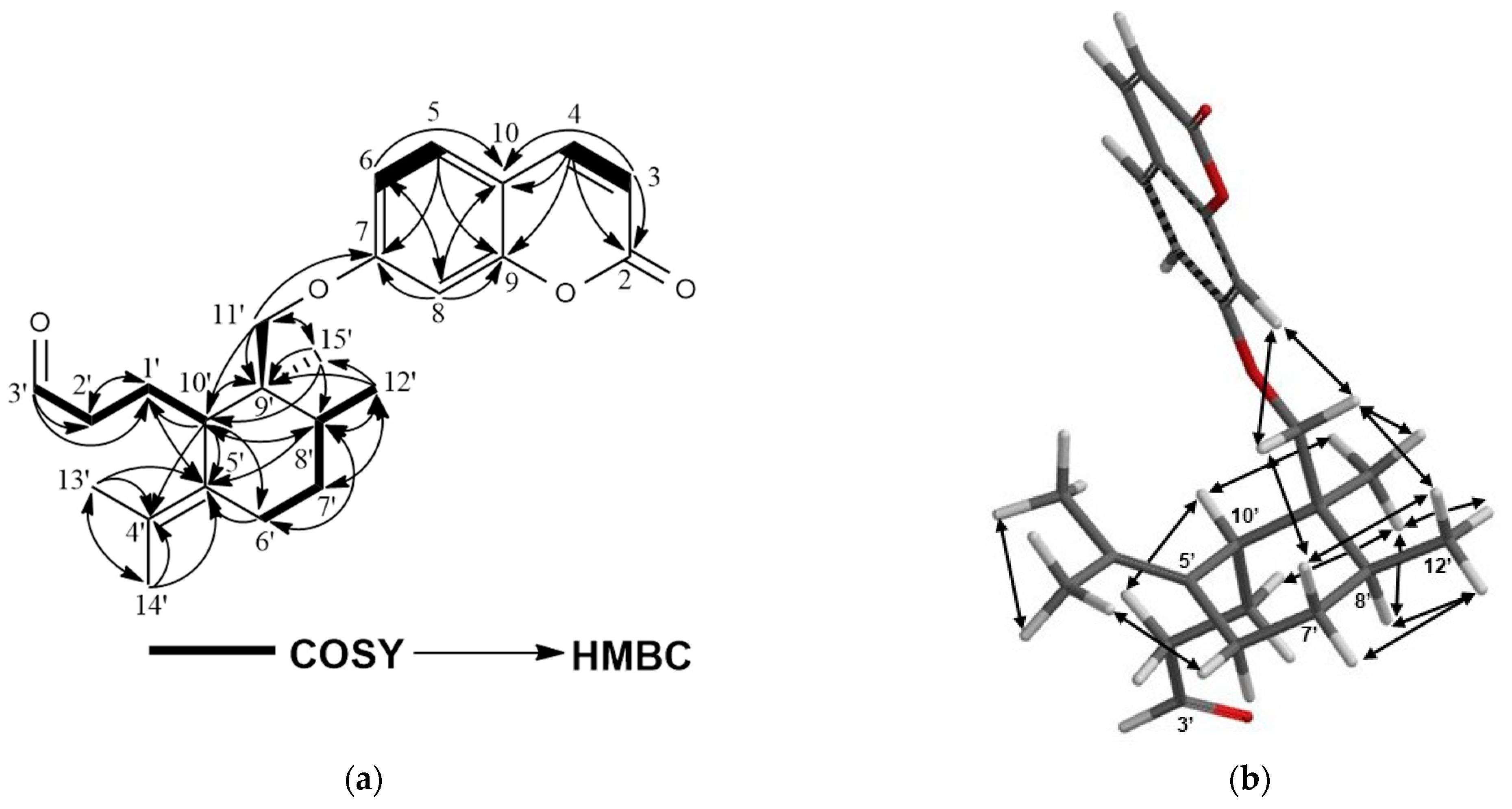
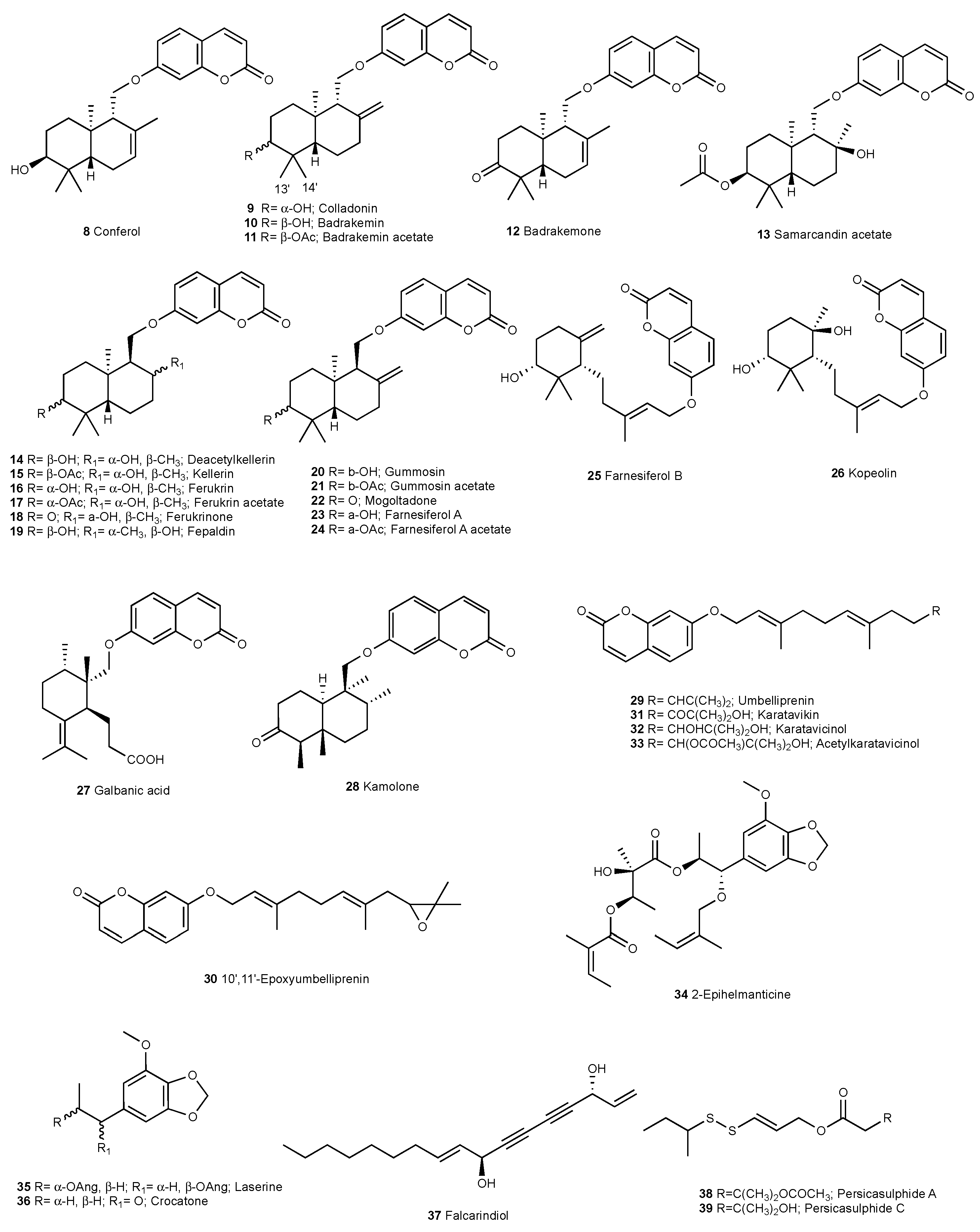
2.1. Characterization of Cytotoxic Compounds
2.2. Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. 2DAY (Colon 2) XTT Cytotoxic Activity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cancer. World Health Organization (WHO) Health Topics. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 June 2023).
- Özhatay, N.; Koçyiğit, M.; Bona, M. İstanbul’un Ballı Bitkileri “Çiçek Varsa Bal Var”, 1st ed.; Türkmenler Matbaacılık Rek. San. Tic. Ltd. Şti. Company: İstanbul, Türkiye, 2010; pp. 18–22. [Google Scholar]
- Zachos, F.E.; Habel, J.C. Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011; p. 171. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs From 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Pimenov, M.G.; Leonov, M.V. The Asian Umbelliferae Biodiversity Database (ASIUM) With Particular Reference To South-West Asian Taxa. Turk. J. Bot. 2004, 28, 139–145. [Google Scholar]
- Tuncay, H.O.; Akalın, E.; Doğru-Koca, A.; Eruçar, F.M.; Miski, M. Two New Ferula (Apiaceae) Species From Central Anatolia: Ferula turcica and Ferula latialata. Horticulturae 2023, 9, 144. [Google Scholar] [CrossRef]
- Baytop, A. Türkçe Bitki Adları Sözlüğü, 1st ed.; Türk Dil Kurumu Yayınları Company: Ankara, Türkiye, 1997; p. 578. [Google Scholar]
- Gunther, R.T. The Greek Herbal of Dioscorides, 3rd ed.; Hafner Publishing Company: London, UK; New York, NY, USA, 1968; pp. 323, 328–332. [Google Scholar]
- Eisenman, S.W.; Zaurov, D.E.; Struwe, L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan; Springer: New York, NY, USA; Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013; p. 10. [Google Scholar]
- Abu-Irmailah, B.; Afifi, F. Treatment with Medicinal Plants in Jordan. Dirasat. Med. Biol. Sci. 2000, 27, 53–74. [Google Scholar]
- Iranshahy, M.; Iranshahi, M. Traditional Uses, Phytochemistry and Pharmacology of Asafoetida (Ferula assa-foetida Oleo-gum-resin)—A review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef]
- Saidkhodzhaev, A.I. Sesquiterpene Derivatives of The Genus Ferula. Chem. Nat. Compd. 1979, 15, 379–404. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J. Daucane Esters From Ferula communis subsp. communis. Phytochemistry 1985, 24, 1735–1741. [Google Scholar] [CrossRef]
- Saidkhodzhaev, A.I.; Mamatkhanov, A.U. Terpenoids of Plants of The Ferula Genus. I. Natural Carotane Derivatives. Chem. Nat. Compd. 1995, 31, 767–780. [Google Scholar]
- Saidkhodzhaev, A.I.; Kadyrov, A.S.; Malikov, V.M. Stereochemistry of Feshurin, Nevskin, and Colladocin. Chem. Nat. Compd. 1979, 15, 266–268. [Google Scholar] [CrossRef]
- Nazari, Z.E.; Iranshahi, M. Biologically Active Sesquiterpene Coumarins From Ferula species. Phytother. Res. 2011, 25, 315–323. [Google Scholar] [CrossRef]
- Iranshahi, M.; Amin, G.R.; Amini, M.; Shafiee, A. Sulfur Containing Derivatives from Ferula persica var. latisecta. Phytochemistry 2003, 63, 965–966. [Google Scholar] [CrossRef]
- Iranshahi, M.; Yazdi, M.C.; Hassanzadeh-Khayyat, M.; Sahebkar, A. Sulfur Containing Compounds In The Volatile Oil of Ferula latisecta Rech. f. & Aell. Leaves. J. Essent. Oil-Bear. Plants 2009, 12, 64–68. [Google Scholar]
- Barthomeuf, C.; Lim, S.; Iranshahi, M.; Chollet, P. Umbelliprenin From Ferula szowitsiana Inhibits The Growth of Human M4Beu Metastatic Pigmented Malignant Melanoma Cells Through Cell-Cycle Arrest In G1 and Induction of Caspase-dependent Apoptosis. Phytomedicine 2008, 15, 103–111. [Google Scholar] [CrossRef]
- Gholami, O.; Jeddi-Tehrani, M.; Iranshahi, M.; Zarnani, A.H.; Ziai, S.A. Umbelliprenin From Ferula szowitsiana Activates Both Intrinsic and Extrinsic Pathways of Apoptosis In Jurkat T-CLL Cell Line. Iran. J. Pharm. Res. 2013, 12, 371–376. [Google Scholar]
- Bahrami, M.; Haji Molla Hoseini, M.; Rezaei, M.; Ziai, S.A. Umbelliprenin Increases The M1/M2 Ratio of Macrophage Polarization and Improves the M1 Macrophage Activity in THP-1 Cells Cocultured with AGS Cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 9927747. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Demeule, M.; Grassi, J.; Saidkhodjaev, A.; Beliveau, R. Conferone From Ferula schtschurowskiana Enhances Vinblastine Cytotoxicity in MDCK-MDR1 Cells by Competitively Inhibiting P-Glycoprotein Transport. Planta Med. 2006, 72, 634–639. [Google Scholar] [CrossRef]
- Bagirov, V.Y.; Sheichenko, V.I.; Veselovskaya, N.V.; Sklyar, Y.E.; Savina, A.A.; Kir’yalov, I.A. Structure and Stereochemistry of Galbanic Acid. Chem. Nat. Compd. 1980, 16, 439–441. [Google Scholar] [CrossRef]
- Corbu, A.; Aquino, M.; Perez, M.; Gandara, Z.; Arseniyadis, S. Natural and Unnatural A-seco Terpenes from Pulegone: Synthesis of Galbanic acid and Marneral Revisited. Eur. J. Org. Chem. 2009, 36, 6386–6392. [Google Scholar] [CrossRef]
- Kasaian, J.; Iranshahy, M.; Iranshahi, M. Synthesis, Biosynthesis and Biological Activities of Galbanic acid—A review. Pharm. Biol. 2014, 52, 524–531. [Google Scholar] [CrossRef]
- Eruçar, F.M.; Kuran, F.K.; Altıparmak Ülbegi, G.; Özbey, S.; Karavuş, Ş.N.; Arcan, G.G.; Yazıcı Tütüniş, S.; Tan, N.; Aksoy Sağırlı, P.; Miski, M. Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities From The Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin. Pharmaceuticals 2023, 16, 792. [Google Scholar] [CrossRef]
- Tosun, F.; Beutler, J.A.; Ransom, T.T.; Miski, M. Anatolicin, A Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin From The root Extract of Heptaptera anatolica. Molecules 2019, 24, 1153. [Google Scholar] [CrossRef]
- Savina, A.A.; Sklyar, Y.E.; Sheichenko, V.I.; Kir’yanova, I.A.; Fesenko, D.A. Migration of The Exocyclic Double Bond In Terpenoid Coumarins of The Iresane Series. Chem. Nat. Compd. 1979, 15, 550–556. [Google Scholar] [CrossRef]
- Xing, Y.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.; Si, Y.; Hou, Y. Sesquiterpene Coumarins From Ferula sinkiangensis Act As Neuroinflammation Inhibitors. Planta. Med. 2017, 83, 135–142. [Google Scholar] [CrossRef]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins: Plants, Structures, Properties, Chapter II. Chem. Nat. Compd. 1998, 34, 517–548. [Google Scholar]
- Tashkhodzhaev, B.; Turgunov, K.K.; Izotova, L.Y.; Kamoldinov, K.S. Stereochemistry of Samarcandin-Type Sesquiterpenoid Coumarins. Crystal Structures of Feshurin and Nevskin. Chem. Nat. Compd. 2015, 51, 242–246. [Google Scholar] [CrossRef]
- Yadav, J.S.; Satyanarayana, K.; Sreedhar, P.; Srihari, P.; Shaik, T.B.; Kalivendi, S.V. Total Synthesis of (+/−)-Elegansidiol, (+/−)-Farnesiferol B, and (+/−)-Farnesiferol D. Bioorg. Med. Chem. Lett. 2010, 20, 3814–3817. [Google Scholar] [CrossRef] [PubMed]
- Miquet, S.; Vanthuyne, N.; Brémond, P.; Audran, G. Enantioselective Syntheses of The Proposed Structures of Kopeolin and Kopeolone. Chemistry 2013, 19, 10632–10642. [Google Scholar] [CrossRef]
- Lee, S.G.; Ryu, S.Y.; Ahn, J.W. Reinvestigation of The Sturcture of Galbanic Acid by 2D NMR Techniques Including 2D INACEQUATE. Bull. Korean Chem. Soc. 1998, 19, 384–386. [Google Scholar]
- Ermatov, N.E.; Ban’kovskii, A.I.; Perel’son, M.E.; Syrova, G.P.; Sheinker, Y.N. Structure of Kamolone and Kamolol-New Coumarins From Ferula penninervis. Chem. Nat. Compd. 1969, 5, 68–71. [Google Scholar] [CrossRef]
- Iranshahi, M.; Amin, G.R.; Jalalizadeh, H.; Shafiee, A. New Germacrane Derivative From Ferula persica. Pharm. Biol. 2003, 41, 431–433. [Google Scholar] [CrossRef]
- Güvenalp, Z.; Özbek, H.; Yerdelen, K.; Yilmaz, G.; Kazaz, C.; Demirezer, L.Ö. Cholinesterase Inhibition and Molecular Docking Studies of Sesquiterpene Coumarin Ethers From Heptaptera cilicica. R. Nat. Prod. 2017, 11, 462–467. [Google Scholar] [CrossRef]
- Kir’yalov, N.P.; Bagirov, V.Y. Structure of Karatavicin. Chem. Nat. Compd. 1967, 3, 185–187. [Google Scholar] [CrossRef]
- Ahmed, A.A. Sesquiterpene Coumarins and Sesquiterpenes From Ferula sinaica. Phytochemistry 1999, 50, 109–112. [Google Scholar] [CrossRef]
- Lee, C.L.; Chiang, L.C.; Cheng, L.H.; Liaw, C.C.; Abd El-Razek, M.H.; Chang, F.R.; Wu, Y.C. Influenza A (H1N1) Antiviral and Cytotoxic Agents from Ferula assa-foetida. J. Nat. Prod. 2009, 72, 1568–1572. [Google Scholar] [CrossRef]
- Iranshahi, M.; Arfa, P.; Ramezani, M.; Jaafari, M.R.; Sadeghian, H.; Bassarello, C.; Piacente, S.; Cosimo, R. Sesquiterpene Coumarins From Ferula szowitsiana and In Vitro Antileishmanial Activity of 7-Prenyloxycoumarins Against Promastigotes. Phytochemistry 2007, 68, 554–561. [Google Scholar] [CrossRef]
- Yang, R.L.; Yan, Z.H.; Lu, Y. Cytotoxic Phenylpropanoids From Carrot. J. Agric. Food. Chem. 2008, 56, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Kadyrov, A.S.; Nikonov, G.K. 3-Methoxy-4,5-methylenedioxypropiophenone—A new component of the roots of Ferula ugamica. Chem. Nat. Compd. 1973, 9, 95. [Google Scholar] [CrossRef]
- Lechner, D.; Stavri, M.; Oluwatuyi, M.; Pereda-Miranda, R.; Gibbons, S. The Anti-staphylococcal Activity of Angelica dahurica (Bai Zhi). Phytochemistry 2004, 65, 331–335. [Google Scholar] [CrossRef]
- Iranshahi, M.; Noroozi, S.; Behravan, J.; Karimi, G.; Schneider, B. Persicasulphide C, A New Sulphur-Containing Derivative From Ferula persica. Nat. Prod. Res. 2009, 23, 1584–1588. [Google Scholar] [CrossRef]
- Iranshahy, M.; Farhadi, F.; Paknejad, B.; Zareian, P.; Iranshahi, M.; Karami, M.; Abtahi, S.R. Gummosin, A Sesquiterpene Coumarin From Ferula assa-foetida Is Preferentially Cytotoxic To Human Breast and Prostate Cancer Cell Lines. Avicenna J. Phytomed. 2019, 9, 446–453. [Google Scholar]
- Hanafi-Bojd, M.Y.; Iranshahi, M.; Mosaffa, F.; Tehrani, S.O.; Kalalinia, F.; Behravan, J. Farnesiferol A From Ferula persica and Galbanic acid from Ferula szowitsiana Inhibit P-Glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011, 77, 1590–1593. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated Multidrug Resistance In MCF-7/Adr Cancer Cells by Sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef]
- Amin, A.; Tuenter, E.; Cos, P.; Maes, L.; Exarchou, V.; Apers, S.; Pieters, L. Antiprotozoal and Antiglycation Activities of Sesquiterpene Coumarins from Ferula narthex Exudate. Molecules 2016, 21, 1287. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.B.; Vistica, D.; Warren, J.T.; Bokesch, H.R.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay For Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]

| Extracts | IC50 (µg/mL) | |||
|---|---|---|---|---|
| COLO 205 | HCT116 | A498 | UO31 | |
| Dichloromethane extract | 6.8 | 16.8 | 20 | 13.9 |
| Methanol extract | >100 | >100 | >100 | >100 |
| Position | Turcicanol A (1) | Turcicanol A Acetate (2) | Turcicanol B (3) | Turcica Ketone (4) | ||||
|---|---|---|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 2 | - | 161.5 | - | 161.5 | - | 161.4 | - | 161.4 |
| 3 | 6.25; d; 9.4; 1H | 113.0 | 6.24; d; 9.5; 1H | 113.1 | 6.25; d; 9.4; 1H | 113.1 | 6.25; d; 9.4; 1H | 113.2 |
| 4 | 7.64; d; 9.4; 1H | 143.5 | 7.64; d; 9.5; 1H | 143.6 | 7.64; d; 9.4; 1H | 143.6 | 7.64; d; 9.4; 1H | 143.5 |
| 5 | 7.36; d; 8.9; 1H | 128.8 | 7.37; d; 8.5; 1H | 128.8 | 7.37; d; 7.5; 1H | 128.7 | 7.37; d; 9.2; 1H | 129.0 |
| 6 | 6.87; dd; 2.3; 8.9; 1H | 113.4 | 6.89; dd; 2.2; 8.5; 1H | 113.2 | 6.87; dd; 2.3; 7.5; 1H | 113.3 | 6.85; dd; 2.4; 9.2; 1H | 113.2 |
| 7 | - | 162.9 | - | 162.5 | - | 162.5 | - | 162.3 |
| 8 | 6.87; d; 2.3; 1H | 101.4 | 6.87; d; 2.2; 1H | 101.6 | 6.86; d; 2.3; 1H | 101.5 | 6.86; d; 2.4; 1H | 101.5 |
| 9 | - | 156.2 | - | 156.0 | - | 156.0 | - | 156.0 |
| 10 | - | 112.5 | - | 112.5 | - | 112.6 | - | 112.7 |
| 1′α | 1.80; td; 3.7; 13.4; 1H | 29.4 | 1.45; dd; 3.1; 9.6; 1H | 30.0 | 1.70; m; 1H ** | 34.5 | 1.91; dd; 6.3; 8.5; 2H | 34.7 |
| 1′β | 1.43; dt; 3.7; 13.4; 1H | 1.67; m; 1H * | 1.49; td; 2.4; 13.1; 1H | |||||
| 2′α | 1.96; tt; 3.3; 14.3; 1H | 25.7 | 1.90; tddd; 1.7; 5.3; 14.1; 1H | 23.3 | 1.70; m; 2H ** | 27.8 | 2.51; m; 2H * | 34.2 |
| 2′β | 1.62; dq; 3.3; 15.3; 1H | 1.64; m; 1H * | ||||||
| 3′ | 3.46; t; 2.8; 1H | 75.8 | 4.69; t; 2.4; 1H | 77.7 | 3.29; dd; 4.5; 11.7; 1H | 78.9 | - | 217.0 |
| 4′ | - | 37.7 | - | 36.9 | - | 38.9 | - | 47.2 |
| 5′ | 1.68; m; 1H * | 44.8 | 1.7; dd; 1.7; 10.7; 1H | 45.9 | 1.24; dd; 2; 12.3; 1H | 50.7 | 1.83; dd; 2.4; 12.4; 1H | 50.8 |
| 6′α | 1.54; qd; 6.5; 12.6; 1H | 18.5 | 1.53; m; 1H * | 18.4 | 1.54; m; 1H * | 18.7 | 1.61; ddd; 6.5; 11.1; 12.7; 1H | 19.9 |
| 6′β | 1.64; m; 1H * | 1.66; m; 1H * | 1.75; dd; 6.8; 13.5; 1H | 1.67; ddt; 2.4; 6.5; 8.6; 1H | ||||
| 7′α | 2.16; dtd; 6.8; 18.2; 2H | 33.8 | 2.16; td; 6.4; 18.5; 1H | 33.6 | 2.17; d; 6.8; 2H | 34.0 | 2.21; m; 2H * | 33.6 |
| 7′β | 2.20; d; 7.6; 1H | |||||||
| 8′ | - | 135.8 | - | 136.0 | - | 136.2 | - | 136.7 |
| 9′ | - | 135.3 | 2.93; t; 5.1; 1H | 135.2 | - | 135.1 | - | 133.7 |
| 10′ | - | 37.8 | - | 37.8 | - | 37.9 | - | 37.67 |
| 11′a | 4.40; d; 9.9; 1H | 64.6 | 4.56; d; 9.9; 1H | 64.7 | 4.38; d; 9.9; 1H | 64.8 | 4.41; d; 10.1; 1H | 64.7 |
| 11′b | 4.55; d; 9.9; 1H | 4.40; d; 9.9; 1H | 4.54; d; 9.9; 1H | 4.55; d; 10.1; 1H | ||||
| 12′ | 1.68; s; 3H | 19.6 | 1.71; s; 3H | 19.7 | 1.69; s; 3H | 19.5 | 1.72; s; 3H | 19.7 |
| 13′ | 0.88; s; 3H | 22.1 | 0.90; s; 3H | 27.7 | 0.83; s; 3H | 15.7 | 1.08; s; 3H | 21.1 |
| 14′ | 1.00; s; 3H | 28.2 | 0.94; s; 3H | 21.7 | 1.04; s; 3H | 28.2 | 1.13; s; 3H | 27.0 |
| 15′ | 1.04; s; 3H | 20.8 | 1.05; s; 3H | 20.7 | 1.03; s; 3H | 20.9 | 1.11; s; 3H | 20.6 |
| CH3–(OAc) | - | - | 2.07; s; 3H | 21.5 | - | - | - | - |
| C=O (OAc) | - | - | - | 171.1 | - | - | - | - |
| Position | 11′-Dehydrokaratavicinol (5) | Galbanaldehyde (6) | Turcicasulphide (7) | |||||
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |||
| 1 | - | - | - | - | 0.97; t; 7.4; 3H | 11.7 | ||
| 2a | - | 161.5 | - | 161.2 | 1.51; m; 1H * | 28.2 | ||
| 2b | 1.68; td; 6.5; 13.2; 1H | |||||||
| 3 | 6.25; d; 9.5; 1H | 113.0 | 6.24; d; 9.4; 1H | 113.0 | 2.79; h; 6.7; 1H | 48.1 | ||
| 4 | 7.64; d; 9.5; 1H | 143.7 | 7.63; d; 9.4; 1H | 143.2 | 1.28; d; 6.9; 3H | 20.2 | ||
| 5 | 7.35; d; 8.6; 1H | 128.8 | 7.34; d; 8.6; 1H | 128.7 | 6.31; dt; 1.3; 14.8; 1H | 133.1 | ||
| 6 | 6.85; dd; 2.4; 8.6; 1H | 113.4 | 6.81; dd; 2.4; 8.6; 1H | 113.3 | 5.96; dt; 6.5; 14.8; 1H | 122.5 | ||
| 7 | - | 162.2 | - | 162.8 | 4.6; dd; 1.3; 6.5; 2H | 64.4 | ||
| 8 | 6.82; d; 2.4 | 101.6 | 6.75; d; 2.4; 1H | 101.2 | - | 171.3 | ||
| 9 | - | 155.8 | - | 156.0 | 3.63; s; 2H | 41.5 | ||
| 10 | - | 112.5 | - | 112.4 | - | - | ||
| 1′ | 4.60; d; 6.5; 2H | 65.4 | 1.84; m; 2H** | 19.6 | - | 134.0 | ||
| 2′ | 5.46; td; 1.2; 6.5; 1H | 118.6 | 2.29; t; 7.6; 2H | 42.3 | 7.29; m; 1H * | 129.4 | ||
| 3′ | - | 142.2 | 9.74; t; 1.5; 1H | 203.5 | 7.32; m; 1H ** | 128.3 | ||
| 4′ | 2.1; dd; 4.6; 11.6; 2H | 39.6 | - | 126.5 | 7.28; m; 1H * | 127.3 | ||
| 5′ | 2.15; q; 6.7; 2H | 26.2 | - | 129.7 | 7.32; m; 1H ** | 128.7 | ||
| 6′α | 5.14; t; 6.7; 1H | 124.1 | 1.86; m; 1H ** | 24.6 | 7.29; m; 1H * | 129.4 | ||
| 6′β | 2.5; dt; 3.1; 14.3; 1H | |||||||
| 7′α | - | 135.4 | 1.2; dd; 4.6; 13.5; 1H | 32.0 | - | - | ||
| 7′β | 1.6; m; 1H * | |||||||
| 8′a | 1.99; ddd; 6.4; 9.1; 14.4; 1H | 35.8 | 1.9; m; 1H ** | 34.8 | - | - | ||
| 8′b | 2.03; ddd; 6.22; 9.1; 15.1; 1H | - | - | |||||
| 9′ | 1.63; m; 2H * | 33.1 | - | 40.8 | - | - | ||
| 10′ | 4.03; dd; 5.4; 7.5; 1H | 75.6 | 2.92; dd; 4.2; 12; 1H | 42.7 | - | - | ||
| 11′a | - | 147.7 | 3.7; d; 8.2; 1H | 71.8 | - | - | ||
| 11′b | 3.87; d; 8.2; 1H | |||||||
| 12′a | 4.83; brs; 1H | 111.2 | 0.91; d; 6.7; 3H | 16.1 | - | - | ||
| 12′b | 4.93; brs; 1H | |||||||
| 13′ | 1.72; s; 3H | 17.8 | 1.43; s; 3H | 20.4 | - | - | ||
| 14′ | 1.61; s; 3H | 16.1 | 1.61; s; 3H | 20.4 | - | - | ||
| 15′ | 1.75; s; 3H | 16.9 | 1.16; s; 3H | 22.6 | ||||
| Compound | IC50 (µM) | ||||
|---|---|---|---|---|---|
| Colo205 | HCT116 | A498 | UO31 | ||
| 1 | Turcicanol A | >50 | 41.1 | >50 | >50 |
| 2 | Turcicanol A acetate | >50 | >50 | >50 | >50 |
| 3 | Turcicanol B | >50 | >50 | >50 | 38.3 |
| 4 | Turcica ketone | 37.3 | 37.1 | >50 | 32.2 |
| 5 | 11′-Dehydrokaratavicinol | >50 | >50 | >50 | >50 |
| 6 | Galbanaldehyde | >50 | 48.8 | >50 | >50 |
| 7 | Turcicasulphide | >50 | >50 | >50 | >50 |
| 8 | Conferol | 16.9 | 36.2 | >50 | >50 |
| 9 | Colladonin | 35.9 | 47.4 | >50 | 33.1 |
| 10 | Badrakemin | >50 | >50 | >50 | >50 |
| 11 | Badrakemin acetate | >50 | 46.1 | >50 | 44.9 |
| 12 | Badrakemone | >50 | >50 | >50 | >50 |
| 13 | Samarcandin acetate | >50 | >50 | >50 | >50 |
| 14 | Deacetylkellerin | >50 | >50 | >50 | >50 |
| 15 | Kellerin | >50 | >50 | >50 | >50 |
| 16 | Ferukrin | >50 | >50 | >50 | >50 |
| 17 | Ferukrin acetate | >50 | >50 | >50 | >50 |
| 18 | Ferukrinone | >50 | >50 | >50 | >50 |
| 19 | Fepaldin | >50 | >50 | >50 | >50 |
| 20 | Gummosin | 12.7 | 18 | >50 | 19.7 |
| 21 | Gummosin acetate | >50 | 32.2 | 43.3 | 31.2 |
| 22 | Mogoltadone | 46.9 | >50 | >50 | >50 |
| 23 | Farnesiferol A | 35.7 | >50 | >50 | 45.3 |
| 24 | Farnesiferol A acetate | >50 | >50 | >50 | >50 |
| 25 | Farnesiferol B | 42.3 | >50 | >50 | >50 |
| 26 | Kopeolin | >50 | >50 | >50 | >50 |
| 27 | Galbanic acid | >50 | >50 | >50 | >50 |
| 28 | Kamolone | >50 | 43.5 | >50 | >50 |
| 29 | Umbelliprenin | 49.5 | >50 | >50 | >50 |
| 30 | 10′,11′-Epoxyumbelliprenin | 44.4 | >50 | >50 | >50 |
| 31 | Karatavikin | >50 | >50 | >50 | >50 |
| 32 | Karatavicinol | >50 | >50 | >50 | 34.4 |
| 33 | 10′-Acetylkaratavicinol | >50 | >50 | >50 | >50 |
| 34 | 2-Epihelmanticine | >50 | >50 | >50 | >50 |
| 35 | Laserine | >50 | >50 | >50 | >50 |
| 36 | Crocatone | >50 | >50 | >50 | >50 |
| 37 | Falcarindiol | >50 | >50 | >50 | >50 |
| 38 | Persicasulphide A | 49.9 | 15.8 | >50 | 42.5 |
| 39 | Persicasulphide C | 19.6 | >50 | >50 | 25.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eruçar, F.M.; Senadeera, S.P.D.; Wilson, J.A.; Goncharova, E.; Beutler, J.A.; Miski, M. Novel Cytotoxic Sesquiterpene Coumarin Ethers and Sulfur-Containing Compounds from the Roots of Ferula turcica. Molecules 2023, 28, 5733. https://doi.org/10.3390/molecules28155733
Eruçar FM, Senadeera SPD, Wilson JA, Goncharova E, Beutler JA, Miski M. Novel Cytotoxic Sesquiterpene Coumarin Ethers and Sulfur-Containing Compounds from the Roots of Ferula turcica. Molecules. 2023; 28(15):5733. https://doi.org/10.3390/molecules28155733
Chicago/Turabian StyleEruçar, Fatma Memnune, Sarath P. D. Senadeera, Jennifer A. Wilson, Ekaterina Goncharova, John A. Beutler, and Mahmut Miski. 2023. "Novel Cytotoxic Sesquiterpene Coumarin Ethers and Sulfur-Containing Compounds from the Roots of Ferula turcica" Molecules 28, no. 15: 5733. https://doi.org/10.3390/molecules28155733
APA StyleEruçar, F. M., Senadeera, S. P. D., Wilson, J. A., Goncharova, E., Beutler, J. A., & Miski, M. (2023). Novel Cytotoxic Sesquiterpene Coumarin Ethers and Sulfur-Containing Compounds from the Roots of Ferula turcica. Molecules, 28(15), 5733. https://doi.org/10.3390/molecules28155733











