A Hierarchical CuO Nanowire@CoFe-Layered Double Hydroxide Nanosheet Array as a High-Efficiency Seawater Oxidation Electrocatalyst
Abstract
1. Introduction
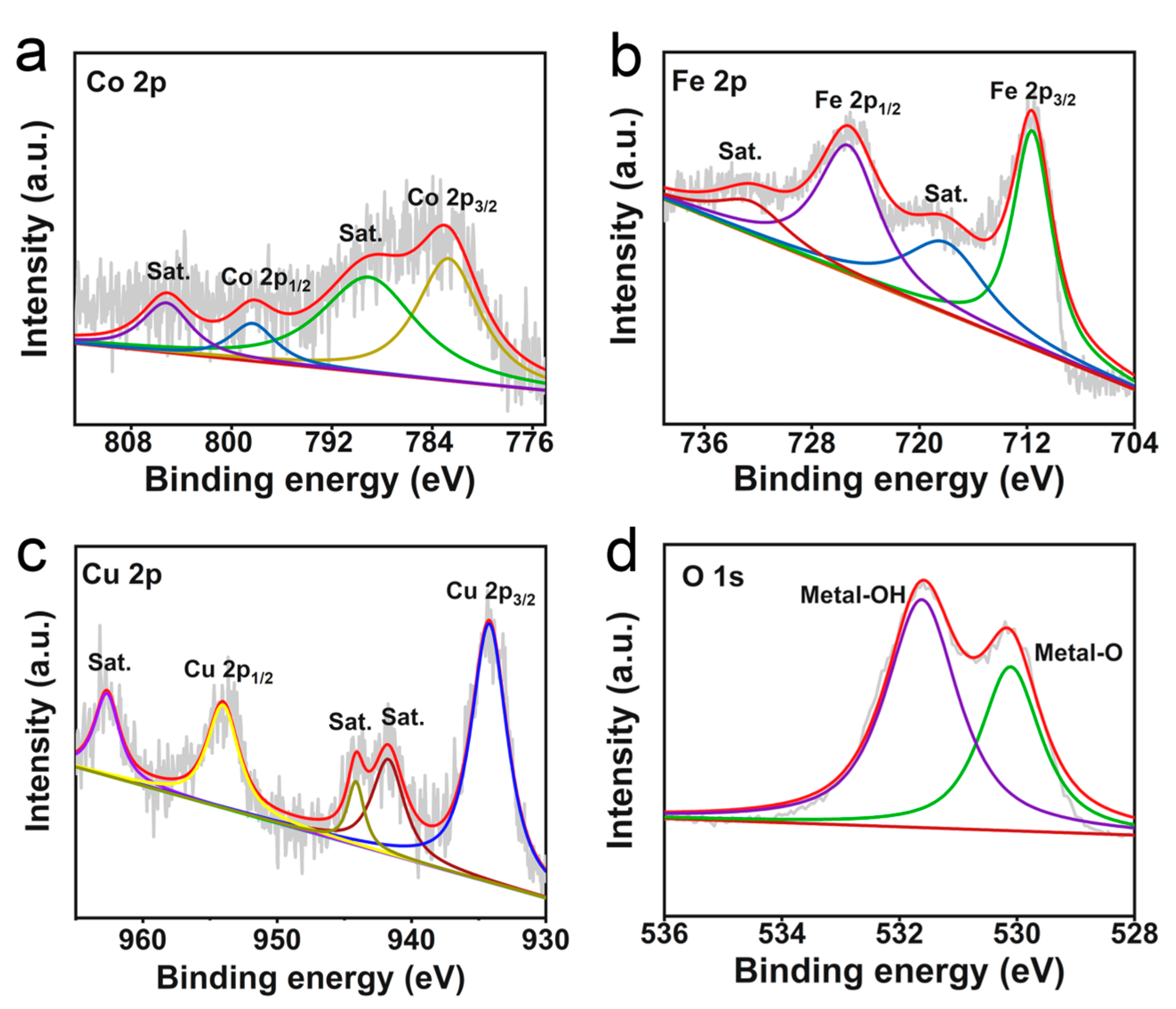
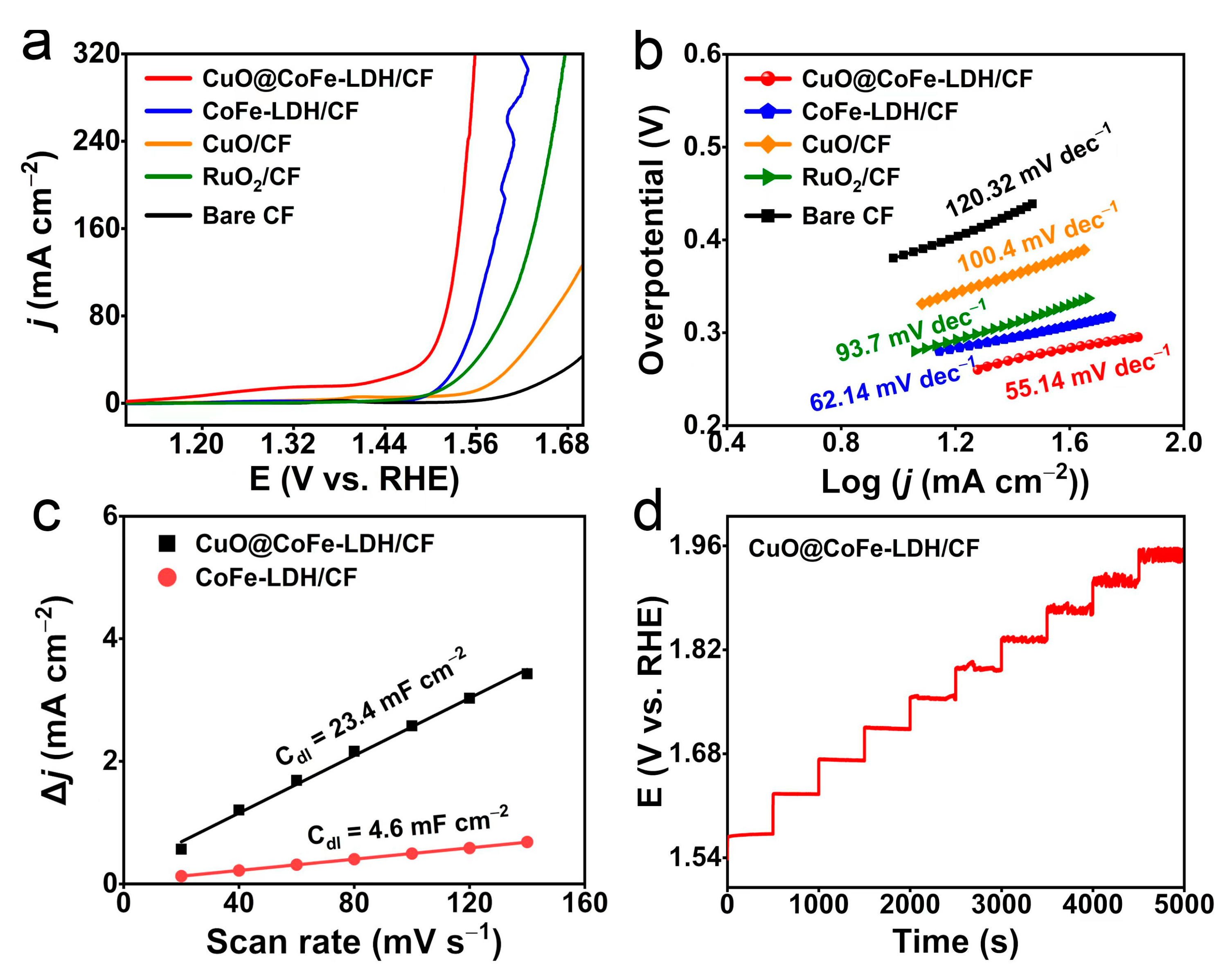
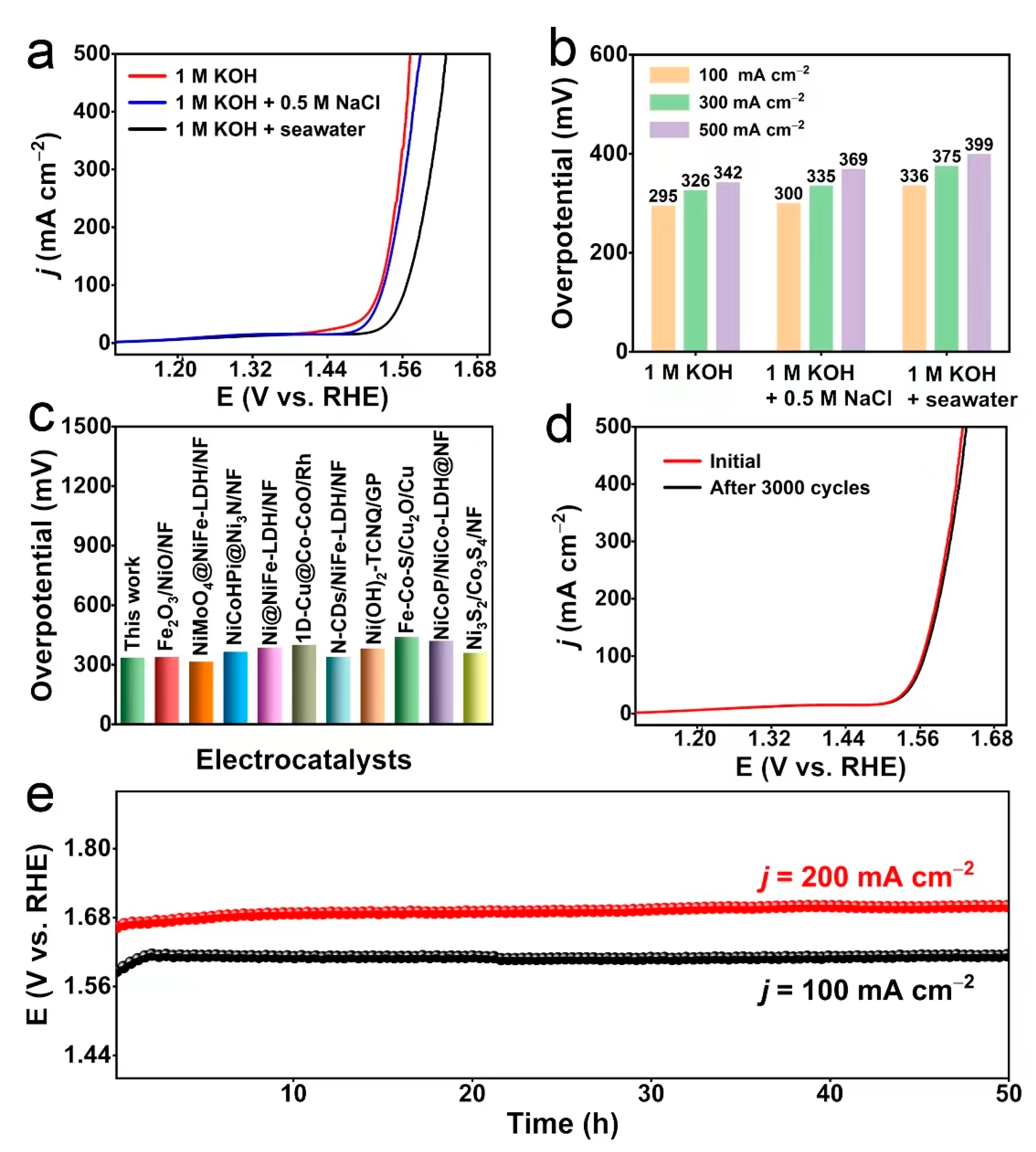
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of CuO/CF, CuO@CoFe-LDH/CF, and CoFe-LDH/CF
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commum. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Shan, J.; Zheng, Y.; Shi, B.; Davey, K.; Qiao, S. Regulating electrocatalysis via surface and interface engineering for acidic water electrooxidation. ACS Energy Lett. 2019, 4, 2719–2730. [Google Scholar] [CrossRef]
- Gao, F.; He, J.; Wang, H.; Lin, J.; Chen, R.; Yi, K.; Huang, F.; Lin, Z.; Wang, M. Te-mediated electro-driven oxygen evolution reaction. Nano Res. Energy 2022, 1, e9120029. [Google Scholar] [CrossRef]
- Fang, X.; Wang, X.; Ouyang, L.; Zhang, L.; Sun, S.; Liang, Y.; Luo, Y.; Zheng, D.; Kang, T.; Liu, Q.; et al. Amorphous Co-Mo-B film: A high-active electrocatalyst for hydrogen generation in alkaline seawater. Molecules 2022, 27, 7617. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z.; et al. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, J.; He, X.; Zheng, Y.; Sun, S.; Fang, X.; Zheng, D.; Luo, Y.; Wang, Y.; et al. High-efficiency overall alkaline seawater splitting: Using a nickel-iron sulfide nanosheet array as a bifunctional electrocatalyst. J. Mater. Chem. A 2023, 11, 1116–1122. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Yue, L.; Dong, K.; Li, J.; Zhao, D.; Li, Z.; Sun, S.; Luo, Y.; Liu, Q.; et al. Benzoate anions-intercalated NiFe-layered double hydroxide nanosheet array with enhanced stability for electrochemical seawater oxidation. Nano Res. Energy 2022, 1, e9120028. [Google Scholar] [CrossRef]
- Ning, M.; Wu, L.; Zhang, F.; Wang, D.; Song, S.; Tong, T.; Bao, J.; Chen, S.; Yu, L.; Ren, Z. One-step spontaneous growth of NiFe layered double hydroxide at room temperature for seawater oxygen evolution. Mater. Today Phys. 2021, 19, 100419. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Shan, B.; Wang, W.; Qi, Y.; Tai, X.; Wang, X.; Zheng, D.; Yan, H.; Ying, B.; et al. Recent advances in self-supported transition-metal-based electrocatalysts for seawater oxidation. Acta Phys.-Chim. Sin. 2023, 39, 2303012. [Google Scholar] [CrossRef]
- Yang, C.; Dong, K.; Zhang, L.; He, X.; Chen, J.; Sun, S.; Yue, M.; Zhang, H.; Zhang, M.; Zheng, D.; et al. Improved alkaline seawater splitting of NiS nanosheets by iron doping. Inorg. Chem. 2023, 62, 7976–7981. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, B.; Vasileff, A.; Jiao, Y.; Qiao, S. Interfacial nickel nitride/sulfide as a bifunctional electrode for highly efficient overall water/seawater electrolysis. J. Mater. Chem. A 2019, 7, 8117–8121. [Google Scholar] [CrossRef]
- Muthurasu, A.; Chhetri, K.; Dahal, B.; Kim, H.K. Ruthenium nanoparticles integrated bimetallic metal-organic framework electrocatalysts for multifunctional electrode materials and practical water electrolysis in seawater. Nanoscale 2022, 14, 6557–6569. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B 2021, 297, 120386. [Google Scholar] [CrossRef]
- Duan, S.; Liu, Z.; Zhuo, H.; Wang, T.; Liu, J.; Wang, L.; Liang, J.; Han, J.; Huang, Y.; Li, Q. Hydrochloric acid corrosion induced bifunctional free-standing NiFe hydroxide nanosheets towards high-performance alkaline seawater splitting. Nanoscale 2020, 12, 21743–21749. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J.; Li, J.; Fan, L.; Liu, Z.; Shi, J.; Cai, W. Surface-growing organophosphorus layer on layered double hydroxides enables boosted and durable electrochemical freshwater/seawater oxidation. Appl. Catal. B 2023, 332, 122749. [Google Scholar] [CrossRef]
- Cheng, F.; Feng, X.; Chen, X.; Lin, W.; Rong, J.; Yang, W. Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta 2017, 251, 336–343. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhu, Q.; McElhenny, B.; Zhang, F.; Wu, C.; Xing, X.; Bao, J.; Chen, S.; Ren, Z. Boron-modified cobalt iron layered double hydroxides for high efficiency seawater oxidation. Nano Energy 2021, 83, 105838. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Ren, P.; Wang, D.; Lu, X.; Xu, R.; Li, Y.; Xue, J.; Zhang, J.; et al. Synergistic interface engineering and structural optimization of non-noble metal telluride-nitride electrocatalysts for sustainably overall seawater electrolysis. Appl. Catal. B 2022, 318, 121834. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Tan, L.; Liu, X.; Wen, Y.; Hou, W.; Zhan, T. Electrodeposition of NiFe-layered double hydroxide layer on sulfur-modified nickel molybdate nanorods for highly efficient seawater splitting. J. Colloid Interface Sci. 2022, 613, 349–358. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, L.; Wu, L.; Luo, D.; Ren, Z. Rational design of oxygen evolution reaction catalysts for seawater electrolysis. Trends Chem. 2021, 3, 485–498. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, K.; Hu, Y.; Li, Q.; Deng, Y.; Bao, J.; Lei, Y. Zr-doped CoFe-layered double hydroxides for highly efficient seawater electrolysis. J. Colloid Interface Sci. 2021, 604, 767–775. [Google Scholar] [CrossRef]
- Qiao, L.; Li, T.; Wei, Q.; Fu, Z.; Cheng, Z.; Wu, J.; Lin, J.; Chen, J.; Chen, Z.; Qi, Y. Cr-doped CoFe layered double hydroxide nanosheets as high-efficiency electrocatalyst for oxygen evolution reaction. J. Phys. Chem. Solids 2022, 171, 111015. [Google Scholar] [CrossRef]
- Wu, J.; Nie, Z.; Xie, R.; Hu, X.; Yu, Y.; Yang, N. Self-assembled Pt-CoFe layered double hydroxides for efficient alkaline water/seawater splitting by spontaneous redox synthesis. J. Power Sources 2022, 532, 231353. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Fei, X.; He, Y.; Zhang, P.; Zhang, G.; Peng, L.; Xie, W. Synthesis of CuO nano- and micro-structures and their Raman spectroscopic studies. CrystEngComm 2010, 12, 2232–2237. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, Y.; You, Z.; Xia, H.; Tuo, Y.; Wang, S.; Jia, C.; Zhang, J. Bi-Functional Fe3O4/Au/CoFe-LDH sandwich-structured electrocatalyst for asymmetrical electrolyzer with low operation voltage. Small 2021, 17, 2103307. [Google Scholar] [CrossRef]
- Faria, D.L.A.; Silva, S.V.; Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Sakita, A.M.P.; Noce, R.D.; Vallés, E.; Benedetti, A.V. Pulse electrodeposition of CoFe thin films covered with layered double hydroxides as a fast route to prepare enhanced catalysts for oxygen evolution reaction. Appl. Surf. Sci. 2018, 434, 1153–1160. [Google Scholar] [CrossRef]
- Tong, R.; Xu, M.; Huang, H.; Wu, C.; Luo, X.; Cao, M.; Li, X.; Hu, X.; Wang, S.; Pan, H. 3D V-Ni3S2@CoFe-LDH core-shell electrocatalysts for efficient water oxidation. Int. J. Hydrogen Energy 2021, 46, 39636–39644. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, T.; Li, X.; Zhang, L.; Luo, Y.; Zhang, F.; Asiri, A.M.; Hu, J.; Liu, Q.; Sun, X. A hierarchical CuO@NiCo layered double hydroxide core-shell nanoarray as an efficient electrocatalyst for the oxygen evolution reaction. Inorg. Chem. Front. 2021, 8, 3049–3054. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Z.; Kim, S.; Baek, M.; Kim, D.; Yong, K. A biomimetic nanoleaf electrocatalyst for robust oxygen evolution reaction. Appl. Catal. B 2019, 259, 118017. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Lee, S.H.; Shin, J.C. CoFe layered double hydroxide for enhanced electrochemical performance. J. Eletroanal. Chem. 2020, 862, 114012. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Zhang, R.; Liu, Q.; Luo, Y.; Cui, G.; Lu, S.; Wang, J.; Ma, Y.; Sun, X. CuO@CoFe layered double hydroxide core-shell heterostructure as an efficient water oxidation electrocatalyst under mild alkaline conditions. Inorg. Chem. 2020, 59, 9491–9495. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Zhu, D.; Liu, D.; Liu, Y.; Yang, S. Fe2O3/NiO Interface for the electrochemical oxygen evolution in seawater and domestic sewage. ACS Appl. Mater. Interfaces 2021, 13, 37152–37161. [Google Scholar] [CrossRef]
- Sun, H.; Sun, J.; Song, Y.; Zhang, Y.; Qiu, Y.; Sun, M.; Tian, X.; Li, C.; Lv, Z.; Zhang, L. Nickel–Cobalt hydrogen phosphate on nickel nitride supported on nickel foam for alkaline seawater electrolysis. ACS Appl. Mater. Interfaces 2022, 14, 22061–22070. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Wu, L.; Ning, M.; Song, S.; Xiao, X.; Hadjiev, V.G.; Fan, D.E.; Wang, D.; Yu, L.; et al. Efficient alkaline seawater oxidation by a three-dimensional core-shell dendritic NiCo@NiFe layered double hydroxide electrode. Mater. Today Phys. 2022, 27, 100841. [Google Scholar] [CrossRef]
- Tran, P.K.L.; Tran, D.T.; Malhotra, D.; Prabhakaran, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Highly effective freshwater and seawater eelectrolysis enabled by atomic rh-modulated Co-CoO lateral heterostructures. Small 2021, 17, 2103826. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Song, H.; Chang, J.; Lu, S. N-doped carbon dots coupled NiFe-LDH hybrids for robust electrocatalytic alkaline water and seawater oxidation. Nano Res. 2022, 15, 7063–7070. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Liu, P.; Liang, J.; Luo, Y.; Cui, G.; Tang, B.; Liu, Q.; Yan, X.; Hao, H.; et al. Ni(OH)2 nanoparticles encapsulated in conductive nanowire array for high-performance alkaline seawater oxidation. Nano Res. 2022, 15, 6084–6090. [Google Scholar] [CrossRef]
- Sun, J.; Song, P.; Zhou, H.; Lang, L.; Shen, X.; Liu, Y.; Cheng, X.; Fu, X.; Zhu, G. A surface configuration strategy to hierarchical Fe-Co-S/Cu2O/Cu electrodes for oxygen evolution in water/seawater splitting. Appl. Surf. Sci. 2021, 567, 150757. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, M.; Cao, Z.; Zhu, P.; Cao, Y.; Xu, X.; Xu, C.; Yin, Z. Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density. Appl. Catal. B 2021, 291, 120071. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, Z.; Yuan, S.; Qi, Z.; Feng, Y.; Wang, Y.; Huang, R.; Zhao, Y.; Sun, J.; Zhao, W.; et al. Solar-driven self-powered alkaline seawater electrolysis via multifunctional earth-abundant heterostructures. Chem. Eng. J. 2021, 411, 128538. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Weng, S.; Lv, H.; Li, P.; Deng, S.; Hao, W. Construction of an “environment-friendly” CuBx@PU self-supporting electrode toward efficient seawater electrolysis. Green Chem. 2022, 24, 5918–5929. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; He, X.; He, L.; Chen, J.; Zhang, L.; Liu, Q.; Cai, Z.; Yang, C.; Sun, S.; Zheng, D.; et al. A Hierarchical CuO Nanowire@CoFe-Layered Double Hydroxide Nanosheet Array as a High-Efficiency Seawater Oxidation Electrocatalyst. Molecules 2023, 28, 5718. https://doi.org/10.3390/molecules28155718
Yang X, He X, He L, Chen J, Zhang L, Liu Q, Cai Z, Yang C, Sun S, Zheng D, et al. A Hierarchical CuO Nanowire@CoFe-Layered Double Hydroxide Nanosheet Array as a High-Efficiency Seawater Oxidation Electrocatalyst. Molecules. 2023; 28(15):5718. https://doi.org/10.3390/molecules28155718
Chicago/Turabian StyleYang, Xiya, Xun He, Lang He, Jie Chen, Longcheng Zhang, Qian Liu, Zhengwei Cai, Chaoxin Yang, Shengjun Sun, Dongdong Zheng, and et al. 2023. "A Hierarchical CuO Nanowire@CoFe-Layered Double Hydroxide Nanosheet Array as a High-Efficiency Seawater Oxidation Electrocatalyst" Molecules 28, no. 15: 5718. https://doi.org/10.3390/molecules28155718
APA StyleYang, X., He, X., He, L., Chen, J., Zhang, L., Liu, Q., Cai, Z., Yang, C., Sun, S., Zheng, D., Farouk, A., Hamdy, M. S., Ren, Z., & Sun, X. (2023). A Hierarchical CuO Nanowire@CoFe-Layered Double Hydroxide Nanosheet Array as a High-Efficiency Seawater Oxidation Electrocatalyst. Molecules, 28(15), 5718. https://doi.org/10.3390/molecules28155718








