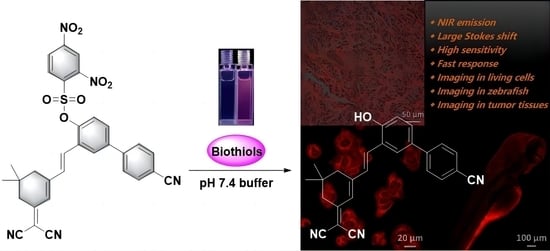
Bioimaging and Sensing Thiols In Vivo and in Tumor Tissues Based on a Near-Infrared Fluorescent Probe with Large Stokes Shift
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of Probe 1
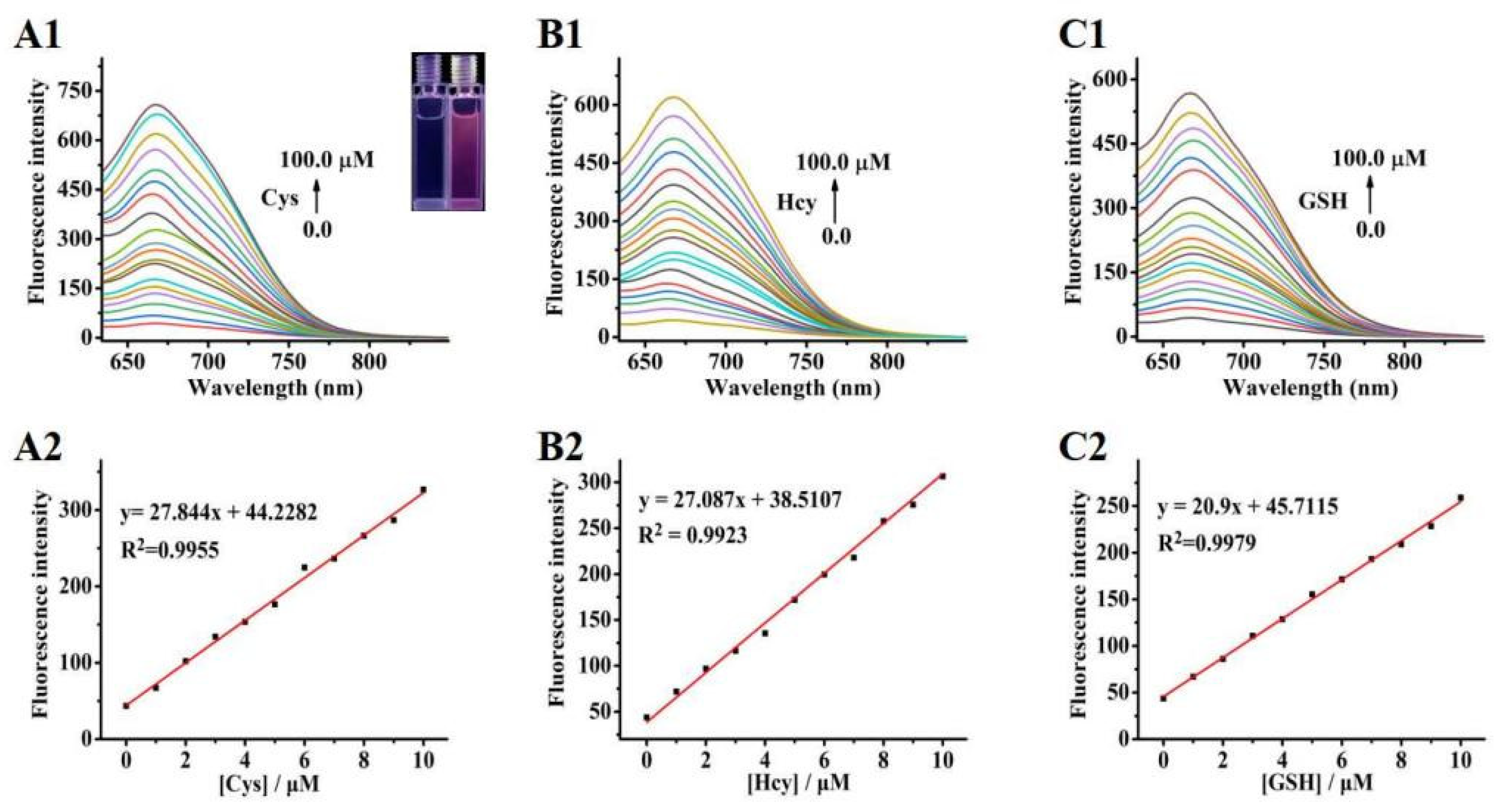
2.2. Fluorescence Properties of Probe 1
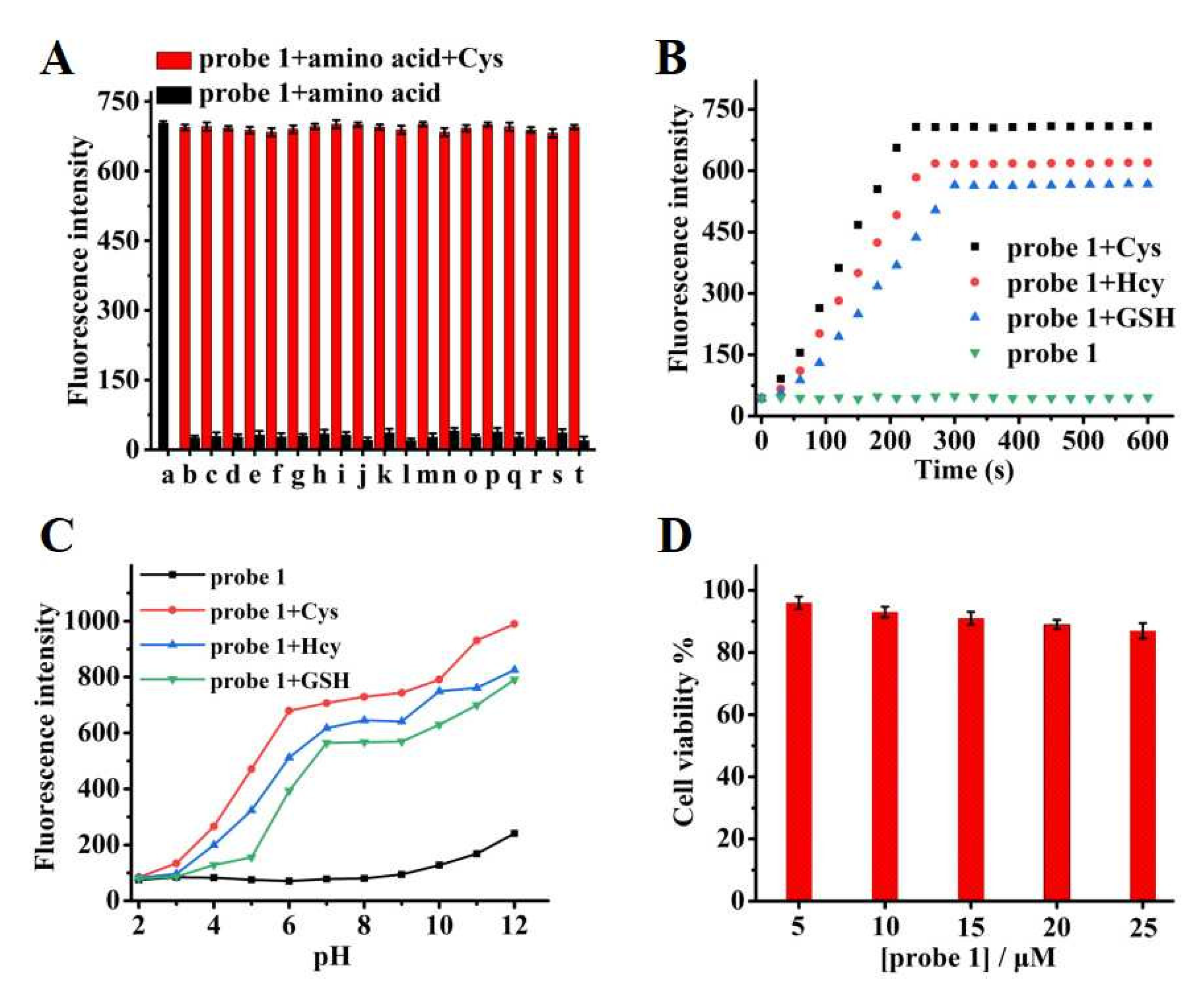
2.3. The Selectivity of Probe 1
2.4. Response Time and pH Study of Probe 1
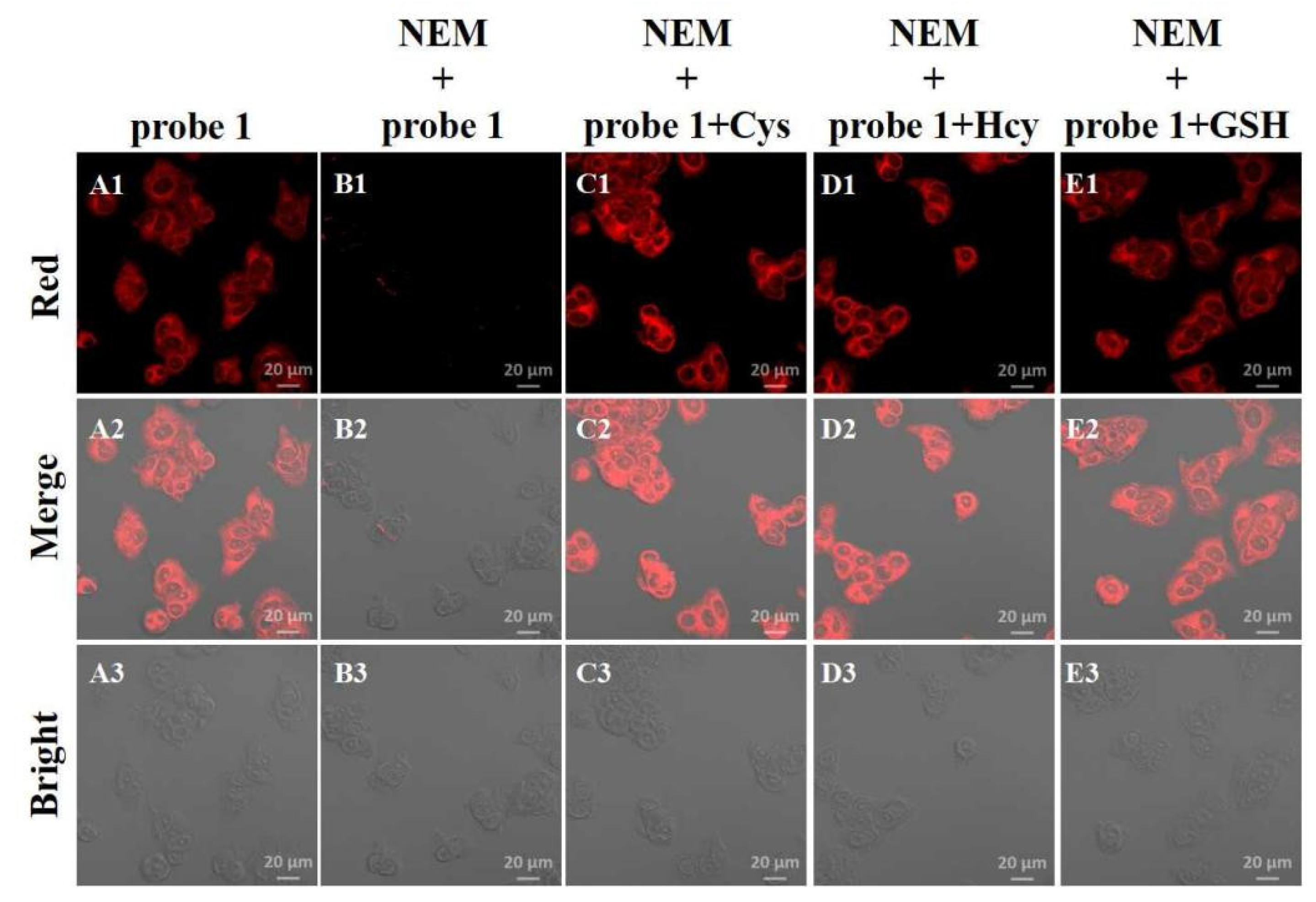
2.5. Imaging of MCF-7 Cells
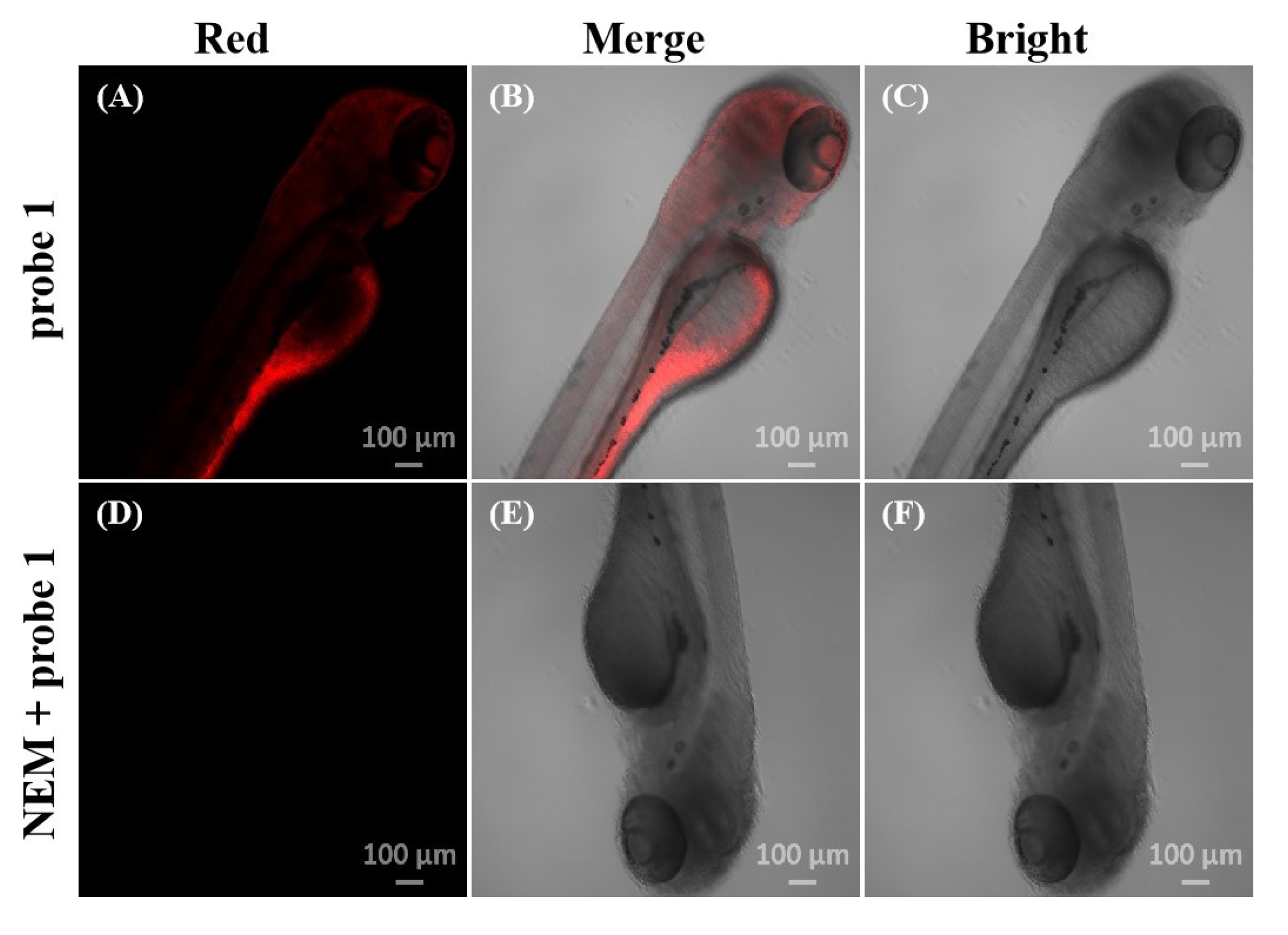
2.6. Fluorescence Imaging of Zebrafish
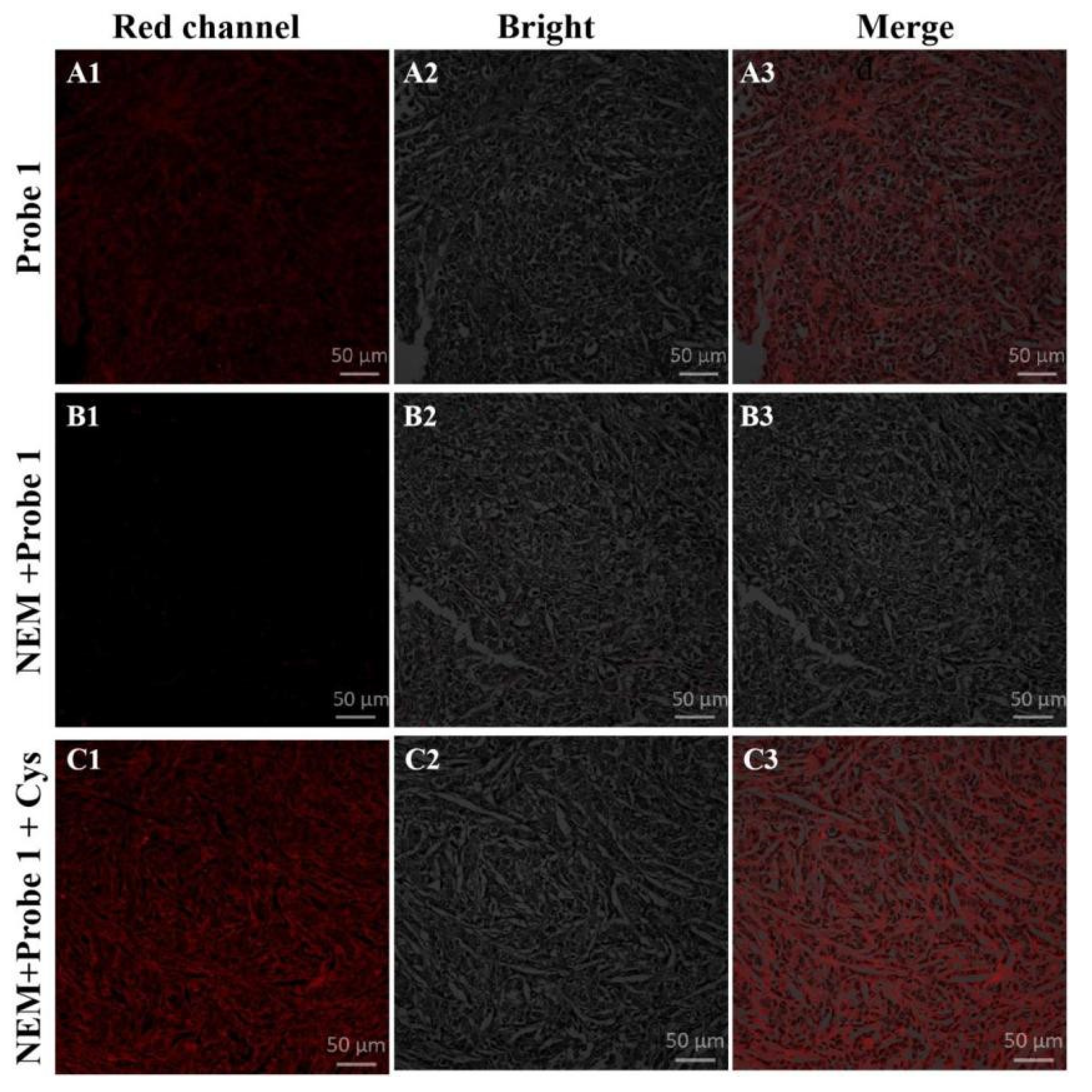
2.7. Confocal Imaging in Fresh Tissue
3. Materials and Methods
3.1. Materials and Instruments
3.2. Spectral Measurements
3.3. Synthesis of Dye 2
3.4. Synthesis of Probe 1
3.5. Cell Culture and Fluorescence Imaging
3.6. Zebrafish Imaging
3.7. Tissue Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Weerapana, E.; Wang, C.; Simon, G.M.; Richter, F.; Khare, S.; Dillon, M.B.D.; Bachovchin, D.A.; Mowen, K.; Baker, D.; Cravatt, B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 2010, 468, 790. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.S.; Qin, J.M.; Li, X.; Cao, Q.Y. A naphthalimide-based fluorescent probe with mitochondria targeting for GSH sensing and cancer cell recognition. Dye. Pigm. 2023, 211, 111089. [Google Scholar] [CrossRef]
- Chen, Y.S.; Zhong, X.L.; Yang, X.R.; Zhu, S.M.; Jiang, Y.L.; Jin, C. A mitochondria-targeted fluorescent probe for monitoring endogenous cysteine in living cells and zebrafish. Chem. Commun. 2021, 57, 8198. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Z.; Cao, X.H.; Ai, K.L.; Cui, Y.; Han, Z.G.; Zhou, B.; Huo, C.D. A dual-reaction-site and dual-channel fluorescent-on probe for selectively monitoring mitochondrial glutathione. Sens. Actuators B Chem. 2023, 382, 133563. [Google Scholar] [CrossRef]
- Ren, H.X.; Huo, F.J.; Zhang, Y.B.; Zhao, S.H.; Yin, C.X. An NIR ESIPT-based fluorescent probe with large stokes shift for specific detection of Cys and its bioimaging in cells and mice. Sens. Actuators B Chem. 2020, 319, 128248. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhang, Y.; Yue, Y.K.; Chao, J.B.; Huo, F.J.; Yin, C.X. Based on morpholine as luminescence mechanism regulation and organelle targeting dual function Cys NIR specific biological imaging probe. Sens. Actuators B Chem. 2020, 320, 128348. [Google Scholar] [CrossRef]
- dos Santos, A.P.A.; da Silva, J.K.; Neri, J.M.; Neves, A.C.O.; de Lima, D.F.; Menezes, F.G. Nucleophilicity of cysteine and related biothiols and the development of fluorogenic probes and other applications. Org. Biomol. Chem. 2020, 18, 9398–9427. [Google Scholar] [CrossRef]
- Yue, Y.K.; Huo, F.J.; Wang, Y.T.; Ma, K.Q.; Li, X.Q.; Yin, C.X. Mutual correlation evaluation of Cys and Hcy in serum through reaction activity regulated fluorescence quantification. Chem. Commun. 2020, 56, 9146–9149. [Google Scholar] [CrossRef]
- Tu, X.Q.; He, L.J.; Huang, H.J.; Ye, H.S.; Sun, L.; Yi, L. Thiolysis of CBD arylethers for development of highly GSH-selective fluorescent probes. Chem. Commun. 2021, 57, 8802–8805. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.S.; Ni, H.; Huang, W.Y.; Xu, P.N.; Ji, M. An off-on quinoxaline-based fluorescent probe for lighting up biothiols in living cells and zebrafish. Tetrahedron 2023, 139, 133433. [Google Scholar] [CrossRef]
- Kubalczyk, P.; Bald, E.; Furmaniak, P.; Głowacki, R. Simultaneous determination of total homocysteine and cysteine in human plasma by capillary zone electrophoresis with pH-mediated sample stacking. Anal. Methods 2014, 6, 4138. [Google Scholar] [CrossRef]
- Yang, M.W.; Huang, J.G.; Fan, J.L.; Du, J.J.; Pu, K.Y.; Peng, X.J. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef]
- Chen, J.; Hu, S.; Cai, Y.L.; Liu, X.; Wu, Y.Q.; Dai, Y.H.; Wang, Z.J. Co-N/C-900 metal-organic framework-derived nanozyme as a H2O2-free oxidase mimic for the colorimetric sensing of L-cysteine. Analyst 2022, 147, 915–922. [Google Scholar] [CrossRef]
- Liu, X.B.; Fu, S.; Zhang, H.G.; Li, S.; Zhu, Z.Z.; Chen, S.; Hou, H.L.; Chen, W.Q.; Hou, P. Rational design of a GSH silent fluorescent probe for simultaneous detection of H2S and Cys/Hcy from distinct channels. Microchem. J. 2023, 184, 108187. [Google Scholar] [CrossRef]
- Yan, D.L.; Liu, L.K.; Liu, X.B.; Liu, Q.; Hou, P.; Wang, H.J.; Xia, C.H.; Li, G.; Ma, C.H.; Chen, S. Simultaneous discrimination of Cys/Hcy and GSH with simple fluorescent probe under a single-wavelength excitation and its application in living cells, tumor tissues, and zebrafish. Front. Chem. 2022, 10, 856994. [Google Scholar] [CrossRef]
- Tian, X.; Murfin, L.C.; Wu, L.L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406. [Google Scholar] [CrossRef]
- Dong, J.N.; Lu, G.W.; He, B.; Tu, Y.Y.; Fan, C.B. A novel NIR fluorescent probe for monitoring cysteine in mitochondria of living cells. Arab. J. Chem. 2023, 16, 104765. [Google Scholar] [CrossRef]
- Chen, X.D.; He, D.; Yang, X.R.; Yuan, F.; Yang, S.X.; Yang, Y.F.; Wang, K.; Qian, J.; Long, L.L. Construction of bifunctional fluorescent probe for two-step cascade recognition of hydrogen sulfide and biothiols in biological system. Sens. Actuators B Chem. 2023, 381, 133440. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, C.Y.; Li, Y.Q.; Zhou, Z.L.; Xie, R.H.; Pang, X.; Xu, H.; Li, H.T.; Zhang, Y.Y. A fluorescent probe for the specific detection of cysteine in human serum samples. Anal. Methods 2019, 11, 3280. [Google Scholar] [CrossRef]
- Yue, X.X.; Chen, J.L.; Chen, W.Q.; Wang, B.H.; Zhang, H.; Song, X.Z. An endoplasmic reticulum-targeting fluorescent probe for discriminatory detection of Cys, Hcy and GSH in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119347. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Huang, H.J.; Kang, X.Y.; Yang, L.; Xi, Z.; Sun, H.Y.; Pluth, M.D.; Yi, L. NBD-based synthetic probes for sensing small molecules and proteins: Design, sensing mechanisms and biological applications. Chem. Soc. Rev. 2021, 50, 7436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.Q.; Mao, G.J.; Jiang, W.L.; Tan, M.; Xu, F.; Li, C.Y. A near-infrared fluorescent probe with large Stokes shift for imaging Cys in tumor mice. Anal. Chim. Acta 2021, 1171, 338655. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.B.; Wang, Z.; Zhang, Y.B.; Huo, F.J.; Yin, C.X.; Li, M.; Duan, Y.X. A Pyrene-Based Fluorescent Probe for Specific Detection of Cysteine and its Application in Living Cell. J. Fluoresc. 2021, 31, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.K.; Huo, F.J.; Yin, C.X. The chronological evolution of small organicmolecular fluorescent probes for thiols. Chem. Sci. 2021, 12, 1220. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, D.S.; Parmar, S.; Kalia, D. Michael addition-elimination-cyclization based turn-on fluorescence (MADELCY TOF) probes for cellular cysteine imaging and estimation of blood serum cysteine and aminoacylase-1. Analyst 2022, 147, 3876–3884. [Google Scholar] [CrossRef]
- Tan, H.Y.; Zou, Y.; Guo, J.M.; Chen, J.; Zhou, L.P. A simple lysosome-targeted fluorescent probe based on flavonoid for detection of cysteine in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121552. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, X.X.; Fu, N.Y. A sensitive fluorescent probe for imaging biothiol in zebrafish. Dye. Pigm. 2020, 174, 107978. [Google Scholar] [CrossRef]
- Xue, X.L.; Wang, Y.; Zhang, H.; Chen, S.J.; Niu, S.Y.; Cui, L.; Wang, K.P.; Hu, Z.Q. A coumarin-based fluorescent probe: Single-wavelength excitation, discrimination of Cys/Hcy and GSH by naked eyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 292, 122410. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, W.J.; Chen, Y.; Wei, S.H.; Zhu, F.; Zhu, X.Z.; Wang, C.Y. An AIE-active fluorescent probe with near-infrared (NIR) emission for selectively monitoring and bioimaging Cys in living cells. Tetrahedron 2023, 140, 133492. [Google Scholar] [CrossRef]
- An, S.X.; Lin, Y.F.; Wang, J.B.; Ye, T.Q.; Mao, Y.Y.; Zhang, J.; Guo, L.; Li, L.H.; Qian, Z.S.; Liu, H.Y. Near-infrared mitochondria-targeted fluorescent probe with a large Stokes shift for rapid and sensitive detection of cysteine/homocysteine and its bioimaging application. Sens. Actuators B Chem. 2023, 374, 132799. [Google Scholar] [CrossRef]
- Liu, H.B.; Xing, H.Z.; Gao, Z.G.; You, M.; Li, B.; Feng, X.Y.; Zhou, B.J.; Cong, Z.J.; Zhu, J.; Jin, M.J. A single-wavelength excited NIR fluorescence probe for distinguishing GSH/H2S and Cys/Hcy in living cells and zebrafish through separated dual-channels. Talanta 2023, 254, 124153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.; Li, H.; Shen, R.; Wang, Y.Z.; Song, X.R.; Cao, C.; Zhang, G.L.; Liu, W.S. Hydro-soluble NIR fluorescent probe with multiple sites and multiple excitations for distinguishing visualization of endogenous Cys/Hcy, and GSH. Sens. Actuators B Chem. 2021, 333, 129189. [Google Scholar] [CrossRef]
- Pham, T.C.; Choi, Y.; Bae, C.; Tran, C.S.; Kim, D.; Jung, O.S.; Kang, Y.C.; Seo, S.; Kim, H.S.; Yun, H.; et al. A molecular design towards sulfonyl aza-BODIPY based NIR fluorescent and colorimetric probe for selective cysteine detection. RSC Adv. 2021, 11, 10154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.C.; Guo, Q.; Wen, G.H.; Xiao, H.Y.; Qi, S.; Wang, Y.; Zhang, H.T.; Wang, L.D.; Sun, H.Y. Photoacoustic/fluorescence dual-modality probe for biothiol discrimination and tumor diagnosis in cells and mice. ACS Sens. 2022, 7, 1105–1112. [Google Scholar] [CrossRef]
- Lu, G.F.; Gao, Y.J.; Wang, X.C.; Zhang, D.Q.; Meng, S.C.; Yu, S.Y.; Zhuang, Y.F.; Duan, L.Y. A near-infrared fluorescent probe based on corrole derivative with large Stokes shift for detection of hydrogen sulfide in water and living cells. Dye. Pigm. 2022, 204, 110445. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.L.; Xie, L.Y.; Pang, M.L.; Zhang, Y.Z.; Ran, H.Y.; Huang, J.J.; Wang, J.Y.; Tao, Y.; Lyu, S.Q. Construction of a robust turn-on fluorescence NIR sensor for rapid detection and imaging of ONOO− in inflammatory models. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 295, 122624. [Google Scholar] [CrossRef]
- Ge, C.P.; Wang, H.Y.; Ni, T.J.; Yang, Z.J.; Chang, K.W. Red-emitting fluorescent turn-on probe with specific isothiocyanate recognition site for cysteine imaging in living systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 259, 119826. [Google Scholar] [CrossRef]
- Qiao, L.Q.; Yang, Y.X.; Li, Y.P.; Lv, X.; Hao, J.S. A fluorescent probe capable of naked eye recognition for the selective detection of biothiols. J. Photochem. Photobiol. A 2022, 425, 113654. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Pan, H.T.; Song, Y.Y.; Jing, C.; Gan, J.A.; Zhang, J.J. Mitochondria-targeted fluorescent probe for rapid detection of thiols and its application in bioimaging. Dye. Pigm. 2021, 191, 109376. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Yan, D.; Hou, P.; Liu, X.; Wang, H.; Xia, C.; Li, G.; Chen, S. Bioimaging and Sensing Thiols In Vivo and in Tumor Tissues Based on a Near-Infrared Fluorescent Probe with Large Stokes Shift. Molecules 2023, 28, 5702. https://doi.org/10.3390/molecules28155702
Ma C, Yan D, Hou P, Liu X, Wang H, Xia C, Li G, Chen S. Bioimaging and Sensing Thiols In Vivo and in Tumor Tissues Based on a Near-Infrared Fluorescent Probe with Large Stokes Shift. Molecules. 2023; 28(15):5702. https://doi.org/10.3390/molecules28155702
Chicago/Turabian StyleMa, Chunhui, Dongling Yan, Peng Hou, Xiangbao Liu, Hao Wang, Chunhui Xia, Gang Li, and Song Chen. 2023. "Bioimaging and Sensing Thiols In Vivo and in Tumor Tissues Based on a Near-Infrared Fluorescent Probe with Large Stokes Shift" Molecules 28, no. 15: 5702. https://doi.org/10.3390/molecules28155702
APA StyleMa, C., Yan, D., Hou, P., Liu, X., Wang, H., Xia, C., Li, G., & Chen, S. (2023). Bioimaging and Sensing Thiols In Vivo and in Tumor Tissues Based on a Near-Infrared Fluorescent Probe with Large Stokes Shift. Molecules, 28(15), 5702. https://doi.org/10.3390/molecules28155702