Recent Developments of Gramine: Chemistry and Biological Activity
Abstract
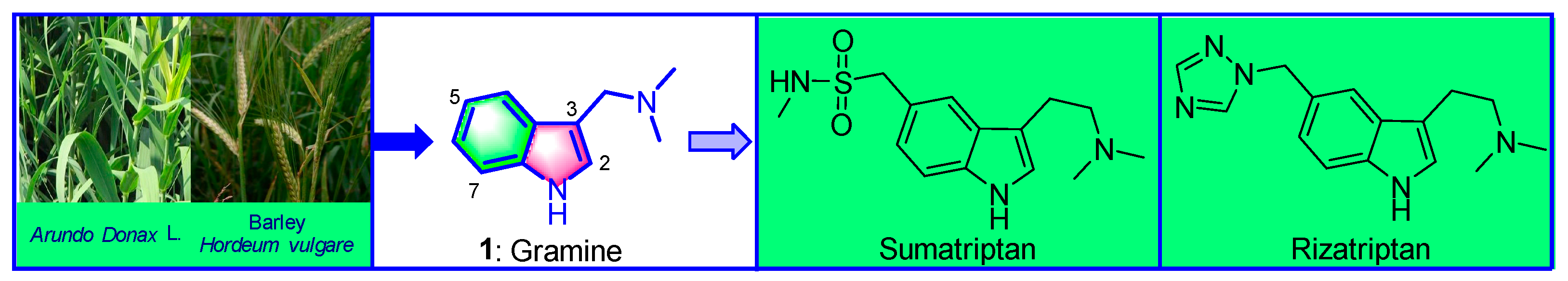
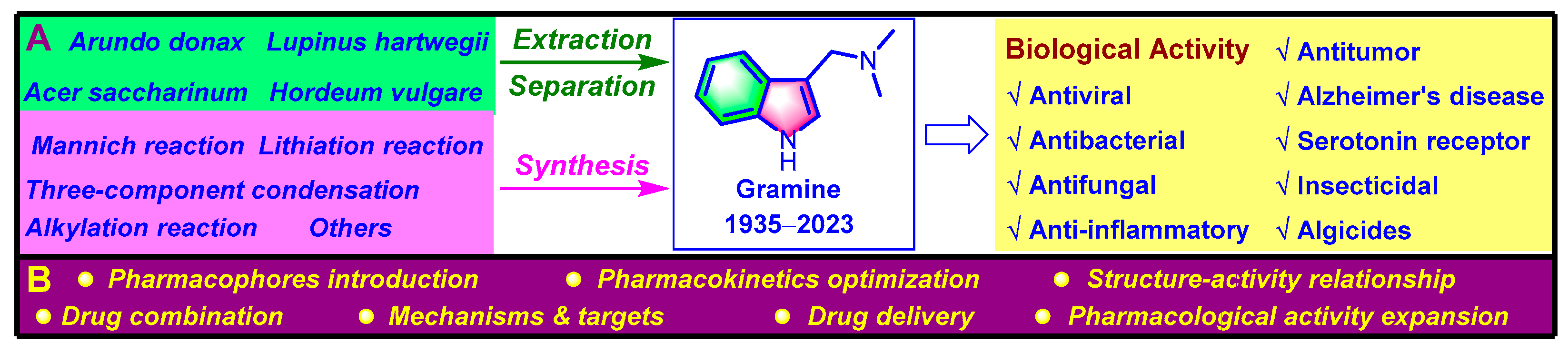
1. Introduction
2. Extraction and Separation
3. Chemical Synthesis
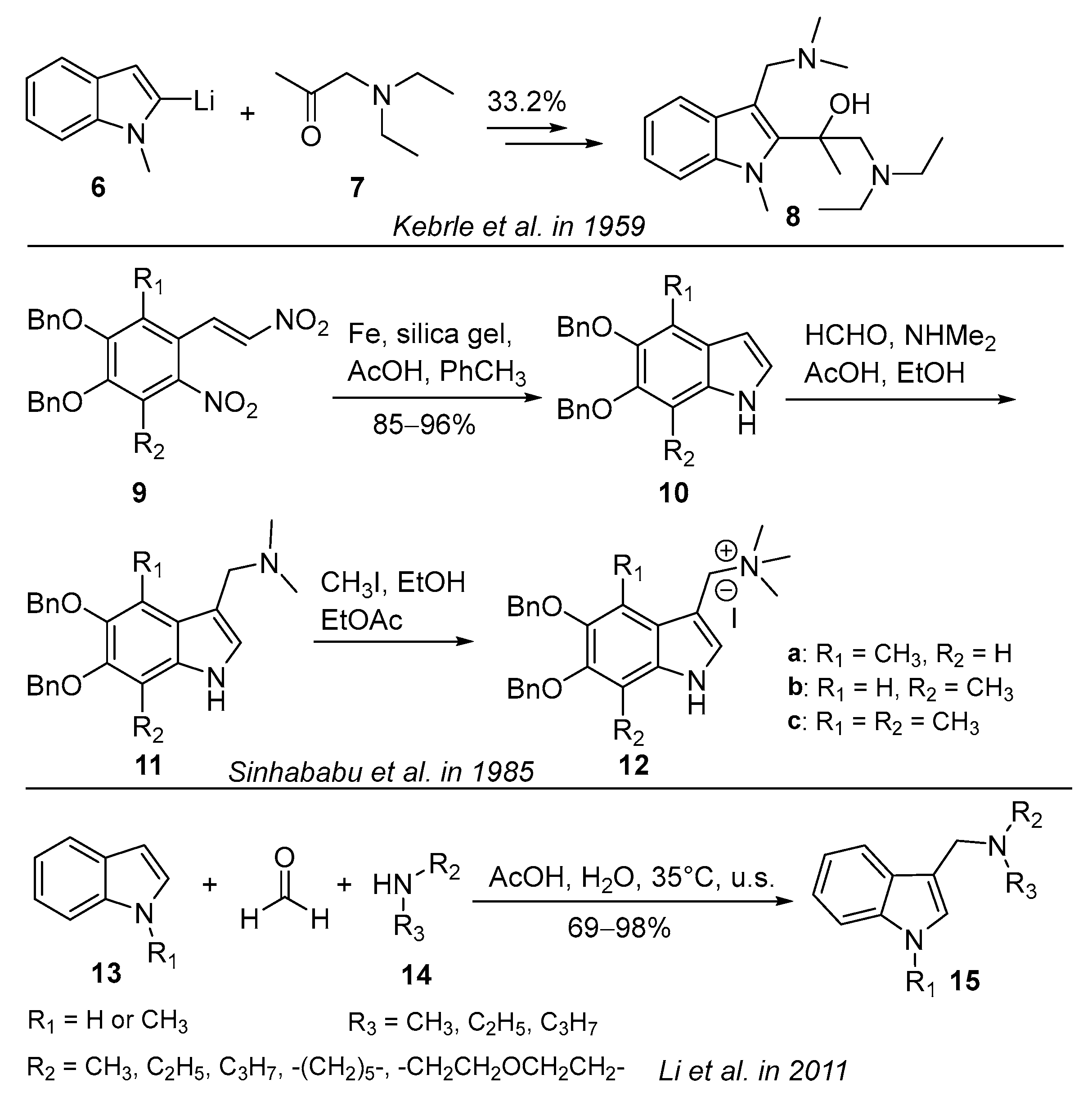
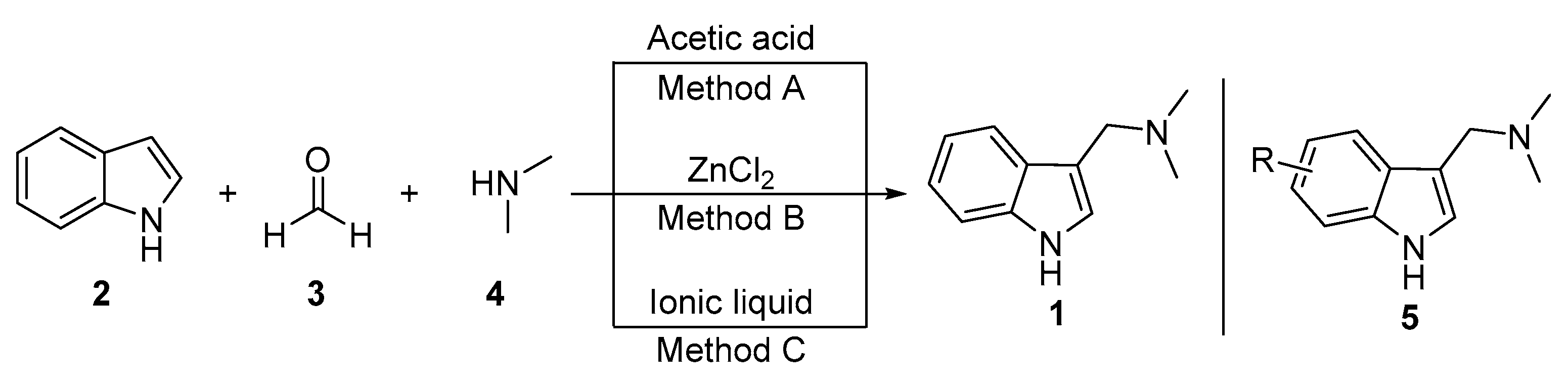
3.1. Mannich Reaction
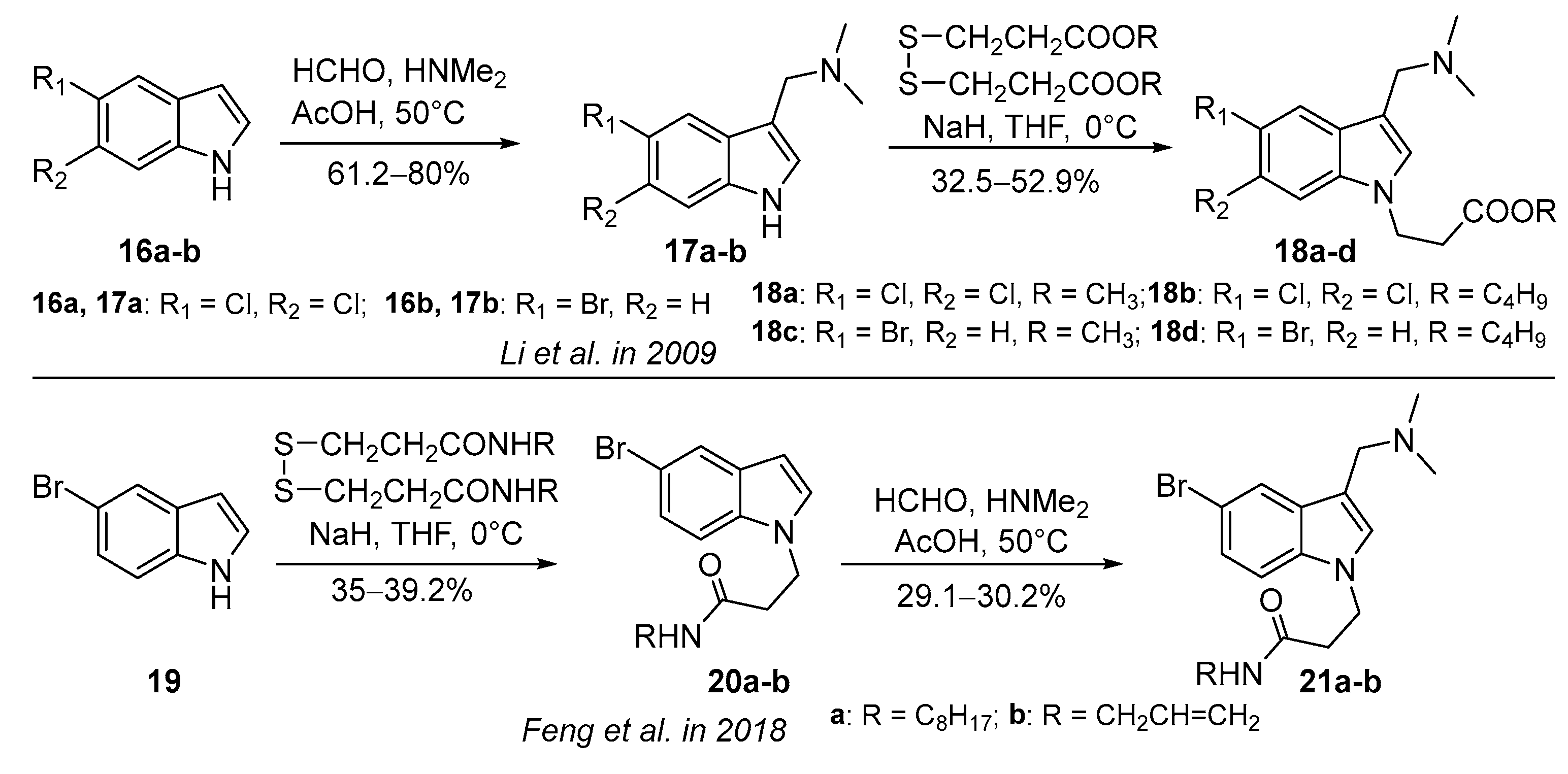
3.1.1. Acetic-Acid-Catalyzed Reaction
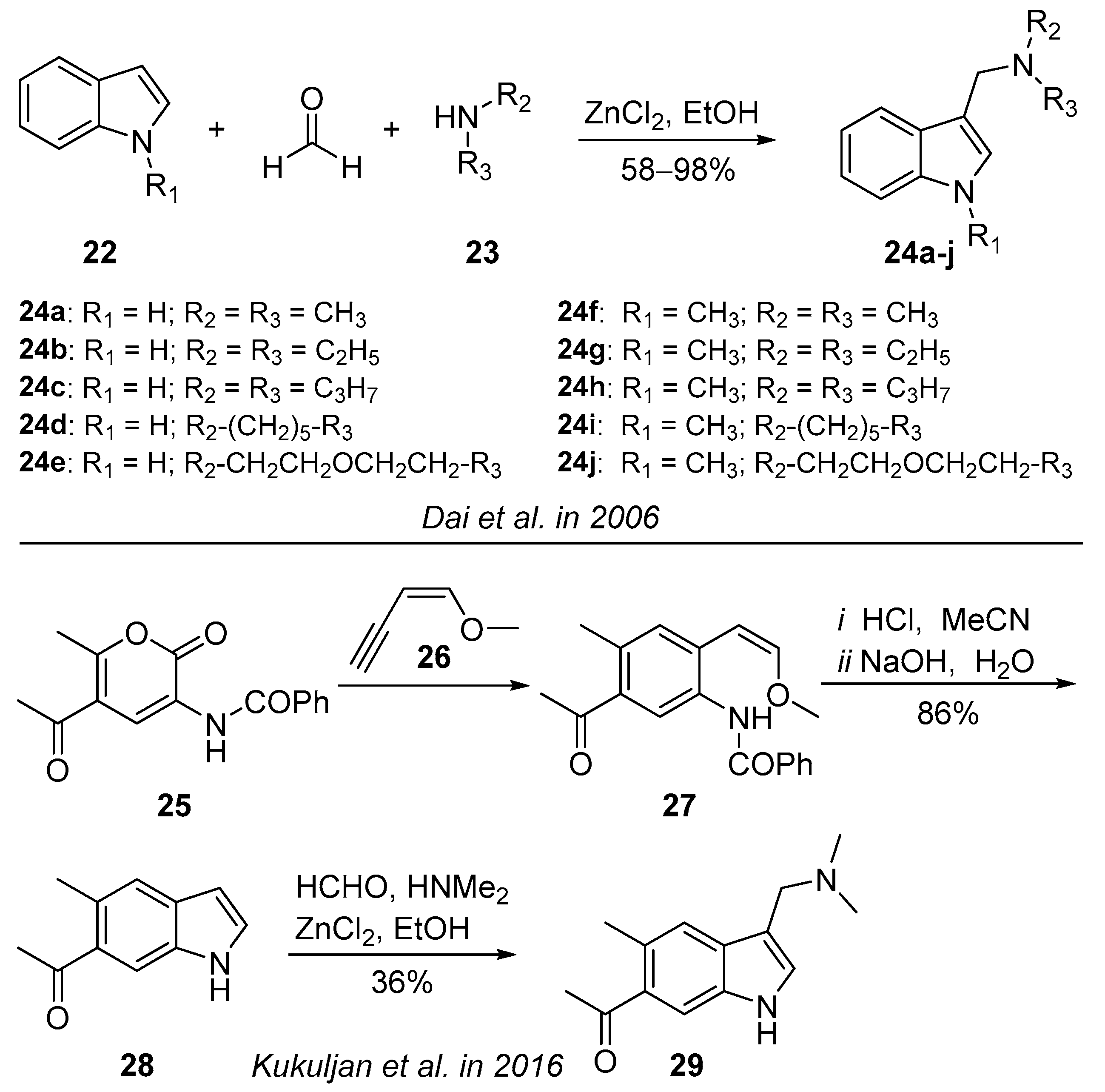
3.1.2. Zinc-Chloride-Catalyzed Reaction
3.1.3. Ionic-Liquid-Catalyzed Reaction
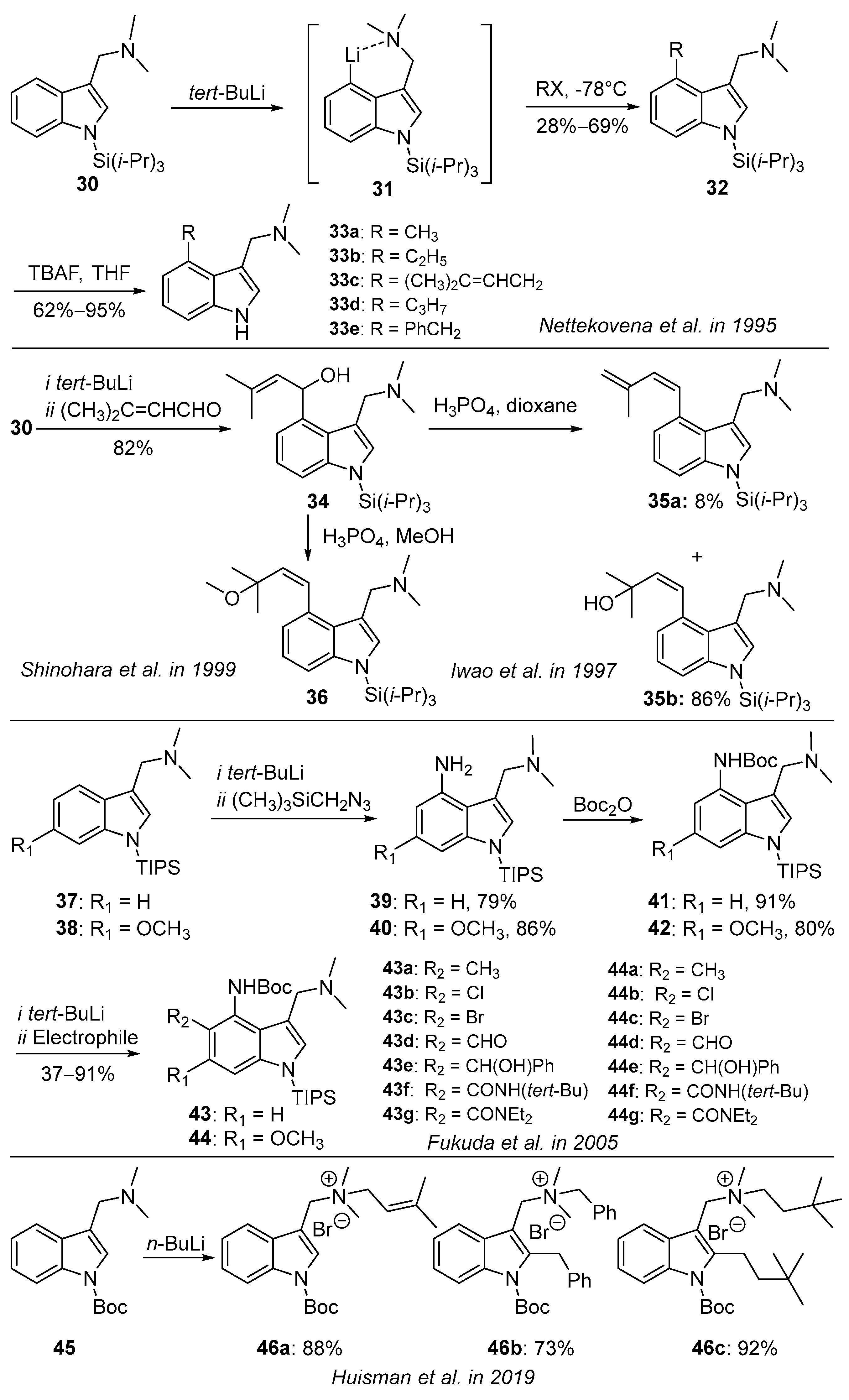
3.2. Lithiation Reaction
3.3. Alkylation Reaction
3.4. Three-Component Condensation Reaction
3.5. Other Reactions
4. Biological Activities
4.1. Antiviral Activity
4.2. Antibacterial Activity
4.3. Antifungal Activity
4.4. Anti-Inflammatory Activity
4.5. Antitumor Activity
4.6. Alzheimer’s Disease Therapy
4.7. Serotonin Receptor-Related Activity
4.8. Insecticidal Activity
4.9. Algicides
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Orechoff, A.; Norkina, S. Über die Alkaloide von Arundo donax L. Ber. Dtsch. Chem. Ges. 1935, 68, 436–437. [Google Scholar] [CrossRef]
- Matsuo, H.; Taniguchi, K.; Hiramoto, T.; Yamada, T.; Ichinose, Y.; Toyoda, K.; Takeda, K.; Shiraishi, T. Gramine increase associated with rapid and transient systemic resistance in barley seedlings induced by mechanical and biological stresses. Plant Cell Physiol. 2001, 42, 1103–1111. [Google Scholar] [CrossRef]
- Hanson, A.D.; Ditz, K.M.; Singletary, G.W.; Leland, T.J. Gramine accumulation in leaves of barley grown under high-temperature stress. Plant Physiol. 1983, 71, 896–904. [Google Scholar] [CrossRef]
- Li, L.; Yang, P.; Li, X.; Pan, F.; Yan, H. Optimization of extracting process of gramine from Arundo donax by response surface method. Food Ind. 2020, 41, 47–51. [Google Scholar]
- Lines, C.R.; Visser, W.H. Rizatriptan: Pharmacological differences from sumatriptan and clinical results. Curr. Med. Res. Opin. 2001, 17, s54–s58. [Google Scholar] [CrossRef]
- Semenov, B.B.; Granik, V.G. Chemistry of N-(1H-indol-3-ylmethyl)-N,N-dimethylamine (gramine): A review. Pharm. Chem. J. 2004, 38, 287–310. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A non-food crop for bioenergy and bio-compound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef]
- Pachter, I.J.; Zacharias, D.E.; Ribeiro, O. Indole alkaloids of Acer saccharinum (the silver maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J. Org. Chem. 1959, 24, 1285–1287. [Google Scholar] [CrossRef]
- Anderson, J.N.; Martin, R.O. Aphylline, epiaphylline, 10,17-dioxosparteine, gramine, and other unexpected alkaloids from Lupinus hartwegii. J. Org. Chem. 1976, 41, 3441–3444. [Google Scholar] [CrossRef] [PubMed]
- Zúǹiga, G.E.; Salgado, M.S.; Corcuera, L.J. Role of an indole alkaloid in the resistance of barley seedlings to aphids. Phytochemistry 1985, 24, 945–947. [Google Scholar] [CrossRef]
- Zúñiga, G.E.; Corcuera, L.J. Effect of gramine in the resistance of barley seedlings to the aphid Rhopalosiphum padi. Entomol. Exp. Appl. 1986, 40, 259–262. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, P. Synthesis and application of gramine, a new plant-derived pesticide. Chin. J. Pestic. Sci. 2004, 43, 76–77. [Google Scholar]
- Zhang, J.; Meng, G. Study on the synthesis of gramine and its derivatives. J. Dali Univ. 2020, 5, 21–26. [Google Scholar]
- Yin, X.; Wu, W.; Wu, J.; Qin, S.H.; Chen, Z. Synthesis of gramine under microwave irradiation reaction. Agrochemicals 2014, 53, 176–178. [Google Scholar]
- Kebrle, J.; Rossi, A.; Hoffmann, K. Beiträge zur Chemie des Indols. Über eine neue Aufbaumethode von γ-Carbolinen. Helv. Chim. Acta 1959, 42, 907–918. [Google Scholar] [CrossRef]
- Sinhababu, A.K.; Ghosh, A.K.; Borchardt, R.T. Molecular mechanism of action of 5,6-dihydroxytryptamine. Synthesis and biological evaluation of 4-methyl-, 7-methyl-, and 4,7-dimethyl-5,6-dihydroxytryptamines. J. Med. Chem. 1985, 28, 1273–1279. [Google Scholar] [CrossRef]
- Li, J.-T.; Sun, S.-F.; Sun, M.-X. Improved synthesis of 3-(dialkylaminomethyl)-indole in acetic acid aqueous solution under ultrasound irradiation. Ultrason. Sonochem. 2011, 28, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, L.; Jiang, X.; Xia, S.; Zhao, H. Synthesis, algal inhibition activities and QSAR studies of novel gramine compounds containing ester functional groups. Chin. J. Oceanol. Limn. 2009, 27, 309–316. [Google Scholar] [CrossRef]
- Feng, K.; Li, X.; Yu, L. Synthesis, antibacterial activity, and application in the antifouling marine coatings of novel acylamino compounds containing gramine groups. Prog. Org. Coat. 2018, 118, 141–147. [Google Scholar] [CrossRef]
- Dai, H.-G.; Li, J.-T.; Li, T.-S. Efficient and practical synthesis of Mannich bases related to gramine mediated by zinc chloride. Synth. Commun. 2006, 36, 1829–1835. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.; Wu, J.; Yin, X.; Chen, Z. Catalytic synthesis of gramine by immobilized montmorillonite under microwave irradiation. Chin. J. Synth. Chem. 2015, 23, 543–546. [Google Scholar]
- Kukuljan, L.; Kranjc, K.; Perdih, F. Synthesis and structural evaluation of 5-methyl-6-acetyl substituted indole and gramine. Acta Chim. Slov. 2016, 63, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Peng, X.; Liu, B.; Guo, X.; Cao, Q.; Chao, J. Synthesis of gramine using acidic ionic liquid as catalyst. CIESC J. 2015, 66, 192–196. [Google Scholar]
- Nettekoven, M.; Psiorz, M.; Waldmann, H. Synthesis of enantiomerically pure 4-alkylsubstituted tryptophan derivatives by a combination of organometallic reactions with enantioselective enzymatic transformations. Tetrahedron Lett. 1995, 36, 1428. [Google Scholar] [CrossRef]
- Iwao, M.; Iwao, F. A concise total synthesis of (±)-cis-and (±)-trans-clavicipitic acids by combinational use of directed lithiation and flouride ion-induced elimination-addition reaction of 1-(triisopropylsilyl)gramine derivatives. Tetrahedron 1997, 53, 51–58. [Google Scholar] [CrossRef]
- Shinohar, H.; Fukuda, T.; Iwao, M. A formal synthesis of optically active clavicipitic acids, unusual azepinoindole-type ergot alkaloids. Tetrahedron 1999, 55, 10989–11000. [Google Scholar] [CrossRef]
- Fukuda, T.; Akashima, H.; Iwao, M. Synthesis of 3,4,5-trisubstituted indoles via iterative directed lithiation of 1-(triisopropylsilyl)gramines. Tetrahedron 2005, 61, 6886–6891. [Google Scholar] [CrossRef]
- Huisman, M.; Rahaman, M.; Asad, S.; Oehm, S.; Novin, S.; Rheingold, A.L.; Hossain, M.M. Total synthesis of tryprostatin B: Synthesis and asymmetric phase-transfer-catalyzed reaction of prenylated gramine salt. Org. Lett. 2019, 21, 134–137. [Google Scholar] [CrossRef]
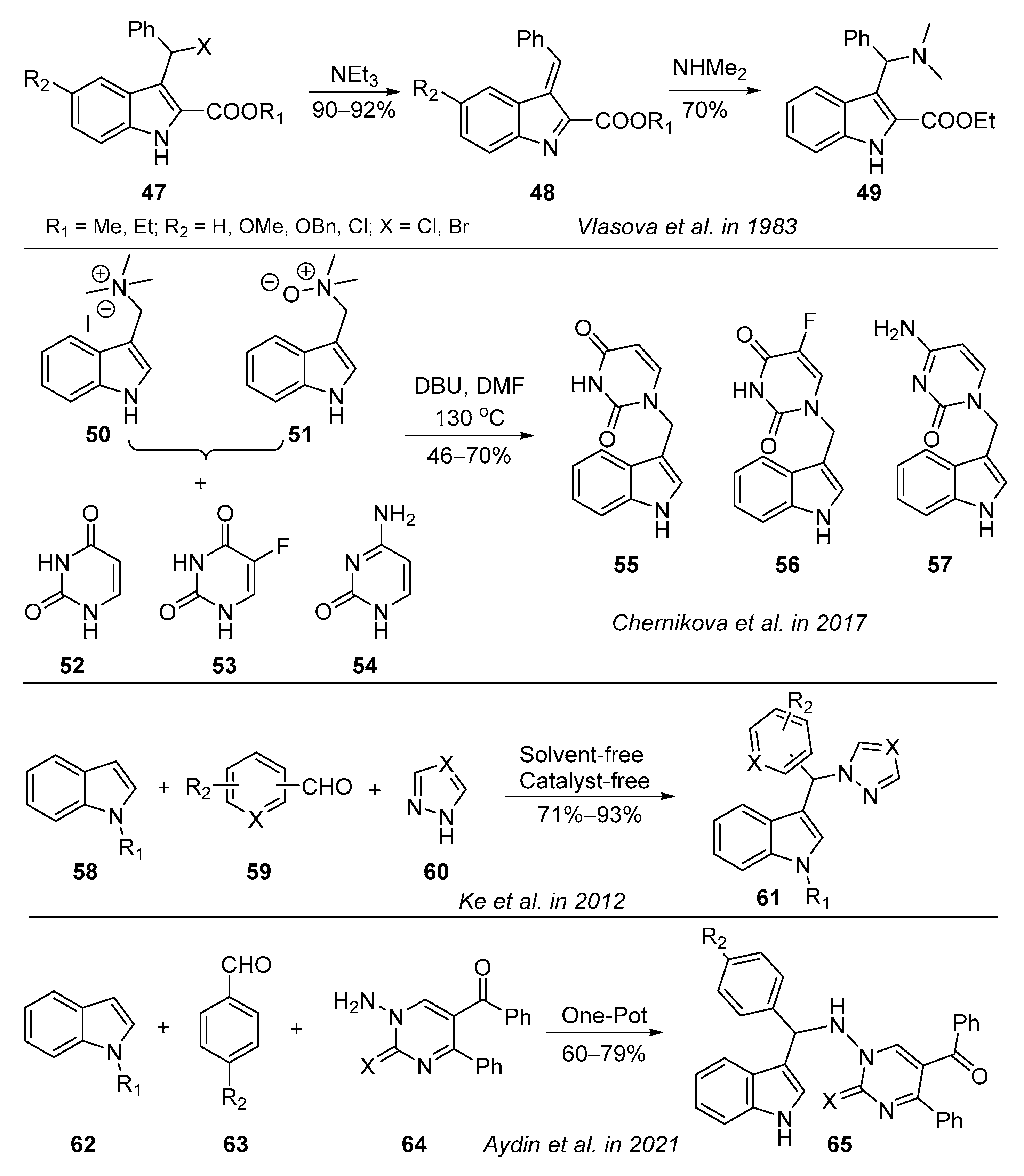
- Vlasova, M.I.; Kogan, N.A. New synthesis of substituted gramines. Chem. Heterocycl. Compd. 1983, 19, 43–48. [Google Scholar] [CrossRef]
- Chernikova, I.B.; Spirikhin, L.V.; Yunusov, M.S. Synthesis of N-1-skatyl uracil derivatives. Chem. Nat. Compd. 2017, 53, 333–337. [Google Scholar] [CrossRef]
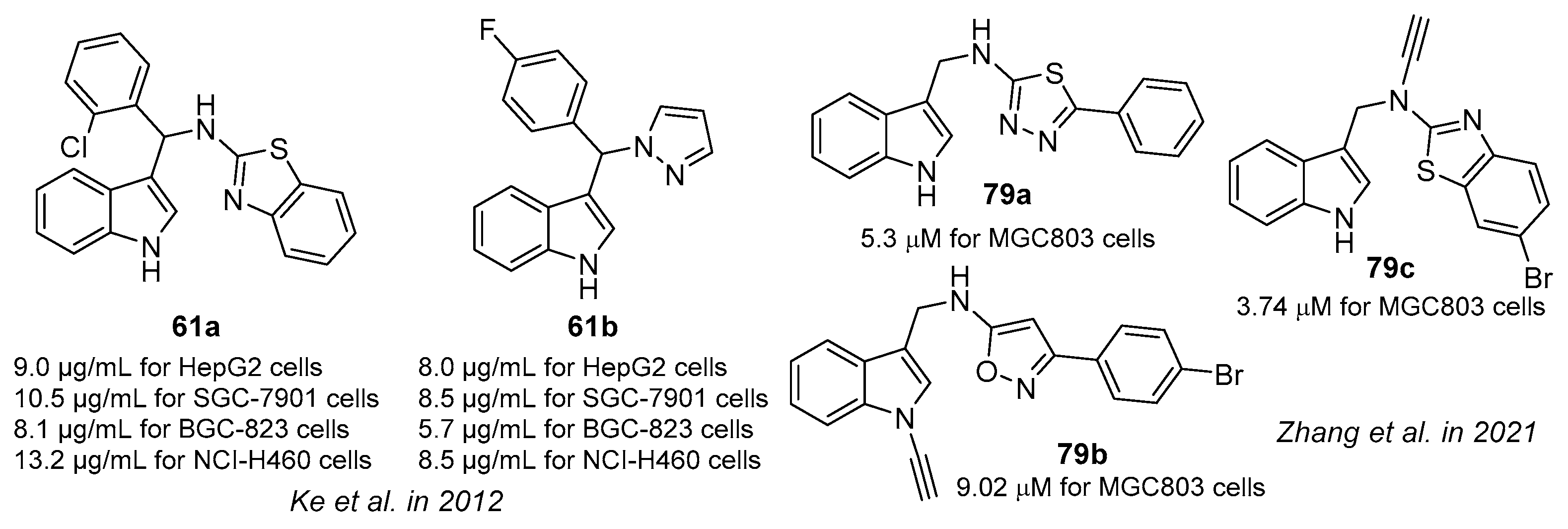
- Ke, S.; Shi, L.; Cao, X.; Yang, Q.; Liang, Y.; Yang, Z. Heterocycle-functional gramine analogues: Solvent- and catalyst-free synthesis and their inhibition activities against cell proliferation. Eur. J. Med. Chem. 2012, 54, 248–254. [Google Scholar] [CrossRef]
- Aydin, H.; Korkusuz, E.; Yildirim, İ. Multicomponent reactions of indoles with 1-amino-5-aroyl-4-aryl-1H-pyrimidin-2-ones/-thiones: One-pot three component synthesis of novel gramine analogues. Lett. Org. Chem. 2021, 18, 703–709. [Google Scholar] [CrossRef]
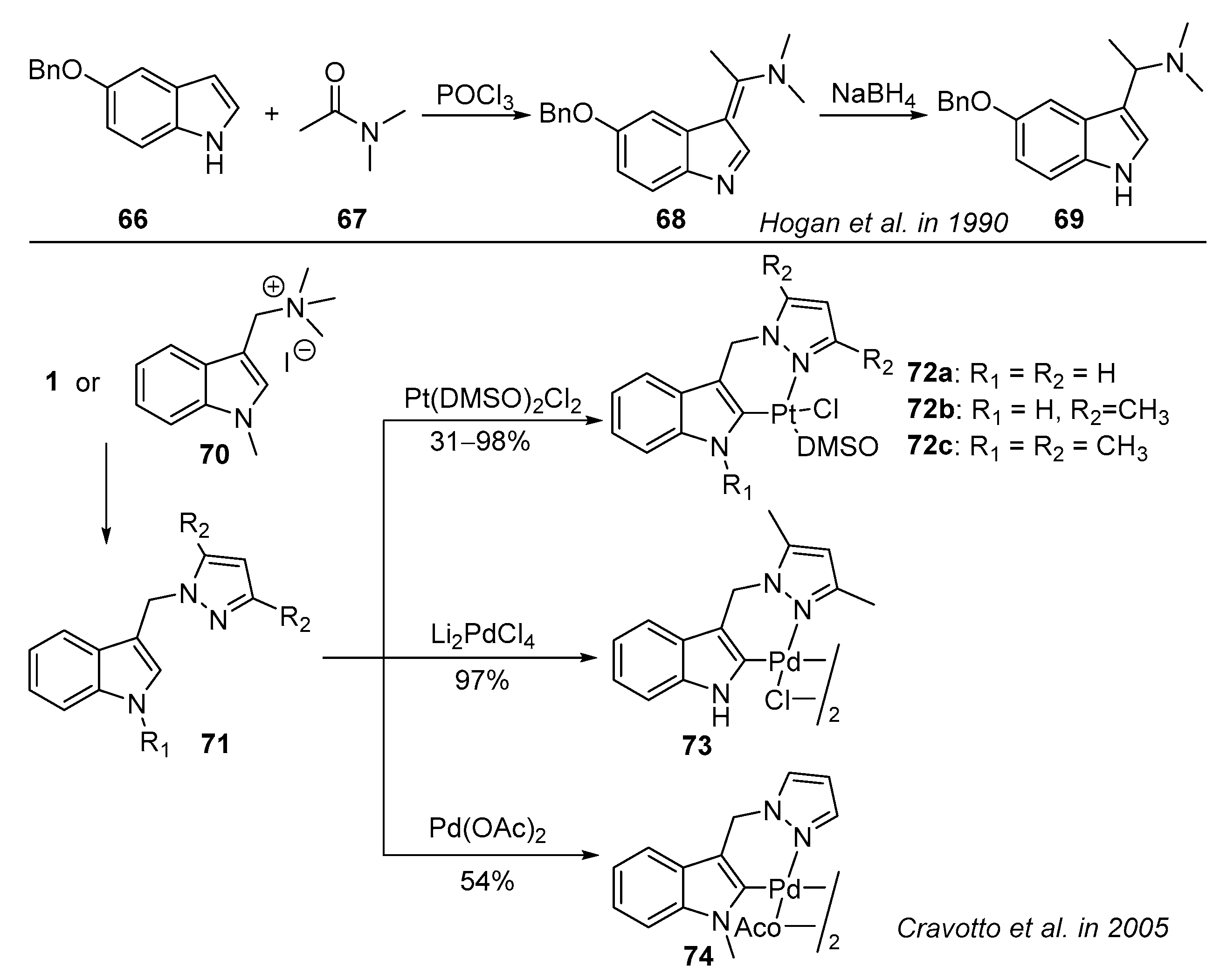
- Hogan, I.; Jenkins, P.D.; Sainsbury, M. The chemistry of the pyrido[4,3-b]carbazoles part 15 the synthesis of unsymmetrically 1,4-disubstituted carbazoles and their use in the synthesis of 6H-Pyrido-[4,3-b]carbazoles. Tetrahedron 1990, 46, 2943–2964. [Google Scholar] [CrossRef]
- Cravotto, G.; Demartin, F.; Palmisano, G.; Penoni, A.; Radice, T.; Tollari, S. Novel cyclometallated Pd(II) and Pt(II) complexes with indole derivatives and their use as catalysts in Heck reaction. J. Organomet. Chem. 2005, 690, 2017–2026. [Google Scholar] [CrossRef]
- Wang, S.-M.; Liu, C.-C. Enterovirus 71: Epidemiology, pathogenesis and management. Expert Rev. Anti-Infect. 2009, 7, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Li, C.M.; Ling, P.; Shen, F.H.; Chen, S.H.; Liu, C.C.; Yu, C.K.; Chen, S.H. Ribavirin reduces mortality in enterovirus 71-infected mice by decreasing viral replication. J. Infect. Dis. 2008, 197, 854–857. [Google Scholar] [CrossRef]
- Liu, M.L.; Lee, Y.P.; Wang, Y.F.; Lei, H.Y.; Liu, C.C.; Wang, S.M.; Su, I.J.; Wang, J.R.; Yeh, T.M.; Chen, S.H. Type I interferons protect mice against enterovirus 71 infection. J. Gen. Virol. 2005, 86, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, L.; Wang, K.; Liu, M.; Yang, Q.; Yang, Z.; Ke, S. Discovery of gramine derivatives that inhibit the early stage of EV71 replication in vitro. Molecules 2014, 19, 8949–8964. [Google Scholar] [CrossRef]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural products for drug discovery: Discovery of gramines as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef]
- Ji, X.F.; Wang, Z.W.; Dong, J.; Liu, Y.X.; Lu, A.D.; Wang, Q.M. Discovery of topsentin alkaloids and their derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2016, 64, 9143–9151. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, L.; Zhao, Y.; Huang, K.; Gong, W.; Yang, Y.; Zhao, L.; Xia, X.; Li, Z.; Sheng, F.; et al. 3-Indoleacetonitrile is highly effective in treating influenza a virus infection in vitro and in vivo. Viruses 2021, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yu, Y.; Sun, W.; Xia, C. Indole derivatives inhibited the formation of bacterial biofilm and modulated Ca2+ efflux in diatom. Mar. Pollut. Bull. 2014, 88, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Lu, L.; Li, Z.; Yu, Q.; Zhang, X. Antibacterial effects of 31 kinds of traditional chinese medicine monomers on MRSA in vitro. Chin. Pharm. 2014, 23, 20–21. [Google Scholar]
- Maver, M.; Escudero-Martinez, C.; Abbott, J.; Morris, J.; Hedley, P.E.; Mimmo, T.; Bulgarelli, D. Applications of the indole-alkaloid gramine modulate the assembly of individual members of the barley rhizosphere microbiota. PeerJ 2021, 9, e12498. [Google Scholar] [CrossRef]
- Schütz, V.; Frindte, K.; Cui, J.; Zhang, P.; Hacquard, S.; Schulze-Lefert, P.; Knief, C.; Schulz, M.; Dörmann, P. Differential impact of plant secondary metabolites on the soil microbiota. Front. Microbiol. 2021, 12, 666010. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Nasmith, C.G.; Allard, G.; Singh, J.; Subramaniam, R.; Desveaux, D. Found in translation: High-throughput chemical screening in Arabidopsis thaliana identifies small molecules that reduce fusarium head blight disease in wheat. Mol. Plant Microbe Interact. 2011, 24, 640–648. [Google Scholar] [CrossRef]
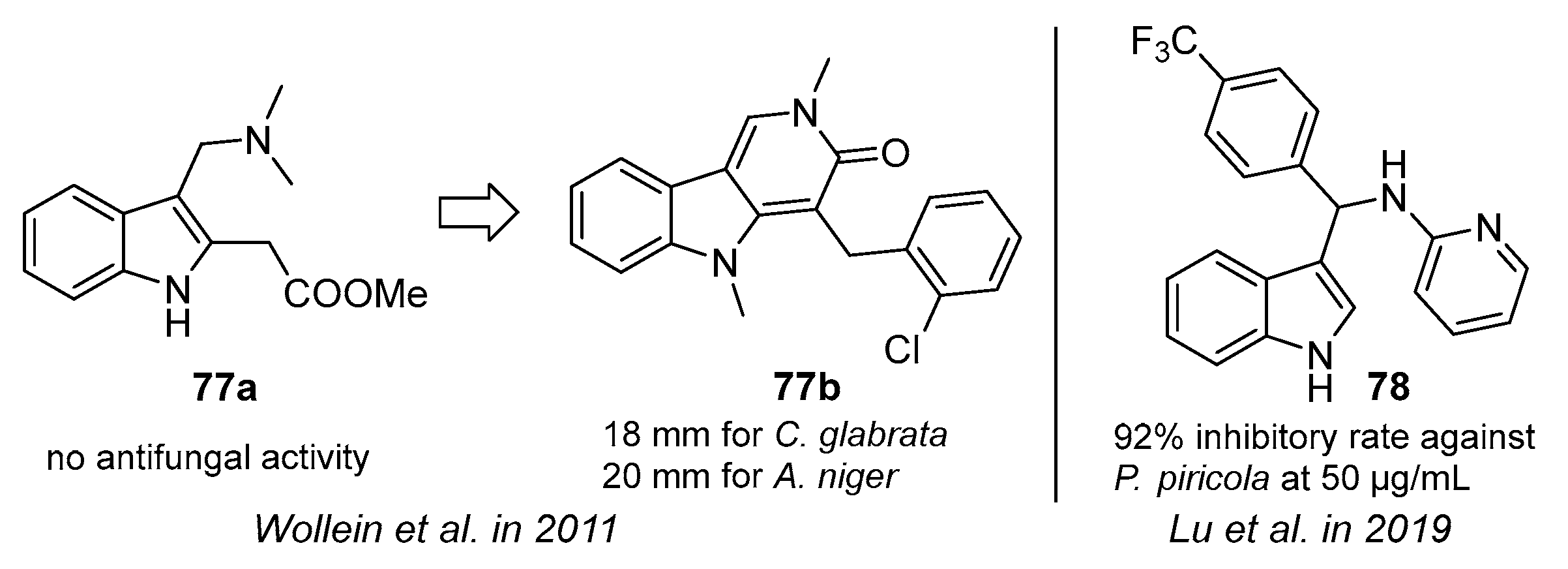
- Wollein, U.; Bracher, F. The gramine route to pyrido[4,3-b]indol-3-ones-identification of a new cytotoxic lead. Sci. Pharm. 2011, 79, 59–68. [Google Scholar] [CrossRef]
- Dan, W.; Cao, Y.; Sun, Y.; Zhang, J.; Liu, J.; Gao, J.; Han, R.; Dai, J. Novel N1 or N9 modified α-carboline analogues as potential ligands in Alzheimer’s disease therapy: Synthesis and neurobiological activity evaluation. Bioorg. Chem. 2023, 133, 106378. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Han, R.; Yuan, T.; Yang, Z.; Zhang, Q.; Wang, W.-W.; Lin, L.-B.; Zhu, M.-Q.; Gao, J.-M. Ulmoidol, an unusual nortriterpenoid from Eucommia ulmoides Oliv. Leaves prevents neuroinflammation by targeting the PU.1 transcriptional signaling pathway. Bioorg. Chem. 2021, 116, 105345. [Google Scholar] [CrossRef]
- Magalhães, S.C.Q.; Fernandes, F.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentão, P.; Andrade, P.B. Alkaloids in the valorization of European Lupinus spp. seeds crop. Ind. Crop. Prod. 2017, 95, 286–295. [Google Scholar] [CrossRef]
- Xie, X.; Xiao, H.; Ding, F.; Zhong, H.; Zhu, J.; Ma, N.; Mei, J. Over-expression of prolyl hydroxylase-1 blocks NF-κB-mediated cyclin D1 expression and proliferation in lung carcinoma cells. Cancer Genet. 2014, 7, 188–194. [Google Scholar] [CrossRef]
- Bernardes, V.F.; Gleber-Netto, F.O.; Sousa, S.F.; Rocha, R.M.; Aguiar, M.C. EGFR status in oral squamous cell carcinoma: Comparing immunohistochemistry, FISH and CISH detection in a case series study. BMJ Open 2013, 3, e002077. [Google Scholar] [CrossRef]
- Ramu, A.; Kathiresan, S.; Ramadoss, H.; Nallu, A.; Kaliyan, R.; Azamuthu, T. Gramine attenuates EGFR-mediated inflammation and cell proliferation in oral carcinogenesis via regulation of NF-κB and STAT3 signaling. Biomed. Pharmacother. 2018, 98, 523–530. [Google Scholar] [CrossRef]
- Lu, X.; Lu, F.; Yu, J.; Xue, X.; Jiang, H.; Jiang, L.; Yang, Y. Gramine promotes functional recovery after spinal cord injury via ameliorating microglia activation. J. Cell. Mol. Med. 2021, 25, 7980–7992. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA-Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Shklar, G. Development of experimental oral carcinogenesis and its impact on current oral cancer research. J. Dent. Res. 1999, 78, 1768–1772. [Google Scholar]
- Kumar, R.A.; Suresh, K. Chemopreventive potential of gramine against 7, 12-dimethylbenz [a] anthracene induced hamster buccal pouch carcinogenesis. Int. J. Mod. Res. Rev. 2014, 2, 188–194. [Google Scholar]
- Ramu, A.; Kathiresan, S.; Ahmed, B.A. Gramine inhibits angiogenesis and induces apoptosis via modulation of TGF-beta signalling in 7,12 dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinoma. Phytomedicine 2017, 33, 69–76. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Guo, Q.; Wang, H.-Y.; Li, Y.-H.; Khamis, M.Y.; Ma, L.-Y.; Wang, B.; Liu, H.-M. Gramine-based structure optimization to enhance anti-gastric cancer activity. Bioorg. Chem. 2021, 107, 104549. [Google Scholar] [CrossRef]
- Alnaim, A.S. Formulation, characterization, and cytotoxic effect of PVA incorporated iron oxide nanoparticles of gramine using HCT-116 cell line in vitro. Indian J. Pharm. Educ. Res. 2023, 57, 1–9. [Google Scholar]
- Cousins, K.A.Q.; Bove, J.; Giannini, L.A.A.; Kinney, N.G.; Balgenorth, Y.R.; Rascovsky, K.; Lee, E.B.; Trojanowski, J.Q.; Grossman, M.; Irwin, D.J. Longitudinal naming and repetition relates to AD pathology and burden in autopsy-confirmed primary progressive aphasia. Alzh. Dement-TRCI 2021, 7, e12188. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016, 12, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yogesha, S.D.; Mayfield, J.E.; Gill, G.N.; Zhang, Y. Viewing serine/threonine protein phosphatases through the eyes of drug designers. FEBS J. 2013, 280, 4739–4760. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Lajarin-Cuesta, R.; Nanclares, C.; Arranz-Tagarro, J.A.; Gonzalez-Lafuente, L.; Arribas, R.L.; Brito, M.A.; Gandia, L.; Rios, C.I. Gramine derivatives targeting Ca2+ channels and ser/thr phosphatases: A new dual strategy for the treatment of neurodegenerative diseases. J. Med. Chem. 2016, 59, 6265–6280. [Google Scholar] [CrossRef]
- Gonzalez, D.; Arribas, R.L.; Viejo, L.; Lajarin-Cuesta, R.; Rios, C.L. Substituent effect of N-benzylated gramine derivatives that prevent the PP2A inhibition and dissipate the neuronal Ca2+ overload, as a multitarget strategy for the treatment of Alzheimer’s disease. Bioorgan. Med. Chem. 2018, 26, 2551–2560. [Google Scholar] [CrossRef]
- Lajarín-Cuesta, R.; Arribas, R.L.; Nanclares, C.; García-Frutos, E.M.; Gandía, L.; Ríos, C.L. Design and synthesis of multipotent 3-aminomethylindoles and 7-azaindoles with enhanced protein phosphatase 2A-activating profile and neuroprotection. Eur. J. Med. Chem. 2018, 157, 294–309. [Google Scholar] [CrossRef]
- Jadhav, G.B.; Sable, R.R. Neuroprotective impact of zingerone and gramine on scopolamine-induced amnesia model. J. Pharm. Negat. Result. 2023, 14, 775–784. [Google Scholar]
- Spitzer, N.; Edwards, D.H.; Baro, D.J. Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkia. J. Exp. Biol. 2008, 211, 92–105. [Google Scholar] [CrossRef]
- Adkins, E.M.; Barker, E.L.; Blakely, R.D. Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain I in substrate recognition. Mol. Pharmacol. 2001, 59, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-J.; Huang, X.-Y.; Ma, Y.-B.; Geng, C.-A.; Li, T.-Z.; Chen, X.-L.; Yang, T.-H.; Zhou, J.; Zhang, X.-M.; Chen, J.-J. Bioactivity-guided synthesis of gramine derivatives as new MT1 and 5-HT1A receptors agonists. J. Asian Nat. Prod. Res. 2017, 19, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.S.; Frost, W.N. Intrinsic neuromodulation in the Tritonia swim CPG: Serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J. Neurophysiol. 1995, 74, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Couper, J.M.; Leise, E.M. Serotonin injections induce metamorphosis in larvae of the gastropod mollusc Ilyanassa obsolete. Biol. Bull. 1996, 191, 178–186. [Google Scholar] [CrossRef][Green Version]
- Litosch, I.; Fradin, M.; Kasaian, M.; Lee, H.S.; Fain, J.N. Regulation of adenylate cyclase and cyclic AMP phosphodiesterase by 5-hydroxytryptamine and calcium ions in blowfly salivary-gland homogenates. Biochem. J. 1982, 204, 153–159. [Google Scholar] [CrossRef]
- Renaud, F.; Parisi, E.; Capasso, A.; Prisco, P. On the role of serotonin and 5-methoxy-tryptamine in the regulation of cell division in sea urchin eggs. Dev. Biol. 1983, 98, 37–46. [Google Scholar] [CrossRef]
- Niacaris, T.; Avery, L. Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J. Exp. Biol. 2003, 206, 223–231. [Google Scholar] [CrossRef]
- Froldi, G.; Silvestrin, B.; Dorigo, P.; Caparrotta, L. Gramine: A vasorelaxing alkaloid acting on 5-HT2A receptors. Planta Med. 2004, 70, 373–375. [Google Scholar]
- Sheng, W.L.; Chen, W.Y.; Yang, X.L.; Zhong, Y.M.; Weng, S.J. Co-expression of two subtypes of melatonin receptor on rat M1-type intrinsically photosensitive retinal ganglion cells. PLoS ONE 2015, 10, e0117967. [Google Scholar] [CrossRef]
- Orchard, I.; Lange, A.B. Pharmacological profile of octopamine receptors on the lateral oviducts of the locust, Locusta migratoria. J. Insect Physiol. 1986, 32, 741–745. [Google Scholar] [CrossRef]
- Yang, J.; Kong, X.-D.; Zhu-Salzman, K.; Qin, Q.-M.; Cai, Q.N. The key glutathione S-transferase family genes involved in the detoxification of rice gramine in brown planthopper Nilaparvata lugens. Insects 2021, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.R.; Strobel, B.W.; Hama, J.R.; Cedergreen, N. Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environ. Sci. Eur. 2021, 33, 10. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar]
- Mary, M.A.; Rashel, R.H.; Patiño, R. Growth inhibition of the harmful alga Prymnesium parvum by plant-derived products and identification of ellipticine as highly potent allelochemical. J. Appl. Phycol. 2021, 33, 3853–3860. [Google Scholar] [CrossRef]
- Xu, L.; Su, Y.; Yang, X.; Bai, X.; Wang, Y.; Zhuo, C.; Meng, Z. Gramine protects against pressure overload-induced pathological cardiac hypertrophy through Runx1-TGFBR1 signaling. Phytomedicine 2023, 114, 154779. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Sierakowska, A.; Kowalczyk, I.; Mrówczyńska, L.; Jasiewicz, B. New triazole-bearing gramine derivatives-synthesis, structural analysis and protective effect against oxidative haemolysis. Nat. Prod. Res. 2022, 36, 3413–3419. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jia, Q.; Li, N.; Gu, L.; Dan, W.; Dai, J. Recent Developments of Gramine: Chemistry and Biological Activity. Molecules 2023, 28, 5695. https://doi.org/10.3390/molecules28155695
Zhang J, Jia Q, Li N, Gu L, Dan W, Dai J. Recent Developments of Gramine: Chemistry and Biological Activity. Molecules. 2023; 28(15):5695. https://doi.org/10.3390/molecules28155695
Chicago/Turabian StyleZhang, Jiaoyue, Qitao Jia, Na Li, Liqiang Gu, Wenjia Dan, and Jiangkun Dai. 2023. "Recent Developments of Gramine: Chemistry and Biological Activity" Molecules 28, no. 15: 5695. https://doi.org/10.3390/molecules28155695
APA StyleZhang, J., Jia, Q., Li, N., Gu, L., Dan, W., & Dai, J. (2023). Recent Developments of Gramine: Chemistry and Biological Activity. Molecules, 28(15), 5695. https://doi.org/10.3390/molecules28155695