Tetraphenylethene Derivatives Bearing Alkylammonium Substituents: Synthesis, Chemical Properties, and Application as BSA, Telomere DNA, and Hydroxyl Radical Sensors
Abstract
1. Introduction
2. Results and Discussion
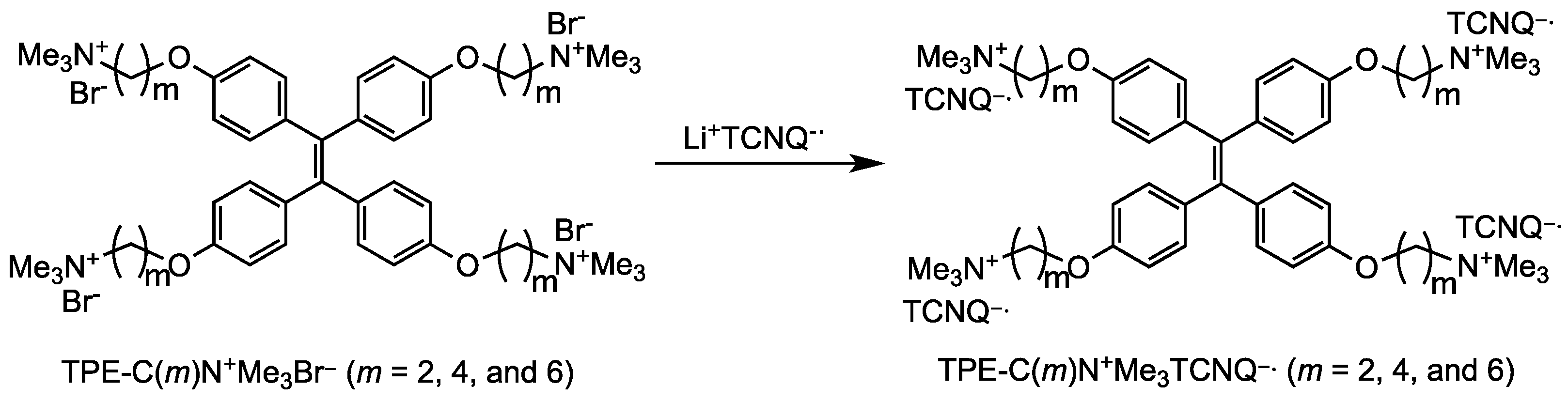
2.1. Synthesis
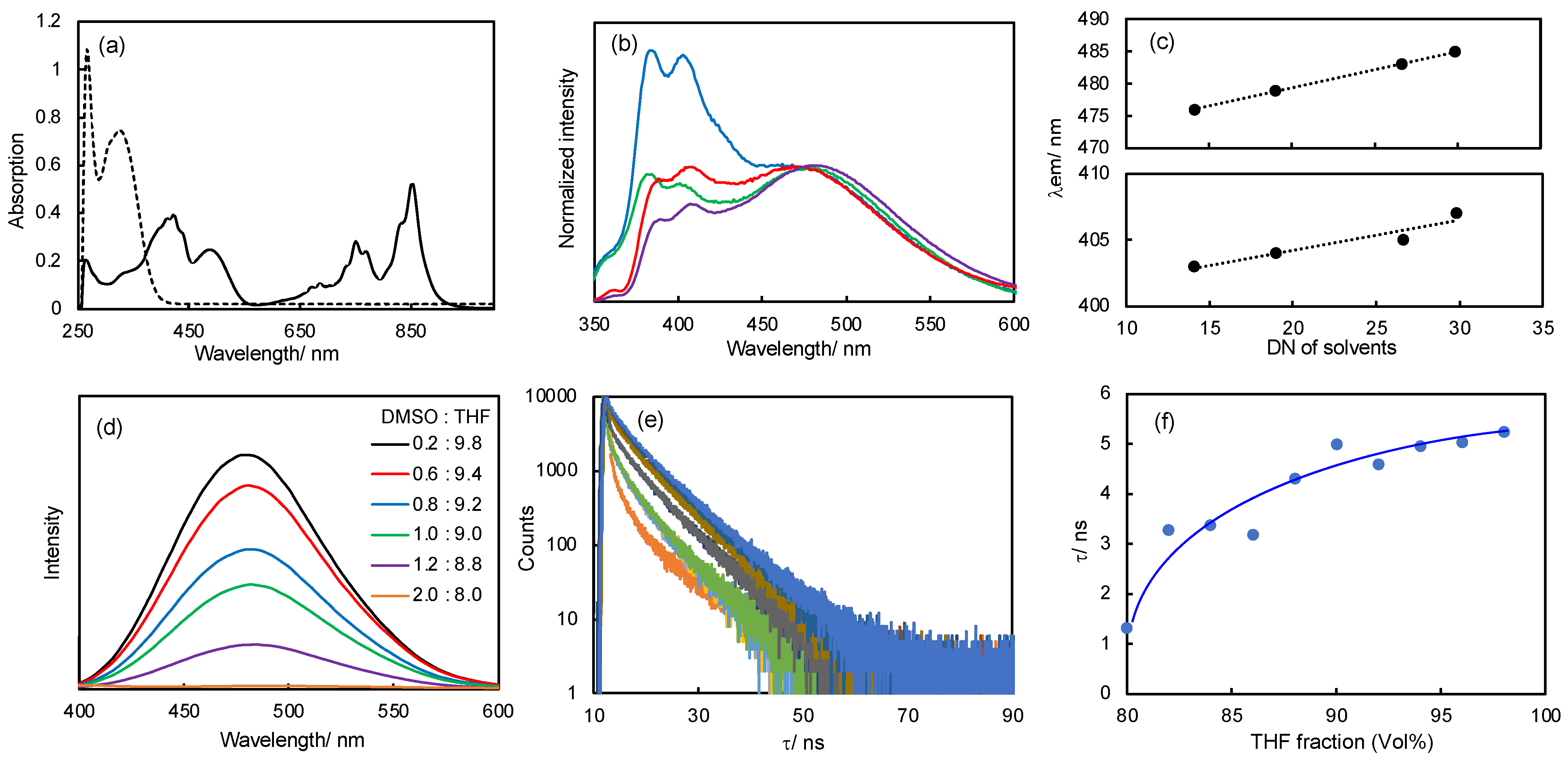
2.2. Optical Properties
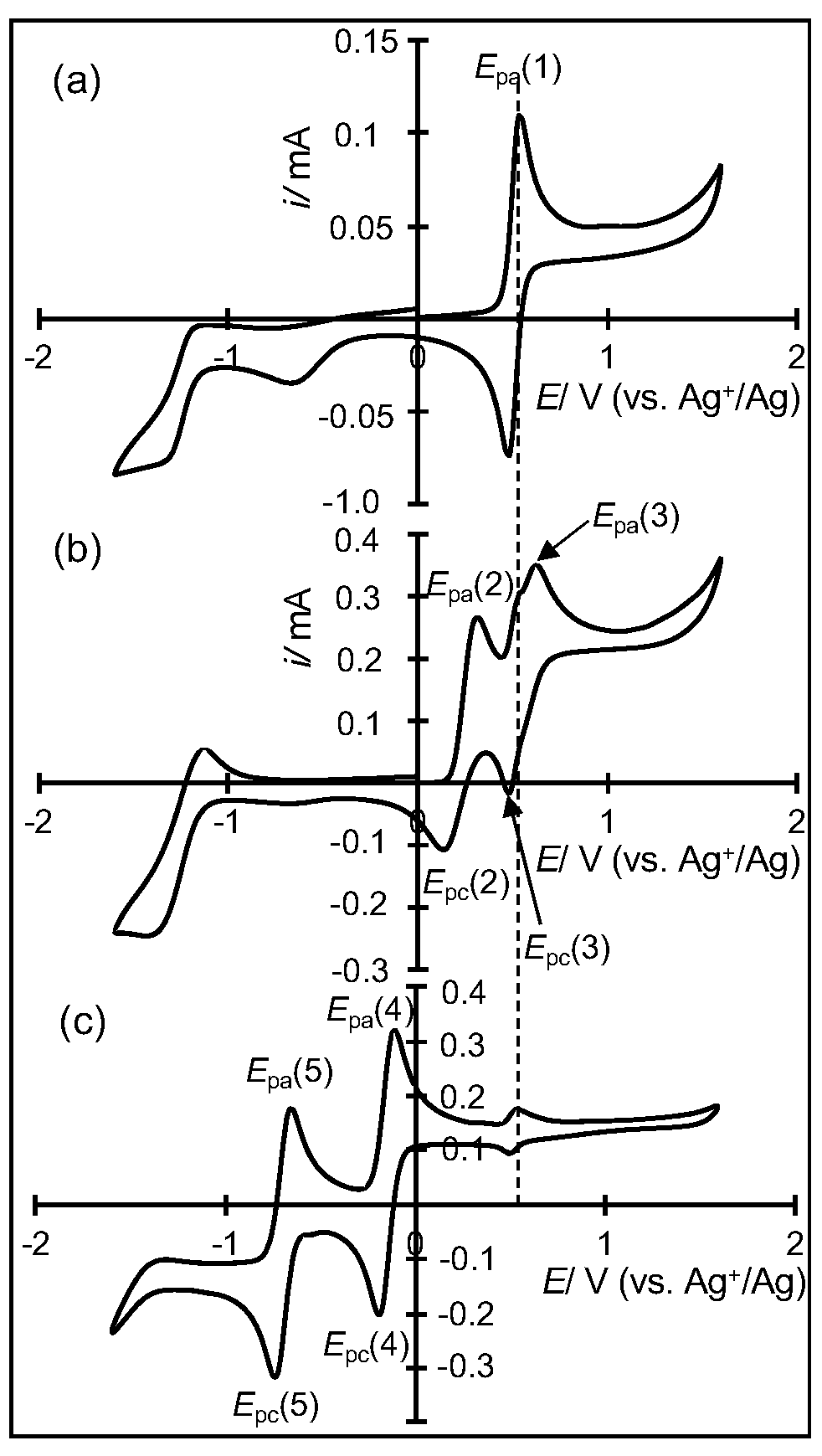
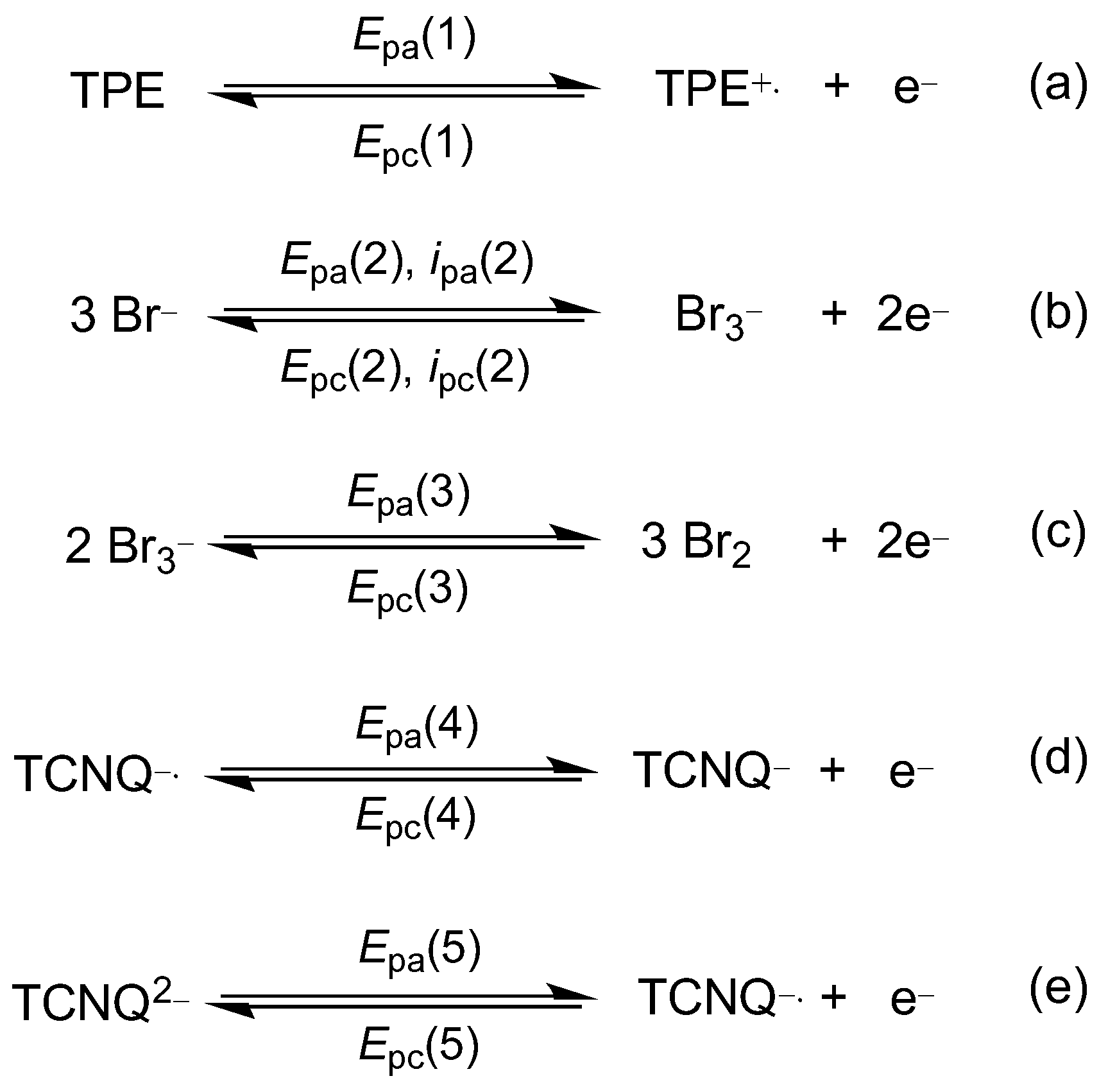
2.3. Electrochemical Properties
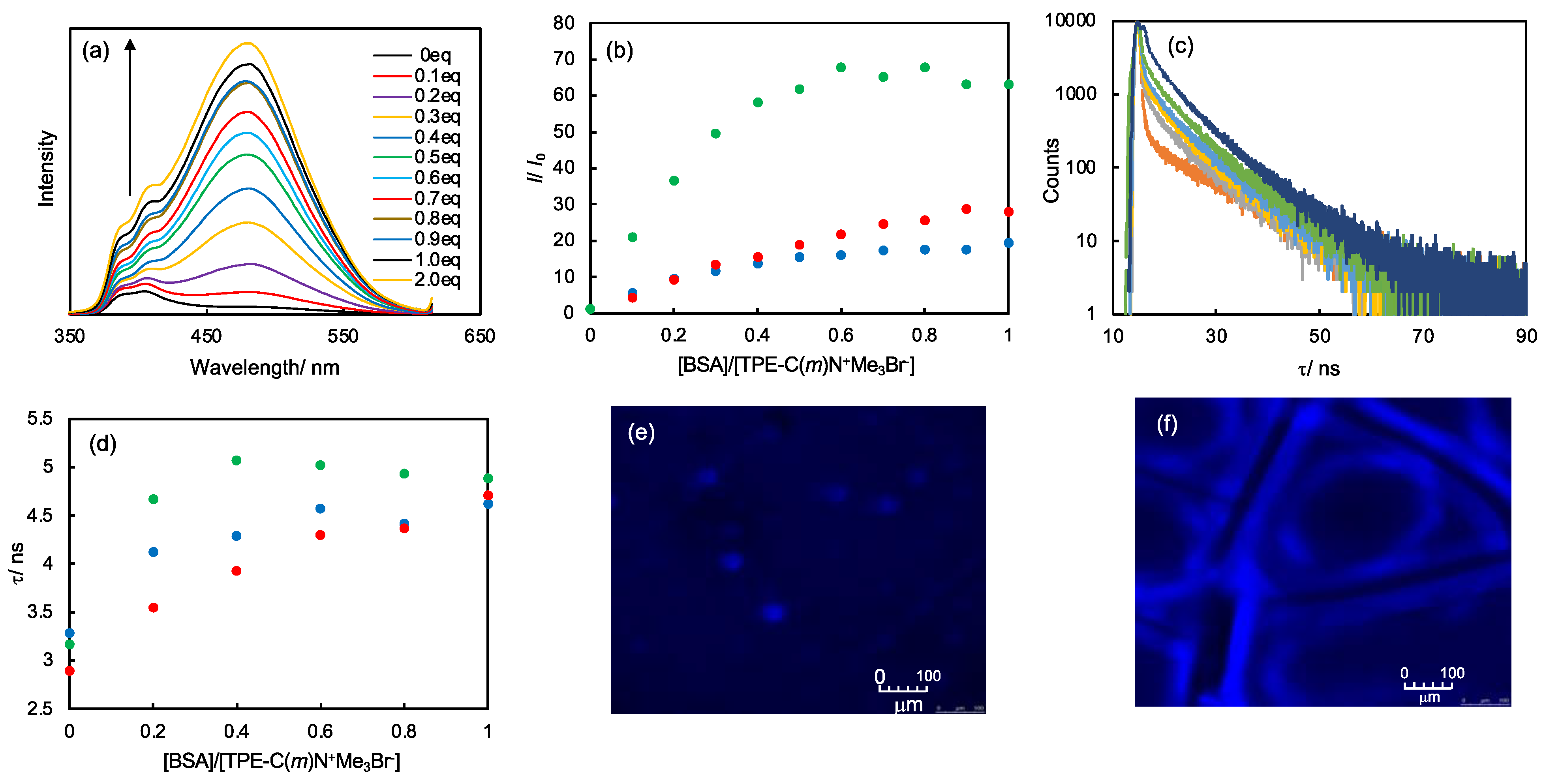
2.4. BSA Sensing
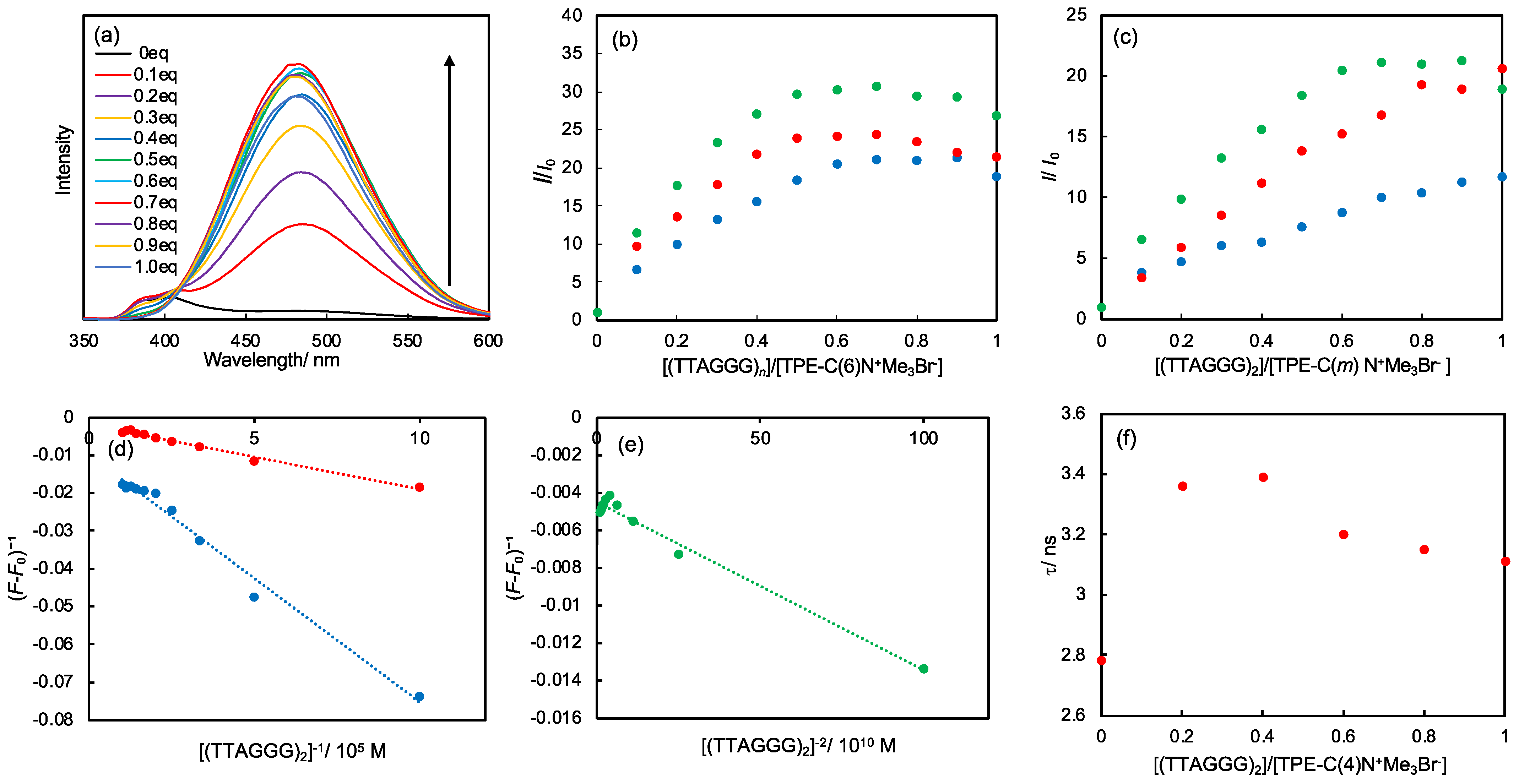
2.5. DNA Sensing
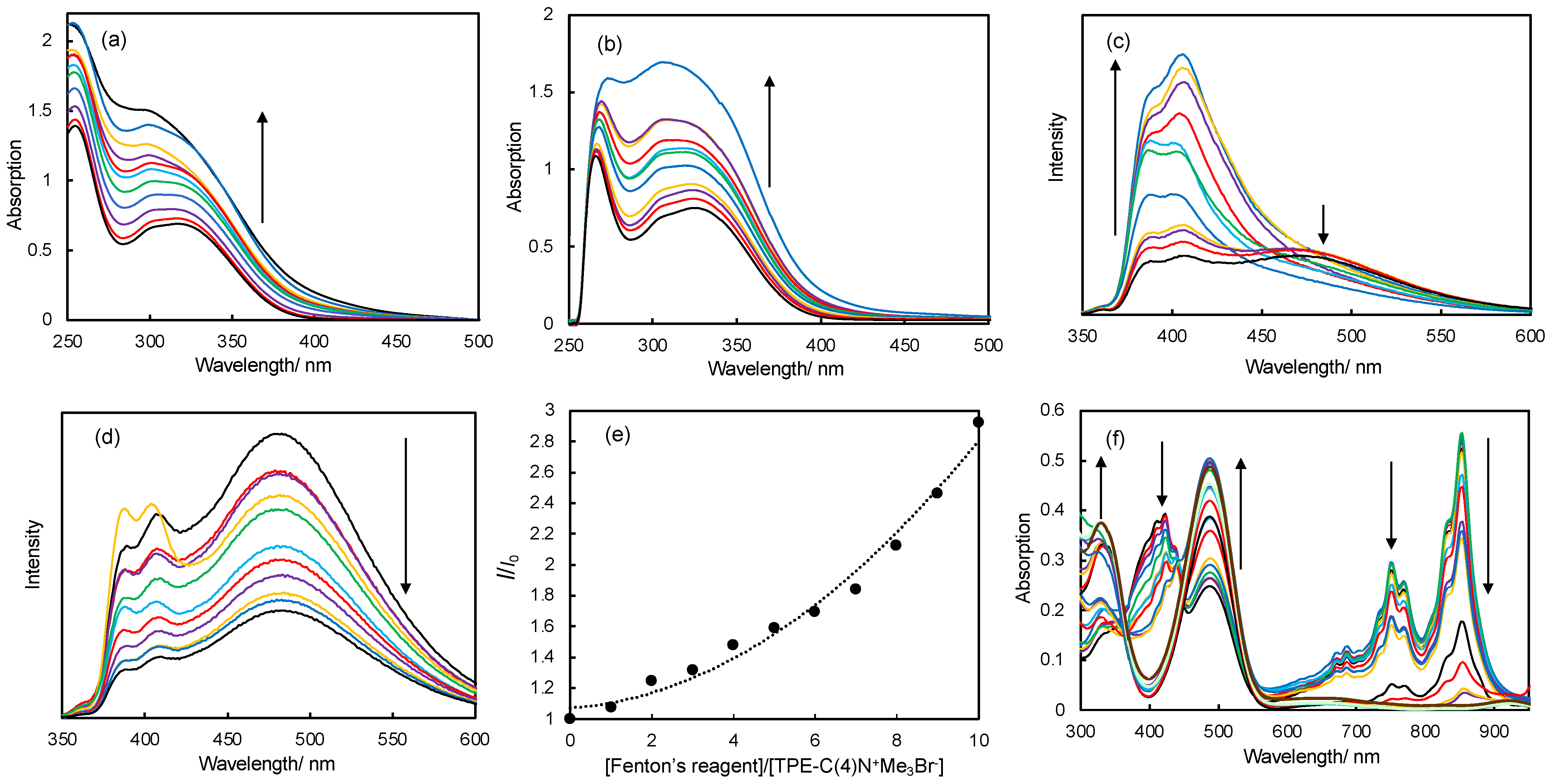
2.6. Hydroxyl Radical Sensing
3. Experimental Section
3.1. General
3.2. Synthesis of TPE-C(m)N+Me3TCNQ−•
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, J.L.; Hu, L.; Wang, J.; Lam, J.W.Y.; Blancafort, L.; Tang, B.Z. Through-space interaction of tetraphenylethene: What, where, and how. J. Am. Chem. Soc. 2022, 144, 7901–7910. [Google Scholar]
- Feng, H.T.; Zou, S.; Chen, M.; Xiong, F.; Lee, M.H.; Fng, L.; Tang, Z.T. Tuning push–pull electronic effects of AIEgens to boost the theranostic efficacy for colon cancer. J. Am. Chem. Soc. 2020, 142, 11442–11450. [Google Scholar] [CrossRef]
- Chen, S.; Han, T.; Zhao, Y.; Luo, W.; Zhang, Z.; Su, H.; Tang, B.Z. A facile strategy to prepare smart coatings with autonomous self-healing and self-reporting functions. ACS Appl. Mater. Interfaces 2020, 12, 4870–4877. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tang, B.Z. Fluorescent sensors based on aggregation-induced emission: Recent advances and perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-based AIE-active probes for sensing applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef]
- Mei, J.; Huang, Y.; Tian, H. Progress and trends in AIE-based bioprobes: A brief overview. ACS Appl. Mater. Interfaces 2018, 10, 12217–12261. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.; Dong, Y.; Ha1ussler, M.; Li, Z.; Lam, J.W.Y.; Dong, Y.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. Protein detection and quantitation by tetraphenylethene-based fluorescent probes with aggregation-induced emission characteristics. J. Phys. Chem. B 2007, 111, 11817–11823. [Google Scholar] [CrossRef]
- Xu, J.P.; Fang, Y.; Song, Z.G.; Mei, J.; Jia, L.; Qin, A.J.; Sun, J.Z.; Ji, J.; Tang, B.Z. BSA–tetraphenylethene derivative conjugates with aggregation-induced emission properties: Fluorescent probes for label-free and homogeneous detection of protease and a1-antitrypsin. Analyst 2011, 136, 2315–2321. [Google Scholar] [CrossRef]
- Lee, W.E.; Kim, J.W.; Oh, C.J.; Sakaguchi, T.; Fujiki, M.; Kwak, G. Correlation of intramolecular excimer emission with lamellar layer distance in liquid-crystalline polymers: Verification by the film-swelling method. Angew. Chem. Int. Ed. 2010, 49, 1406–1409. [Google Scholar] [CrossRef]
- Wang, Y.; Zappas, A.J., II; Wilson, J.N.; Kim, I.B.; Solntsev, K.M.; Tolbert, L.M.; Bunz, U.H.F. Optical spectroscopy of grafted poly(p-phenyleneethynylene)s in water and water-DMF mixtures. Macromolecules 2008, 41, 1112–1117. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.C.; Kong, D.M.; Guo, D.S. Tetraphenylethene derivatives with different numbers of positively charged side arms have different multimeric G-quadruplex recognition specificity. Chem. Eur. J. 2015, 21, 13253–13260. [Google Scholar] [CrossRef]
- Kotras, C.; Fossépré, M.; Roger, M.; Gervais, V.; Richeter, S.; Gerbier, P.; Ulrich, S.; Surin, M.; Clément, S. A cationic tetraphenylethene as a light-up supramolecular probe for DNA G-quadruplexes. Front. Chem. 2019, 7, 493. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Matsumoto, Y.; Miyoshi, K.; Wang, A. Synthesis, properties and graft polymerization of ionic conjugated polymers with TCNQ anion radical. Polymer 2021, 219, 123552. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Makrigiorgos, G.M.; Baranowska-Korttlewicz, J.; Bump, E.; Sahu, S.K.; Berman, R.M.; Kassis, A.I. A method for detection of hydroxyl radicals in the vicinity of biomolecules using radiation-induced fluorescence of coumarin. Int. J. Radiat. Biol. 1993, 63, 445–458. [Google Scholar] [CrossRef]
- Makrigiorgos, G.M.; Folkard, M.; Huang, C.; Bump, E.; Baranowska-Kortylewicz, J.; Sahu, S.K.; Michael, B.D.; Kassis, A.I. Quantification of radiation-induced hydroxyl radicals within nucleohistones using a Molecular fluorescent probe. Radiat. Res. 1994, 138, 177–185. [Google Scholar] [CrossRef]
- Makrigiorgos, G.M.; Bump, E.; Huang, C.; Baranowska-Kortylewicz, J.; Kassis, A.I. A fluorimetric method for the detection of copper-mediated hydroxyl free radicals in the immediate proximity of DNA. Free Radic. Biol. Med. 1995, 18, 669–678. [Google Scholar] [CrossRef]
- Soh, N.; Mmakihara, K.; Ariyoshi, T.; Seto, D.; Maki, T.; Nakajima, H.; Nakano, K.; Imato, T. Phospholipid-linked coumarin: A fluorescent probe for sensing hydroxyl radicals in lipid membranes. Anal. Sci. 2008, 24, 293–296. [Google Scholar] [CrossRef]
- Chen, L.N.; Yu, W.S.; Wang, T.; Yang, X.D.; Yang, H.J.; Chen, Z.X.; Wang, T.; Tian, N.; Zhou, Z.Y.; Sun, S.G. Fluorescence detection of hydroxyl radical generated from oxygen reduction on Fe/N/C catalyst. Sci. China Chem. 2020, 63, 198–202. [Google Scholar] [CrossRef]
- Soh, N.; Makihara, K.; Sakoda, E.; Imato, T. A ratiometric fluorescent probe for imaging hydroxyl radicals in living cells. Chem. Commun. 2004, 40, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, G.G.; Martin, I.; Zhao, Q.; Amoscato, A.A.; Tyurina, Y.Y.; Kagan, V.E. Glutathione propagates oxidative stress triggered by myeloperoxidase in HL-60 cells. J. Biol. Chem. 2004, 279, 23453–23462. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.G.; Babbs, C.F. Quantitation of the hydroxyl radical by reaction with dimethyl sulfoxide. Arch. Biochem. Biophys. 1990, 278, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.B.; Foley, J.F.; Korzeniewki, C.; Pons, S. An infrared spectroelectrochemical investigation of the ion pairing reactions of the anions and dianions of TCNE and TCNQ. J. Electroanal. Chem. 1987, 233, 223–236. [Google Scholar] [CrossRef]
- Melby, L.R.; Harder, R.J.; Hertler, W.R.; Mahler, W.; Benson, R.E.; Mochel, W.E. Substituted quinodimethans. II. Anion-radical derivatives and complexes of 7,7,8,8-tetracyanoquinodimethan. J. Am. Chem. Soc. 1962, 84, 3374–3387. [Google Scholar] [CrossRef]
- Chang, J.; Bennett, B.; Bard, A.J. Detection of an unstable intermediate in Br− electro-oxidation to Br3− on a platinum electrode in nitrobenzene by scanning electrochemical microscopy. Electrochim. Acta 2017, 238, 74–80. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Atifi, A.; Ryan, M.D. Voltammetry and spectroelectrochemistry of TCNQ in acetonitrile/RTIL mixtures. Molecules 2020, 25, 303. [Google Scholar] [CrossRef]
- Curvale, R.; Masuelli, M.; Padilla, A.P. Intrinsic viscosity of bovine serum albumin conformers. Int. J. Biol. Macromol. 2008, 42, 133–137. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Oliveros, E.; Göb, S.; Siegwart, S.; Straub, M.; Wörner, M.; Braun, A.M. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J. Phys. Chem. A 1998, 102, 5542–5550. [Google Scholar] [CrossRef]
- Fang, M.; Wei, W.; Li, R.; Mao, L.; Wang, Y.; Guan, Y.; Chen, Q.; Shuai, Z.; Wei, Y. The variance of photophysical properties of tetraphenylethene and its derivatives during their transitions from dissolved states to solid states. Polymers 2022, 14, 2880. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; Chapter 8. [Google Scholar]
- Jonkman, H.T.; Kommandeur, J. The UV spectra and their calculation of TCNQ and its mono- and di-valent anion. Chem. Phys. Lett. 1972, 15, 496–499. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Zhong, C.; Shi, J.; Tong, B.; Feng, X.; Zhi, J.; Dong, Y. Investigating the effects of side chain length on the AIE properties of water-soluble TPE derivatives. Tetrahedron Lett. 2014, 55, 1496–1500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, I.; Ikawa, K.; Takimiya, N.; Wang, A. Tetraphenylethene Derivatives Bearing Alkylammonium Substituents: Synthesis, Chemical Properties, and Application as BSA, Telomere DNA, and Hydroxyl Radical Sensors. Molecules 2023, 28, 5663. https://doi.org/10.3390/molecules28155663
Yamaguchi I, Ikawa K, Takimiya N, Wang A. Tetraphenylethene Derivatives Bearing Alkylammonium Substituents: Synthesis, Chemical Properties, and Application as BSA, Telomere DNA, and Hydroxyl Radical Sensors. Molecules. 2023; 28(15):5663. https://doi.org/10.3390/molecules28155663
Chicago/Turabian StyleYamaguchi, Isao, Kensuke Ikawa, Nobuto Takimiya, and Aohan Wang. 2023. "Tetraphenylethene Derivatives Bearing Alkylammonium Substituents: Synthesis, Chemical Properties, and Application as BSA, Telomere DNA, and Hydroxyl Radical Sensors" Molecules 28, no. 15: 5663. https://doi.org/10.3390/molecules28155663
APA StyleYamaguchi, I., Ikawa, K., Takimiya, N., & Wang, A. (2023). Tetraphenylethene Derivatives Bearing Alkylammonium Substituents: Synthesis, Chemical Properties, and Application as BSA, Telomere DNA, and Hydroxyl Radical Sensors. Molecules, 28(15), 5663. https://doi.org/10.3390/molecules28155663





