Electrochemical Determination of the Drug Colchicine in Pharmaceutical and Βiological Samples Using a 3D-Printed Device
Abstract
1. Introduction
2. Results and Discussion
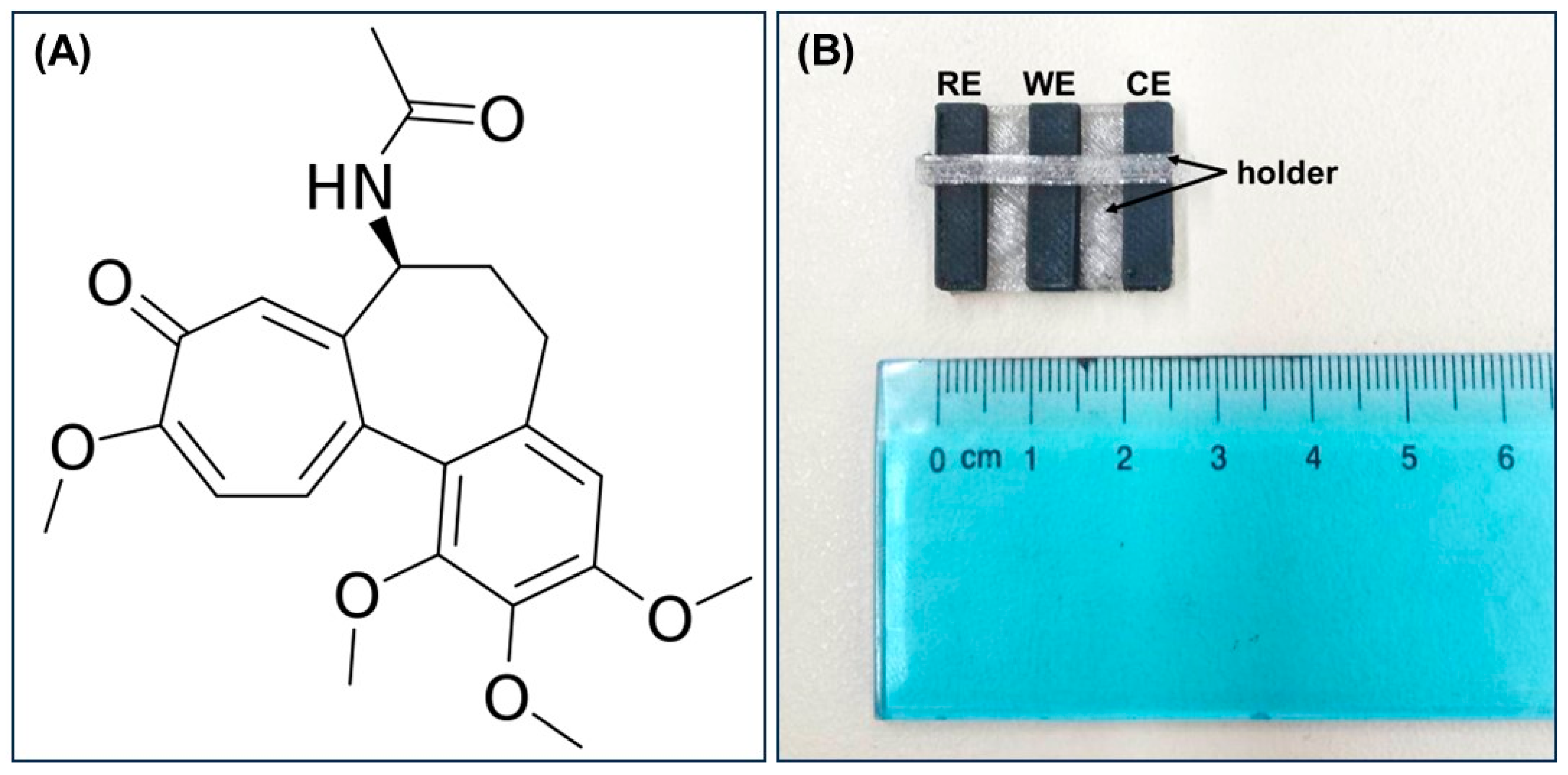
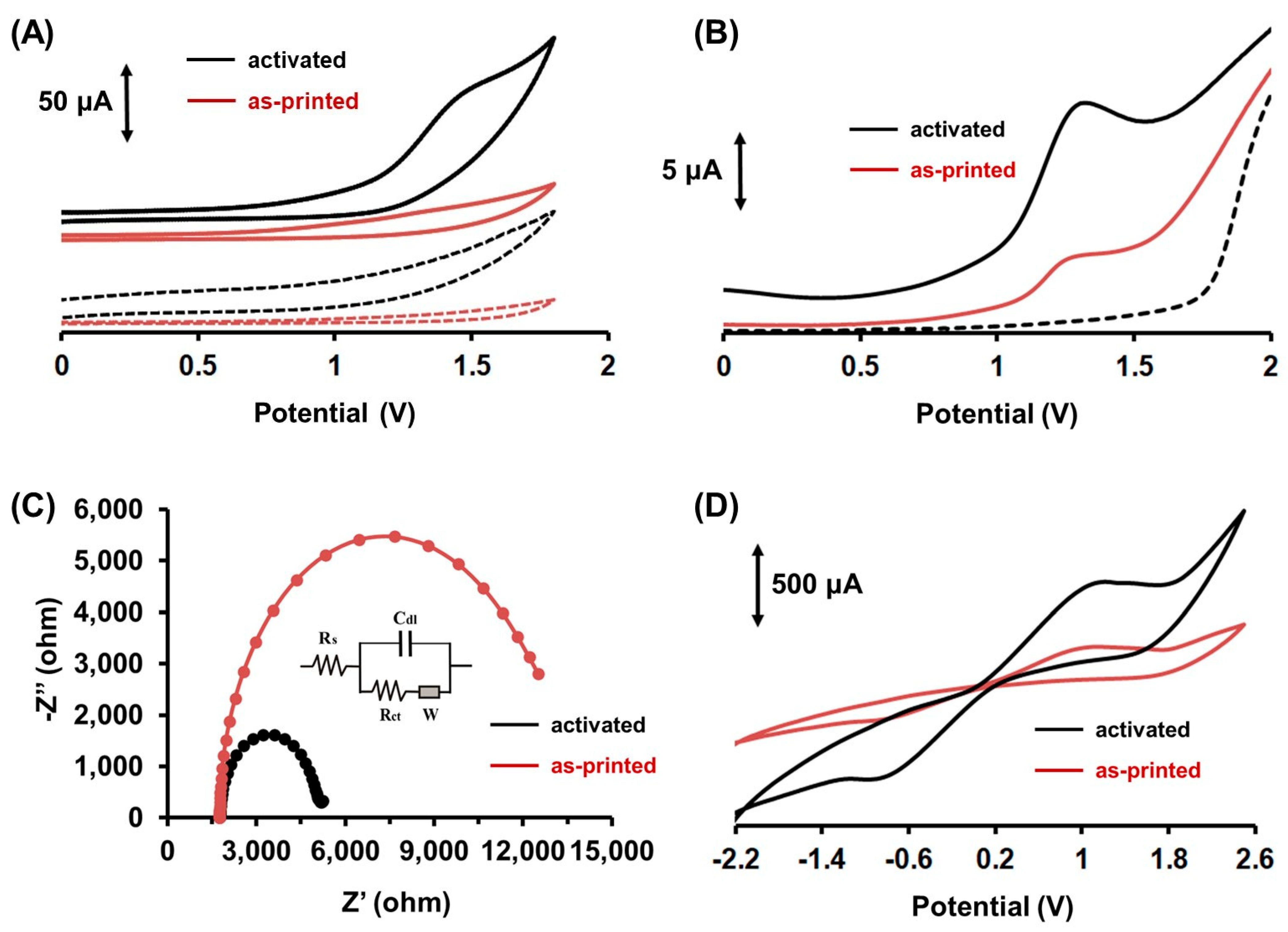
2.1. Electrochemical Behavior of Colchicine at the 3D-Printed Device
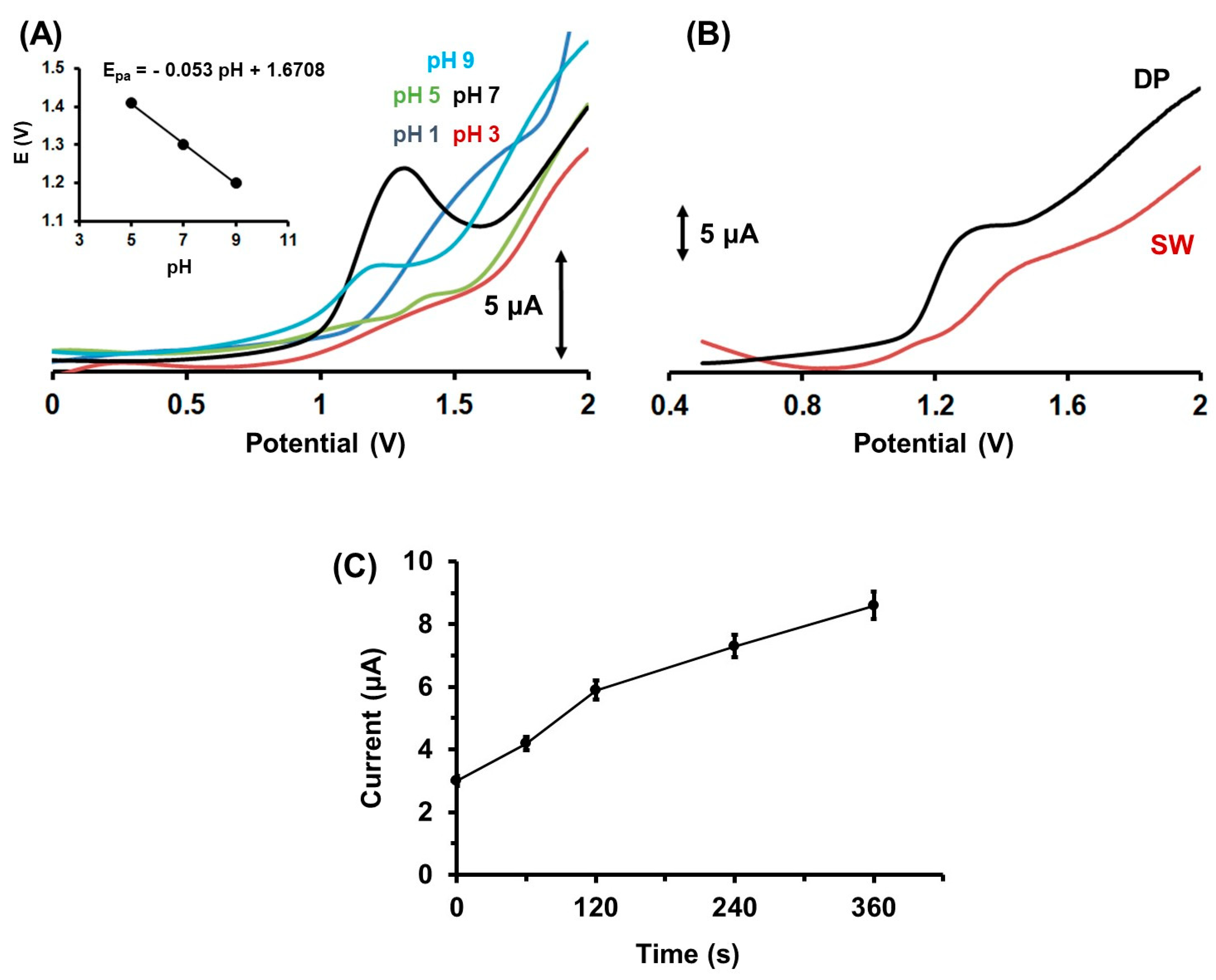
2.2. Influence of the pH, the Accumulation Conditions, the Voltammetric Waveforms on the Determination of Colc
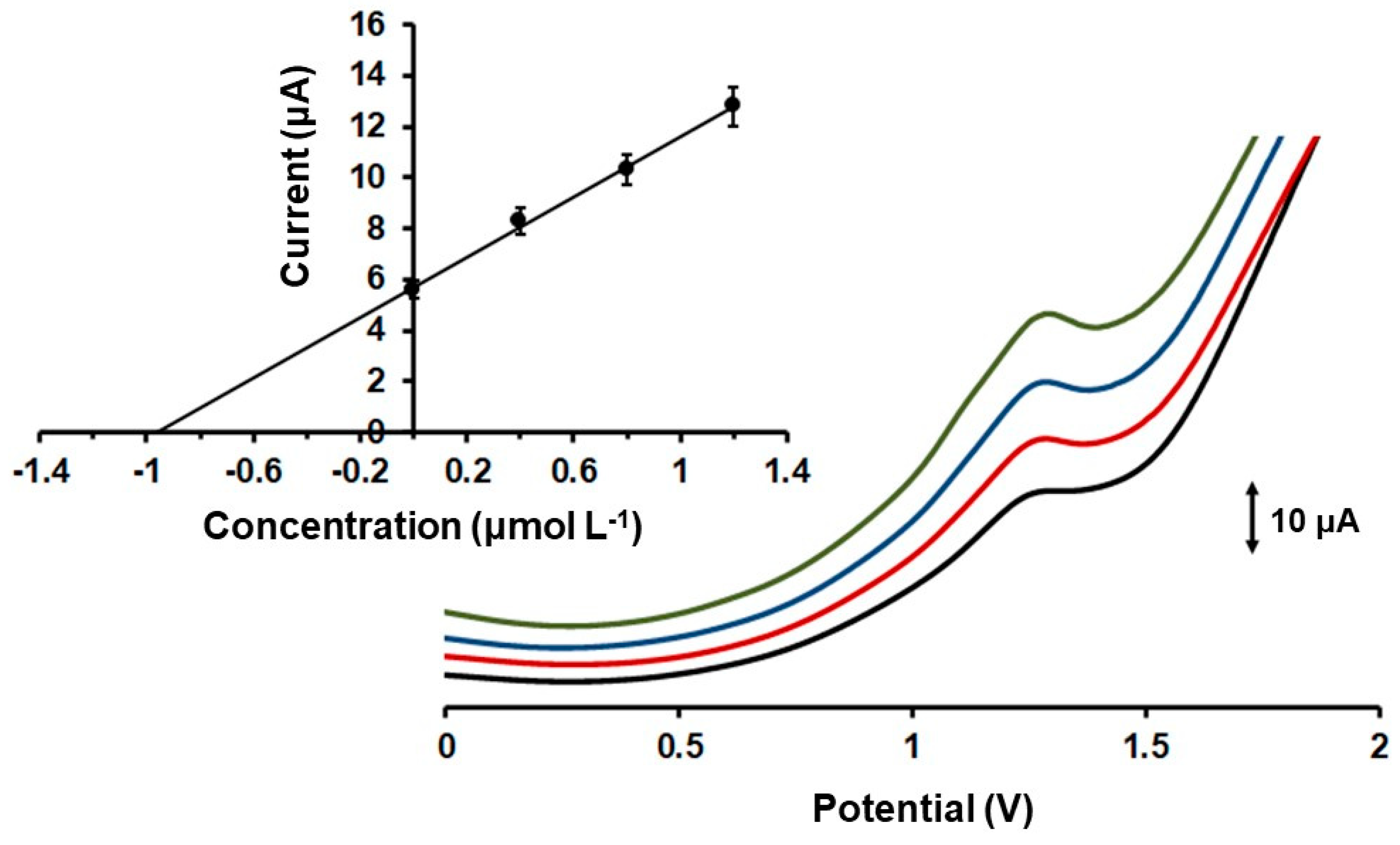
2.3. Analytical Performance and Interferences
2.4. Applications
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Fabrication of the 3D-Printed Device
3.3. Electrochemical Measurements and Samples Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chabert, J.F.D.; Vinader, V.; Santos, A.R.; Horcajo, M.R.; Dreneau, A.; Basak, R.; Cosentino, L.; Marston, G.; Rahman, H.A.; Loadman, P.M.; et al. Synthesis and biological evaluation of colchicine C-ring analogues tethered with aliphatic linkers suitable for prodrug derivatization. Bioorg. Med. Chem. Lett. 2012, 22, 7693–7696. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Lauterio, T.J.; Wolfe, H.R. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 2014, 36, 1465–1479. [Google Scholar] [CrossRef]
- Sonmez, A.O.; Sonmez, H.E.; Çakan, M.; Yavuz, M.; Keskindemirci, G.; Ayaz, N.A. The evaluation of anxiety, depression and quality of life scores of children and adolescents with familial Mediterranean fever. Rheumatol. Int. 2020, 40, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Kone-Paut, I.; Gul, A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin. Arthritis Rheum. 2017, 47, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharmaceut. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Worachartcheewan, A.; Songtawee, N.; Siriwong, S.; Prachayasittikul, S.; Nantasenamat, C.; Prachayasittikul, V. Rational Design of Colchicine Derivatives as anti-HIV Agents via QSAR and Molecular Docking. Med. Chem. 2019, 15, 328–340. [Google Scholar] [CrossRef]
- Toro-Huamanchumo, C.J.; Benites-Meza, J.K.; Mamani-Garcia, C.S.; Bustamante-Paytan, D.; Gracia-Ramos, A.E.; Diaz-Velez, C.; Barboza, J.J. Efficacy of Colchicine in the Treatment of COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2615. [Google Scholar] [CrossRef]
- Slobodnick, A.; Shah, B.; Krasnokutsky, S.; Pillinger, M.H. Update on colchicine, 2017. Rheumatology 2018, 57, i4–i11. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Li, J.; Wei, Y.L.; Tang, J.; Man, X.; Liu, F. Colchicine improves severe acute pancreatitis-induced acute lung injury by suppressing inflammation, apoptosis and oxidative stress in rats. Biomed. Pharmacother. 2022, 153, 113461. [Google Scholar] [CrossRef]
- Hammadi, S.H.; Hassan, M.A.; Allam, E.A.; Elsharkawy, A.M.; Shams, S.S. Effect of sacubitril/valsartan on cognitive impairment in colchicine-induced Alzheimer’s model in rats. Fundam. Clin. Pharmacol. 2023, 37, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Maciel, T.R.; de Oliveira Pacheco, C.; Ramos, P.F.; Ribeiro, A.C.F.; dos Santos, R.B.; Haas, S.E. Simultaneous determination of chloroquine and colchicine co-nanoencapsulated by HPLC-DAD. J. Appl. Pharm. Sci. 2023, 13, 106–112. [Google Scholar] [CrossRef]
- Dimitrijevic, D.; Fabian, E.; Nicol, B.; Funk-Weyer, D.; Landsiedel, R. Toward Realistic Dosimetry In Vitro: Determining Effective Concentrations of Test Substances in Cell Culture and Their Prediction by an In Silico Mass Balance Model. Chem. Res. Toxicol. 2022, 35, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Sajitha, T.P.; Siva, R.; Manjunatha, B.L.; Rajani, P.; Navdeep, G.; Kavita, D.; Ravikanth, G.; Shaanker, R.U. Sequestration of the plant secondary metabolite, colchicine, by the noctuid moth Polytela gloriosae (Fab.). Chemoecology 2019, 29, 135–142. [Google Scholar] [CrossRef]
- Izidoro, M.A.; Cecconi, A.; Panadero, M.I.; Mateo, J.; Godzien, J.; Vilchez, J.P.; Lopez-Gonzalvez, A.; Ruiz-Cabello, J.; Ibanez, B.; Barbas, C.; et al. Plasma Metabolic Signature of Atherosclerosis Progression and Colchicine Treatment in Rabbits. Sci. Rep. 2020, 10, 7072. [Google Scholar] [CrossRef]
- Cankaya, N.; Bulduk, I.; Colak, A.M. Extraction, development and validation of HPLC-UV method for rapid and sensitive determination of colchicine from Colchicum autumnale L. Bulbs. Saudi J. Biol. Sci. 2019, 26, 345–351. [Google Scholar] [CrossRef]
- Wang, J.; Ozsoz, M. Trace measurements of colchicine by adsorptive stripping voltammetry. Talanta 1990, 37, 783–787. [Google Scholar] [CrossRef]
- Bodoki, E.; Sandulescu, R.; Roman, L. Method validation in quantitative electrochemical analysis of colchicine using glassy carbon electrode. Cent. Eur. J. Chem. 2007, 5, 766–778. [Google Scholar] [CrossRef]
- Kasim, E.A. Voltammetric behavior of anti-infammatory alkaloid colchicine at a glassy carbon electrode and a hanging mercury electrode and its determination at ppb levels. Anal. Lett. 2002, 35, 1987–2004. [Google Scholar] [CrossRef]
- Bodoki, E.; Laschi, S.; Palchetti, I.; Sandulescu, R.; Mascini, M. Electrochemical behavior of colchicine using graphite-based screen-printed electrodes. Talanta 2008, 76, 288–294. [Google Scholar] [CrossRef]
- Moreira, D.A.R.; de Oliveira, F.M.; Pimentel, D.M.; Guedes, T.J.; Luz, R.C.S.; Damos, F.S.; Pereira, A.C.; da Silva, R.A.B.; dos Santos, W.T.P. Determination of Colchicine in Pharmaceutical Formulations and Urine by Multiple-Pulse Amperometric Detection in an FIA System Using Boron-Doped Diamond Electrode. J. Braz. Chem. Soc. 2018, 29, 1796–1802. [Google Scholar] [CrossRef]
- Stankovic, D.M.; Svorc, L.; Mariano, J.F.M.L.; Ortner, A.; Kalcher, K. Electrochemical Determination of Natural Drug Colchicine in Pharmaceuticals and Human Serum Sample and its Interaction with DNA. Electroanalysis 2017, 29, 2276–2281. [Google Scholar] [CrossRef]
- Bishop, E.; Hussein, W. Anodic voltammetry of colchicine. Analyst 1984, 109, 623–625. [Google Scholar] [CrossRef]
- Pang, T.T.; Zhang, X.Y.; Xue, Y.B. Supramolecular Detoxification of Colchicine through Electrochemical Recognition. Anal. Lett. 2017, 50, 1743–1753. [Google Scholar] [CrossRef]
- Pang, T.T.; Zhang, X.Y. Detection of Colchicine using p-Sulfonated Calix[4]arene Modified Electrode. Asian J. Chem. 2019, 31, 2663–2668. [Google Scholar]
- Bodoki, E.; Chira, R.; Zaharia, V.; Sandulescu, R. Mechanistic study of colchicine’s electrochemical oxidation. Electrochim. Acta 2015, 178, 624–630. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, S.M.; Jia, L.; Xu, Z.X.; Zeng, Y. Electrochemical properties of colchicine on the PoPD/SWNTs composite-modified glassy carbon electrode. Sens. Actuators B Chem. 2008, 134, 477–482. [Google Scholar] [CrossRef]
- Zhang, H. Electrochemistry and voltammetric determination of colchicine using an acetylene black-dihexadecyl hydrogen phosphate composite film modified glassy carbon electrode. Bioelectrochemistry 2006, 68, 197–201. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Shamspur, T. Sensitive detection of colchicine at a glassy carbon electrode modified with magnetic ionic liquid/CuO nanoparticles/carbon nanofibers in pharmaceutical and plasma samples. J. Iran. Chem. Soc. 2020, 17, 1753–1764. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, J.; Liu, J.; Li, K.; Li, Y.; Yang, L.; Ye, B. Sensitive determination of colchicine at carbon paste electrode doped with multiwall carbon nanotubes. Anal. Methods 2013, 5, 1830–1836. [Google Scholar] [CrossRef]
- Carrasco-Correa, E.J.; Simo-Alfonso, E.F.; Herrero-Martínez, J.M.; Miro, M. The Emerging Role of 3D Printing in the Fabrication of Detection Systems. Trends Anal. Chem. 2021, 136, 116177. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-printed biosensor arrays for medical diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.; Patel, B.A. 3D-Printed Electrochemical Sensors: A New Horizon for Measurement of Biomolecules. Curr. Opin. Electrochem. 2020, 20, 78–81. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.L.; dos Santos, P.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-Manufactured (3D-printed) Electrochemical Sensors: A Critical Review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cao, Q.; Venton, B.J. 3D printing for customized carbon electrodes. Curr. Opin. Electrochem. 2023, 38, 101228. [Google Scholar] [CrossRef]
- Omar, M.H.; Razak, K.A.; Wahab, M.N.A.; Hamzah, H.H. Recent progress of conductive 3D-printed electrodes based upon polymers/carbon nanomaterials using a fused deposition modelling (FDM) method as emerging electrochemical sensing devices. RSC Adv. 2021, 11, 16557. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Bonanni, A. How 3D printing can boost advances in analytical and bioanalytical chemistry. Microchim. Acta 2021, 188, 265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Song, J.; Liang, X.; Zhang, Z.; Men, D.; Wang, D.; Zhang, X.E. Chemical nature of electrochemical activation of carbon electrodes. Biosens. Bioelectron. 2019, 144, 111534. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Katic, V.; Loureiro, H.C.; dos Santos, M.F.; dos Santos, D.P.; Formiga, A.L.B.; Bonacin, J.A. Enhanced performance of 3D printed graphene electrodes after electrochemical pre-treatment: Role of exposed graphene sheets. Sens. Actuators B Chem. 2019, 281, 837–848. [Google Scholar] [CrossRef]
- Rocha, D.P.; Squissato, A.L.; da Silva, S.M.; Richter, E.M.; Munoz, R.A.A. Improved electrochemical detection of metals in biological samples using 3D-printed electrode: Chemical/electrochemical treatment exposes carbon-black conductive sites. Electrochim. Acta 2020, 335, 135688. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filopoulou, M.; Michail, G.; Katseli, V.; Economou, A.; Kokkinos, C. Electrochemical Determination of the Drug Colchicine in Pharmaceutical and Βiological Samples Using a 3D-Printed Device. Molecules 2023, 28, 5539. https://doi.org/10.3390/molecules28145539
Filopoulou M, Michail G, Katseli V, Economou A, Kokkinos C. Electrochemical Determination of the Drug Colchicine in Pharmaceutical and Βiological Samples Using a 3D-Printed Device. Molecules. 2023; 28(14):5539. https://doi.org/10.3390/molecules28145539
Chicago/Turabian StyleFilopoulou, Maria, Giorgios Michail, Vasiliki Katseli, Anastasios Economou, and Christos Kokkinos. 2023. "Electrochemical Determination of the Drug Colchicine in Pharmaceutical and Βiological Samples Using a 3D-Printed Device" Molecules 28, no. 14: 5539. https://doi.org/10.3390/molecules28145539
APA StyleFilopoulou, M., Michail, G., Katseli, V., Economou, A., & Kokkinos, C. (2023). Electrochemical Determination of the Drug Colchicine in Pharmaceutical and Βiological Samples Using a 3D-Printed Device. Molecules, 28(14), 5539. https://doi.org/10.3390/molecules28145539








