Abstract
The process of Fischer–Tropsch synthesis is commonly described as a series of reactions in which CO and H2 are dissociated and adsorbed on the metals and then rearranged to produce hydrocarbons and H2O. However, CO dissociation adsorption is regarded as the initial stage of Fischer–Tropsch synthesis and an essential factor in the control of catalytic activity. Several pathways have been proposed to activate CO, namely direct CO dissociation, activation hydrogenation, and activation by insertion into growing chains. In addition, H2O is considered an important by-product of Fischer–Tropsch synthesis reactions and has been shown to play a key role in regulating the distribution of Fischer–Tropsch synthesis products. The presence of H2O may influence the reaction rate, the product distribution, and the deactivation rate. Focus on H2O molecules and H2O-derivatives (H*, OH* and O*) can assist CO activation hydrogenation on Fe- and Co-based catalysts. In this work, the intermediates (C*, O*, HCO*, COH*, COH*, CH*, etc.) and reaction pathways were analyzed, and the H2O and H2O derivatives (H*, OH* and O*) on Fe- and Co-based catalysts and their role in the Fischer–Tropsch synthesis reaction process were reviewed.
1. Introduction
Fischer–Tropsch synthesis (FTS) is a catalytic chemical reaction in which syngas, a mixture of carbon monoxide (CO) and hydrogen (H2) derived from coal, biomass, or natural gas, is converted into mainly hydrocarbons (light olefins, liquid fuels, linear α-olefins, etc.), as well as oxygenated compounds (alcohols, aldehydes, ketones, and acids) [1,2,3,4]. The synthesis of methanation, paraffins, olefins, and oxygenated compounds can be represented by Equations (1)–(4). Compared with other metals, Fe- and Co-based catalysts are suitable and often used for FTS due to their high activity and target products selectivity, low water gas shift (WGS) activity, and relatively low cost [5,6,7]. At the same time, Fe- and Co-based catalysts have differences such as the product distribution being very dependent on the type of catalysts (Fe, Co) used and on the reaction conditions. For instance, Fe has a high water gas shift activity and is used when the syngas is produced from coal, i.e., when the water gas shift reaction is desirable due to low H2/CO ratios in this syngas; Co is the preferred catalyst for the FTS of long chain paraffins from natural gas due to their high activity and selectivity, low water gas shift activity, and comparatively low price. To explain the production and distributions of different products, many researchers have worked to identify and clarify the mechanism underlying the FTS response. However, the details of some mechanisms remain unclear and speculative [8,9].
Methanation: CO + 3H2 → CH4 + H2O
Olefins: 2nH2 + nCO → CnH2n+ nH2O
Paraffins: (2n+1)H2 + nCO → CnH2n+2 + nH2O
Oxygenates: 2nH2 + nCO → CnH2n+2O + (n − 1)H2O
In a series of recent papers on FTS deactivation [10], selectivity towards higher hydrocarbons [11], and the influence of the catalyst supports [12], the H2O was found to be not a silent spectator but to play a significant role in all parts of FTS [1]. H2O can exist in all three physical states of matter, but in chemistry, on the surface of catalysts, the states of H2O are diverse and dynamic, depending on the surface types and the thermodynamic conditions. H2O is not only prevalent in most chemical reactions as solvents, impurities, reactants, intermediates, or products, but it also plays a special role in its promotion or inhibition as shown in Figure 1 [13,14,15,16,17,18,19,20,21]. The effect of H2O on catalyst performance was initially described by Minderhoud et al. [22] and Kim et al. [23]. The adsorption of H2O can create favorable conditions for a number of surface reactions. Several studies have been conducted to determine the mechanism of the H2O action and some viable theories have been proposed. For example, H2O can inhibit the secondary hydrogenation of primary olefins [24,25], oxidize exposed CO molecules during H2O treatment [26], alter the active carbon concentration [27], facilitate the transport of syngas and hydrocarbons within the particles [24], and the addition of reactive H2O and produced H2O during synthesis leads to higher selectivity for higher hydrocarbons [11,12,28,29,30,31,32]. Studies have demonstrated that H2O is always present in various forms during the FTS reaction, altering the activity and selectivity of the FTS in various ways [33,34,35,36].
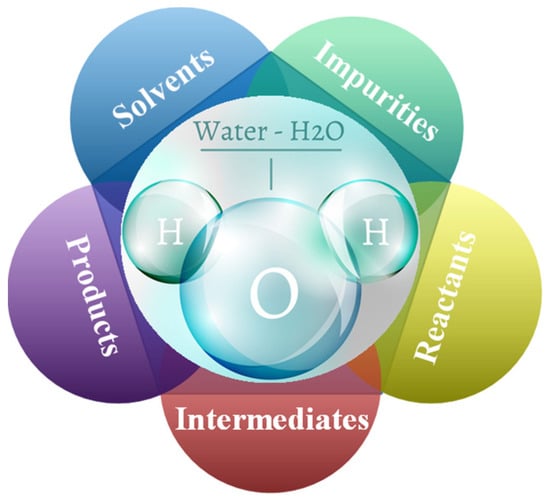
Figure 1.
The role of water in the Fischer–Tropsch synthesis reaction.
It is well known that one of the undesirable byproducts of the FTS process is H2O, and several experimental studies have shown that both H2O generated during the reaction and H2O introduced from outside can reduce or increase the reaction activity [8,13,26,37]. For example, it was observed that H2O reversibly enhances the activity of some catalysts but not all. Reversible selectivity changes induced by H2O include decreased methane selectivity, higher product olefinicity at a given carbon number, and an increased fraction of higher molecular weight products [38]. The role of H2O in FTS reactivity, reaction pathway, and product distribution has been reported in the literature, but the effects of H2O and H2O derivation (H*, OH* and O*) on CO activation hydrogenation on Fe- and Co-based catalysts have hardly been reported in detail. Here, we only study the effect of H2O on CO dissociation activation on Fe- and Co-based catalysts. Elucidating the mechanism of CO activation on the surface of Fe- and Co-based catalysts is essential for understanding the initial process of FTS reaction. In this study, a comprehensive review of research activities on H2O derivatives mediating CO activation hydrogenation in FTS is presented. A detailed summary and analysis of the H2O molecule, H-assisted, OH-assisted, and O-assisted CO activation are presented, which helps to elucidate the effect of H2O. Finally, the field is presented for conclusions and outlook.
2. H2O Derivatives Mediate CO Activation
2.1. CO Dissociation Activation
Here, we present a short review on the mechanism of CO dissociation, which is regarded as the initial step in the FTS. As a very important and useful basic chemical, CO has found a wide range of applications in the energy society as well as in the production of value-added bulk and fine chemicals. To provide further insight into these key processes, it is essential to study the mechanisms of CO adsorption and dissociation on the surfaces of heterogeneous catalysts. More specifically, the adsorption and desorption of CO on Fe- and Co-based catalyst surfaces are very essential steps related to catalytic activity.
In order to fundamentally understand the process of FTS reaction, it is essential to investigate the mechanism of CO activation hydrogenation on the surface of Fe- and Co-based catalysts, and several pathways for CO activation have been proposed, including direct CO dissociation, hydrogenation, and insertion into the growth chain for activation [33,39,40,41,42,43,44,45]. It is generally accepted that CO dissociation is the rate-determining step [42,46]. Up to now, the general consensus derived from experimental and theoretical studies about the elementary steps involved in the FTS can be categorized into (a) initiation, which involves the CO dissociation and CHx formation; (b) propagation via the C-C coupling reactions; and finally, (c) the termination and desorption of the hydrocarbons. This process involves contrary reactions of bond breaking and forming on the metal surfaces [39]. Although the mentioned mechanisms are detailed, the exact paths that explain the formation of transition species between reagents and products are not entirely understood. After reviewing the literature, we found out that for Fe- and Co-based catalysts surfaces, the direct dissociation and the H-assisted CO dissociation are the main proposed mechanisms [45]. Next, we will focus on the CO-activated dissociation part of the direct dissociation and H-assisted mechanisms.
In general, the FTS can be described as a polymerization reaction, where three essential steps are identified: the first step is the initiation, which involves the adsorption of CO and H2 on the metal surface; the second step is the polymerization and chain formation; and the third step is products desorption [39]. As shown in Figure 2, direct dissociation pathways and H-assisted pathways have been proven to play an important role in kinetic-related CO dissociation steps on Fe- and Co-based catalysts. In the direct dissociation pathway, CO directly dissociate on the Fe surface to C* and O* species for subsequent reactions. In the H-assisted pathway, CO molecules are not directly dissociated on the surface of Fe- and Co-based catalysts, and CO forms COH* or HCO* intermediates with H* species. Intermediate species formed in these pathways can be continuous reactions, such as chemically adsorbing hydrogen (H*) on the surface of Fe- and Co-based surfaces to interact with CO* hydrogenated to form HxCO species, or directly forming OH* precursors, resulting in the preferential repulsion of H2O by O atoms in CO. On the Fe-based surface, CO* interacts with O* to form CO2. These assisted pathways represent the exclusive CO activation routes on Co surfaces and the predominant one on Fe catalysts at relevant FTS conditions. H-assisted pathways occur concurrently with unassisted CO dissociation on Fe-based catalysts with oxygen rejection as CO2 [45]. Both pathways produce intermediates with significant chain growth under the desired reaction conditions.
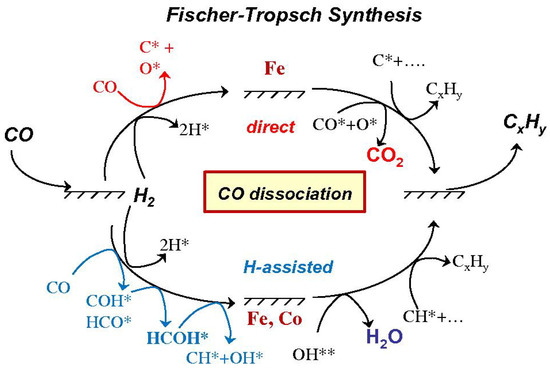
Figure 2.
CO activation reacts with chemisorbed hydrogen before and after C-O bond cleavage on Fe and Co-based catalysts. Reproduced with permission from ref. [45]. Copyright (2010) Elsevier.
2.2. H2O Molecules-Assisted CO Activation
In recent years, there has been a growing interest in understanding reactions at the molecular level to improve energy efficiency, product selectivity and renewability of chemical processes, thus making the description of each reaction step a key aspect for improving such processes [4,8,10]. Adsorption and reaction of H2O on solid surfaces are fundamental ingredients for numerous chemical reactions in many practical applications such as heterogeneous catalysis, electrochemistry, and corrosion, to which various analytical techniques have been applied [36]. For most cases of heterogeneous catalysis, H2O is found to have an important role as a promoter or inhibitor, which is typically unrecognized or underestimated but is critical for understanding the essence of catalytic processes [21]. Particularly, FTS is often viewed as a sequence of reactions in which CO and H2 are dissociatively adsorbed and subsequently rearranged to form hydrocarbons and H2O [47]. The H2O molecules are thought to be a byproduct of the FTS reaction, but the presence of H2O has been shown to play an important role in regulating the distribution of products in FTS. We will analyze how H2O molecules affect CO dissociation activation. The mechanism responsible for the positive effects of H2O has been reported to facilitate the mass transfer of syngas and hydrocarbons to influence the kinetics of the reaction [25,48,49,50,51,52,53]. H2O molecules are adsorbed on contact with the surface of metallic Fe- and Co-based catalysts, and they can dissociate into OH* and H* species, as well as OH* radical into O* and H* species as intermediates in a subsequent reaction [8,54,55]. The role of H2O in FTS, such as the degree of adsorption and dissociation, chain initiation, chain growth, methanation, and olefins hydrogenation, are all possible effects of H2O molecules on the catalyst that may affect syngas conversion, hydrocarbon selectivity, FTS product distribution, and catalyst stability [8,39,54,56,57,58]. When H2O molecules dissociate and adsorb, they can polymerize and hydrogenate with CH2, leading to alkane chain growth [7,59]. H2O molecules inhibit the secondary hydrogenation of olefin products, which may be the result of competitive adsorption of H2O molecules [24,25].
This work only discusses the effect of H2O on the CO-activated hydrogenation process. The present results show that H2O has a dramatic effect on the dynamic equilibrium around C* species: the H2O increases the coverage of C* species by preferentially increasing the rate of CO activation, which also slightly affects the production of branched chain hydrocarbons and internal olefin isomers [60]. As shown in Figure 3, H2O may influence formate route: (1) as a H-source (H2O* reacts with HCO* to form *HCOH* and OH*); (2) as a “solvent” to stabilize the FTS for H-addition to the O atom of HCO* (through H-bonding to the primary O-H bonds); or (3) as a H-closing agent (as a H2O molecule or extended phase) for H* transfer to the O-atom in HCO* [21,61]. The HCO* intermediate corresponds to a lower barrier than the direct CO dissociation, since the formation of HCO* species is an endothermic process. As shown in Figure 4, the increase in CO activity in the presence of H2O may be explained by a direct interaction between the weak hydrogen bond of H2O and the oxygen of CO, reducing the barrier to dissociation of the CO barrier [27] and enhancing the surface coverage of polymeric intermediates [62]. Both direct adsorbate–adsorbate (e.g., weak hydrogen bonding of H2O to the oxygen of CO) and metal-mediated mechanisms (e.g., increased back-bonding to the π* orbital of CO) are explained by the interaction between CO and H2O [34,36,63,64]. Kim [23] found higher CO conversion and higher C5+ and lower methane selectivities when a small amount of external H2O was added during FTS on Co-based catalysts. Therefore, H2O molecules not only affect CO activation and dissociation in FTS reactions but also affect the reaction path and product distribution. This article reviews the intrinsic mechanism of the role of H2O in the surface reaction of Fe- and Co-based catalysts, so that researchers can better understand the H2O effect.
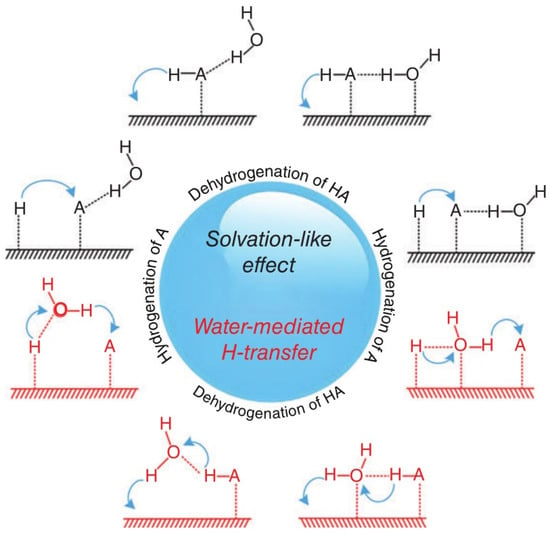
Figure 3.
Schematic of the promotional role of water molecule: solvation-like effect (in black) and water-mediated H-transfer (in red). A refers to reactants of hydrogenation reactions, and H-A refers to reactants of dehydrogenation reactions. Reproduced with permission from ref. [21]. Copyright (2016) Wires.
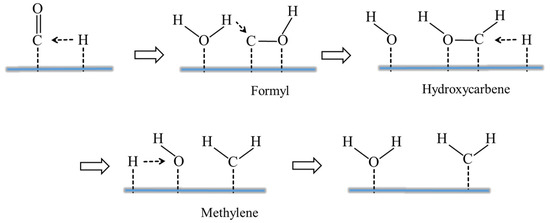
Figure 4.
H2O- and H-assisted activation of CO to methylene. Reproduced with permission from ref. [65]. Copyright (2018) Springer Link.
2.3. H-Assisted CO Activation
There are two parallel CO activation pathways on the surface of Fe and Co-based catalysts, which are unassisted activation and H-assisted activation [39,40,41,42,43,44,66]. Reaction paths and products vary under different reaction conditions. Here, we have reviewed experimental and theoretical evidence that the H-assisted pathway plays an important role in the kinetically relevant CO activation steps on both Fe- and Co-based catalysts under the required reaction conditions [67,68]. It is necessary to understand all possible competing and successive surface reactions starting from the H-assisted CO dissociation activation. In the FTS reaction, one H is derived from H2 in the syngas, and the other H from H2O produced [65]. This review is an attempt to understand the role of H-assisted CO activation and the initial stages of the formation of essential surface species such as CH*, CH2*, and OH* intermediates in FTS. Therefore, we summarize some key points that illustrate the importance of the H-assisted pathways for significant chain growth in the relevant CO activation steps over Fe- and Co-based catalysts under reaction conditions [45]. The results indicate that co-adsorption H* has a stabilizing effect in the system, while the increase in CO adsorption energy favors its reorganization.
It has been previously reported that the preferential adsorption site for CO activation reaction occurs at hollow sites on the surface of Fe- and Co-based catalysts, forming CO* species [43,44] (step 1, Figure 5). The dissociative adsorption of H2 molecules requires the formation of H* species at two hollow adsorption sites on the Fe-based surface [69] (step 2, Figure 5). In the unassisted CO dissociation pathway, CO* is directly dissociated to form C* and O* species (step 3, Figure 5), and both C* and O* species are hydrogenated to form the intermediate products CH2 (carbene) and H2O, i.e., the carbide mechanism (step 4, Figure 5) [45]. The O* species formed in step 3 are removed via reaction with CO* as CO2 molecule (step 5, Figure 5). For H-assisted CO activation, Storch et al. [4,43] proposed a mechanism by which a hydrogen atom is added directly to the adsorbed CO* species, leading to the formation of formyl (HCO*) species (step 6, Figure 5). HCO* species formation or dissociation into CH* and O* was performed with the presence of an H* and a coadsorbed CO or an HCO* species and an empty neighbor site, respectively. Subsequently, the HCO* species undergoes hydrogenation on the O-atom to form hydroxycarbene (HCOH*) intermediates (step 7, Figure 5). The HCOH* intermediate is unstable and subsequently dissociates to form the chain growth monomer CH* and initiator OH* [39] (step 8, Figure 5). The OH* species formed in step 8 continue to hydrogenate and are scavenged as H2O molecules (step 9, Figure 5). This indicates that step 9 is the primary mechanism for the removal of OH* in the FTS reaction. Alternatively, OH* may react with CO* to form carboxyl (COOH*) species, which subsequently decompose to produce CO2 and H* [68]. DFT calculation indicates that the formation of COOH* has a higher activation energy barrier than the formation of H2O [68]. The unassisted CO activation and H-assisted activation pathways produce CH* species, and both pathways form CH* monomers that are successively hydrogenated to form CH2 and CH3 for alkyl chain growth (step 10 and 11, Figure 5). In the unassisted CO activation pathway, CO removes the chemisorbed oxygen (O*) into CO2 molecules, while the H-assisted CO activation pathway forms only H2O molecules. The results indicate that the presence of co-adsorbed hydrogen has a stabilizing effect in the system, increasing the adsorption energy of CO and facilitating its recombination. In these cases, the H-assisted route provides energetically more favorable alternatives [45,59]. Indeed, the dissociation of H2O molecules has the potential to various chemicals with other species on the surfaces.
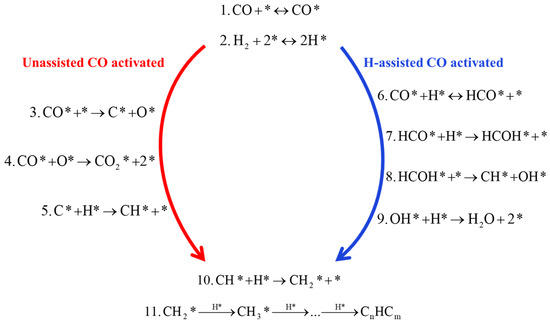
Figure 5.
The H-assisted CO-activated hydrogenation in the FTS reaction elementary steps. Reproduced with permission from ref. [45]. Copyright (2010) Elsevier.
2.4. OH-Assisted CO Activation
On the surface of metal oxide Fe- and Co-based catalysts, H2O could adsorb at both metal cations and oxygen anions or dissociate into two hydroxyl groups, one from H2O and one from surface oxygen plus dissociated H [28,33]. These OH groups could substantially influence the wetting behavior, thermal stability, and catalytic activity of metal oxides, and so forth, some of which arise from the hydrogen bonding between OH groups and molecules and OH-induced electronic structure changes of catalyst surfaces [33]. Therefore, investigation of the interaction of adsorbed H2O and/or OH groups with CO on metal surfaces can help in understanding water gas shift reactions [70]. However, the OH-assisted pathway should start with the co-adsorption of CO*, OH*, and H*. OH groups, as an active hydrogen species derived from H2 or H2O derivatives, have been reported to play an important role in FTS activity and selectivity as well as in CO2 hydrogenation [71]. The role of OH groups as surface hydride species may be universal and involved in several oxygenated compound conversion reactions [33].
The presence of an OH group contributes to improve the C-O bond breaking ability compared to the co-adsorption with H* species, which leads to an increase in CHX [33,61]. Therefore, in addition to unassisted CO activation and H-assisted CO activation pathway, Gunasooriya et al. [33] reported an OH-assisted CO pathway. As shown in Figure 6, CO* intermediate can be directly dissociated into C* and O* [33] (i.e., the carbide mechanism, step 1, Figure 6). Meanwhile, both hydrogenated CO* and RCO* preferentially hydrogenate at the C* species due to the geometry of the OH groups, forming the intermediate HCO* species [33] (step 2, Figure 6). The intermediates of HCO* and formaldehyde (H2CO*) intermediates are the co-adsorbed CO* and H* formation routes demonstrated by Mitchell et al. (step 4, Figure 6) [72]. The reaction forming CH* and O* from the HCO* intermediate dissociate directly (step 5, Figure 6), and CH* species continue to hydrogenate to form CH2*. HCO* species are hydrogenated to hydroxylmethylene (HCOH*), a reaction mechanism recently proposed by Gunasooriya et al. [33,73]. HCOH* species form CH* and OH* by double-assisted dissociation (step 7, Figure 6). Another alternative pathway is through hydrogenation of the oxygen end of CO* to form hydroxyl-carbene (COH*) as an intermediate (step 3, Figure 6), further dissociating into OH* and C* species [74] (step 9, Figure 6). This pathway avoids the formation of H2CO. CO* may be hydrogenated by the C* or O* species to form HCO* or COH* species as reaction intermediates, respectively. With respect to the H-assisted routes, the formations of HCO* and COH* are an endothermic process, and the formation of COH* is kinetically more favorable than the formation of CHO* species and the direct dissociation CO. In both cases, the formation of intermediates (CHO* and COH*) has a subsequent dissociation step leading to the formation of CH* or OH* species. In the FTS process, the OH* group on the surface is the effective hydrogenated substance.
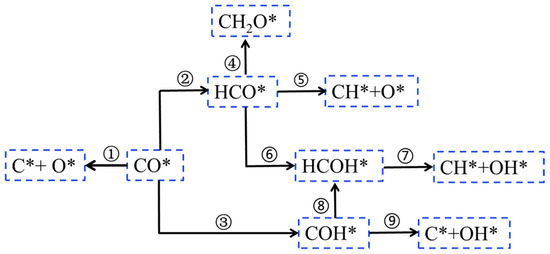
Figure 6.
The elementary steps of OH-assisted CO-activated hydrogenation in the FTS reaction. Reproduced with permission from ref. [45]. Copyright (2010) Elsevier.
2.5. O-Assisted CO Activation
H2O dissociates more readily on the surface of Fe- and Co-based catalysts with the O-assisted, and removing the oxygen atoms in an adsorbed state would be beneficial for refreshing the surface and preventing the surface from oxidating. Moreover, the breaking of the O-H bond is facilitated by O atoms and H2O molecules. In the CO activation reaction, it has recently been proposed that H2O plays a key role due to its ability to supply hydrogen to the O atoms in CO [65]. H2O and CO2 act as the main deoxygenation pathways in the FTS reaction, leading to the belief that most oxygenated products (CO activation) leave the catalyst surface in the form of H2O [75,76,77]. The use of H2O and CO2 as supports of O atoms in the unassisted CO activation and H-assisted CO activation steps is worthy of discussion. H-assisted CO dissociation removes O atoms as H2O, while direct dissociation forms chemisorbed oxygen atoms that desorb as CO2. Direct CO dissociation routes are minor contributors to monomer formation on Fe-based and may become favored at high temperatures on alkali-promoted catalysts, but not on Co-basted catalysts, which remove oxygen predominantly as H2O because of the preponderance of H-assisted CO dissociation routes [45]. Therefore, it is necessary to explore the potential mechanism for the removal of surface O* on CO-activated hydrogenation. Here, four kinds of O removal pathways under an FTS environment were roughly investigated. In the removing routes through H2O molecules, O-H bond formation was followed by two viable pathways: one is that the OH hydrogenates sequentially, the other is that the OH interacts with another OH [49]. The removal of CO2 form could occur in a direct way in which surface O atoms react with adsorbed CO, or in an indirect way with COOH* as an intermediate followed by dehydrogenation [33,49].
H2O molecules can dissociate directly into OH* and H* species (Equation (5)). OH* species react rapidly and disproportionately to produce H2O molecules (Equation (6)), which immediately dissociate into OH* and H* species [18,19]. Provided that Equations (5) and (6) react fast enough, O* and OH* species are considered to be quasi-equilibrium under the FTS reactions. It is worth noting that when O-H forms a bond, the reaction between an OH* intermediate and another OH* is superior to that with H, which is due to the latter needing higher barrier and endothermic energy. The OH* disproportionation reactions are kinetically unfavorable and thermodynamically more favorable. Therefore, it is the OH* rather than the O* species that is pre-existing on the catalyst surface, which is readily hydrogenated to form OH* (Equation (7)). Meanwhile, the conversion of O* to OH* is faster than the reaction of O* with CO* to form CO2 (Equation (8)) [34]. As in the pathways of H2O formation, a hanging chemisorbed H* and O* are produced by rapid reaction on the catalyst surface. One is that OH* species disproportionation react to form H2O molecules and O* species [17]; the other is that the OH* hydrogenates sequentially. This suggests that H2O is one of the main pathways for the removal of O* species. In addition, CO2 is also one of the important pathways for the removal of oxygen. The removal of surface oxygen in the form of CO2 was explored, in which the direct reaction of surface O* with CO* and indirect route through COOH* intermediate was included (COOH* dehydrogenation) [33]. In addition, COOH* and HCOOH* intermediates are formed through OH* and CO* species coupling and CO2 hydrogenation in two paths, in which the barrier and reaction energy are overcome differently (Equations (9) and (10)). As an alternative way to form CO2, the reaction between CO* and O* is the main pathway for the formation of CO2. Herein, we explored the removal of pre-adsorbed oxygen on Fe- and Co-based catalyst surfaces, and several pathways were investigated, including both direct and indirect routes for generating H2O and CO2. The removal mechanism shows diversity over different catalysts. The removal of O atoms is different on the surface of Fe- and Co-based catalysts, where O atoms are removed to form H2O and/or CO2 on Fe-based catalysts, while Co-based catalysts only generate H2O as O atom removal products [78]. Finally, the CO*-derived intermediates can also form CO2 via Boudouard reaction (CO* + CO* → C* + CO2*). These reaction pathways facilitate the exchange of chemisorbed O* species between CO2 and H2O. However, in actual reactions, the surface species are mobile and interact with each other, stabilizing reaction intermediates to different extents and thus likely influencing the preferred CO activation mechanisms [36,43]. Our work provides knowledge about the mechanisms of O removal and CO2 and H2O formation: the removal of adsorbed oxygen on the surface may be necessary for refreshing the catalyst surfaces and protecting the catalysts from further oxidation.
H2O ↔ OH* + H*
2OH* ↔ H2O + O*
O* + H* ↔ OH* + *
CO* + O* ↔ CO2* + O*
CO* + OH* ↔ COOH* + *
CO2 + 2H* ↔ HCOOH*
3. Concluding Remarks and Future Perspectives
The mechanism of the effect of H2O on the FTS performance of Fe- and Co-based catalyst surfaces remains unclear and requires further investigation. It is worth noting that in-depth understanding and accurate control of the intermediates formed in the reaction and the reaction path formation are required to significantly improve catalytic performance. In view of the current problems, this paper uses the intrinsic mechanism of the H2O molecule and derivatives as an accelerator on the surface of Fe- and Co-based catalysts to stimulate a better understanding of various H2O effects in various reaction systems. In this paper, the effects of H2O molecules and H2O derivatives (H*, OH* and O* species) on CO activation reactions and reaction intermediates are reviewed. We can conclude form this review article as follows:
- The positive mechanism of action of H2O facilitates partial transport of syngas and hydrocarbons and influences the kinetics of the reaction. H2O increased the coverage C* species by preferentially increasing the rate of CO activation.
- H-assisted CO dissociation activation is easier on the surface of Fe- and Co-based catalysts than unassisted CO. H-assisted pathways are more advantageous for kinetic formation of COH* species than direct dissociation of CO. The co-adsorption H* has a stabilizing effect in the system, and increasing the adsorption energy of CO helps its recombination.
- OH groups can induce changes in the electronic structure of catalyst surfaces. The presence of OH group contributes to improve the C-O bond breaking ability and can also be used as a surface hydrogenated species of active hydrogen species and participate in the conversion reaction of oxygen-containing compounds.
- The O atoms promote the breaking of O-H bond. H2O and CO2 molecules were discussed as carriers of O atoms. The removal pathways of four oxygen species in FTS were analyzed.
Since H2O may be present in FTS as H2O molecules, it may also dissociate into OH/OH− and H/H+ on the catalyst surface, affecting the secondary reaction in FTS as well as the activity and selectivity of the product. We believe that studies on the H2O-derivatives need to help understand the mechanism of the effect of H2O on the activity and selectivity of FTS. This paper reviews CO activation on the surface of Fe- and Co-based catalysts from four aspects: H2O-assisted, H-assisted, OH-assisted, and O-assisted. Our group reported a series of work such as hydrophilic modification of catalyst surfaces, functional group modification, and preparation of catalysts with special morphology and achieved good experimental results [79,80,81,82,83]. Based on the experimental work of our group, combined with theoretical calculations, the influence of H2O-derivative (H2O molecules, H*, OH*, O*) intermediates on the formation and product distribution of FTS reaction intermediates was explored. By using different methods to prepare catalysts, surface modification, pre-treatment conditions, and reaction conditions, the formation and quantity of H2O molecule intermediates, H*, OH*, and O* species on the surface of Fe- and Co-based catalysts will be controlled, and the product distribution will be regulated. The current work explores the addition of reagents containing hydrophobic groups to the pretreatment solution for the preparation of Fe-based catalysts. The material preparation has been completed, and application and characterization data are being compiled. Finally, more research work has to be conducted to show clearly how the structure and surface properties of the supports could play a role in the way H2O affects the FTS performance of Fe- and Co-based catalysts.
Funding
This work was financially supported in part by the National Natural Science Foundation of China (No. 21968025), Innovation Leadership Program in Sciences and Technologies of Ningxia (2020GKLRLX09), Fourth Batch of Ningxia Youth Talents Supporting Program (TJGC2019022), and the Central Guidance on Local Science and Technology Development Fund of Ningxia (2023FRD05030).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was financially supported in part by the National Natural Science Foundation of China (No. 21968025), Innovation Leadership Program in Sciences and Technologies of Ningxia (2020GKLRLX09), Fourth Batch of Ningxia Youth Talents Supporting Program (TJGC2019022), and the Central Guidance on Local Science and Technology Development Fund of Ningxia (2023FRD05030).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Z.; Mai, K.; Kumar, N.; Elder, T.; Groom, L.H.; Spivey, J.J. Effect of steam during Fischer–Tropsch synthesis using biomass-derived syngas. Catal. Lett. 2017, 147, 62–70. [Google Scholar] [CrossRef]
- Eschemann, T.O.; De Jong, K.P. Deactivation behavior of Co/TiO2 catalysts during Fischer–Tropsch synthesis. ACS Catal. 2015, 5, 3181–3188. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; de Jong, K.P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Schulz, H. Short history and present trends of Fischer−Tropsch synthesis. Appl. Catal. A-Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Mubenesha, S. The use of iron are as a catalyst in Fischer–Tropsch synthesis-A review. Crystals 2022, 12, 1349. [Google Scholar] [CrossRef]
- De Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.H. Fischer–Tropsch synthesis: Comparison of performances of iron and cobalt catalysts. Ind. Eng. Chem. Res. 2007, 46, 8938–8945. [Google Scholar] [CrossRef]
- Mousavi, S.; Zamaniyan, A.; Irani, M.; Rashidzadeh, M. Generalized kinetic model for iron and cobalt based Fischer–Tropsch synthesis catalysts: Review and model evaluation. Appl. Catal. A-Gen. 2015, 506, 57–66. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Xie, J.; Wang, Y.; Weinberg, W.H. Carbon monoxide hydrogenation on the Ru(001) surface at low temperature using gas-phase atomic hydrogen. J. Electron Spectrosc. Relat. Phenom. 1993, 64–65, 427–433. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Deactivation and regeneration of commercial type Fischer–Tropsch Co-catalysts-A mini-review. Catalysts 2015, 5, 478–499. [Google Scholar] [CrossRef]
- Rytter, E.; Tsakoumis, N.E.; Holmen, A. On the selectivity to higher hydrocarbons in Co-based Fischer–Tropsch synthesis. Catal. Today 2016, 261, 3–16. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. On the support in cobalt Fischer–Tropsch synthesis−emphasis on alumina and aluminates. Catal. Today 2016, 275, 11–19. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Ye, R.; Zhang, R.; Feng, G.; Zhang, J. Theoretical investigation of high coverage water adsorption on Co and Ni doped γ-Al2O3 surface. J. Mater. Sci. 2022, 57, 16710–16724. [Google Scholar] [CrossRef]
- Dry, M.E. Fischer–Tropsch synthesis over iron catalysts. Catal. Lett. 1990, 7, 241–251. [Google Scholar] [CrossRef]
- Dry, M.E. Practical and theoretical aspects of the catalytic FTS. Appl. Catal. A-Gen. 1996, 138, 319–344. [Google Scholar] [CrossRef]
- Michaelides, A.; Hu, P. A density functional theory study of hydroxyl and the intermediate in the water formation reaction on Pt. J. Chem. Phys. 2001, 114, 513. [Google Scholar] [CrossRef]
- Michaelides, A.; Hu, P. Catalytic water formation on platinum: A first-principles study. J. Am. Chem. Soc. 2001, 123, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Clay, C.; Hodgson, A. Water and mixed OH/water adsorption at close packed metal surfaces. Curr. Opin. Solid State Mater. Sci. 2005, 9, 11–18. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; DS Cordeiro, M.N.; Gomes, J.R.B. Density functional theory study of the water dissociation on platinum surfaces: General trends. J. Phys. Chem. A 2014, 118, 5832–5840. [Google Scholar] [CrossRef]
- Karlberg, G.S.; Olsson, F.E.; Persson, M.; Wahnström, G. Energetics, vibrational spectrum, and scanning tunneling microscopy images for the intermediate in water production reaction on Pt(111) from density functional calculations. J. Chem. Phys. 2003, 119, 4865–4872. [Google Scholar] [CrossRef]
- Chang, C.R.; Huang, Z.Q.; Li, J. The promotional role of water in heterogeneous catalysis: Mechanism insights from computational modeling. Wires. Comput. Mol. Sci. 2016, 6, 679–693. [Google Scholar] [CrossRef]
- Minderhoud, J.K.; Post, M.F.M.; Sie, S.T.; Sudholter, E.J.R. Process for the Preparation of Hydrocarbons from a Mixture of CO and H. sub. 2. U.S. Patent 4,628,133, 9 December 1986. [Google Scholar]
- Kim, C.J. Process for catalytic hydrocarbon synthesis from CO and H2 over metallic cobalt. Eur. Pat. Appl. 1990, 218, A1. [Google Scholar]
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer–Tropsch synthesis catalysts. Appl. Catal. A-Gen. 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Rothaemel, M.; Hanssen, K.F.; Blekkan, E.A.; Schanke, D.; Holmen, A. The effect of water on cobalt Fischer–Tropsch catalysts studied by steady-state isotopic transient kinetic analysis (ssitka). Catal. Today 1997, 38, 79–84. [Google Scholar] [CrossRef]
- Banerjee, A.; Van Bavel, A.P.; Kuipers, H.P.; Saeys, M. CO activation on realistic cobalt surfaces: Kinetic role of hydrogen. ACS Catal. 2017, 7, 5289–5293. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Perspectives on the effect of water in cobalt Fischer–Tropsch synthesis. ACS Catal. 2017, 7, 5321–5328. [Google Scholar] [CrossRef]
- Borg, Ø.; Storsæter, S.; Eri, S.; Wigum, H.; Rytter, E.; Holmen, A. The effect of water on the activity and selectivity for γ-alumina supported cobalt Fischer–Tropsch catalysts with different pore sizes. Catal. Lett. 2006, 107, 95–102. [Google Scholar] [CrossRef]
- Lögdberg, S.; Boutonnet, M.; Walmsley, J.C.; Järås, S.; Holmen, A.; Blekkan, E.A. Effect of water on the space-time yield of different supported cobalt catalysts during Fischer–Tropsch synthesis. Appl. Catal. A-Gen. 2011, 393, 109–121. [Google Scholar] [CrossRef]
- Enger, B.C.; Fossan, Å.; Borg, Ø.; Rytter, E.; Holmen, A. Modified alumina as catalyst support for cobalt in the Fischer–Tropsch synthesis. J. Catal. 2011, 284, 9–22. [Google Scholar] [CrossRef]
- Storsæter, S.; Borg, Ø.; Blekkan, E.A.; Tøtdal, B.; Holmen, A. Fischer–Tropsch synthesis over re-promoted Co supported on Al2O3, SiO2 and TiO2: Effect of water. Catal. Today 2005, 100, 343–347. [Google Scholar] [CrossRef]
- Gunasooriya, G.K.K.; Van Bavel, A.P.; Kuipers, H.P.; Saeys, M. Key role of surface hydroxyl groups in C-O activation during Fischer–Tropsch synthesis. ACS Catal. 2016, 6, 3660–3664. [Google Scholar] [CrossRef]
- Visconti, C.G.; Tronconi, E.; Lietti, L.; Forzatti, P.; Rossini, S.; Zennaro, R. Detailed kinetics of the Fischer–Tropsch synthesis on cobalt catalysts based on H-assisted CO activation. Top. Catal. 2011, 54, 786–800. [Google Scholar] [CrossRef]
- Jung, S.C.; Kang, M.H. Adsorption of a water molecule on Fe(100): Density-functional calculations. Phys. Rev. B 2010, 81, 115460. [Google Scholar] [CrossRef]
- Yuzawa, T.; Higashi, T.; Kubota, J.; Kondo, J.N.; Domen, K.; Hirose, C. CO coadsorption-induced recombination of surface hydroxyls to water on Ni(110) surface by iras and tpd. Surf. Sci. 1995, 325, 223–229. [Google Scholar] [CrossRef]
- Li, J.; Zhan, X.; Zhang, Y.; Jacobs, G.; Das, T.; Davis, B.H. Fischer–Tropsch synthesis: Effect of water on the deactivation of Pt promoted Co/Al2O3 catalysts. Appl. Catal. A-Gen. 2002, 228, 203–212. [Google Scholar] [CrossRef]
- Hilmen, A.M.; Lindvåg, O.A.; Bergene, E.; Schanke, D.; Eri, S.; Holmen, A. Selectivity and activity changes upon water addition during Fischer-Tropsch synthesis. Stud. Surf. Sci. Catal. 2001, 136, 295–300. [Google Scholar]
- Shetty, S.; van Santen, R.A. CO dissociation on Ru and Co surfaces: The initial step in the Fischer–Tropsch synthesis. Catal. Today 2011, 171, 168–173. [Google Scholar] [CrossRef]
- Bromfield, T.C.; Curulla Ferré, D.; Niemantsverdriet, J.W. A DFT study of the adsorption and dissociation of CO on Fe(100): Influence of surface coverage on the nature of accessible adsorption states. ChemPhysChem 2005, 6, 254–260. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Thompson, D.L.; Hurley, M.M.; Chabalowski, C.F. First-principles calculations of the adsorption, diffusion, and dissociation of a CO molecule on the Fe(100) surface. Phys. Rev. B 2002, 66, 35416. [Google Scholar] [CrossRef]
- Wang, T.; Tian, X.; Li, Y.; Wang, J.; Beller, M.; Jiao, H. Coverage-dependent CO adsorption and dissociation mechanisms on iron surfaces from DFT computations. ACS Catal. 2014, 4, 1991–2005. [Google Scholar] [CrossRef]
- Zhang, C.H.; Chen, B.; Sun, D.B. A DFT study of H2O dissociation on metal-precovered Fe(100) surface. Surf. Interface Anal. 2018, 50, 420–429. [Google Scholar] [CrossRef]
- Helden, P.V.; Steen, E.V. Coadsorption of CO and H on Fe(100). J. Phys. Chem. C 2008, 112, 16505–16513. [Google Scholar] [CrossRef]
- Ojeda, M.; Nabar, R.; Nilekar, A.U.; Ishikawa, A. CO activation pathways and the mechanism of Fischer–Tropsch synthesis. J. Catal. 2010, 272, 287–297. [Google Scholar] [CrossRef]
- Li, J.; Jacobs, G.; Das, T.; Zhang, Y.; Davis, B. Fischer–Tropsch synthesis: Effect of water on the catalytic properties of a Co/SiO2 catalyst. Appl. Catal. A-Gen. 2002, 236, 67–76. [Google Scholar] [CrossRef]
- Gong, X.; Raval, R.; Hu, P. Co dissociation and O removal on Co(0001): A density functional theory study. Surf. Sci. 2004, 562, 247–256. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Tu, M.; Ojeda, M.P.; Pinna, D.; Iglesia, E. An investigation of the effects of water on rate and selectivity for the Fischer–Tropsch synthesis on cobalt-based catalysts. J. Catal. 2002, 211, 422–433. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, J.; Wang, T. Theoretical study about adsorbed oxygen reduction over χ-Fe5C2: Formation of H2O and CO2. Mol. Catal. 2022, 524, 112236. [Google Scholar] [CrossRef]
- Van Steen, E.; Schulz, H. Polymerisation kinetics of the Fischer–Tropsch CO hydrogenation using iron and cobalt based catalysts. Appl. Catal. A-Gen. 1999, 186, 309–320. [Google Scholar] [CrossRef]
- Das, T.K.; Conner, W.A.; Li, J.; Jacobs, G.; Dry, M.E.; Davis, B.H. Fischer–Tropsch synthesis: Kinetics and effect of water for a Co/SiO2 catalyst. Energy Fuels 2005, 19, 1430–1439. [Google Scholar] [CrossRef]
- Rohde, M.P.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer–Tropsch synthesis with in situ H2O removal–directions of membrane development. Microporous Mesoporous Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Dalai, A.K.; Das, T.K.; Chaudhari, K.V.; Jacobs, G.; Davis, B.H. Fischer–Tropsch synthesis: Water effects on Co supported on narrow and wide-pore silica. Appl. Catal. A-Gen. 2005, 289, 135–142. [Google Scholar] [CrossRef]
- Blekkan, E.A.; Borg, Ø.; Frøseth, V.; Holmen, A. Fischer–Tropsch synthesis on cobalt catalysts: The effect of water. Catalysis 2007, 20, 13–32. [Google Scholar]
- Henderson, M.A. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Rofer-depoorter, C.K. A comprehensive mechanism for the Fischer–Tropsch synthesis. Chem. Rev. 1981, 81, 447–474. [Google Scholar] [CrossRef]
- Gorimbo, J.; Muleja, A.; Liu, X.; Hildebrandt, D. Fischer–Tropsch synthesis: Product distribution, operating conditions, iron catalyst deactivation and catalyst speciation. Int. J. Ind. Chem. 2018, 9, 317–333. [Google Scholar] [CrossRef]
- Dalai, A.K.; Davis, B.H. Fischer–Tropsch synthesis: A review of water effects on the performances of unsupported and supported Co catalysts. Appl. Catal. A-Gen. 2008, 348, 1–15. [Google Scholar] [CrossRef]
- Inderwildi, O.R.; Jenkins, S.J.; King, D.A. Fischer–Tropsch mechanism revisited: Alternative pathways for the production of higher hydrocarbons from synthesis gas. J. Phys. Chem. C 2008, 112, 1305–1307. [Google Scholar] [CrossRef]
- Bertole, C.J.; Mims, C.A.; Kiss, G. The effect of water on the cobalt-catalyzed Fischer–Tropsch synthesis. J. Catal. 2002, 210, 84–96. [Google Scholar] [CrossRef]
- Hibbitts, D.D.; Loveless, B.T.; Neurock, M.; Iglesia, E. Mechanistic role of water on the rate and selectivity of Fischer–Tropsch synthesis on ruthenium catalysts. Angew. Chem. Int. Ed. 2013, 52, 12273–12278. [Google Scholar] [CrossRef]
- Bertole, C. Support and rhenium effects on the intrinsic site activity and methane selectivity of cobalt Fischer–Tropsch catalysts. J. Catal. 2004, 221, 191–203. [Google Scholar] [CrossRef]
- Leung, L.W.H.; Goodman, D.W. Modeling the metal-solution interface under ultrahigh vacuum: Vibrational studies of the coadsorption of water and carbon monoxide on Rh(100). Langmuir 1991, 7, 493–496. [Google Scholar] [CrossRef]
- Nakamura, M.; Ito, M. Coadsorption of water monomers with Co on Ru(001) and charge transfer during hydration processes. Chem. Phys. Lett. 2001, 335, 170–175. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Consorted vinylene mechanism for cobalt Fischer–Tropsch synthesis encompassing water or hydroxyl assisted CO-activation. Top. Catal. 2018, 61, 1024–1034. [Google Scholar] [CrossRef]
- Filot, I.A.W.; Van Santen, R.A.; Hensen, E.J.M. The optimally performing Fischer–Tropsch catalyst. Angew. Chem. Int. Ed. 2014, 53, 12746–12750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, X.; Yu, Y.; Zhang, M. Hydrogen-assisted versus hydroxyl-assisted CO dissociation over co-doped Cu(111): A DFT study. Surf. Sci. 2018, 669, 114–120. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Wang, J.; Jiao, H. Mechanisms of H-and OH-assisted co activation as well as C-C coupling on the flat Co(0001) surface-revisited. Catal. Sci. Technol. 2016, 6, 8336–8343. [Google Scholar] [CrossRef]
- Benziger, J.B.; Madix, R.J. The coadsorption of CO and H2 on Fe(100). Surf. Sci. 1982, 115, 279–289. [Google Scholar] [CrossRef]
- Freund, H.J.; Roberts, M.W. Surface chemistry of carbon dioxide. Surf. Sci. Rep. 1996, 8, 225–273. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Luo, L.; Du, J.; Zeng, J. Symmetry-breaking sites for activating linear carbon dioxide molecules. Acc. Chem. Res. 2021, 54, 1454–1464. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Xie, J.; Jachimowski, T.A.; Weinberg, W.H. Carbon monoxide hydrogenation on the Ru(001) surface at low temperature using gas-phase atomic hydrogen: Spectroscopic evidence for the carbonyl insertion mechanism on a transition metal surface. J. Am. Chem. Soc. 1995, 117, 2606–2617. [Google Scholar] [CrossRef]
- Amaya-Roncancio, S.; Linares, D.H.; Duarte, H.A.; Sapag, K. DFT study of hydrogen-assisted dissociation of CO by HCO, COH, and HCOH formation on Fe(100). J. Phys. Chem. C 2016, 120, 10830–10837. [Google Scholar] [CrossRef]
- Elahifard, M.R.; Jigato, M.P.; Niemantsverdriet, J.W. Direct versus hydrogen-assisted CO dissociation on the Fe(100) surface: A DFT study. ChemPhysChem 2012, 13, 89–91. [Google Scholar] [CrossRef]
- Hilmen, A.M.; Schanke, D.; Hanssen, K.F.; Holmen, A. Study of the effect of water on alumina supported cobalt Fischer–Tropsch catalysts. Appl. Catal. A-Gen. 1999, 186, 169–188. [Google Scholar] [CrossRef]
- Li, T.; Wen, X.; Li, Y.W.; Jiao, H. Mechanisms of CO activation, surface oxygen removal, surface carbon hydrogenation, and C–C coupling on the stepped Fe(710) surface from computation. J. Phys. Chem. C 2018, 122, 15505–15519. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, X.; Yu, Y. Theoretical insights into the removal pathways of adsorbed oxygen on the surface of χ-Fe5C2(510). Chem. Eng. Sci. 2023, 271, 118576. [Google Scholar] [CrossRef]
- Gracia, J.M.; Prinsloo, F.F.; Niemantsverdriet, J.W. Mars-van krevelen-like mechanism of CO hydrogenation on an iron carbide surface. Catal. Lett. 2009, 133, 257–261. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Wang, X.; Ma, Q.; Gao, X.; Xia, H.; Lai, X.; Fan, S.; Zhao, T. Fischer–Tropsch synthesis over methyl modified Fe2O3@SiO2 catalysts with low CO2 selectivity. App. Catal. B-Environ. 2018, 232, 420–428. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Gao, X.; Ma, Q.; Zhang, J.; Zhao, T. Highly selective formation of linear α-olefins over layered and hydrophilic Fe3O4/MAG catalysts in CO hydrogenation. Fuel 2022, 326, 125054. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Liu, B.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T. Cellulose modified iron catalysts for enhanced light olefins and linear C5+ α-olefins from CO hydrogenation. Fuel 2021, 294, 120504. [Google Scholar] [CrossRef]
- Yan, B.; Ma, L.; Gao, X.; Zhang, J.; Ma, Q.; Zhao, T. Amphiphobic surface fabrication of iron catalyst and effect on product distribution of Fischer–Tropsch synthesis. Appl. Catal. A-Gen. 2019, 585, 117184. [Google Scholar] [CrossRef]
- Guo, X.; Liu, B.; Gao, X.; He, F.; Ma, Q.; Fan, S.; Zhao, T.; Tian, J.; Reubroycharoen, P.; Zhang, J. Improved olefin selectivity during CO hydrogenation on hydrophilic Fe/HAP catalysts. Catal. Today 2023, 410, 193–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).