Semi-Interpenetrating Network Anion Exchange Membranes by Thiol–Ene Coupling Reaction for Alkaline Fuel Cells and Water Electrolyzers
Abstract
1. Introduction
2. Results and Discussion
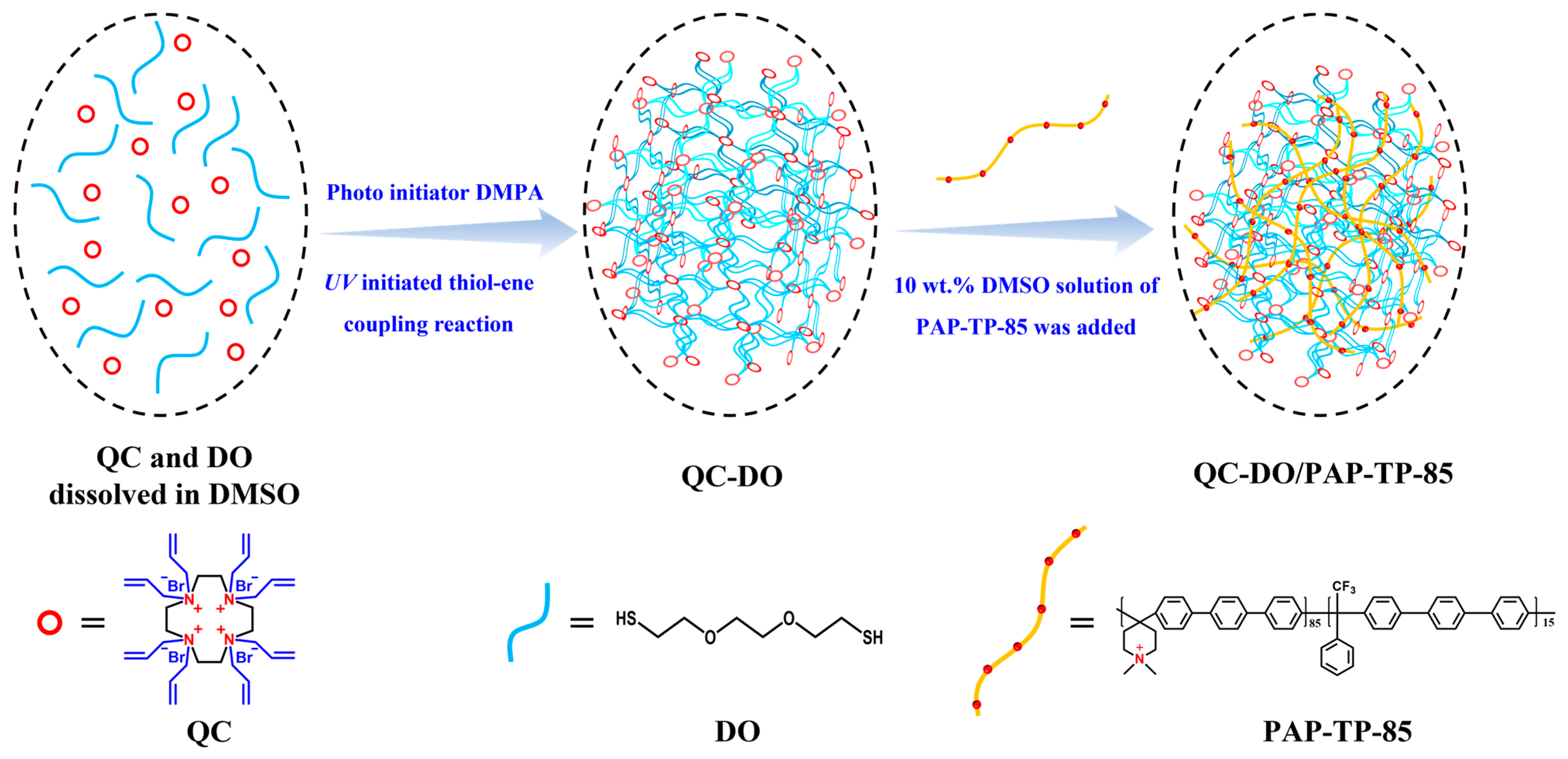
2.1. Synthesis and Structure Characterization
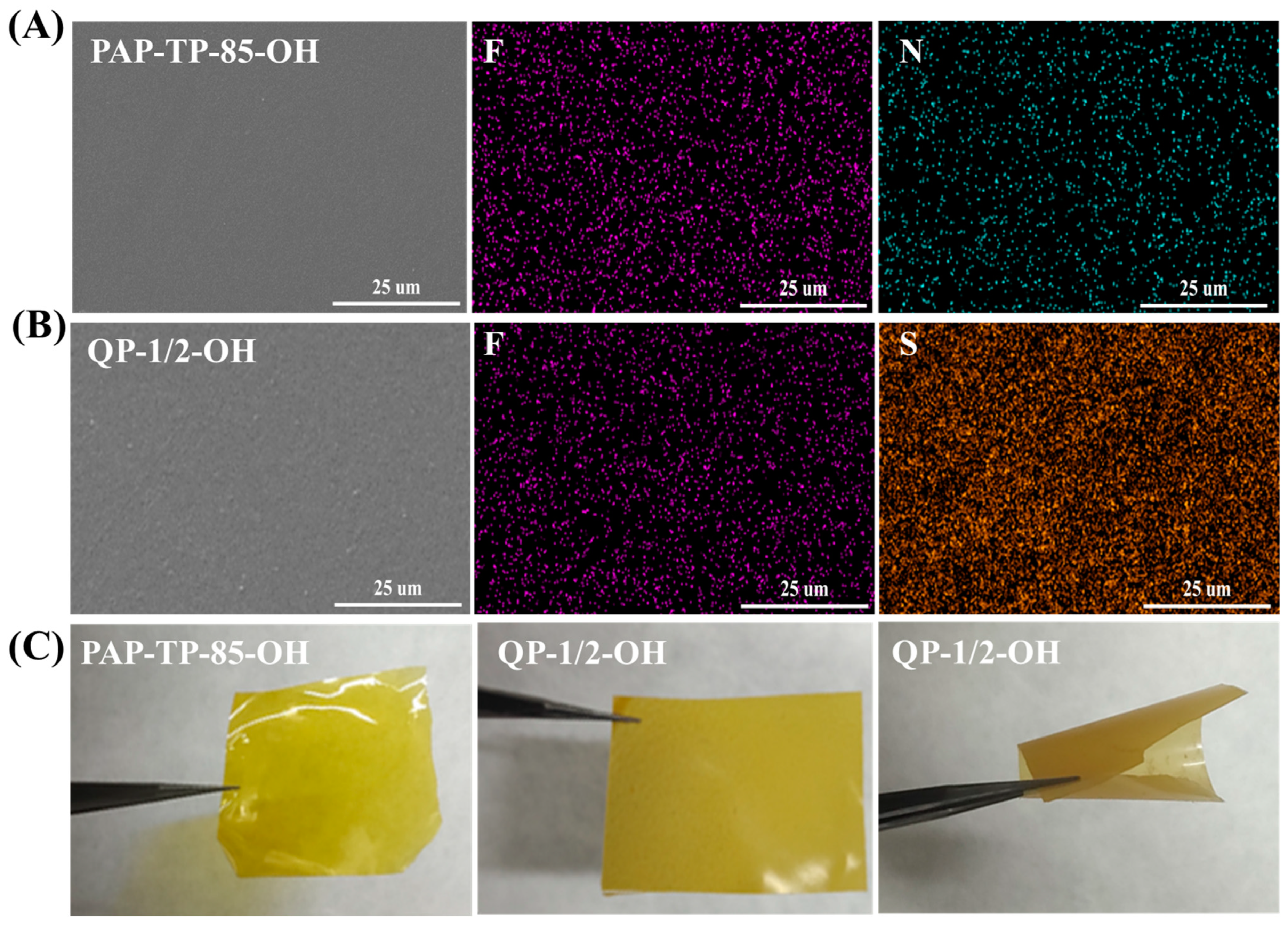
2.2. Morphological Characterization of sIPN AEMs
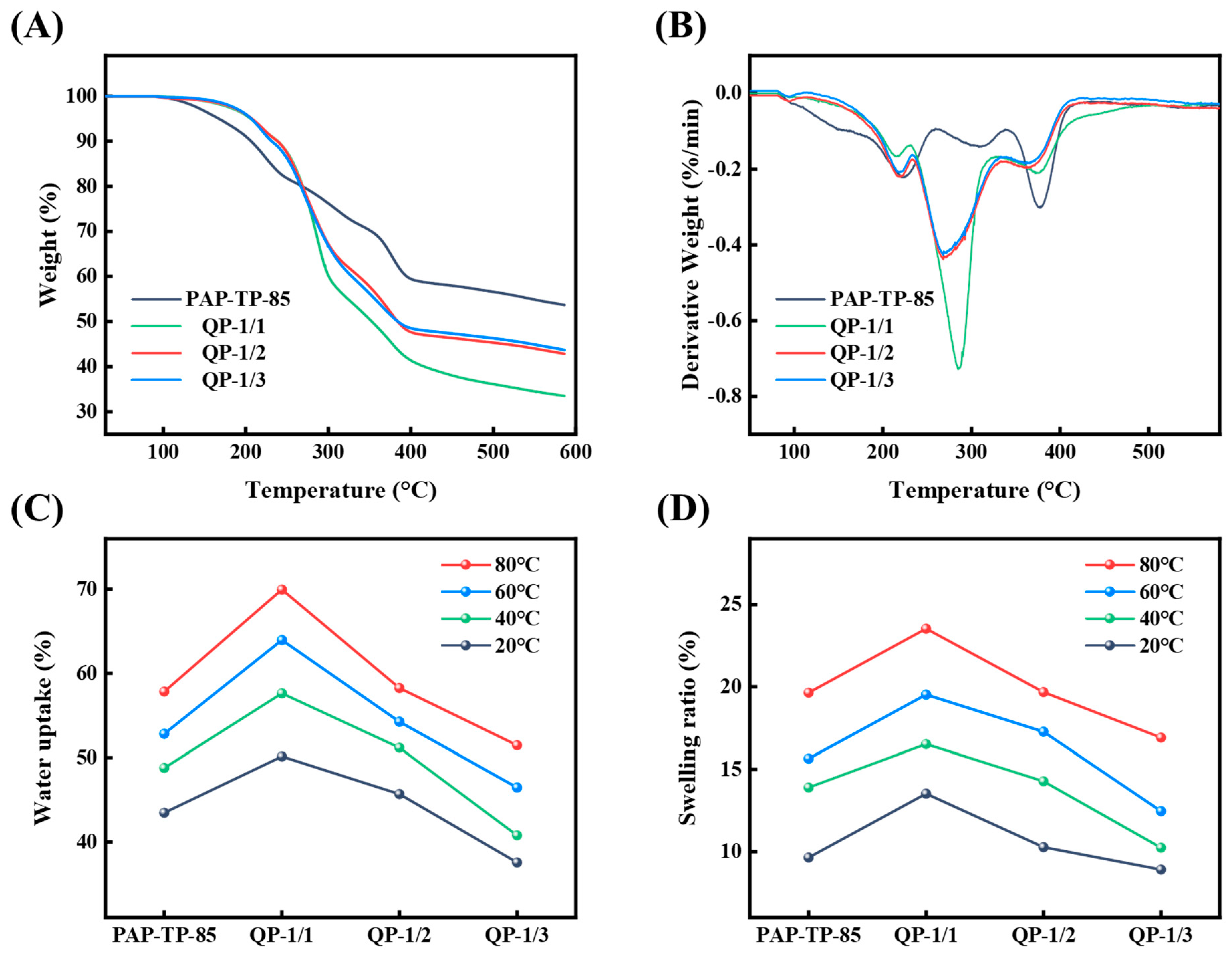
2.3. Thermal Stability of sIPN AEMs
2.4. Water Uptake, Swelling Ratio, and Ion Exchange Capacity (IEC) of sIPN AEMs
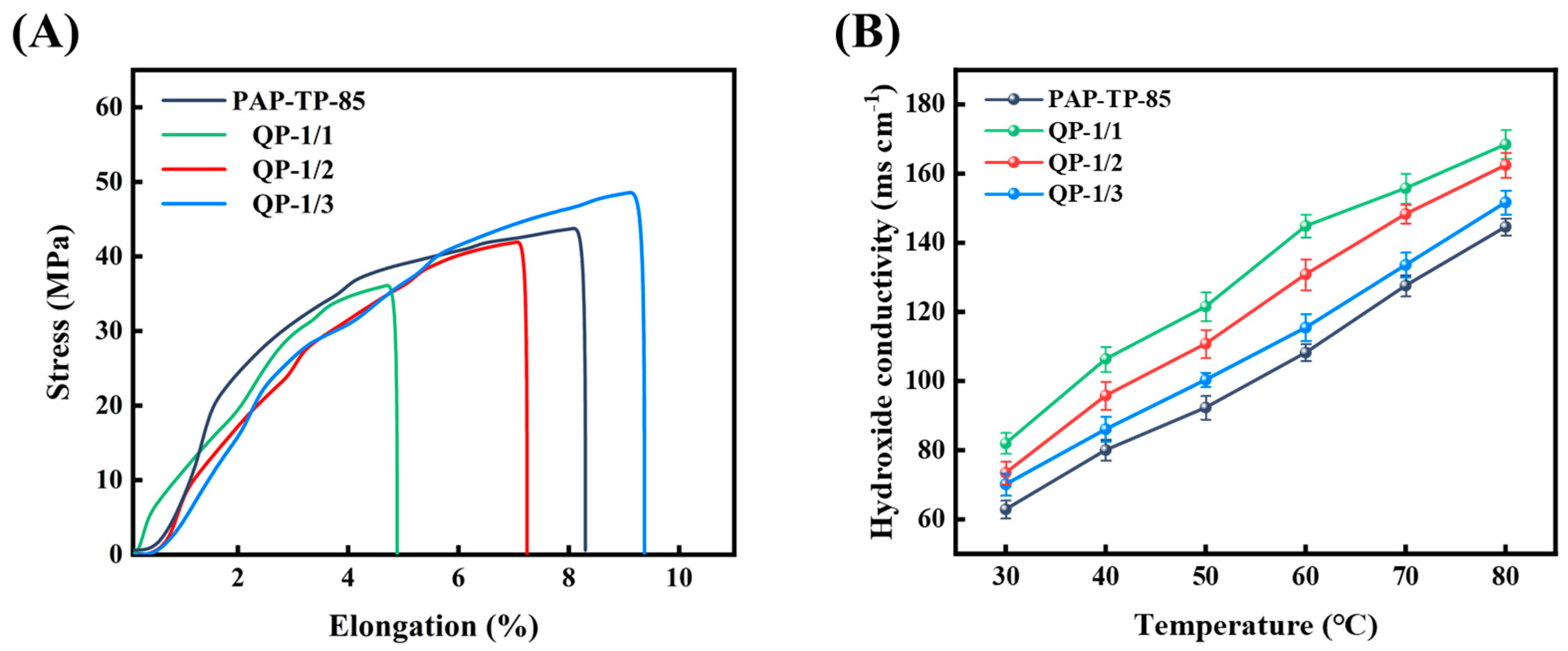
2.5. Mechanical Property and Hydroxide Conductivity of sIPN AEMs
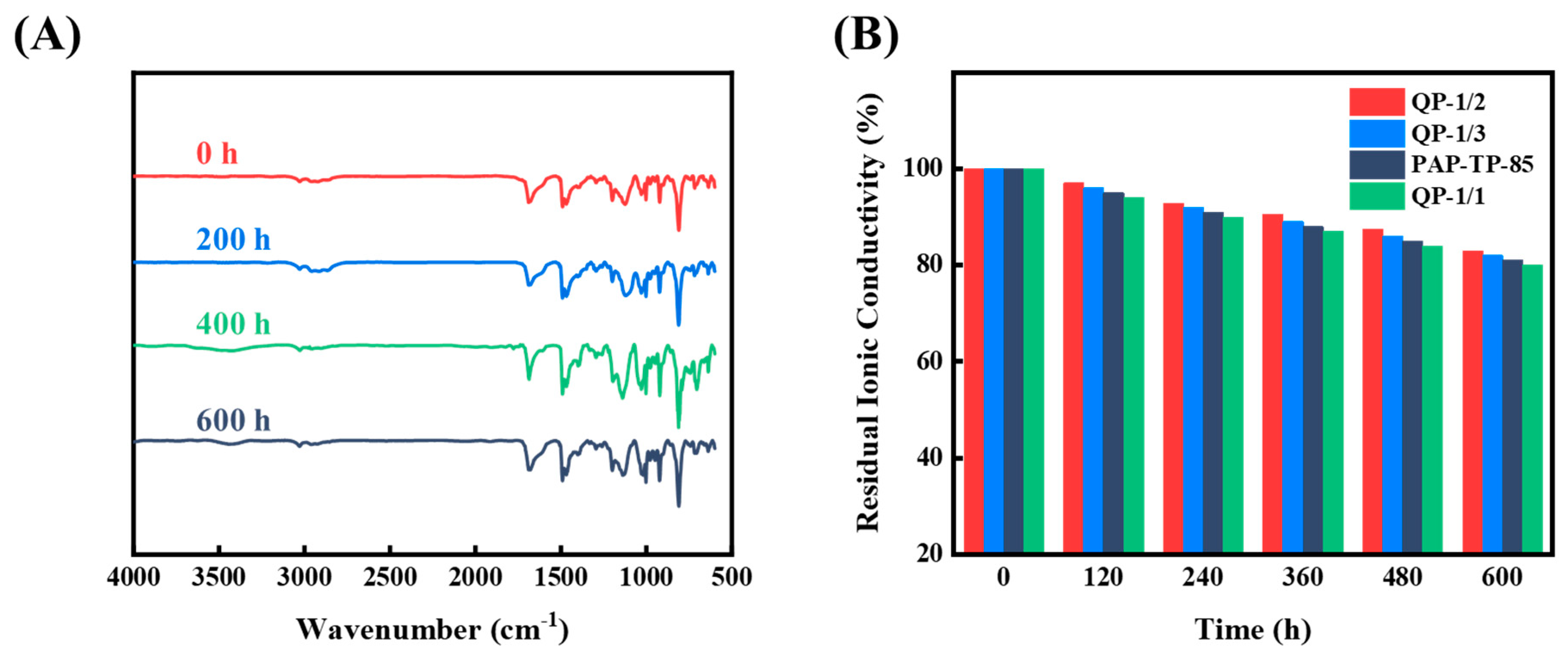
2.6. Chemical Stability of sIPN AEMs
2.7. Fuel Cell and Alkaline Water Electrolysis Applications
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Quaternized Cyclen (QC)
3.3. Synthesis of Hypercrosslinked Polymers by QC
3.4. Synthesis of the Poly(aryl piperidinium) (PAP) Polymers
3.5. Preparation of QC-DO/PAP-TP-85 Anion Exchange Membranes
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kanchanakul, I.; Srinophakun, T.R.; Kuboon, S.; Kaneko, H.; Kraithong, W.; Miyauchi, M.; Yamaguchi, A. Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study. Molecules 2023, 28, 4578. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Nam, S.Y. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, J.; Liu, X.; Huang, T.; Jiang, H.; Yin, Y.; Qin, Y.; Guiver, M.D. Toward alkaline-stable anion exchange membranes in fuel cells: Cycloaliphatic quaternary ammonium-based anion conductors. Electrochem. Energy Rev. 2022, 5, 348–400. [Google Scholar] [CrossRef]
- Aili, D.; Wright, A.G.; Kraglund, M.R.; Jankova, K.; Holdcroft, S.; Jensen, J.O. Towards a stable ion-solvating polymer electrolyte for advanced alkaline water electrolysis. J. Mater. Chem. A 2017, 5, 5055–5066. [Google Scholar] [CrossRef]
- Park, E.J.; Arges, C.G.; Xu, H.; Kim, Y.S. Membrane Strategies for Water Electrolysis. ACS Energy Lett. 2022, 7, 3447–3457. [Google Scholar] [CrossRef]
- Chand, K.; Paladino, O. Recent developments of membranes and electrocatalysts for the hydrogen production by anion exchange membrane water electrolysers: A review. Arab. J. Chem. 2023, 16, 104451. [Google Scholar] [CrossRef]
- Xue, B.; Wang, Q.; Zheng, J.; Li, S.; Zhang, S. Bi-guanidinium-based crosslinked anion exchange membranes: Synthesis, characterization, and properties. J. Membr. Sci. 2020, 601, 117923. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Q.; Gao, X.; Wu, H.; Zhang, Q.; Zhu, A.; Liu, Q. Anion exchange membranes with dense N-spirocyclic cations as side-chain. J. Membr. Sci. 2020, 595, 117560. [Google Scholar] [CrossRef]
- Karibayev, M.; Kalybekkyzy, S.; Wang, Y.; Mentbayeva, A. Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications. Molecules 2022, 27, 3574. [Google Scholar] [CrossRef]
- Chen, J.; Choo, Y.S.L.; Wang, X.; Liu, Y.; Yue, X.; Gao, X.; Gao, W.; Zhang, Q.; Zhu, A.; Liu, Q. Effects of the crown ether cavity on the performance of anion exchange membranes. J. Colloid Interface Sci. 2023, 643, 62–72. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Zhao, Z.; Zhang, X.; Fan, M. Construction of Quaternized Polysulfone/Polyquaternium-10 Anion Exchange Membrane with Semi-Interpenetrating Network for Alkaline Fuel Cell. Macromol. Mater. Eng. 2022, 307, 2100539. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Chen, N.; Hu, C.; Wang, H.H.; Kim, S.P.; Kim, H.M.; Lee, W.H.; Bae, J.Y.; Park, J.H.; Lee, Y.M. Poly(Alkyl-Terphenyl Piperidinium) Ionomers and Membranes with an Outstanding Alkaline-Membrane Fuel-Cell Performance of 2.58 W cm−2. Angew. Chem. Int. Ed. Engl. 2021, 60, 7710–7718. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(arylene piperidinium) Hydroxide Ion Exchange Membranes: Synthesis, Alkaline Stability, and Conductivity. Adv. Funct. Mater. 2018, 28, 1702758. [Google Scholar] [CrossRef]
- Dong, D.; Xiao, Y.; Zhang, M.; Yang, Z.; Wang, K.; Fan, M. Crosslinked anion exchange membranes with regional intensive ion clusters prepared from quaternized branched polyethyleneimine/quaternized polysulfone. Int. J. Hydrogen Energy 2022, 47, 24991–25006. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, M.; Xiao, Y.; Yang, Z.; Wang, K.; Fan, M. Quaternized Branched Polyethyleneimine Modified Nitrogen-Doped Graphene Quantum Dots/Quaternized Polysulfone Composite Anion Exchange Membranes with Improved Performance. Macromol. Mater. Eng. 2022, 307, 2100787. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Liu, P.; Wang, X.; Zhu, X. Photo-cross-linked poly(N-allylisatin biphenyl)-co-poly(alkylene biphenyl)s with pendant N-cyclic quaternary ammonium as anion exchange membranes for direct borohydride/hydrogen peroxide fuel cells. React. Funct. Polym. 2020, 152, 104576. [Google Scholar] [CrossRef]
- Chen, W.; Shen, H.; Gong, Y.; Li, P.; Cheng, C. Anion exchange membranes with efficient acid recovery obtained by quaternized poly epichlorohydrin and polyvinyl alcohol during diffusion dialysis. J. Membr. Sci. 2023, 674, 21514. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, W.; Shen, H.; Cheng, C. Semi-interpenetrating Polymer-Network Anion Exchange Membrane Based on Quaternized Polyepichlorohydrin and Polyvinyl Alcohol for Acid Recovery by Diffusion Dialysis. Ind. Eng. Chem. Res. 2023, 13, 5624–5634. [Google Scholar] [CrossRef]
- Zhu, L.; Zimudzi, T.J.; Li, N.; Pan, J.; Lin, B.; Hickner, M.A. Crosslinking of comb-shaped polymer anion exchange membranes via thiol–ene click chemistry. Polym. Chem. 2016, 7, 2464–2475. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, C.; He, G.; Zhang, F.; Wu, X. Pendent piperidinium-functionalized blend anion exchange membrane for fuel cell application. Int. J. Hydrogen Energy 2019, 44, 15482–15493. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Feng, C.; Wu, X.; Bao, R.; Liu, Z.; Yang, M.; Yang, W. Direct modification of polyketone resin for anion exchange membrane of alkaline fuel cells. J. Colloid Interface Sci. 2019, 556, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Z.; Zhang, M.; Du, W.; Lan, W.; Zhang, X.; Fan, M. Tripartite Cationic Interpenetrating Polymer Network Anion Exchange Membranes for Fuel Cells. ACS Appl. Energy Mater. 2023, 6, 1488–1500. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, C.; Liu, F.; Li, L.; Zhang, Q.; Zhu, A.; Liu, Q. Poly (2,6-dimethyl-1,4-phenylene oxide)/ionic liquid functionalized graphene oxide anion exchange membranes for fuel cells. J. Membr. Sci. 2018, 552, 367–376. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Liu, F.; Wang, X.; Chen, H.; Mao, T.; Wang, Z. Poly (aryl ether ketone)/polymeric ionic liquid with anisotropic swelling behavior for anion exchange membranes. J. Membr. Sci. 2019, 581, 303–311. [Google Scholar] [CrossRef]
- Zhao, T.; Long, C.; Wang, Z.; Zhu, H. Multication Cross-Linked Poly(p-terphenyl isatin) Anion Exchange Membranes for Fuel Cells: Effect of Cross-Linker Length on Membrane Performance. ACS Appl. Energy Mater. 2021, 4, 14476–14487. [Google Scholar] [CrossRef]
- Zeng, L.; Yuan, W.; Ma, X.; He, Q.; Zhang, L.; Wang, J.; Wei, Z. Dual-Cation Interpenetrating Polymer Network Anion Exchange Membrane for Fuel Cells and Water Electrolyzers. Macromolecules 2022, 55, 4647–4655. [Google Scholar] [CrossRef]
- Dong, J.; Li, H.; Ren, X.; Che, X.; Yang, J.; Aili, D. Anion exchange membranes of bis-imidazolium cation crosslinked poly(2,6-dimethyl-1,4-phenylene oxide) with enhanced alkaline stability. Int. J. Hydrogen Energy 2019, 44, 22137–22145. [Google Scholar] [CrossRef]
- Li, G.; Chuang, P.-Y.A. Identifying the forefront of electrocatalytic oxygen evolution reaction: Electronic double layer. Appl. Catal. B 2018, 239, 425–432. [Google Scholar] [CrossRef]
- Gao, W.; Gao, X.; Choo, Y.; Wang, J.; Cai, Z.; Zhang, Q.; Zhu, A.; Liu, Q. Durable dual-methylpiperidinium crosslinked poly(binaphthyl-co-terphenyl piperidinium) anion exchange membranes with high ion transport and electrochemical performance. Chem. Eng. J. 2023, 466, 143107. [Google Scholar] [CrossRef]

| Samples | Theoretical IEC | Titration IEC |
|---|---|---|
| PAP-TP-85 | 2.35 | 2.29 |
| QP-1/1 | 2.72 | 2.62 |
| QP-1/2 | 2.60 | 2.47 |
| QP-1/3 | 2.54 | 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Zou, X.; Xu, G.; Sun, Z.; Yan, F. Semi-Interpenetrating Network Anion Exchange Membranes by Thiol–Ene Coupling Reaction for Alkaline Fuel Cells and Water Electrolyzers. Molecules 2023, 28, 5470. https://doi.org/10.3390/molecules28145470
Jin Z, Zou X, Xu G, Sun Z, Yan F. Semi-Interpenetrating Network Anion Exchange Membranes by Thiol–Ene Coupling Reaction for Alkaline Fuel Cells and Water Electrolyzers. Molecules. 2023; 28(14):5470. https://doi.org/10.3390/molecules28145470
Chicago/Turabian StyleJin, Zhiyu, Xiuyang Zou, Guodong Xu, Zhe Sun, and Feng Yan. 2023. "Semi-Interpenetrating Network Anion Exchange Membranes by Thiol–Ene Coupling Reaction for Alkaline Fuel Cells and Water Electrolyzers" Molecules 28, no. 14: 5470. https://doi.org/10.3390/molecules28145470
APA StyleJin, Z., Zou, X., Xu, G., Sun, Z., & Yan, F. (2023). Semi-Interpenetrating Network Anion Exchange Membranes by Thiol–Ene Coupling Reaction for Alkaline Fuel Cells and Water Electrolyzers. Molecules, 28(14), 5470. https://doi.org/10.3390/molecules28145470








