UPLC-MS/MS Method for Simultaneous Determination of Valnemulin and Its Metabolites in Crucian Carp: In Vivo Metabolism and Tissue Distribution Analyses
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Validation
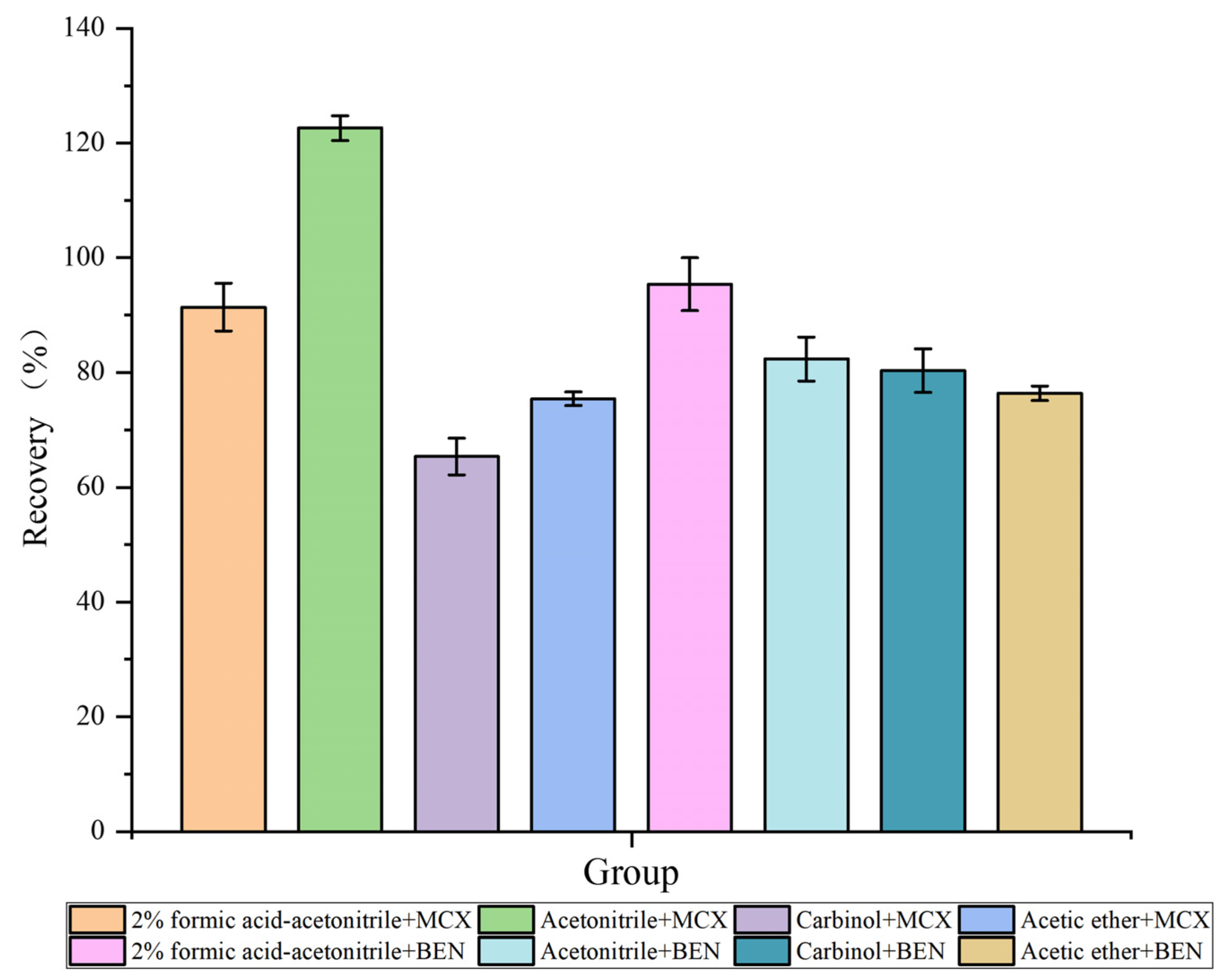
2.2. Optimization of the Extraction Conditions
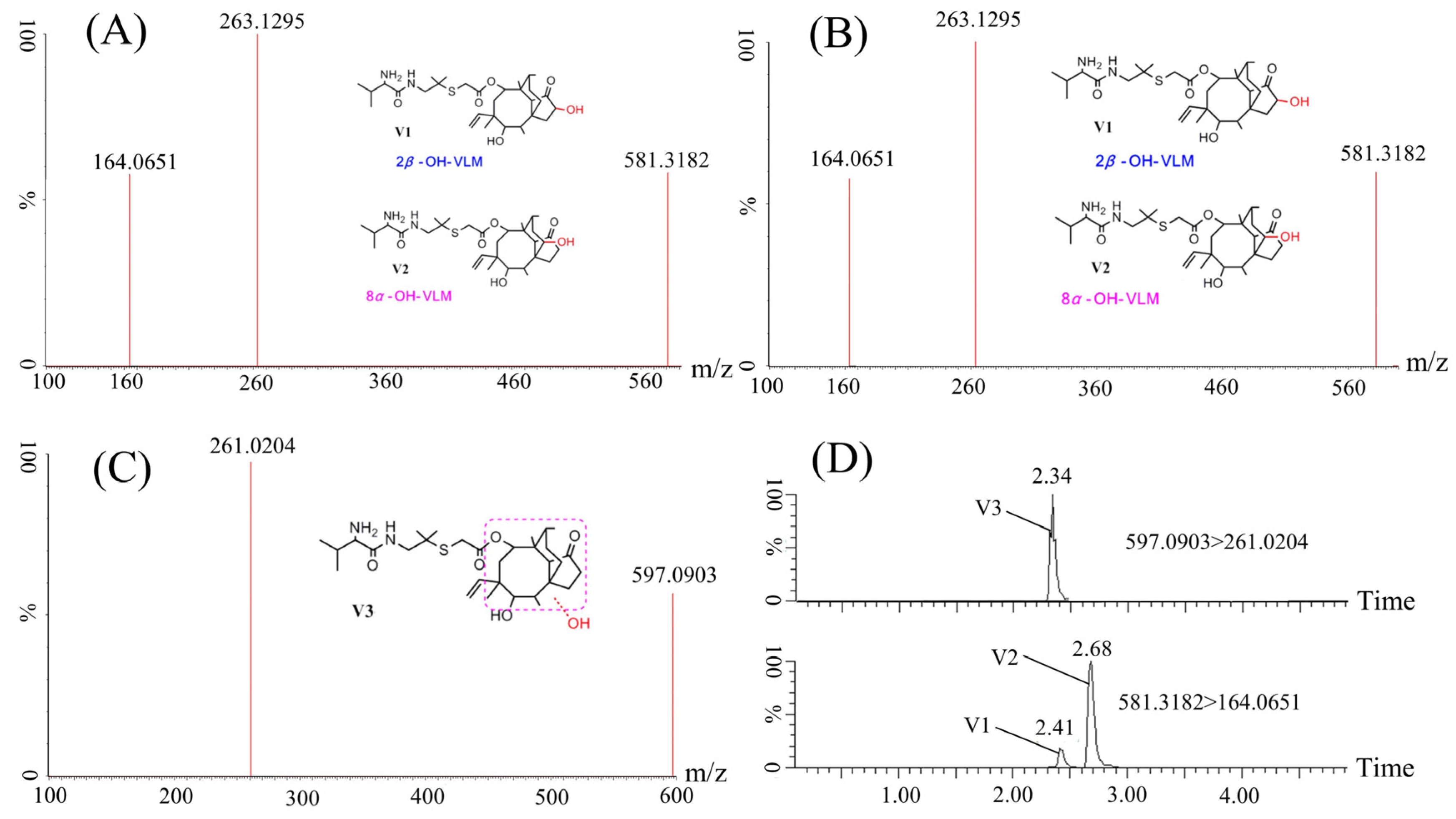
2.3. Identification of VML Metabolites in Crucian Carp
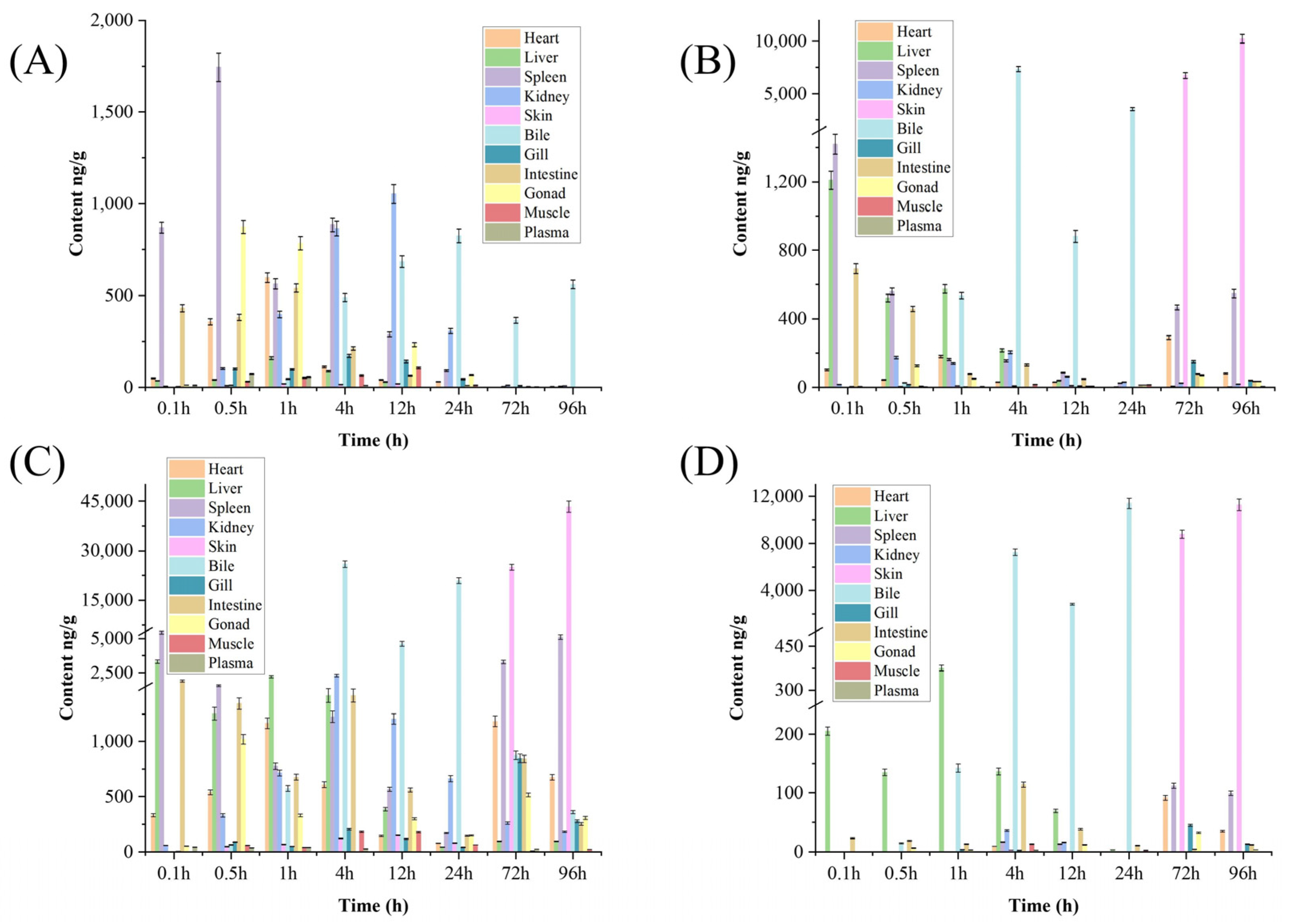
2.4. Distribution of VML and Its Metabolites in Tissues
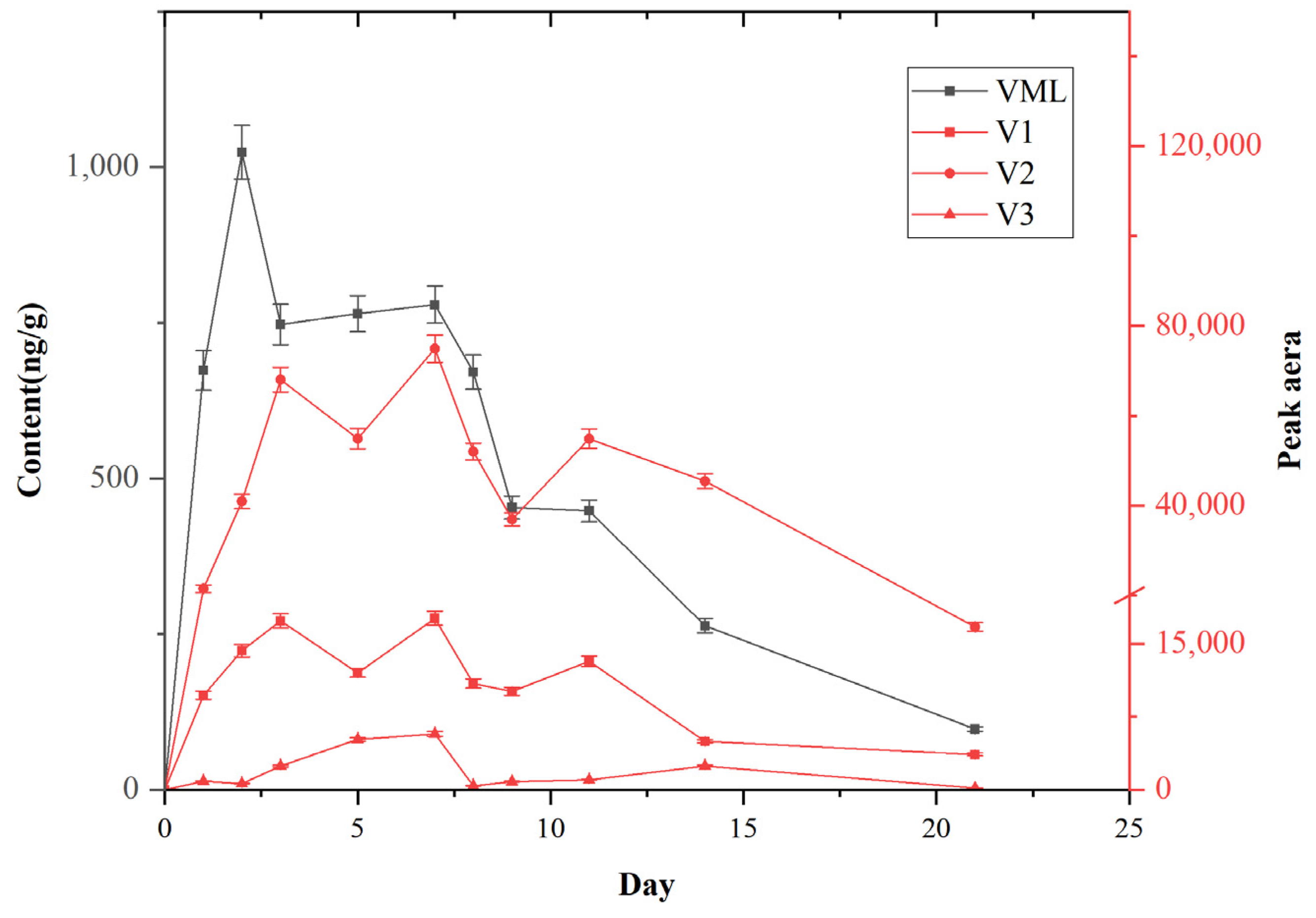
2.5. Kinetic Investigation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals
3.3. Sample Preparation
3.4. Instrumental Conditions
3.5. Method Validation
3.6. Pharmacokinetic and Tissue Distribution
3.7. Kinetic Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Long, K.S.; Poehlsgaard, J.; Hansen, L.H.; Hobbie, S.N.; Bottger, E.C.; Vester, B. Single 23S rRNA mutations at the ribosomal peptidyl transferase centre confer resistance to valnemulin and other antibiotics in Mycobacterium smegmatis by perturbation of the drug binding pocket. Mol. Microbiol. 2009, 71, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Hansen, L.H.; Jakobsen, L.; Vester, B. Interaction of pleuromutilin derivatives with the ribosomal peptidyl transferase center. Antimicrob. Agents Chemother. 2006, 50, 1458–1462. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Singer, R.S.; Gebhart, C.J. In vitro antimicrobial activity against 10 North American and European Lawsonia intracellularis isolates. Vet. Microbiol. 2009, 134, 305–310. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Greko, C.; Pringle, M.; Baptiste, K.E.; Catry, B.; Jukes, H.; Moreno, M.A.; Pomba, M.C.M.F.; Pyörälä, S.; Rantala, M.; et al. Pleuromutilins: Use in food-producing animals in the European Union, development of resistance and impact on human and animal health. J. Antimicrob. Chemother. 2014, 69, 2022–2031. [Google Scholar] [CrossRef]
- Wang, R.; Yuan, L.G.; He, L.M.; Zhu, L.X.; Luo, X.Y.; Zhang, C.Y.; Yu, J.J.; Fang, B.H.; Liu, Y.H. Pharmacokinetics and bioavailability of valnemulin in broiler chickens. J. Vet. Pharmacol. Ther. 2011, 34, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, J.; Xia, L.; Xia, X.; Duan, P.; Shen, J.; Ding, S. Residue depletion of valnemulin in swine tissues after oral administration. Anal. Chim. Acta 2010, 664, 62–67. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, X.; Fu, Q.; Shan, Y.; Liu, T.; Xia, X. Determination of valnemulin in swine and bovine tissues by ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2016, 1014, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, W.; Hu, D.; Zhang, S.; Zhang, H.; Wang, Z.; Cheng, L.; Sun, F.; Shen, J.; Cao, X. In vitro and in vivo metabolite profiling of valnemulin using ultraperformance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. J. Agric. Food Chem. 2014, 62, 9201–9210. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Su, Y.; Yang, J.; Bian, K.; Wang, Z.; He, L.-M. High-Throughput Screening and Confirmation of 22 Banned Veterinary Drugs in Feedstuffs Using LC-MS/MS and High-Resolution Orbitrap Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 516–527. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, L.; Zhu, L.; He, L.; Luo, X.; Wang, R.; Liu, Y. Pharmacokinetics and bioavailability of valnemulin in Muscovy ducks (Cairina moschata). Br. Poult. Sci. 2012, 53, 374–378. [Google Scholar] [CrossRef]
- Sun, F.; Fan, R.; Wang, J.; Xiong, L.; Shen, J.; Zhang, S.; Cao, X. Pharmacokinetics of valnemulin after intravenous, intramuscular, and oral administration in layer chickens. J. Vet. Pharmacol. Ther. 2017, 40, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cho, B.-H.; Lim, C.-M.; Kim, D.-G.; Yune, S.Y.; Shin, J.Y.; Bong, Y.H.; Kang, J.; Kim, M.-A.; Son, S.-W. Chemical residues and contaminants in foods of animal origin in korea during the past decade. J. Agric. Food Chem. 2013, 61, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Jingjing, Z.; Zhongyong, Y.; Xiaojun, Z.; Tingting, L.I.; Xuehui, G. Determination of tiamulin and valnemulin drug residues in aquatic products by ion exchange olid phase extraction-ultra performance liquid chromatography-tandem mass spectrometry. J. Zhejiang Univ. (Sci. Ed.) 2019, 46, 466–473. [Google Scholar]
- Guo, H.; Liu, Y.; Fang, B.; Ding, H.; Wang, M.; He, L. Determination of valnemulin residues in porcine tissues by molecularly imprinted solid-phase extraction coupling with high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1597–1613. [Google Scholar] [CrossRef]
- Patyra, E.; Nebot, C.; Gavilán, R.E.; Cepeda, A.; Kwiatek, K. Development and validation of an LC-MS/MS method for the quantification of tiamulin, trimethoprim, tylosin, sulfadiazine and sulfamethazine in medicated feed. Food Addit. Contam. Part A 2018, 35, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Gajda, A.; Nowacka-Kozak, E.; Gbylik-Sikorska, M.; Posyniak, A. Multi-residues UHPLC–MS/MS analysis of 53 antibacterial compounds in poultry feathers as an analytical tool in food safety assurance. J. Chromatogr. B 2019, 1104, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Hu, X.; Sun, Y.; Kwee, S.; Xing, G.; Xing, Y.; Zhang, G. A highly sensitive monoclonal antibody-based paper sensor for simultaneously detecting valnemulin and tiamulin in porcine liver. J. Food Sci. 2020, 85, 1681–1688. [Google Scholar] [CrossRef]
- Song, Z.-W.; Yang, F.; Liu, Y.; Shao, H.-T.; Zhang, M.; Chen, J.-C.; Ma, K.-L.; Yang, F. Residue depletion of danofloxacin in Yellow River carp (Cyprinus carpio haematopterus) following multiple oral administration. Aquaculture 2022, 562, 738789. [Google Scholar] [CrossRef]
- Kolanczyk, R.C.; Serrano, J.A.; Tapper, M.A.; Schmieder, P.K. A comparison of fish pesticide metabolic pathways with those of the rat and goat. Regul. Toxicol. Pharmacol. 2018, 94, 124–143. [Google Scholar] [CrossRef]
- Shan, Q.; Huang, H.; Zheng, G.; Yin, Y.; Zhu, X.; Ma, L.; Zhou, H.; Xie, W.; Li, L.; Liu, S.; et al. Pharmacokinetics and tissue residue profiles of Enrofloxacin in crucian carp (Carassius auratus gibelio) following single and multiple oral administration. Front. Vet. Sci. 2022, 9, 872828. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, G.W.; Kwon, M.-G.; Seo, J.S. Temperature-dependent tissue residue depletion and withdrawal time of orally administered tylosin tartrate in starry flounder, Platichthys stellatus. Aquaculture 2022, 561, 738644. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Zheng, G.; Yin, Y.; Zhu, X.; Shan, Q.; Yang, Y.; Ma, L.; Li, L.; Liu, S. Pharmacokinetics, tissue distribution, and depletion of enrofloxacin and its metabolite ciprofloxacin in the northern snakehead (Channa argus) following multiple oral administration. Aquaculture 2021, 533, 736183. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, C.-S.; Duan, M.-H.; Wang, H.; Song, Z.-W.; Shao, H.-T.; Ma, K.-L.; Yang, F. Pharmacokinetics and tissue distribution of Enrofloxacin following single oral administration in yellow river carp (Cyprinus carpio haematoperus). Front. Vet. Sci. 2022, 9, 822032. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, X.; Wang, F.; Yang, H.; Xu, N.; Yang, Q. Comparative pharmacokinetics and tissue distribution of quinocetone in crucian carp (Carassius auratus), common carp (Cyprinus carpio L.), and grass carp (Ctenopharyngodon idella) following the same experimental conditions. J. Vet. Pharmacol. Ther. 2015, 38, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Bioanalytical Method Validation Guidance for Industry. U.S. Department of Health and Human Services. Food and Drug Administration. 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 1 June 2023).
| Tissues | Spiked Concentration (μg/kg) | Average Recovery Rate (%) | Intra-Day RSD (%) | Inter-Day RSD (%) |
|---|---|---|---|---|
| Muscle | 0.1 | 102.63 ± 4.93 | 2.0 | 5.2 |
| 1.0 | 113.78 ± 4.06 | 3.7 | 5.9 | |
| 5.0 | 105.30 ± 2.25 | 3.0 | 5.7 | |
| 10.0 | 104.77 ± 3.21 | 3.3 | 7.3 | |
| 50.0 | 101.42 ± 4.05 | 1.7 | 3.0 | |
| Liver | 0.1 | 93.65 ± 4.02 | 6.1 | 7.5 |
| 1.0 | 115.43 ± 5.17 | 5.8 | 7.0 | |
| 5.0 | 111.37 ± 4.25 | 3.7 | 6.8 | |
| 10.0 | 107.26 ± 4.93 | 5.5 | 6.5 | |
| 50.0 | 103.41 ± 4.68 | 4.0 | 5.6 | |
| Intestine | 0.1 | 103.68 ± 3.63 | 6.2 | 7.3 |
| 1.0 | 101.22 ± 2.93 | 4.7 | 5.2 | |
| 5.0 | 102.56 ± 4.69 | 4.5 | 6.2 | |
| 10.0 | 98.61 ± 3.46 | 3.0 | 5.3 | |
| 50.0 | 111.41 ± 5.05 | 6.2 | 7.4 |
| Analyte | Precursor Ion (m/z) | Product Ions (m/z) | Cone Voltage (V) | Collision Energy (ev) | Internal Standard |
|---|---|---|---|---|---|
| VML | 565.32 | 263.09 a 146.80 | 10 | 1540 | Tiamulin-13C4 |
| V1 | 581.32 | 164.07 | 30 | 15 | Tiamulin-13C4 |
| V2 | 581.32 | 164.07 | 30 | 15 | Tiamulin-13C4 |
| V3 | 597.10 | 261.02 | 30 | 15 | Tiamulin-13C4 |
| Tiamulin-13C4 | 498.08 | 196.32 | 30 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhang, X.; Wang, Q.; Zhong, Y.; Liu, W. UPLC-MS/MS Method for Simultaneous Determination of Valnemulin and Its Metabolites in Crucian Carp: In Vivo Metabolism and Tissue Distribution Analyses. Molecules 2023, 28, 5430. https://doi.org/10.3390/molecules28145430
Yang Q, Zhang X, Wang Q, Zhong Y, Liu W. UPLC-MS/MS Method for Simultaneous Determination of Valnemulin and Its Metabolites in Crucian Carp: In Vivo Metabolism and Tissue Distribution Analyses. Molecules. 2023; 28(14):5430. https://doi.org/10.3390/molecules28145430
Chicago/Turabian StyleYang, Qiyu, Xiaojun Zhang, Qianfeng Wang, Yaqian Zhong, and Wenjing Liu. 2023. "UPLC-MS/MS Method for Simultaneous Determination of Valnemulin and Its Metabolites in Crucian Carp: In Vivo Metabolism and Tissue Distribution Analyses" Molecules 28, no. 14: 5430. https://doi.org/10.3390/molecules28145430
APA StyleYang, Q., Zhang, X., Wang, Q., Zhong, Y., & Liu, W. (2023). UPLC-MS/MS Method for Simultaneous Determination of Valnemulin and Its Metabolites in Crucian Carp: In Vivo Metabolism and Tissue Distribution Analyses. Molecules, 28(14), 5430. https://doi.org/10.3390/molecules28145430






