Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America
Abstract
1. Introduction
2. Results and Discussion
2.1. Alkaloid Identification and Quantification
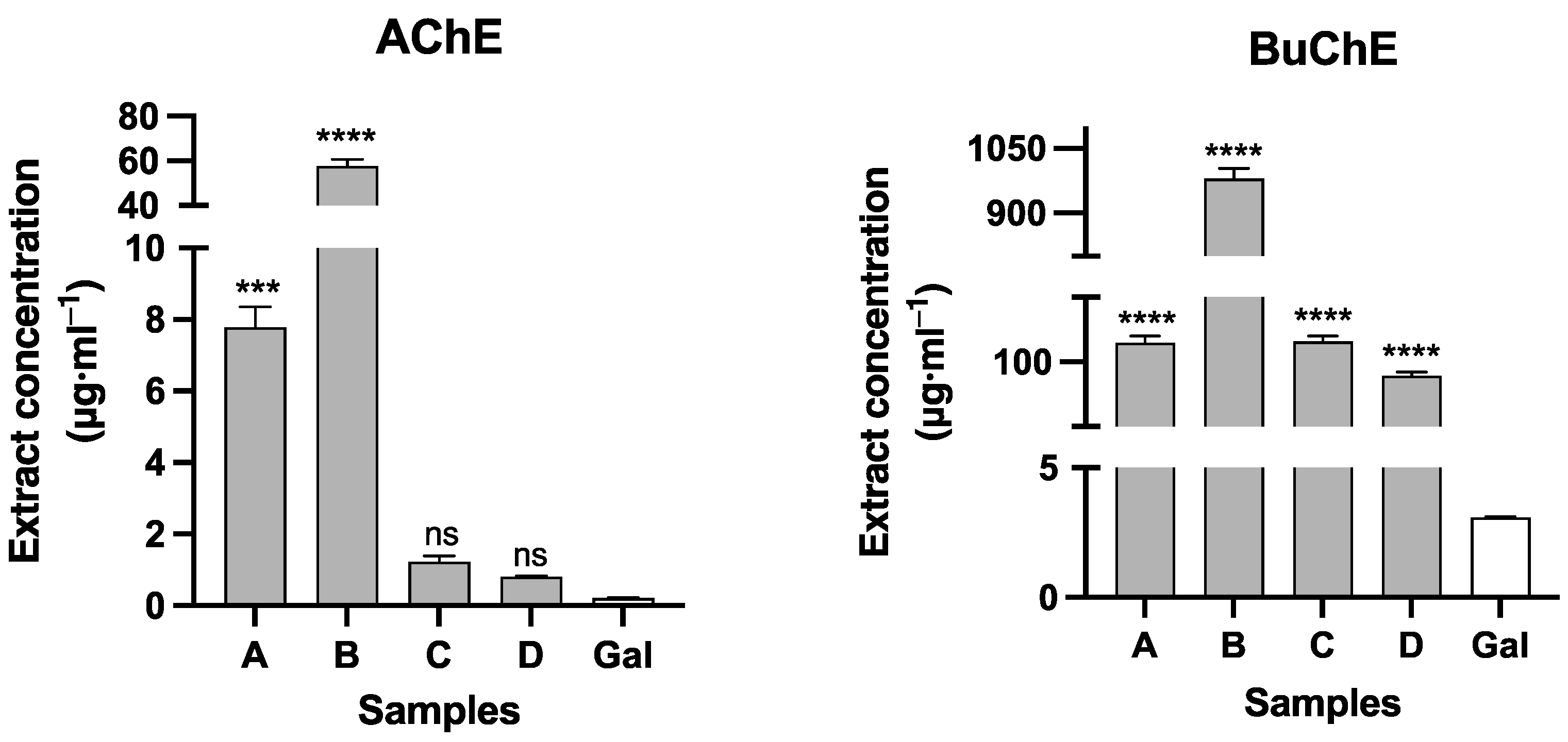
2.2. Cholinesterase Inhibitory Activity
3. Materials and Methods
3.1. Plant Material
3.2. Alkaloid Extraction
3.3. GC-MS Analysis
3.4. Alkaloid Identification
3.5. Alkaloid Quantification
3.6. AChE and BuChE Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Esquerre-Ibañez, B.; Meerow, A.W. A new species of Clinanthus (Amaryllidaceae: Amaryllidoideae: Clinantheae) from Cordillera de los Tarros, Northwest Peru, and notes on related species. Phytotaxa 2020, 438, 2. [Google Scholar] [CrossRef]
- León, B.; Sagástegui, A.; Sánchez, I.; Zapata, M.; Meerow, A.; Cano, A. Amaryllidaceae endémicas del Perú. Rev. Peru. Biol. 2006, 13, 690–697. [Google Scholar] [CrossRef]
- Meerow, A.W.; Guy, C.L.; Li, Q.B.; Yang, S.L. Phylogeny of the American Amaryllidaceae based on nrDNA ITS sequences. Syst. Bot. 2000, 25, 708–726. [Google Scholar] [CrossRef]
- Meerow, A.W. Convergence or reticulation? Mosaic evolution in the canalized American Amaryllidaceae. In Diversity, Phylogeny and Evolution in the Monocotyledons; Seberg, O., Petersen, G., Barfod, A.S., Davis, J.I., Eds.; Aarhus University Press: Aarhus, Denmark, 2010; pp. 145–168. [Google Scholar]
- Meerow, A.W.; Nakamura, K. Two new species of Peruvian Amaryllidaceae, an expanded concept of the genus Paramongaia, and taxonomic notes in Stenomesson. Phytotaxa 2019, 416, 184–196. [Google Scholar] [CrossRef]
- Meerow, A.W.; Gardner, E.M.; Nakamura, K. Phylogenomics of the Andean tetraploid clade of the American Amaryllidaceae (subfamily Amaryllidoideae): Unlocking a polyploid generic radiation abetted by continental geodynamics. Front. Plant Sci. 2020, 11, 582422. [Google Scholar] [CrossRef]
- Ravenna, P. Stenomesson subgen. Fulgituba Ravenna. Plant Life 1974, 30, 77. [Google Scholar]
- Meerow, A.W. A review of Stenomesson. Plant Life 1987, 43, 42–49. [Google Scholar]
- Meerow, A.W.; Cano, A. Taxonomic novelties in Amaryllidaceae from the Department of Ancash, Peru, and a new combination in Clinanthus. PhytoKeys 2019, 131, 115–126. [Google Scholar] [CrossRef]
- Gonzáles, P.; Meerow, A.W. Two new species of Clinanthus (Asparagales: Amaryllidaceae: Clinantheae) from northern Peru. Phytotaxa 2020, 472, 18. [Google Scholar] [CrossRef]
- Meerow, A.W. 202, Amaryllidaceae. In Flora of Ecuador 41; Harling, G., Ed.; University of Göteborg: Göteborg, Sweden, 1990. [Google Scholar]
- Ruiz, H.; Pavon, J. Flora Peruviana et Chilensis; Typis Gabrielis de Sancha: Madrid, Spain, 1802; Volume 3, p. 226. [Google Scholar]
- Likhitwitayawuid, K.; Angerhofer, C.K.; Chai, H.; Pezzuto, J.M.; Cordell, G.A.; Ruangrungsi, N. Cytotoxic and antimalarial alkaloids from the bulbs of Crinum amabile. J. Nat. Prod. 1993, 56, 1331–1338. [Google Scholar] [CrossRef]
- Weniger, B.; Italiano, L.; Beck, J.P.; Bastida, J.; Bergoñón, S.; Codina, C.; Lobstein, A.; Anton, R. Cytotoxic activity of Amaryllidaceae alkaloids. Planta Med. 1995, 61, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Meerow, A.W.; Snijman, D.A. Amaryllidaceae. In Flowering Plants: Monocotyledons; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 3, pp. 83–110. [Google Scholar] [CrossRef]
- Yui, S.; Mikami, M.; Mimaki, Y.; Sashida, Y.; Yamazaki, M. Inhibition effect of Amaryllidaceae alkaloids, lycorine and lycoricidinol on macrophage TNF-α production. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2001, 121, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chemical and Biological Aspects of Narcissus Alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Elsevier: San Diego, CA, USA, 2006; Volume 63, pp. 87–179. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Tang, L.J.; Zhang, G.P.; Hu, W.X. Treatment of lycorine on SCID mice model with human APL cells. Biomed. Pharmacother. 2007, 61, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bremer, B.; Bremer, K.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Bailey, L.H.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F.; Anderberg, A.A.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Chase, M.W.; Reveal, J.L.; Fay, M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2009, 161, 132–136. [Google Scholar] [CrossRef]
- Leiva, S.; Meerow, A.W. A new species of Clinanthus from northern Peru (Asparagales, Amaryllidaceae, Amarylloideae, Clinantheae). PhytoKeys 2016, 63, 99–106. [Google Scholar] [CrossRef]
- Beltran, H. Catalogue of vascular flora of the district of Laraos (Yauyos, Lima). Arnaldoa 2018, 25, 565–596. [Google Scholar] [CrossRef]
- Adessi, T.G.; Borioni, J.L.; Pigni, N.B.; Bastida, J.; Cavallaro, V.; Murray, A.P.; Puiatti, M.; Oberti, J.C.; Leiva, S.; Nicotra, V.E.; et al. Clinanthus microstephium, an Amaryllidaceae species with cholinesterase inhibitor alkaloids: Structure−activity analysis of haemanthamine skeleton derivatives. Chem. Biodivers. 2019, 16, e1800662. [Google Scholar] [CrossRef]
- Soto-Vásquez, M.R.; Pinedo, M.V.H.; Tallini, L.R.; Bastida, J. Chemical composition and in vitro antiplasmodial activity of the total alkaloids of the bulbs of two Amaryllidaceae species from Northern Peru. Pharmacogn. J. 2021, 13, 1046–1052. [Google Scholar] [CrossRef]
- Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. N-Alkylated galanthamine derivatives: Potent acetylcholinesterase inhibitors from Leucojum aestivum. Bioorg. Med. Chem. Lett. 2008, 18, 2263–2266. [Google Scholar] [CrossRef]
- Ghosal, S.; Saini, S.S.; Razdan, S. Crinum alkaloids: Their chemistry and biology. Phytochemistry 1985, 24, 2141–2156. [Google Scholar] [CrossRef]
- Berkov, S.; Viladomat, F.; Codina, C.; Suárez, S.; Ravelo, A.; Bastida, J. GC-MS of amaryllidaceous galanthamine-type alkaloids. J. Mass Spectrom. 2012, 47, 1065–1073. [Google Scholar] [CrossRef]
- Berkov, S.; Torras-Claveria, L.; Viladomat, F.; Suárez, S.; Bastida, J. GC-MS of some lycorine-type Amaryllidaceae alkaloids. J. Mass Spectrom. 2021, 56, e4704. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Denev, R.; Sidjimova, B.; Zarev, Y.; Shkondrov, A.; Torras-Claveria, L.; Viladomat, F.; Bastida, J. Gas chromatography-mass spectrometry of some homolycorine-type Amaryllidaceae alkaloids. Rapid Commun. Mass Spectrom. 2023, 37, e9506. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qi, W.B.; Tian, J.; Jiao, P.R.; Liu, G.Q.; Zhang, C.H.; Liao, M. Amaryllidaceae alkaloids exhibit anti-influenza activity in MDCK cells, an investigation of Amaryllidaceae alkaloids and mdck cells insight. J. Anim. Vet. Adv. 2012, 11, 2485–2492. [Google Scholar] [CrossRef]
- He, J.; Qi, W.B.; Wang, L.; Tian, J.; Jiao, P.R.; Liu, G.Q.; Ye, W.C.; Liao, M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir. Viruses 2013, 7, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, L.F.; Wang, Q.Y.; Shang, L.Q.; Shi, P.Y.; Yin, Z. Anti-dengue virus activity and structure-activity relationship studies of lycorine derivatives. ChemMedChem 2014, 9, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cai, J.; Cheng, J.; Jing, C.; Yin, J.; Jiang, J.; Peng, Z.; Hao, X. Design, synthesis and structure- activity relationship optimization of lycorine derivatives for HCV inhibition. Sci. Rep. 2015, 5, 14972. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yu, D.; Fu, S.; Zhang, G.; Pan, Y.; Bao, M.; Tu, J.; Shang, B.; Guo, P.; Yang, P.; et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol. Lett. 2013, 218, 174–185. [Google Scholar] [CrossRef]
- Doskočil, I.; Hošťálková, A.; Šafratová, M.; Benešová, N.; Havlík, J.; Havelek, R.; Kuneš, J.; Královec, K.; Chlebek, J.; Cahlíková, L. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochemistry 2015, 13, 394–398. [Google Scholar] [CrossRef]
- Hu, M.; Peng, S.; He, Y.; Qin, M.; Cong, X.; Xing, Y.; Liu, M.; Yi, Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget 2015, 6, 15348–15361. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.; Liu, X.M.; Huang, X.J.; Zhang, D.M.; Zhang, W.; Wang, L.; Ye, W.C. Four new Amaryllidaceae alkaloids from Lycoris radiata and their cytotoxicity. Planta Med. 2015, 81, 1712–1718. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, S.; Zhang, Y.; Zhang, G.; Liu, S. Lycorine induces apoptosis in human pancreatic cancer cell line PANC-1 via ROS-mediated inactivation of the PI3K/Akt/mTOR signaling pathway. Int. J. Clin. Exp. Med. 2016, 9, 21048–21056. [Google Scholar]
- Hao, B.; Shen, S.F.; Zhao, Q.J. Cytotoxic and antimalarial amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Molecules 2013, 18, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Huang, X.Y.; Cui, M.R.; Zhang, X.D.; Chen, Z.; Yang, B.S.; Zhao, X.K. Amaryllidaceae alkaloids from the bulbs of Lycoris radiata with cytotoxic and anti-inflammatory activities. Fitoterapia 2015, 101, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Mathieu, V.; Lefranc, F.; Kornienko, A.; Evidente, A.; Kiss, R. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013, 33, 439–455. [Google Scholar] [CrossRef]
- Cedrón, J.; Ravelo, Á.; León, L.; Padrón, J. Relationships of antiproliferative and structural activity of Amaryllidaceae alkaloids. Molecules 2015, 20, 13854–13863. [Google Scholar] [CrossRef]
- Roy, M.; Liang, L.; Xiao, X.; Feng, P.; Ye, M.; Liu, J. Lycorine: A prospective natural lead for anticancer drug discovery. Biomed. Pharmacother. 2018, 107, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Osorio, E.J.; Berkov, S.; Brun, R.; Codina, C.; Viladomat, F.; Cabezas, F.; Bastida, J. In vitro antiprotozoal activity of alkaloids from Phaedranassa dubia (Amaryllidaceae). Phytochem. Lett. 2010, 3, 161–163. [Google Scholar] [CrossRef]
- Rojas-Vera, J.; Buitrago-Díaz, A.A.; Possamai, L.M.; Timmers, L.F.S.M.; Tallini, L.R.; Bastida, J. Alkaloid profile and cholinesterase inhibition activity of five species of Amaryllidaceae family collected from Mérida state-Venezuela. S. Afr. J. Bot. 2021, 136, 126–136. [Google Scholar] [CrossRef]
- Konrath, E.L.; Passos, C.D.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef]
- Cedrón, J.C.; Gutiérrez, D.; Flores, N.; Ravelo, Á.G.; Estévez-Braun, A. Synthesis and antiplasmodial activity of lycorine derivatives. Bioorg. Med. Chem. 2010, 18, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Antiplasmodial constituents in the minor alkaloid groups of the Amaryllidaceae. S. Afr. J. Bot. 2019, 126, 362–370. [Google Scholar] [CrossRef]
- Martinez-Peinado, N.; Ortiz, J.E.; Cortes-Serra, N.; Pinazo, M.J.; Gascon, J.; Tapia, A.; Roitman, G.; Bastida, J.; Ferensin, G.E.; Alonso-Padilla, J. Anti-Trypanosoma cruzi activity of alkaloids isolated from Habranthus brachyandrus (Amaryllidaceae) from Argentina. Phytomedicine 2022, 101, 154126. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peinado, N.; Cortes-Serra, N.; Torras-Claveria, L.; Pinazo, M.J.; Gascon, J.; Bastida, J.; Alonso-Padilla, J. Amaryllidaceae alkaloids with anti-Trypanosoma cruzi activity. Parasites Vectors 2020, 13, 299. [Google Scholar] [CrossRef]
- Bores, G.M.; Huger, F.P.; Petko, W.; Mutlib, A.E.; Camacho, F.; Rush, D.K.; Selk, D.E.; Wolf, V.; Kosley, R.W.; Davis, L.; et al. Pharmacological evaluation of novel Alzheimer’s disease therapeutics: Acetylcholinesterase inhibitors related to galanthamine. J. Pharmacol. Exp. Ther. 1996, 277, 728–738. [Google Scholar]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC-MS combined with k-means cluster analysis. Ind. Crop. Prod. 2014, 56, 211–222. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2022, 71, 2521–2529. [Google Scholar] [CrossRef]

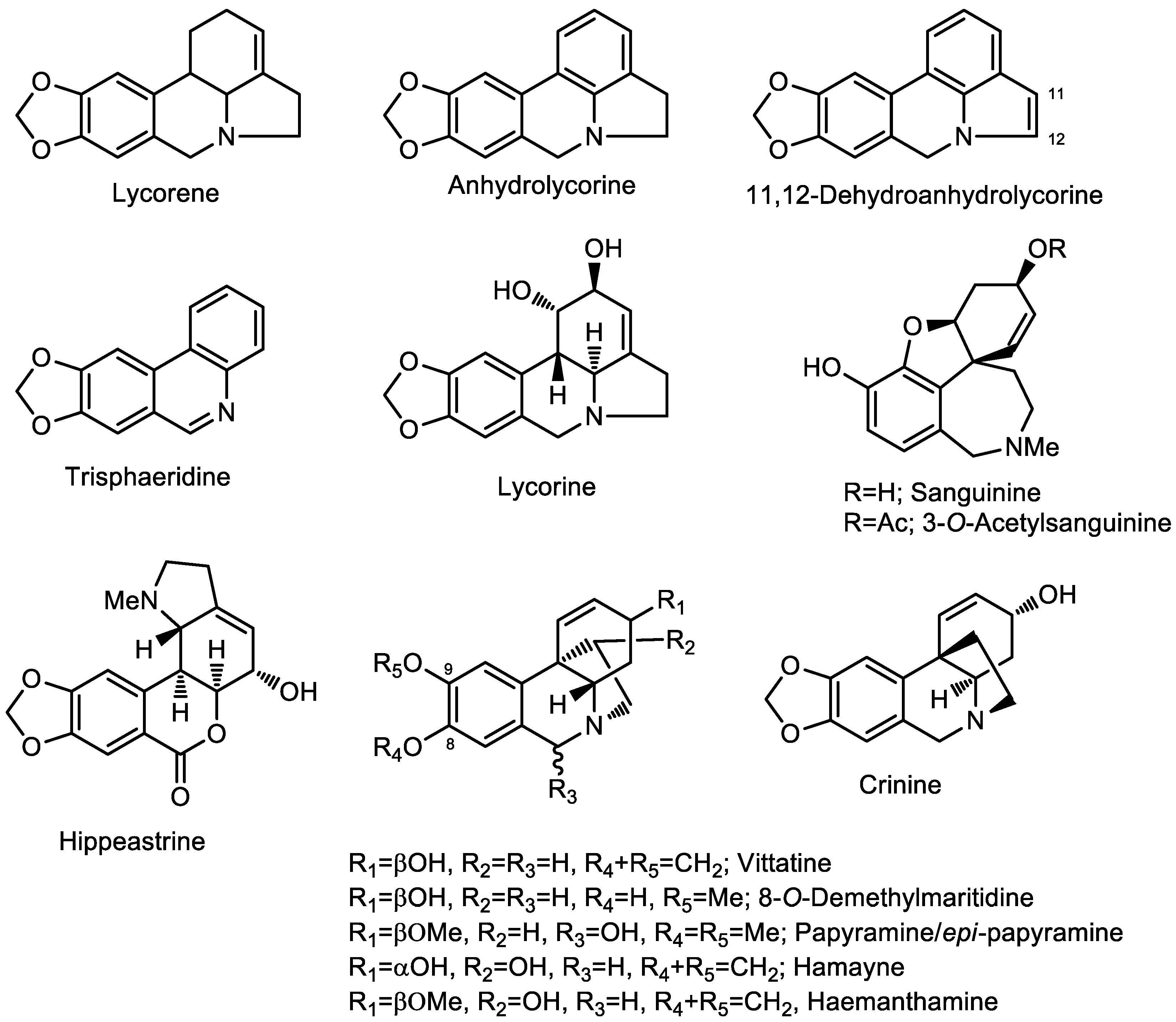
| Alkaloid | RI | MS | A | B | C | D |
|---|---|---|---|---|---|---|
| Lycorine-type (total) | 71.9 | 39.5 | 29.5 | 40.3 | ||
| Lycorene (1) | 2245.2 | 255 (60), 254(100), 227 (17), 226 (19), 211 (13), 183 (11) | - | - | 16.4 | 12.2 |
| Anhydrolycorine (2) | 2519.6 | 251 (46), 250 (100), 192 (12), 191 (11), 124 (7), 95 (7) | 39.6 | - | - | 7.7 |
| 11,12-Dehydroanhydrolycorine (3) | 2613.9 | 249 (61), 248 (100), 191 (10), 190 (25), 189 (7), 95 (15) | 26.1 | 20.7 | 6.0 | 7.1 |
| Lycorine (4) | 2735.2 | 287 (27), 286 (18), 268 (22), 250 (19), 227 (67), 226 (100) | 6.2 | 18.8 | 7.1 | 13.3 |
| Haemanthamine/crinine-type (total) | - | - | 67.7 | 110.8 | ||
| Vittatine/crinine (5a/5b) | 2467.2 | 271 (100), 200 (24), 199 (72), 187 (64), 128 (25), 115 (26) | - | - | 7.0 | 10.5 |
| 8-O-Demethylmaritidine (6) | 2501.3 | 273 (100), 230 (28), 202 (25), 201 (82), 189 (55), 175 (23) | - | - | 24.8 | 44.8 |
| Papyramine/epi-papyramine (7a/7b) | 2545.5 | 317 (100), 286 (50), 259 (46), 230 (83), 187 (36), 186 (43) | - | - | 4.7 | 6.6 |
| Haemanthamine (8) | 2628.9 | 301 (17), 273 (23), 272 (100), 242 (18), 240 (20), 181 (28) | - | - | 26.3 | 35.8 |
| Hamayne (9) | 2698.8 | 287 (5), 259 (20), 258 (100), 214 (8), 211 (16), 186 (11) | - | - | 4.9 | 13.1 |
| Galanthamine-type (total) | - | - | 14.2 | 26.5 | ||
| Sanguinine (10) | 2429.9 | 273 (100), 272 (81), 256 (20), 202 (37), 160 (44), 115 (19) | - | - | 9.9 | 19.4 |
| 3-O-Acetylsanguinine (11) | 2515.0 | 315 (52), 256 (100), 255 (60), 254 (40), 212 (27), 96 (60) | - | - | 4.3 | 7.1 |
| Narciclassine-type (total) | 3.6 | - | 4.6 | 5.2 | ||
| Trisphaeridine (12) | 2304.1 | 223 (100), 222 (36), 164 (15), 138 (22), 137 (10), 111 (13) | 3.6 | - | 4.6 | 5.2 |
| Homolycorine-type (total) | - | - | 19.9 | 29.1 | ||
| Hippeastrine (13) | 2881.2 | 315 (<1), 162 (5), 126 (14), 125 (100), 124 (15), 96 (51) | - | - | 19.9 | 29.1 |
| Unidentified Alkaloid (total) | 8.9 | 90.1 | 6.0 | 12.1 | ||
| UI (14) Haemanthamine/crinine-type * | 2599.5 | 315 (100), 286 (9), 272 (27), 256 (89), 254 (57), 218 (58) | - | - | - | 6.8 |
| UI (15) Haemanthamine/crinine-type * | 2670.1 | 297 (98), 296 (36), 278 (53), 252 (36), 251 (22), 132 (100) | - | - | 6.0 | 5.3 |
| UI (16) Lycorine-type * | 2690.8 | 373 (60), 342 (59), 250 (31), 225 (53), 212 (100) 131 (51) | - | 11.2 | - | - |
| UI (17) Ismine-type * | 2732.4 | 299 (32), 250 (20), 225 (65), 224 (100), 212 (8), 166 (9) | - | 14.8 | - | - |
| UI (18) Lycorine-type * | 2851.8 | 279 (79), 278 (100), 280 (14), 263 (9), 235 (16), 178 (12) | 8.9 | 30.5 | - | - |
| UI (19) Lycorine-type * | 2854.9 | 359 (2), 358 (4), 299 (43), 268 (100), 250 (51), 212 (26) | - | 33.6 | - | |
| Total Alkaloids | 84.4 | 129.6 | 141.9 | 224.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Escobar, M.L.; Tallini, L.R.; Lisa-Molina, J.; Berkov, S.; Viladomat, F.; Meerow, A.; Bastida, J.; Torras-Claveria, L. Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America. Molecules 2023, 28, 5408. https://doi.org/10.3390/molecules28145408
Rodríguez-Escobar ML, Tallini LR, Lisa-Molina J, Berkov S, Viladomat F, Meerow A, Bastida J, Torras-Claveria L. Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America. Molecules. 2023; 28(14):5408. https://doi.org/10.3390/molecules28145408
Chicago/Turabian StyleRodríguez-Escobar, María Lenny, Luciana R. Tallini, Julia Lisa-Molina, Strahil Berkov, Francesc Viladomat, Alan Meerow, Jaume Bastida, and Laura Torras-Claveria. 2023. "Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America" Molecules 28, no. 14: 5408. https://doi.org/10.3390/molecules28145408
APA StyleRodríguez-Escobar, M. L., Tallini, L. R., Lisa-Molina, J., Berkov, S., Viladomat, F., Meerow, A., Bastida, J., & Torras-Claveria, L. (2023). Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America. Molecules, 28(14), 5408. https://doi.org/10.3390/molecules28145408











