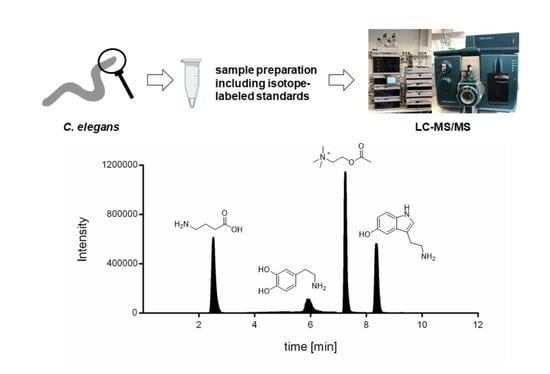
A Reliable Method Based on Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Quantification of Neurotransmitters in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
2.1. Method Development for Neurotransmitter Quantification via LC-MS/MS
2.2. Sample Preparation and Neurotransmitter Extraction
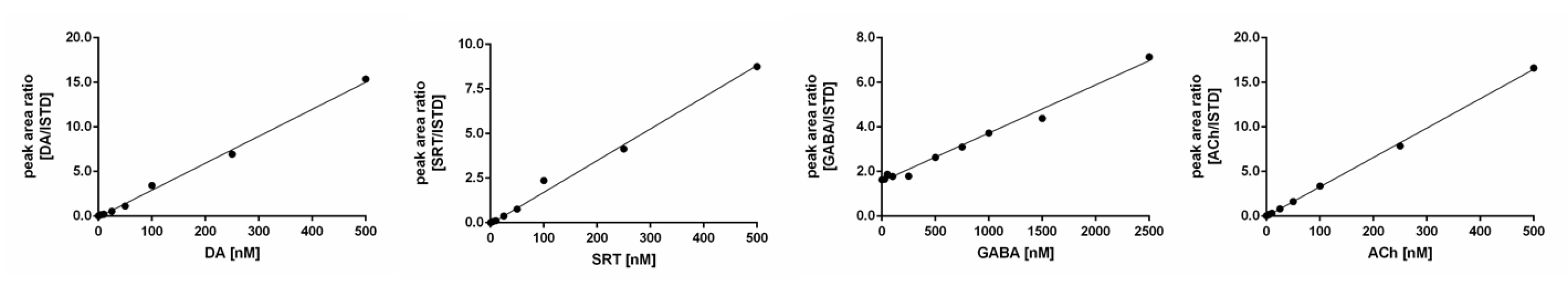
2.3. Method Validation
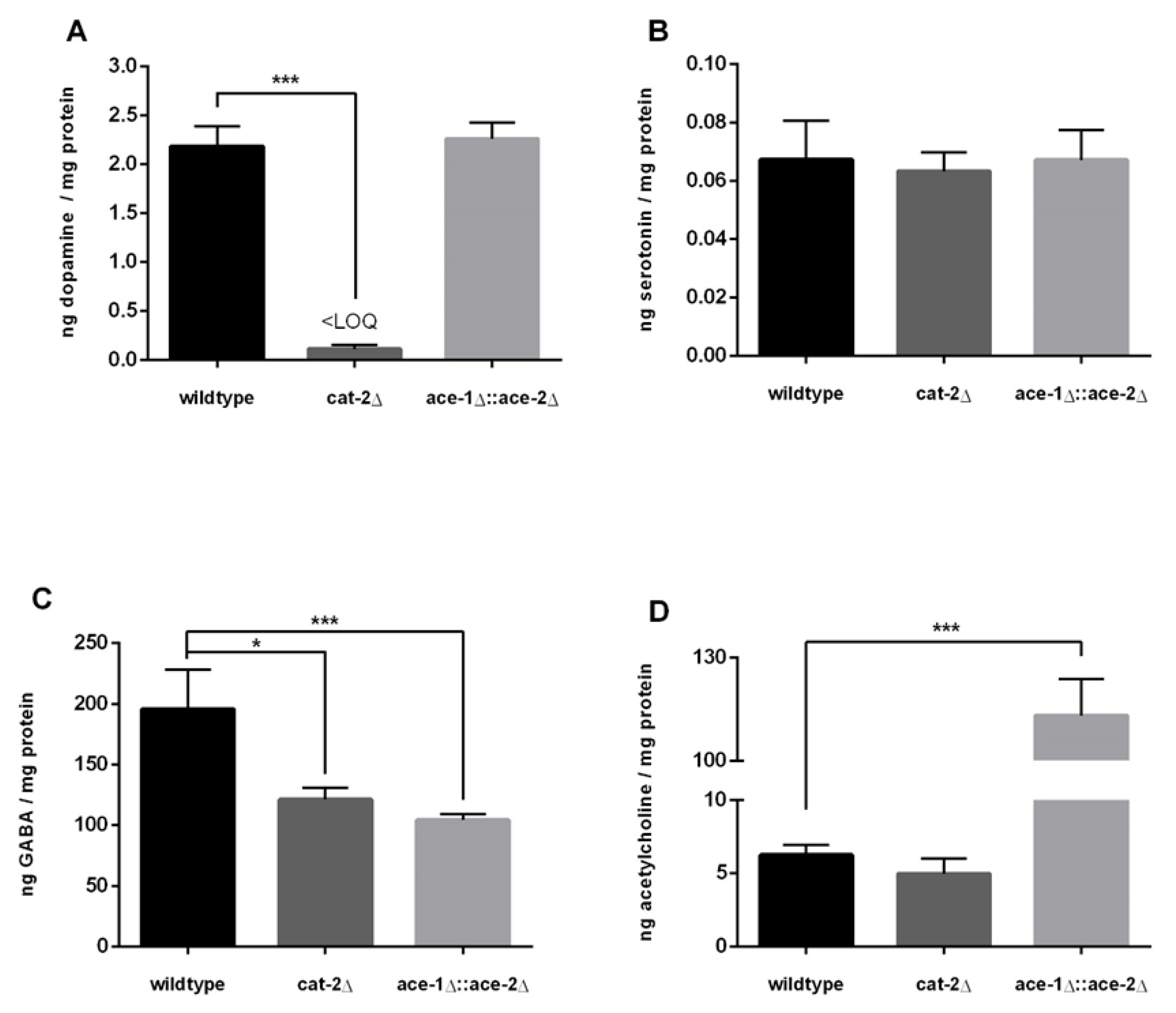
2.4. Neurotransmitter Levels in Wildtype Worms and cat-2Δ and ace-1Δ::ace-2Δ Deletion Mutants
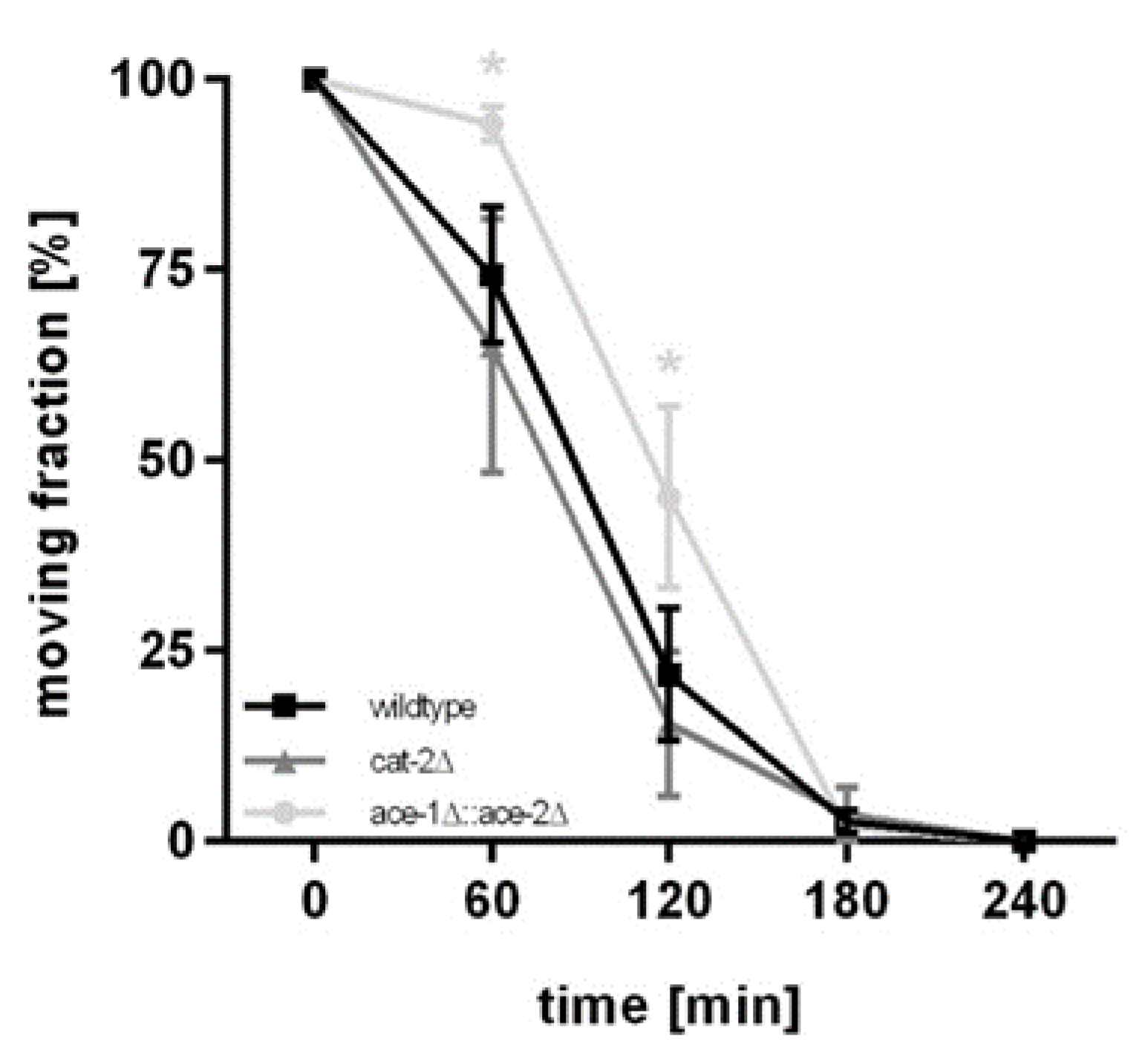
2.5. Aldicarb-Induced Paralysis Assay
3. Discussion
4. Materials and Methods
4.1. C. elegans Handling and Cultivation
4.2. Neurotransmitter Standard Solutions
4.3. Sample Preparation and Neurotransmitter Extraction
4.4. LC-MS/MS Parameters
4.5. Method Validation
4.6. Aldicarb-Induced Paralysis Assay
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Meder, D.; Herz, D.M.; Rowe, J.B.; Lehéricy, S.; Siebner, H.R. The role of dopamine in the brain–Lessons learned from Parkinson’s disease. Neuroimage 2019, 190, 79–93. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Di Giovanni, G. Serotonin in Health and Disease. Int. J. Mol. Sci. 2020, 21, 3500. [Google Scholar] [CrossRef] [PubMed]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A Brief Review on the Non-protein Amino Acid, Gamma-amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef]
- Sood, A.; Preeti, K.; Fernandes, V.; Khatri, D.K.; Singh, S.B. Glia: A major player in glutamate-GABA dysregulation-mediated neurodegeneration. J. Neurosci. Res. 2021, 99, 3148–3189. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Natural Products in Alzheimer’s Disease Therapy: Would Old Therapeutic Approaches Fix the Broken Promise of Modern Medicines? Molecules 2019, 24, 1519. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Tretter, F.; Voit, E.O. A heuristic model of alcohol dependence. PLoS ONE 2014, 9, e92221. [Google Scholar] [CrossRef] [PubMed]
- Huber, N.; Korhonen, S.; Hoffmann, D.; Leskelä, S.; Rostalski, H.; Remes, A.M.; Honkakoski, P.; Solje, E.; Haapasalo, A. Deficient neurotransmitter systems and synaptic function in frontotemporal lobar degeneration-Insights into disease mechanisms and current therapeutic approaches. Mol. Psychiatry 2022, 27, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Zhang, M.-D.; Tao, X.; Zhou, Y.-F.; Liu, X.-M.; Pan, R.-L.; Liao, Y.-H.; Chang, Q. LC-MS/MS-based quantification of tryptophan metabolites and neurotransmitters in the serum and brain of mice. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1112, 24–32. [Google Scholar] [CrossRef]
- Blanco, M.E.; Mayo, O.B.; Bandiera, T.; de Pietri Tonelli, D.; Armirotti, A. LC-MS/MS analysis of twelve neurotransmitters and amino acids in mouse cerebrospinal fluid. J. Neurosci. Methods 2020, 341, 108760. [Google Scholar] [CrossRef]
- Fernandez-Funez, P.; Sanchez-Garcia, J.; Rincon-Limas, D.E. Drosophila models of prionopathies: Insight into prion protein function, transmission, and neurotoxicity. Curr. Opin. Genet. Dev. 2017, 44, 141–148. [Google Scholar] [CrossRef]
- Taylor, C.A.; Tuschl, K.; Nicolai, M.M.; Bornhorst, J.; Gubert, P.; Varão, A.M.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. Maintaining Translational Relevance in Animal Models of Manganese Neurotoxicity. J. Nutr. 2020, 150, 1360–1369. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.A.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M. C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 2018, 354, 126–135. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Cooper, J.F.; van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Parkinsons. Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, X.; Liu, C.; Yan, W.; Ma, J.; Petersen, R.B.; Peng, A.; Huang, K. Modelling Parkinson’s Disease in C. elegans: Strengths and Limitations. Curr. Pharm. Des. 2022, 28, 3033–3048. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.A.; Willicott, C.W.; Caldwell, G.A. Modeling neurodegeneration in Caenorhabditiselegans. Dis. Model. Mech. 2020, 13, dmm046110. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Galindo, F.J.; Ai, R.; Fang, E.F.; Nilsen, H.L.; SenGupta, T.C. elegans as an Animal Model to Study the Intersection of DNA Repair, Aging and Neurodegeneration. Front. Aging 2022, 3, 916118. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H.R.C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef]
- Trojanowski, N.F.; Raizen, D.M.; Fang-Yen, C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci. Rep. 2016, 6, 22940. [Google Scholar] [CrossRef]
- Mahoney, T.R. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat. Protoc. 2006, 1, 1772–1777. [Google Scholar] [CrossRef]
- Ke, T.; Tsatsakis, A.; Santamaría, A.; Antunes Soare, F.A.; Tinkov, A.A.; Docea, A.O.; Skalny, A.; Bowman, A.B.; Aschner, M. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology 2020, 77, 105–113. [Google Scholar] [CrossRef]
- Yan, Z.; Cheng, X.; Li, Y.; Su, Z.; Zhou, Y.; Liu, J. Sexually Dimorphic Neurotransmitter Release at the Neuromuscular Junction in Adult Caenorhabditis elegans. Front. Mol. Neurosci. 2021, 14, 780396. [Google Scholar] [CrossRef]
- Schumacher, F.; Chakraborty, S.; Kleuser, B.; Gulbins, E.; Schwerdtle, T.; Aschner, M.; Bornhorst, J. Highly sensitive isotope-dilution liquid-chromatography-electrospray ionization-tandem-mass spectrometry approach to study the drug-mediated modulation of dopamine and serotonin levels in Caenorhabditis elegans. Talanta 2015, 144, 71–79. [Google Scholar] [CrossRef]
- Patel, B.A.; Arundell, M.; Parker, K.H.; Yeoman, M.S.; O’Hare, D. Simple and rapid determination of serotonin and catecholamines in biological tissue using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 818, 269–276. [Google Scholar] [CrossRef]
- Baek, J.Y.; Trinh, T.A.; Huh, W.; Song, J.H.; Kim, H.Y.; Lim, J.; Kim, J.; Choi, H.J.; Kim, T.-H.; Kang, K.S. Electro-Acupuncture Alleviates Cisplatin-Induced Anorexia in Rats by Modulating Ghrelin and Monoamine Neurotransmitters. Biomolecules 2019, 9, 624. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Mu, H.; Bai, Y.-H.; Yu, H.; Hu, Y.-M. A rapid method for the determination of dopamine in porcine muscle by pre-column derivatization and HPLC with fluorescence detection. J. Pharm. Anal. 2011, 1, 208–212. [Google Scholar] [CrossRef]
- Şanlı, N.; Tague, S.E.; Lunte, C. Analysis of amino acid neurotransmitters from rat and mouse spinal cords by liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2015, 107, 217–222. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chen, M.; Hu, N.; Hu, Y.; Chen, R.; Lyu, Y.; Guo, W.; Li, L.; Liu, Y. Carbon dots-based dual-emission ratiometric fluorescence sensor for dopamine detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 243, 118804. [Google Scholar] [CrossRef]
- Sun, F.; Zeng, J.; Jing, M.; Zhou, J.; Feng, J.; Owen, S.F.; Luo, Y.; Li, F.; Wang, H.; Yamaguchi, T.; et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174, 481–496.e19. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Feng, A.; Wu, X.; Che, X.; Zhou, W. Enhanced Tb(III) fluorescence on gelatin-coated silver nanoparticles in dopamine detection. Talanta 2021, 231, 122334. [Google Scholar] [CrossRef]
- Wu, J.; Wiegand, R.; LoRusso, P.; Li, J. A stable isotope-labeled internal standard is essential for correcting for the interindividual variability in the recovery of lapatinib from cancer patient plasma in quantitative LC-MS/MS analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 941, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Carreño, F.; Helfer, V.E.; Staudt, K.J.; Olivo, L.B.; Barreto, F.; Herrmann, A.P.; Rates, S.M.K.; Dalla Costa, T. Quantification of neurotransmitters in microdialysate samples following quetiapine dosing to schizophrenia phenotyped rats using a validated LC-MS/MS method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1155, 122282. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Z.; Zhu, L.; Sui, Z.; Bi, W.; Liu, R.; Bi, K.; Li, Q. Targeted Neurotransmitters Profiling Identifies Metabolic Signatures in Rat Brain by LC-MS/MS: Application in Insomnia, Depression and Alzheimer’s Disease. Molecules 2018, 23, 2375. [Google Scholar] [CrossRef]
- Tufi, S.; Leonards, P.; Lamoree, M.; de Boer, J.; Legler, J.; Legradi, J. Changes in Neurotransmitter Profiles during Early Zebrafish (Danio rerio) Development and after Pesticide Exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef]
- Fuertes, I.; Barata, C. Characterization of neurotransmitters and related metabolites in Daphnia magna juveniles deficient in serotonin and exposed to neuroactive chemicals that affect its behavior: A targeted LC-MS/MS method. Chemosphere 2021, 263, 127814. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- McHardy, S.F.; Wang, H.-Y.L.; McCowen, S.V.; Valdez, M.C. Recent advances in acetylcholinesterase Inhibitors and Reactivators: An update on the patent literature (2012-2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef]
- Oh, K.H.; Kim, H. Aldicarb-induced paralysis assay to determine defects in synaptic transmission in Caenorhabditis elegans. Bio-protocol 2017, 7, e2400. [Google Scholar] [CrossRef]
- Rand, J.B. Acetylcholine. WormBook 2007, 72, 362. [Google Scholar] [CrossRef]
- Bhat, S.; El-Kasaby, A.; Freissmuth, M.; Sucic, S. Functional and Biochemical Consequences of Disease Variants in Neurotransmitter Transporters: A Special Emphasis on Folding and Trafficking Deficits. Pharmacol. Ther. 2021, 222, 107785. [Google Scholar] [CrossRef]
- Rizzi, G.; Tan, K.R. Dopamine and Acetylcholine, a Circuit Point of View in Parkinson’s Disease. Front. Neural Circuits 2017, 11, 110. [Google Scholar] [CrossRef]
- Sanchez-Catasus, C.A.; Bohnen, N.I.; D’Cruz, N.; Müller, M.L.T.M. Striatal Acetylcholine-Dopamine Imbalance in Parkinson Disease: In Vivo Neuroimaging Study with Dual-Tracer PET and Dopaminergic PET-Informed Correlational Tractography. J. Nucl. Med. 2022, 63, 438–445. [Google Scholar] [CrossRef]
- Umek, N.; Geršak, B.; Vintar, N.; Šoštarič, M.; Mavri, J. Dopamine Autoxidation Is Controlled by Acidic pH. Front. Mol. Neurosci. 2018, 11, 467. [Google Scholar] [CrossRef]
- Huang, F.; Li, J.; Shi, H.-L.; Wang, T.; Muhtar, W.; Du, M.; Zhang, B.; Wu, H.; Yang, L.; Hu, Z.; et al. Simultaneous quantification of seven hippocampal neurotransmitters in depression mice by LC-MS/MS. J. Neurosci. Methods 2014, 229, 8–14. [Google Scholar] [CrossRef]
- Olesti, E.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Ramaekers, J.G.; de la Torre, R.; Pozo, O.J. Quantification of endogenous neurotransmitters and related compounds by liquid chromatography coupled to tandem mass spectrometry. Talanta 2019, 192, 93–102. [Google Scholar] [CrossRef]
- Han, X.-M.; Qin, Y.-J.; Zhu, Y.; Zhang, X.-L.; Wang, N.-X.; Rang, Y.; Zhai, X.-J.; Lu, Y.-N. Development of an underivatized LC-MS/MS method for quantitation of 14 neurotransmitters in rat hippocampus, plasma and urine: Application to CUMS induced depression rats. J. Pharm. Biomed. Anal. 2019, 174, 683–695. [Google Scholar] [CrossRef]
- McLafferty, F.W.; Turecek, F. Interpretation of Mass Spectra; University Science Books: Mill Welley, CA, USA, 1993; ISBN 978-0-935702-25-5. [Google Scholar]
- Zhang, M.; Fang, C.; Smagin, G. Derivatization for the simultaneous LC/MS quantification of multiple neurotransmitters in extracellular fluid from rat brain microdialysis. J. Pharm. Biomed. Anal. 2014, 100, 357–364. [Google Scholar] [CrossRef]
- Caito, S.W.; Aschner, M. NAD+ Supplementation Attenuates Methylmercury Dopaminergic and Mitochondrial Toxicity in Caenorhabditis Elegans. Toxicol. Sci. 2016, 151, 139–149. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Miah, M.R.; Akingbade, G.T.; Bucinca, H.; Aschner, M. Nickel-Induced Developmental Neurotoxicity in C. elegans Includes Cholinergic, Dopaminergic and GABAergic Degeneration, Altered Behaviour, and Increased SKN-1 Activity. Neurotox. Res. 2020, 37, 1018–1028. [Google Scholar] [CrossRef]
- Defaix, C.; Solgadi, A.; Pham, T.H.; Gardier, A.M.; Chaminade, P.; Tritschler, L. Rapid analysis of glutamate, glutamine and GABA in mice frontal cortex microdialysis samples using HPLC coupled to electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 152, 31–38. [Google Scholar] [CrossRef]
- Muñoz, A.; Lopez-Lopez, A.; Labandeira, C.M.; Labandeira-Garcia, J.L. Interactions Between the Serotonergic and Other Neurotransmitter Systems in the Basal Ganglia: Role in Parkinson’s Disease and Adverse Effects of L-DOPA. Front. Neuroanat. 2020, 14, 26. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Moussaif, M.; Kullyev, A.; Nguyen, K.C.Q.; McDonald, T.V.; Hall, D.H.; Sze, J.Y. A homolog of FHM2 is involved in modulation of excitatory neurotransmission by serotonin in C. elegans. PLoS ONE 2010, 5, e10368. [Google Scholar] [CrossRef]
- Suthakaran, N.; Brock, T.; Naraine, A.; Gonzalez-Lerma, P.; Hopkins, C.; Dawson-Scully, K. Atropine reduces aldicarb-induced sensitivity to C. elegans electroshock model. MicroPubl. Biol. 2022, 2022. [Google Scholar] [CrossRef]
- Giles, A.C.; Desbois, M.; Opperman, K.J.; Tavora, R.; Maroni, M.J.; Grill, B. A complex containing the O-GlcNAc transferase OGT-1 and the ubiquitin ligase EEL-1 regulates GABA neuron function. J. Biol. Chem. 2019, 294, 6843–6856. [Google Scholar] [CrossRef]
- Opperman, C.H.; Chang, S. Effects of Aldicarb and Fenamiphos on Acetycholinesterase and Motility of Caenorhabditis elegans. J. Nematol. 1991, 23, 20–27. [Google Scholar]
- Nass, R. The nematode C. elegans as an animal model to explore toxicology in vivo: Solid and axenic growth culture conditions and compound exposure parameters. Curr. Protoc. Toxicol. 2007, 31, 1.9.1–1.9.18. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef] [PubMed]
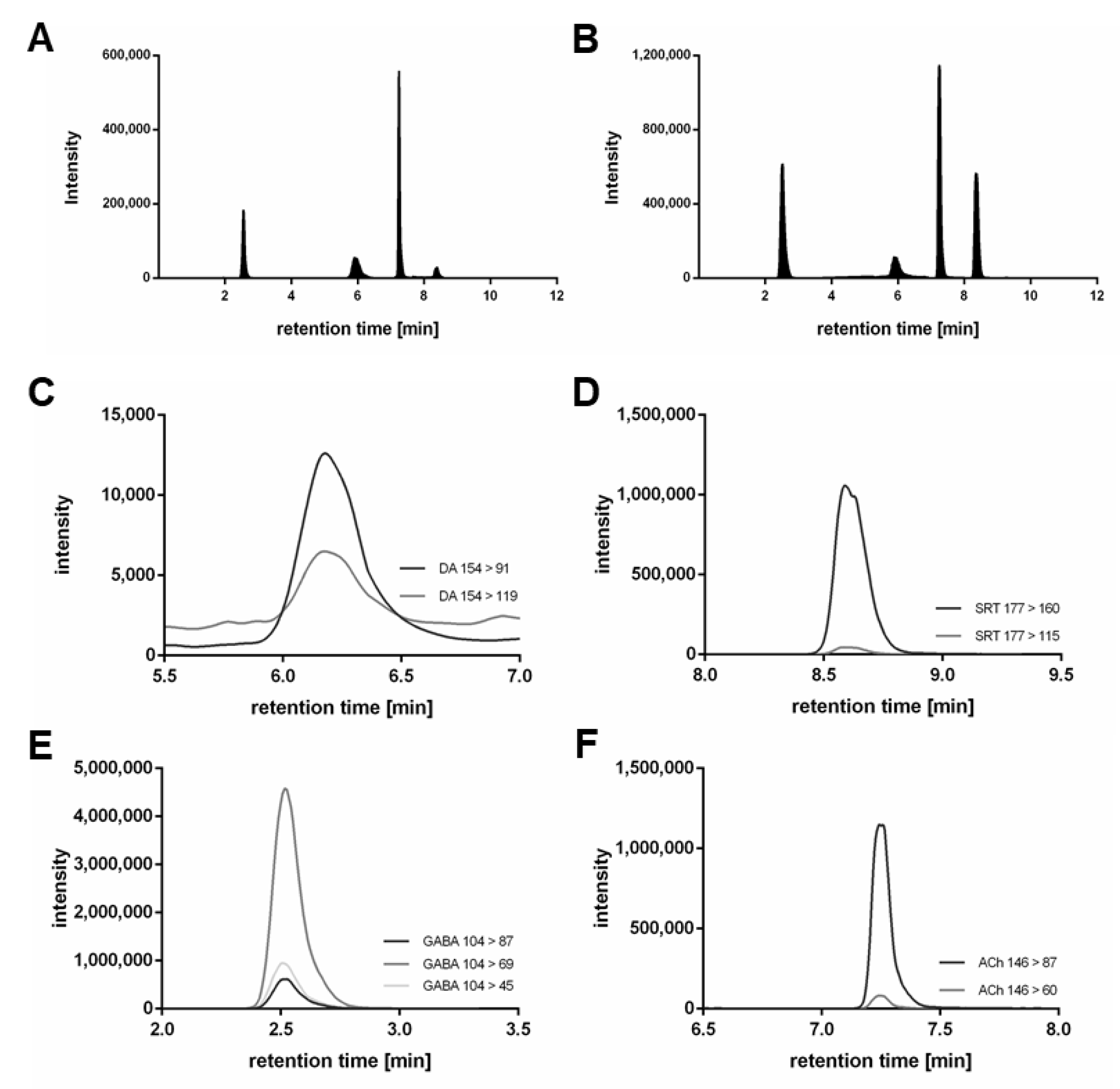
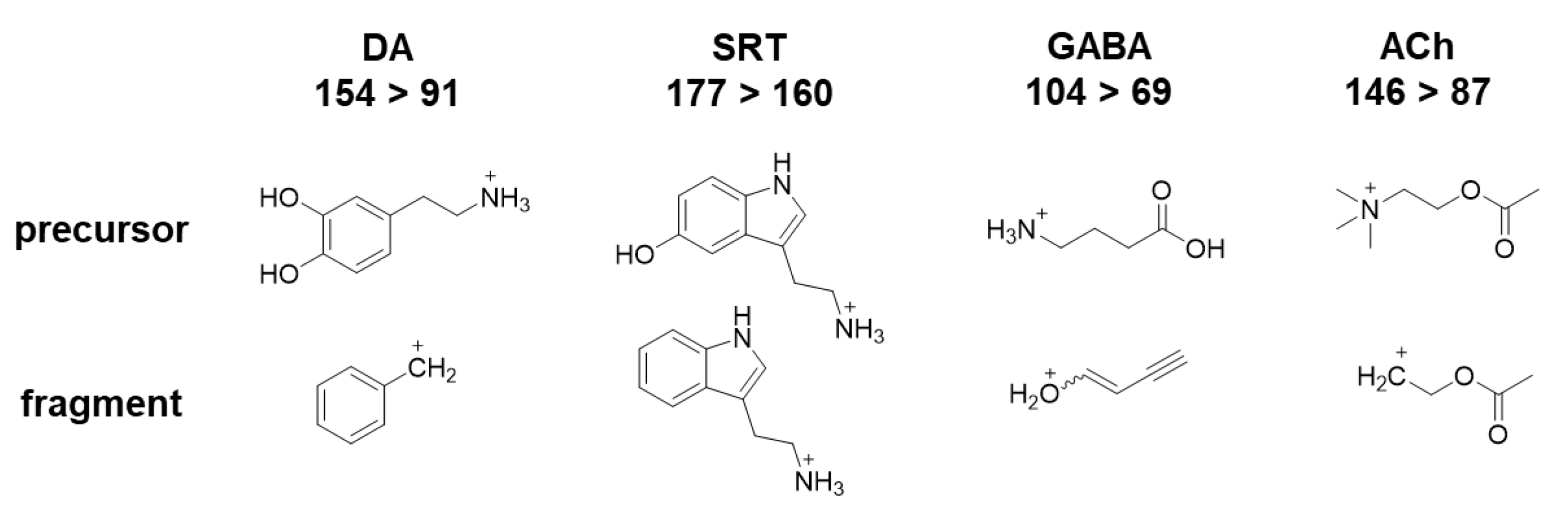
| Compound | Q1 | Q3 | CE | DP | CXP | Retention Time (min) |
|---|---|---|---|---|---|---|
| DA | 154 | 137 | 15 | 30 | 15 | 5.92 |
| 119 | 25 | 30 | 15 | |||
| 91 | 32 | 30 | 15 | |||
| DAd4 | 158 | 141 | 15 | 30 | 15 | |
| 123 | 25 | 30 | 15 | |||
| 95 | 32 | 30 | 15 | |||
| SRT | 177 | 160 | 15 | 15 | 17 | 8.38 |
| 115 | 51 | 30 | 41 | |||
| SRTd4 | 181 | 164 | 15 | 15 | 17 | |
| 118 | 51 | 30 | 41 | |||
| GABA | 104 | 87 | 15 | 17 | 10 | 2.50 |
| 69 | 21 | 18 | 10 | |||
| 45 | 28 | 25 | 11 | |||
| GABAd6 | 110 | 93 | 15 | 17 | 10 | |
| 73 | 21 | 18 | 10 | |||
| 49 | 28 | 25 | 11 | |||
| ACh | 146 | 87 | 19 | 27 | 13 | 7.22 |
| 60 | 16 | 32 | 9 | |||
| AChd4 | 150 | 91 | 19 | 27 | 13 | |
| 60 | 16 | 32 | 9 |
| DA | SRT | GABA | ACh | |
|---|---|---|---|---|
| Concentration in samples # | 11.9 nM | 2.2 nM | 2.6 µM | 38.8 nM |
| Correlation coefficient (R2) | 0.9966 | 0.9939 | 0.9873 | 0.9993 |
| Limit of detection (nM) | 0.204 | 0.097 | 15.628 | 0.0009 |
| Limit of quantification (nM) | 0.679 | 0.324 | 52.094 | 0.0029 |
| Recovery (%) | 103 ± 2.7 | 64 ± 2.3 | 80 ± 4.1 | 56 ± 11.9 |
| Accuracy [%] | Precision [RSD%] | ||||
|---|---|---|---|---|---|
| Low | Middle | High | Intraday | Interday | |
| DA | 114.8 ± 8.8 | 111.1 ± 7.9 | 112.7 ± 4.1 | 3.1 | 2.6 |
| SRT | 84.9 ± 1.3 | 85.6 ± 1.5 | 81.1 ± 1.8 | 6.1 | 14.0 |
| GABA | 95.3 ± 8.7 | 108.2 ± 5.2 | 116.4 ± 5.4 | 3.4 | 3.2 |
| ACh | 98.5 ± 4.9 | 96.6 ± 1.0 | 99.8 ± 0.6 | 7.6 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weishaupt, A.-K.; Kubens, L.; Ruecker, L.; Schwerdtle, T.; Aschner, M.; Bornhorst, J. A Reliable Method Based on Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Quantification of Neurotransmitters in Caenorhabditis elegans. Molecules 2023, 28, 5373. https://doi.org/10.3390/molecules28145373
Weishaupt A-K, Kubens L, Ruecker L, Schwerdtle T, Aschner M, Bornhorst J. A Reliable Method Based on Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Quantification of Neurotransmitters in Caenorhabditis elegans. Molecules. 2023; 28(14):5373. https://doi.org/10.3390/molecules28145373
Chicago/Turabian StyleWeishaupt, Ann-Kathrin, Laura Kubens, Lysann Ruecker, Tanja Schwerdtle, Michael Aschner, and Julia Bornhorst. 2023. "A Reliable Method Based on Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Quantification of Neurotransmitters in Caenorhabditis elegans" Molecules 28, no. 14: 5373. https://doi.org/10.3390/molecules28145373
APA StyleWeishaupt, A.-K., Kubens, L., Ruecker, L., Schwerdtle, T., Aschner, M., & Bornhorst, J. (2023). A Reliable Method Based on Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Quantification of Neurotransmitters in Caenorhabditis elegans. Molecules, 28(14), 5373. https://doi.org/10.3390/molecules28145373