Engineered Lipidic Nanomaterials Inspired by Sphingomyelin Metabolism for Cancer Therapy
Abstract
1. Introduction
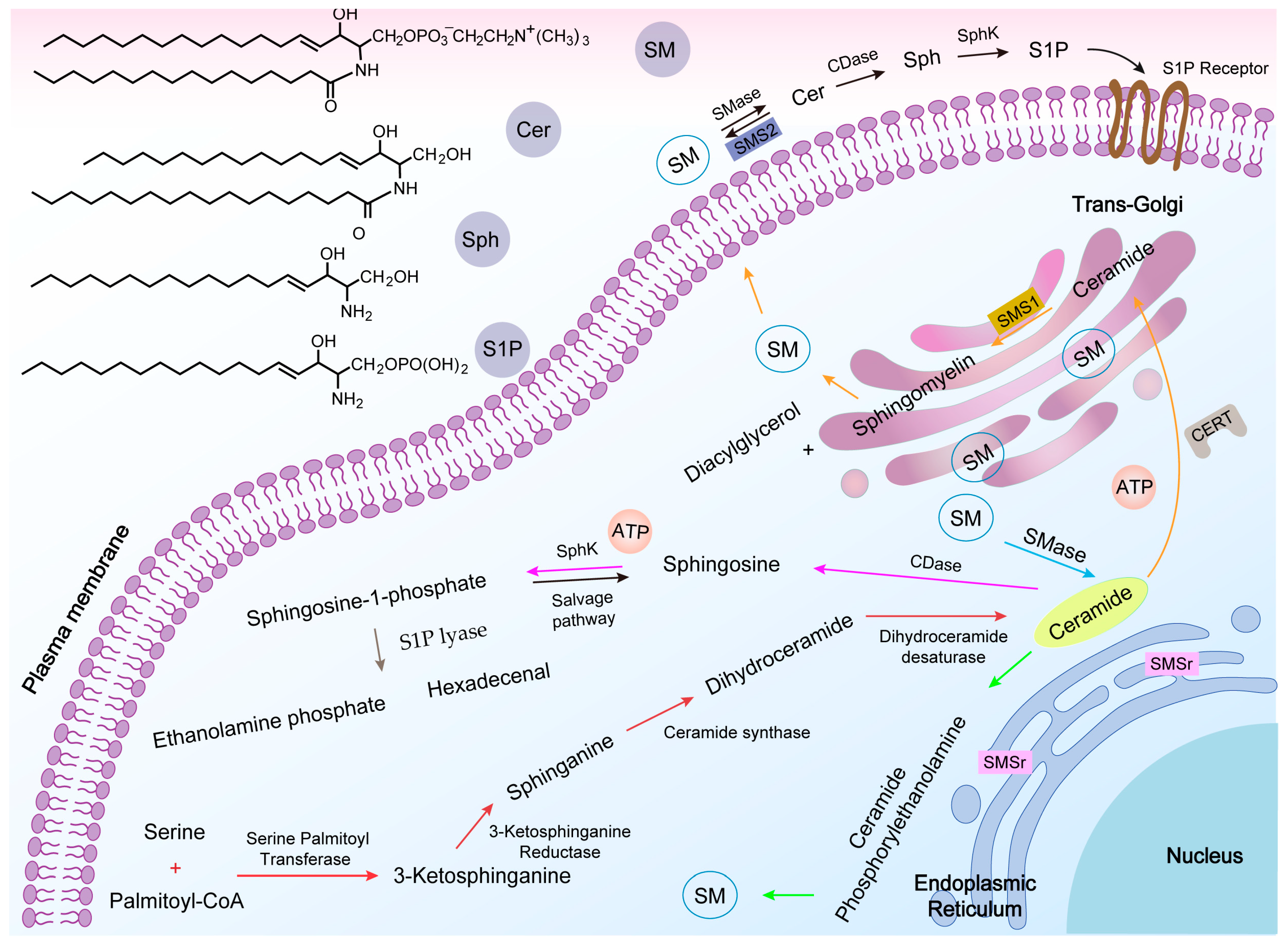
2. Synthesis and Metabolism of SM
2.1. Structure and Distribution of SM
2.2. SM Synthesis
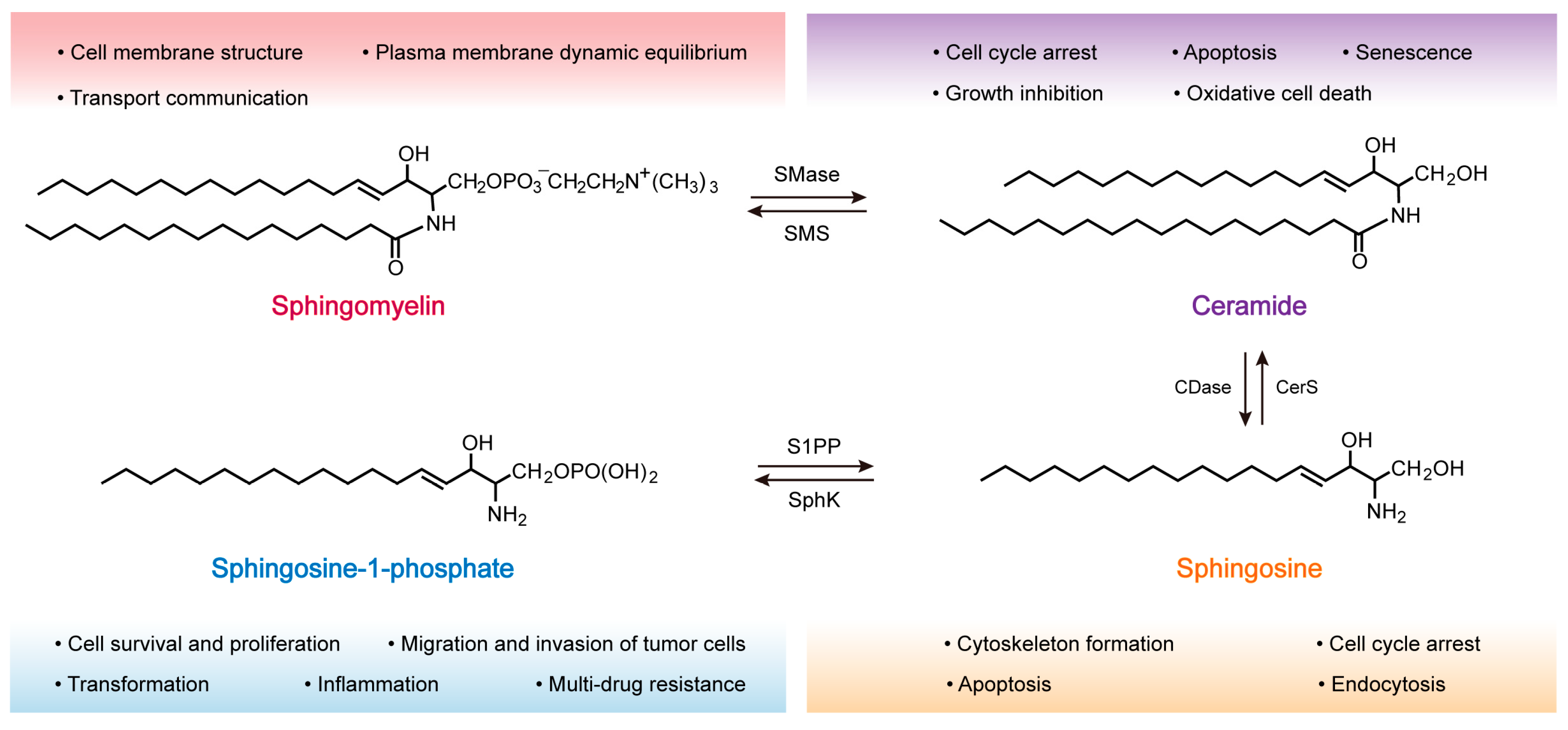
2.3. SM Metabolism
2.3.1. Ceramide (Cer)
2.3.2. Sphingosine (Sph)
2.3.3. Sphingosine-1-Phosphate (S1P)
3. Role of SM in the Development of Tumors
3.1. Oncogenesis
3.2. Proliferation and Metastasis
3.3. Multidrug Resistance
4. SM Metabolism-Based Lipidic Nanomaterials for Cancer Therapy
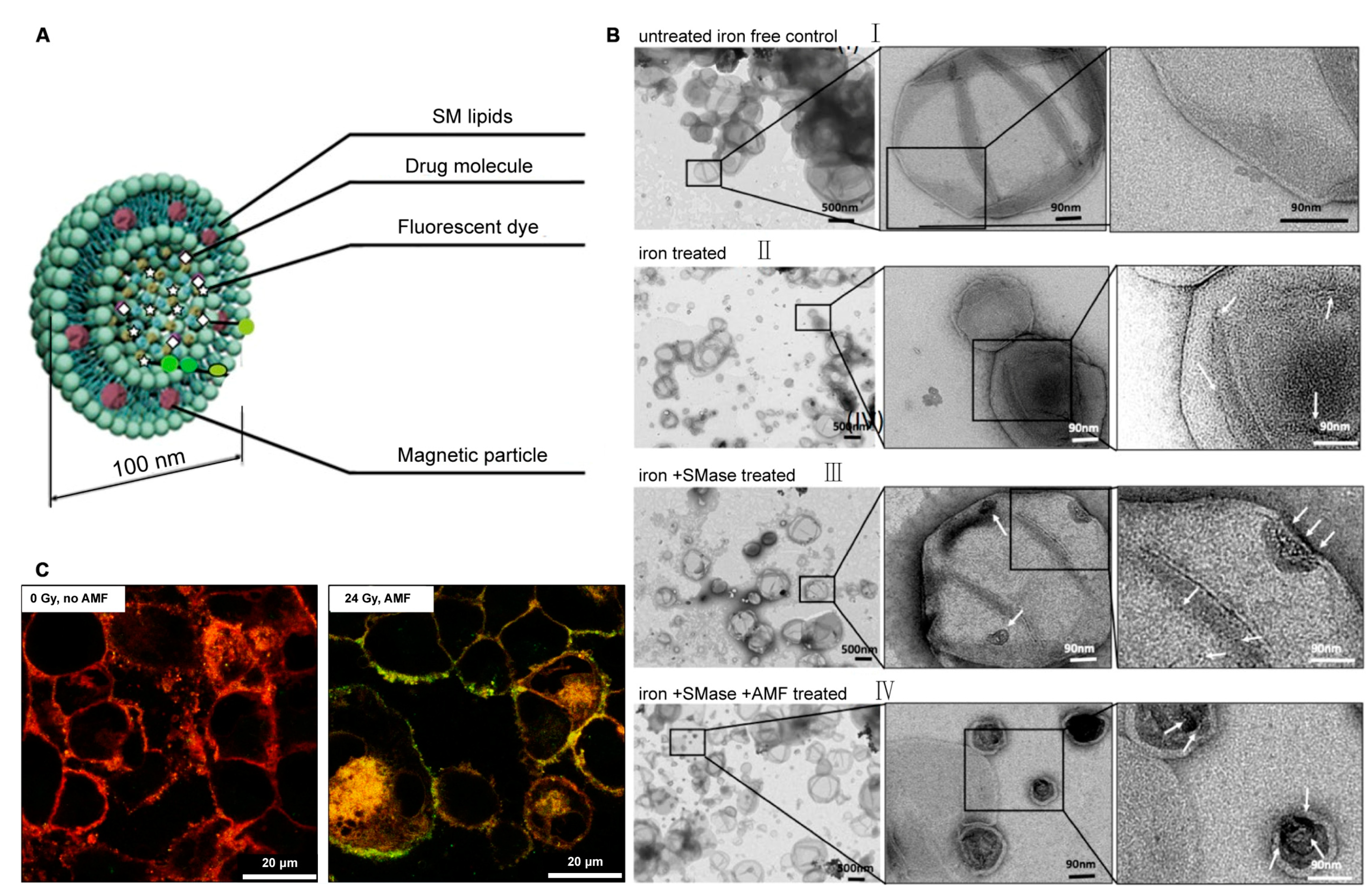
4.1. SM-Based Lipidic Nanomaterials for Cancer Therapy
4.2. Cer-Based Lipidic Nanomaterials for Cancer Therapy
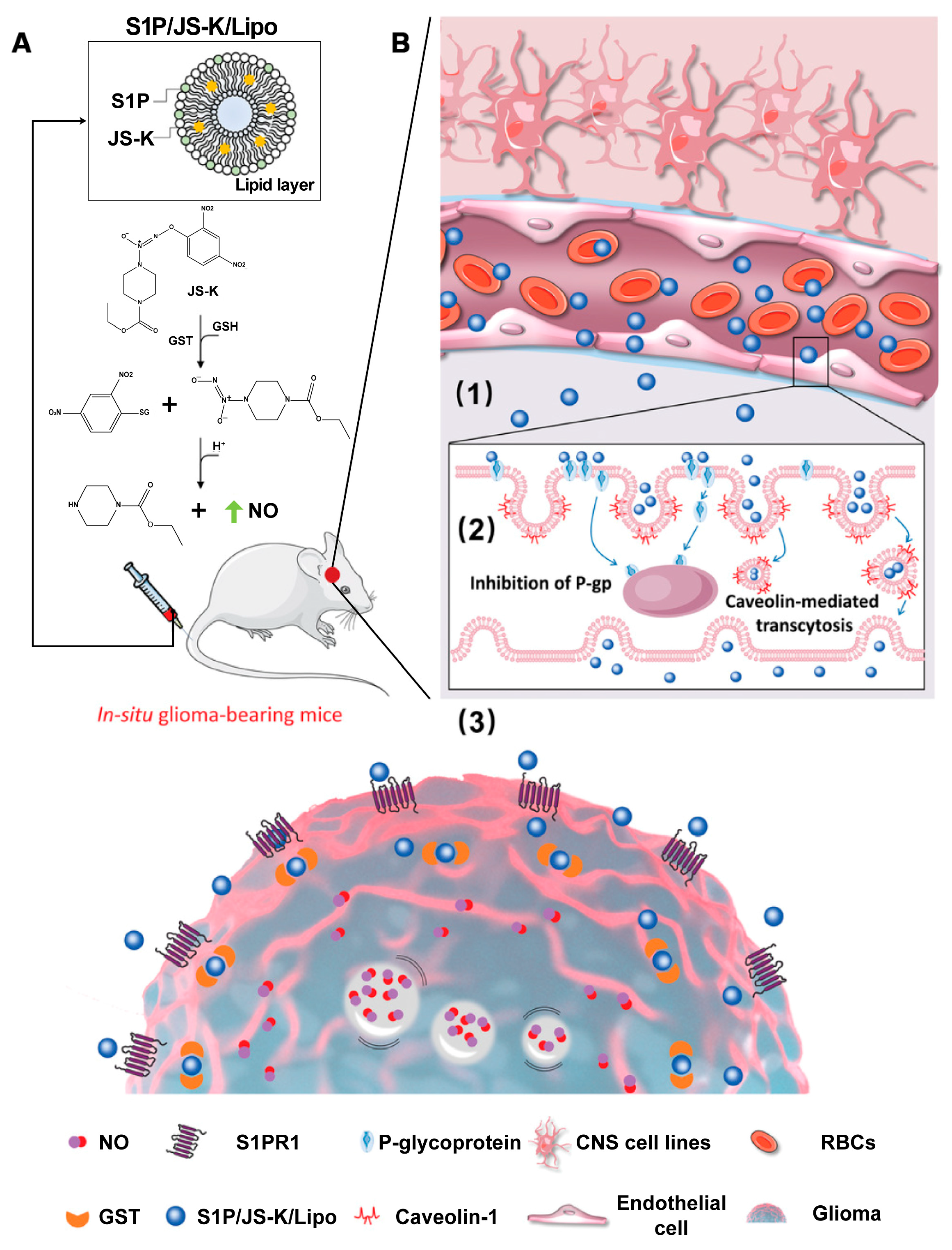
4.3. S1P-Based Lipidic Nanomaterials for Cancer Therapy
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine--challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for cancer therapies. Nanotechnol. Rev. 2017, 6, 473–496. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvara, P.; Metselaar, J.; Banala, S.; Straub, M.; Guvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Li, M.; Huang, Y.; Wu, J.; Li, S.; Mei, M.; Chen, H.; Wang, N.; Wu, W.; Zhou, B.; Tan, X.; et al. A PEG-lipid-free COVID-19 mRNA vaccine triggers robust immune responses in mice. Mater. Horiz. 2023, 10, 466–472. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Murty, U.S.; Banerjee, S. Lipidic nanomaterials to deliver natural compounds against cancer: A review. Environ. Chem. Lett. 2020, 18, 1803–1812. [Google Scholar] [CrossRef]
- Limongi, T.; Susa, F.; Marini, M.; Allione, M.; Torre, B.; Pisano, R.; di Fabrizio, E. Lipid-based nanovesicular drug delivery systems. Nanomaterials 2021, 11, 3391. [Google Scholar] [CrossRef]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Wolrab, D.; Jirasko, R.; Cifkova, E.; Horing, M.; Mei, D.; Chocholouskova, M.; Peterka, O.; Idkowiak, J.; Hrnciarova, T.; Kuchar, L.; et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022, 13, 124. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, R.Q.; Wang, Z.G.; Liu, S.L. In situ quantification of lipids in live cells by using lipid-binding domain-based biosensors. Bioconjug. Chem. 2022, 33, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Radin, N.S. Cancer progression in the kidney and prostate: Vital roles of sphingolipids in chemotherapy. Urology 2002, 60, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Vykoukal, J.; Fahrmann, J.F.; Gregg, J.R.; Tang, Z.; Basourakos, S.; Irajizad, E.; Park, S.; Yang, G.; Creighton, C.J.; Fleury, A.; et al. Caveolin-1-mediated sphingolipid oncometabolism underlies a metabolic vulnerability of prostate cancer. Nat. Commun. 2020, 11, 4279. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Moorthi, S.; Luberto, C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv. Cancer Res. 2018, 140, 61–96. [Google Scholar]
- Bienias, K.; Fiedorowicz, A.; Sadowska, A.; Prokopiuk, S.; Car, H. Regulation of sphingomyelin metabolism. Pharmacol. Rep. 2016, 68, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef]
- Modrak, D.E.; Gold, D.V.; Goldenberg, D.M. Sphingolipid targets in cancer therapy. Mol. Cancer Ther. 2006, 5, 200–208. [Google Scholar] [CrossRef]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. Role of ceramide/sphingomyelin (SM) balance regulated through “SM cycle” in cancer. Cell. Signal. 2021, 87, 110119. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef]
- Taha, T.A.; Mullen, T.D.; Obeid, L.M. A house divided: Ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta 2006, 1758, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Investig. 2002, 110, 3–8. [Google Scholar] [CrossRef]
- Huwiler, A.; Pfeilschifter, J. Altering the sphingosine-1-phosphate/ceramide balance: A promising approach for tumor therapy. Curr. Pharm. Des. 2006, 12, 4625–4635. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Schmelz, E.M.; Dillehay, D.L.; Spiegel, S.; Shayman, J.A.; Schroeder, J.J.; Riley, R.T.; Voss, K.A.; Wang, E. Sphingolipids--the enigmatic lipid class: Biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 1997, 142, 208–225. [Google Scholar] [CrossRef]
- Filippov, A.; Oradd, G.; Lindblom, G. Sphingomyelin structure influences the lateral diffusion and raft formation in lipid bilayers. Biophys. J. 2006, 90, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Christie; William, W. Lipids: Their structures and occurrence. In Lipid Analysis, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–19. [Google Scholar]
- Furland, N.E.; Zanetti, S.R.; Oresti, G.M.; Maldonado, E.N.; Aveldano, M.I. Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells. J. Biol. Chem. 2007, 282, 18141–18150. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Allan, D.; Quinn, P. Resynthesis of sphingomyelin from plasma-membrane phosphatidylcholine in BHK cells treated with staphylococcus aureus sphingomyelinase. Biochem. J. 1988, 254, 765–771. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Futerman, A.H.; Stieger, B.; Hubbard, A.L.; Pagano, R.E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J. Biol. Chem. 1990, 265, 8650–8657. [Google Scholar] [CrossRef] [PubMed]
- Huitema, K.; van den Dikkenberg, J.; Brouwers, J.F.; Holthuis, J.C. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004, 23, 33–44. [Google Scholar] [CrossRef]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef]
- Hanada, K. Regulation of CERT-mediated trafficking of ceramide. Chem. Phys. Lipids 2007, 149, S7. [Google Scholar] [CrossRef]
- Mitsutake, S.; Zama, K.; Yokota, H.; Yoshida, T.; Tanaka, M.; Mitsui, M.; Ikawa, M.; Okabe, M.; Tanaka, Y.; Yamashita, T.; et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 2011, 286, 28544–28555. [Google Scholar] [CrossRef]
- Vacaru, A.M.; Tafesse, F.G.; Ternes, P.; Kondylis, V.; Hermansson, M.; Brouwers, J.F.; Somerharju, P.; Rabouille, C.; Holthuis, J.C. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J. Cell Biol. 2009, 185, 1013–1027. [Google Scholar] [CrossRef]
- Saddoughi, S.A.; Song, P.; Ogretmen, B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell. Biochem. 2008, 49, 413–440. [Google Scholar] [CrossRef]
- Haddadi, N.; Lin, Y.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. “Dicing and splicing” sphingosine kinase and relevance to cancer. Int. J. Mol. Sci. 2017, 18, 1891. [Google Scholar] [CrossRef]
- Turpin-Nolan, S.M.; Bruning, J.C. The role of ceramides in metabolic disorders: When size and localization matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A. Functions of ceramide in coordinating cellular responses to stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Testai, F.D.; Landek, M.A.; Dawson, G. Regulation of sphingomyelinases in cells of the oligodendrocyte lineage. J. Neurosci. Res. 2004, 75, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Draeger, A. Fluorescent annexin a1 reveals dynamics of ceramide platforms in living cells. Traffic 2010, 9, 1757–1775. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Beutel, O.; Ebell, K.; Korneev, S.; Holthuis, J.C. Diverting CERT-mediated ceramide transport to mitochondria triggers Bax-dependent apoptosis. J. Cell. Sci. 2017, 130, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Chalfant, C. Sphingolipids as Signaling and Regulatory Molecules; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Gomez-Munoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Trueba, M.; Ordonez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Goi, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar]
- Marchesini, N.; Hannun, Y.A. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef]
- Clarke, C.J.; Wu, B.X.; Hannun, Y.A. The neutral sphingomyelinase family: Identifying biochemical connections. Adv. Enzyme Regul. 2011, 51, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tashima, M.; Takahashi, A.; Uchiyama, T.; Okazaki, T. Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3. J. Biol. Chem. 1999, 274, 10654–10660. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Nakashima, S.; Kiyono, T.; Sawada, M.; Yoshimura, S.; Iwama, T.; Banno, Y.; Shinoda, J.; Sakai, N. p53-independent ceramide formation in human glioma cells during gamma-radiation-induced apoptosis. Cell Death Differ. 2004, 11, 853–861. [Google Scholar] [CrossRef]
- Schütze, S.; Machleidt, T.; Krönke, M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J. Leukoc. Biol. 1994, 56, 533–541. [Google Scholar] [CrossRef]
- Santana, P.; Pena, L.A.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; McLoughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef]
- Nilsson, A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim. Biophys. Acta 1969, 176, 339–347. [Google Scholar] [CrossRef]
- Duan, R.D. Alkaline sphingomyelinase: An old enzyme with novel implications. BBA-Bioenerg. 2006, 1761, 281–291. [Google Scholar] [CrossRef]
- Machala, M.; Prochazkova, J.; Hofmanova, J.; Kralikova, L.; Slavik, J.; Tylichova, Z.; Ovesna, P.; Kozubik, A.; Vondracek, J. Colon Cancer and Perturbations of the Sphingolipid Metabolism. Int. J. Mol. Sci. 2019, 20, 6051. [Google Scholar] [CrossRef]
- Novgorodov, S.A.; Wu, B.X.; Gudz, T.I.; Bielawski, J.; Ovchinnikova, T.V.; Hannun, Y.A.; Obeid, L.M. Novel pathway of ceramide production in mitochondria: Thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J. Biol. Chem. 2011, 286, 25352–25362. [Google Scholar] [CrossRef]
- Maceyka, M.; Payne, S.G.; Milstien, S.; Spiegel, S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta 2002, 1585, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Spiegel, S. Sphingosine kinase: A mediator of vital cellular functions. Prostag. Other Lipid Mediat. 2001, 64, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase in immunity and cancer: Silencing the siren. Trends Mol. Med. 2007, 13, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta 2006, 1758, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Yatomi, Y.; Ohmori, T.; Ge, R.L.; Kazama, F.; Okamoto, H.; Sano, T.; Satoh, K.; Kume, S.; Tigyi, G.; Igarashi, Y.; et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood 2000, 96, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Kluk, M.J.; Hla, T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ. Res. 2001, 89, 496–502. [Google Scholar] [CrossRef]
- Adada, M.M.; Canals, D.; Jeong, N.; Kelkar, A.D.; Hernandez-Corbacho, M.; Pulkoski-Gross, M.J.; Donaldson, J.C.; Hannun, Y.A.; Obeid, L.M. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 2015, 29, 4654–4669. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Alvarez, S.E.; Milstien, S.; Spiegel, S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 2007, 18, 300–307. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2019, 195, 85–99. [Google Scholar] [CrossRef]
- Camaré, C.; Trayssac, M.; Garmy-Susini, B.; Mucher, E.; Sabbadini, R.; Salvayre, R.; Negre-Salvayre, A. Oxidized LDL-induced angiogenesis involves sphingosine 1-phosphate: Prevention by anti-S1P antibody. Br. J. Pharmacol. 2014, 172, 106–118. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Ramanathan, R.; Takabe, K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast Cancer Manag. 2015, 4, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, S.; Abdolalizadeh, J.; Ostadrahimi, A.; Mohammadi, S.A.; Barzegari, A.; Lotfi, H.; Bonabi, E.; Zarghami, N. Apoptotic effect of saccharomyces cerevisiae on human colon cancer SW480 cells by regulation of Akt/NF-kB signaling pathway. Probiotics Antimicrob. Proteins 2020, 12, 311–319. [Google Scholar] [CrossRef]
- Nagahashi, M.; Yamada, A.; Katsuta, E.; Aoyagi, T.; Huang, W.C.; Terracina, K.P.; Hait, N.C.; Allegood, J.C.; Tsuchida, J.; Yuza, K.; et al. Targeting the SphK1/S1P/S1PR1 axis that links obesity, chronic inflammation, and breast cancer metastasis. Cancer Res. 2018, 78, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhuang, T.; Liang, Z.; Li, L.; Xue, M.; Liu, J.; Liang, H. Breast cancer suppression by aplysin is associated with inhibition of PI3K/AKT/FOXO3a pathway. Oncotarget 2017, 8, 63923–63934. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Li, K.; Guo, Y.; Wang, Q.; Li, Z.; Yang, Y.; Chen, Z.; Wang, J.; Zhao, W.; Zhang, H.; et al. Tumor suppressor PRSS8 targets Sphk1/S1P/Stat3/Akt signaling in colorectal cancer. Oncotarget 2016, 7, 26780–26792. [Google Scholar] [CrossRef]
- Zheng, X.D.; Zhang, Y.; Qi, X.W.; Wang, M.H.; Sun, P.; Zhang, Y.; Jiang, J. Role of Sphk1 in the malignant transformation of breast epithelial cells and breast cancer progression. Indian J. Cancer 2014, 51, 524–529. [Google Scholar] [CrossRef]
- Kawahara, S.; Otsuji, Y.; Nakamura, M.; Murakami, M.; Murate, T.; Matsunaga, T.; Kanoh, H.; Seishima, M.; Banno, Y.; Hara, A. Sphingosine kinase 1 plays a role in the upregulation of CD44 expression through extracellular signal-regulated kinase signaling in human colon cancer cells. Anticancer Drugs 2013, 24, 473–483. [Google Scholar] [CrossRef]
- Ader, I.; Brizuela, L.; Bouquerel, P.; Malavaud, B.; Cuvillier, O. Sphingosine kinase 1: A new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res. 2008, 68, 8635–8642. [Google Scholar] [CrossRef]
- Pchejetski, D.; Golzio, M.; Bonhoure, E.; Calvet, C.; Doumerc, N.; Garcia, V.; Mazerolles, C.; Rischmann, P.; Teissie, J.; Malavaud, B.; et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005, 65, 11667–11675. [Google Scholar] [CrossRef]
- Alshaker, H.; Wang, Q.; Kawano, Y.; Arafat, T.; Bohler, T.; Winkler, M.; Cooper, C.; Pchejetski, D. Everolimus (RAD001) sensitizes prostate cancer cells to docetaxel by down-regulation of HIF-1alpha and sphingosine kinase 1. Oncotarget 2016, 7, 80943–80956. [Google Scholar] [CrossRef]
- Sauer, L.; Nunes, J.; Salunkhe, V.; Skalska, L.; Kohama, T.; Cuvillier, O.; Waxman, J.; Pchejetski, D. Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. Int. J. Cancer 2009, 125, 2728–2736. [Google Scholar] [CrossRef]
- Sukocheva, O.; Wang, L.; Verrier, E.; Vadas, M.A.; Xia, P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology 2009, 150, 4484–4492. [Google Scholar] [CrossRef]
- Giussani, P.; Bassi, R.; Anelli, V.; Brioschi, L.; De Zen, F.; Riccitelli, E.; Caroli, M.; Campanella, R.; Gaini, S.M.; Viani, P.; et al. Glucosylceramide synthase protects glioblastoma cells against autophagic and apoptotic death induced by temozolomide and Paclitaxel. Cancer Investig. 2012, 30, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Han, T.Y.; Giuliano, A.E.; Cabot, M.C. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J. Biol. Chem. 1999, 274, 1140–1146. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Patwardhan, G.A.; Xie, P.; Gu, X.; Giuliano, A.E.; Cabot, M.C. Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. Int. J. Oncol. 2011, 39, 425–431. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, F.R.; Pearson, J.M.; Tan, S.F.; Cheon, H.; Xing, J.C.; Dunton, W.; Feith, D.J.; Loughran, T.P., Jr. Sphingosine kinase-2 is overexpressed in large granular lymphocyte leukaemia and promotes survival through Mcl-1. Br. J. Heaematol. 2020, 190, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Oskouian, B.; Sooriyakumaran, P.; Borowsky, A.D.; Crans, A.; Dillard-Telm, L.; Tam, Y.Y.; Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 17384–17389. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, Z.; Feng, H.; Chen, Y.; Zhang, C.; Yu, J.; Luo, Y.; Zhao, L.; Jiang, X.; Shi, F. Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin. Cell Death Dis. 2019, 10, 157. [Google Scholar] [CrossRef]
- Lafont, E.; Milhas, D.; Carpentier, S.; Garcia, V.; Jin, Z.X.; Umehara, H.; Okazaki, T.; Schulze-Osthoff, K.; Levade, T.; Benoist, H.; et al. Caspase-mediated inhibition of sphingomyelin synthesis is involved in FasL-triggered cell death. Cell Death Differ. 2010, 17, 642–654. [Google Scholar] [CrossRef]
- Grassme, H.; Schwarz, H.; Gulbins, E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem. Biophys. Res. Commun. 2001, 284, 1016–1030. [Google Scholar] [CrossRef]
- Eroica, S.; Susan, C.E.; Cynthia, C.; Elroy, F. Characterizing the sphingomyelinase pathway triggered by PRIMA-1 derivatives in lung cancer cells with differing p53 status. Anticancer Res. 2014, 34, 3271. [Google Scholar]
- Kohno, M.; Momoi, M.; Oo, M.L.; Paik, J.H.; Lee, Y.M.; Venkataraman, K.; Ai, Y.; Ristimaki, A.P.; Fyrst, H.; Sano, H.; et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 2006, 26, 7211–7223. [Google Scholar] [CrossRef] [PubMed]
- Senkal, C.E.; Ponnusamy, S.; Manevich, Y.; Meyers-Needham, M.; Saddoughi, S.A.; Mukhopadyay, A.; Dent, P.; Bielawski, J.; Ogretmen, B. Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. J. Biol. Chem. 2011, 286, 42446–42458. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, C.Y.; Lin, Y.C.; Wu, M.T.; Su, K.L.; Yuan, S.S.; Wang, H.D.; Fong, Y.; Lin, Y.H.; Chiu, C.C. C(2)-ceramide-induced rb-dominant senescence-like phenotype leads to human breast cancer Mcf-7 escape from p53-dependent cell death. Int. J. Mol. Sci. 2019, 20, 4292. [Google Scholar] [CrossRef]
- Tallima, H.; Azzazy, H.M.E.; El Ridi, R. Cell surface sphingomyelin: Key role in cancer initiation, progression, and immune evasion. Lipids Health Dis. 2021, 20, 150. [Google Scholar] [CrossRef]
- Mombelli, E.; Morris, R.; Taylor, W.; Fraternali, F. Hydrogen-bonding propensities of sphingomyelin in solution and in a bilayer assembly: A molecular dynamics study. Biophys. J. 2003, 84, 1507–1517. [Google Scholar] [CrossRef]
- Barenholz, Y.; Thompson, T.E. Sphingomyelin: Biophysical aspects. Chem. Phys. Lipids 1999, 102, 29–34. [Google Scholar] [CrossRef]
- Migliardo, F.; Tallima, H.; Ridi, R.E. Is there a sphingomyelin-based hydrogen bond barrier at the mammalian host-schistosome parasite interface? Cell Biochem. Biophys. 2014, 68, 359–367. [Google Scholar] [CrossRef]
- Slotte, J.P. The importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-lipids. Biochim. Biophys. Acta 2016, 1858, 304–310. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Liu, D.; Wang, C. Ceramide glycosylation and related enzymes in cancer signaling and therapy. Biomed. Pharmacother. 2021, 139, 111565. [Google Scholar] [CrossRef]
- Sheridan, M.; Ogretmen, B. The role of ceramide metabolism and signaling in the regulation of mitophagy and cancer therapy. Cancers 2021, 13, 2475. [Google Scholar] [CrossRef] [PubMed]
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238. [Google Scholar] [CrossRef]
- Chan, W.K.; Lee, H.M.; Lee, T.H.; Kang, C.; Yong, S.G. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002, 62, 6312–6317. [Google Scholar]
- Riboni, L.; Viani, P.; Bassi, R.; Giussani, P.; Tettamanti, G. Basic fibroblast growth factor-induced proliferation of primary astrocytes. evidence for the involvement of sphingomyelin biosynthesis. J. Biol. Chem. 2001, 276, 12797–12804. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xiao, J.; Dong, M.; Qiu, Z.; Jin, J. Human alkaline ceramidase 2 promotes the growth, invasion, and migration of hepatocellular carcinoma cells via sphingomyelin phosphodiesterase acid-like 3B. Cancer Sci. 2020, 111, 2259–2274. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Zama, K.; Mitsutake, S.; Okazaki, T.; Igarashi, Y. Sphingomyelin in microdomains of the plasma membrane regulates amino acid-stimulated mTOR signal activation. Cell Biol. Int. 2018, 42, 823–831. [Google Scholar] [CrossRef]
- Jing, F.; Jing, C.; Dai, X.; Zhou, G.; Hong, L. Sphingomyelin synthase 2 but not sphingomyelin synthase 1 is upregulated in ovarian cancer and involved in migration, growth and survival via different mechanisms. Am. J. Transl. Res. 2021, 13, 4412–4421. [Google Scholar]
- Don, A.S.; Lim, X.Y.; Couttas, T.A. Re-configuration of sphingolipid metabolism by oncogenic transformation. Biomolecules 2014, 4, 315–353. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ueda, Y.; Matsushita, M.; Nagaya, S.; Hashizume, C.; Arai, K.; Kabayama, K.; Fukase, K.; Watanabe, K.; Wardhani, L.O.; et al. Deficiency of sphingomyelin synthase 2 prolongs survival by the inhibition of lymphoma infiltration through ICAM-1 reduction. FASEB J. 2020, 34, 3838–3854. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, J.C.; He, S.H.; Lou, B.; Ding, T.B.; Yang, J.T.; Mo, M.G.; Ye, D.Y.; Zhou, L.; Jiang, X.C.; et al. Sphingomyelin synthase 2 facilitates M2-like macrophage polarization and tumor progression in a mouse model of triple-negative breast cancer. Acta Pharmacol. Sin. 2021, 42, 149–159. [Google Scholar] [CrossRef]
- Chongsathidkiet, P.; Jackson, C.; Koyama, S.; Loebel, F.; Cui, X.; Farber, S.H.; Woroniecka, K.; Elsamadicy, A.A.; Dechant, C.A.; Kemeny, H.R.; et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 2018, 24, 1459–1468. [Google Scholar] [CrossRef]
- Montfort, A.; Bertrand, F.; Rochotte, J.; Gilhodes, J.; Filleron, T.; Milhes, J.; Dufau, C.; Imbert, C.; Riond, J.; Tosolini, M.; et al. Neutral sphingomyelinase 2 heightens anti-melanoma immune responses and anti-pd-1 therapy efficacy. Cancer Immunol. Res. 2021, 9, 568–582. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Gu, J.; Yu, J.; Guo, S.; Zhu, Y.; Ye, D. Abnormal methylation status of FBXW10 and SMPD3, and associations with clinical characteristics in clear cell renal cell carcinoma. Oncol. Lett. 2015, 10, 3073–3080. [Google Scholar] [CrossRef]
- Jabalee, J.; Towle, R.; Lawson, J.; Dickman, C.; Garnis, C. Sphingomyelin phosphodiesterase 3 methylation and silencing in oral squamous cell carcinoma results in increased migration and invasion and altered stress response. Oncotarget 2020, 11, 523–534. [Google Scholar] [CrossRef]
- Tsuruo, T. Mechanism of multidrug resistance and implication for therapy. Pathophysiology 1994, 1, 285–296. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Emma, B.; Gfn, B.; Fms, B.; Asc, B.; Bs, B.; Rcf, A.; Ono, B. Role of sphingomyelin on the interaction of the anticancer drug gemcitabine hydrochloride with cell membrane models. Colloids Surf. B 2020, 196, 111357. [Google Scholar]
- Xu, J.X.; Morii, E.; Liu, Y.; Nakamichi, N.; Ikeda, J.; Kimura, H.; Aozasa, K. High tolerance to apoptotic stimuli induced by serum depletion and ceramide in side-population cells: High expression of CD55 as a novel character for side-population. Exp. Cell Res. 2007, 313, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Cervia, D.; Assi, E.; De Palma, C.; Giovarelli, M.; Bizzozero, L.; Pambianco, S.; Di Renzo, I.; Zecchini, S.; Moscheni, C.; Vantaggiato, C.; et al. Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin. Oncotarget 2016, 7, 24995–25009. [Google Scholar] [CrossRef]
- Grammatikos, G.; Teichgraber, V.; Carpinteiro, A.; Trarbach, T.; Weller, M.; Hengge, U.R.; Gulbins, E. Overexpression of acid sphingomyelinase sensitizes glioma cells to chemotherapy. Antioxid. Redox. Signal. 2007, 9, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.; Hammann, A.; Grazide, S.; Lagadic-Gossmann, D.; Athias, A.; Sergent, O.; Laurent, G.; Gambert, P.; Solary, E.; Dimanche-Boitrel, M.-T. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004, 64, 3593–3598. [Google Scholar] [CrossRef] [PubMed]
- Maurmann, L.; Belkacemi, L.; Adams, N.R.; Majmudar, P.M.; Moghaddas, S.; Bose, R.N. A novel cisplatin mediated apoptosis pathway is associated with acid sphingomyelinase and FAS proapoptotic protein activation in ovarian cancer. Apoptosis 2015, 20, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Schuchman, E.H. Acid sphingomyelinase overexpression enhances the antineoplastic effects of irradiation in vitro and in vivo. Mol. Ther. 2008, 16, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Gran, M.L.; Peppas, N.A. Targeted nanodelivery of drugs and diagnostics. Nano Today 2010, 5, 143–159. [Google Scholar] [CrossRef]
- Wang, Z.; Little, N.; Chen, J.; Lambesis, K.T.; Le, K.T.; Han, W.; Scott, A.J.; Lu, J. Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy. Nat. Nanotechnol. 2021, 16, 1130–1140. [Google Scholar] [CrossRef]
- Bouzo, B.L.; Lores, S.; Jatal, R.; Alijas, S.; Alonso, M.J.; Conejos-Sanchez, I.; de la Fuente, M. Sphingomyelin nanosystems loaded with uroguanylin and etoposide for treating metastatic colorectal cancer. Sci. Rep. 2021, 11, 17213. [Google Scholar] [CrossRef]
- Medina, O.P.; Tower, R.J.; Medina, T.P.; Ashkenani, F.; Appold, L.; Bötcher, M.; Huber, L.; Will, O.; Ling, Q.; Hauser, C.; et al. Multimodal targeted nanoparticle-based delivery system for pancreatic tumor imaging in cellular and animal models. Curr. Pharm. Des. 2020, 28, 313–323. [Google Scholar] [CrossRef]
- Penate Medina, T.; Gerle, M.; Humbert, J.; Chu, H.; Kopnick, A.L.; Barkmann, R.; Garamus, V.M.; Sanz, B.; Purcz, N.; Will, O.; et al. Lipid-iron nanoparticle with a cell stress release mechanism combined with a local alternating magnetic field enables site-activated drug release. Cancers 2020, 12, 3767. [Google Scholar] [CrossRef]
- Masoumi, F.; Saraiva, S.M.; Bouzo, B.L.; Lopez-Lopez, R.; Esteller, M.; Diaz-Lagares, A.; de la Fuente, M. Modulation of colorectal tumor behavior via lncrna tp53tg1-lipidic nanosystem. Pharmaceutics 2021, 13, 1507. [Google Scholar] [CrossRef]
- Nagachinta, S.; Bouzo, B.L.; Vazquez-Rios, A.J.; Lopez, R.; Fuente, M. Sphingomyelin-based nanosystems (sns) for the development of anticancer miRNA therapeutics. Pharmaceutics 2020, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Mutter, N.L.; Volaric, J.; Szymanski, W.; Feringa, B.L.; Maglia, G. Reversible photocontrolled nanopore assembly. J. Am. Chem. Soc. 2019, 141, 14356–14363. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Park, H.R.; Yoon, Y.M.; Kim, H.I.; Yoo, S.Y.; Lee, K.W.; Lee, Y.S. Antiproliferative 3-deoxysphingomyelin analogs: Design, synthesis, biological evaluation and molecular docking of pyrrolidine-based 3-deoxysphingomyelin analogs as anticancer agents. Bioorganic Chem. 2019, 84, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Messner, M.C.; Levin, J.C.; Abdelmageed, N.; Park, H.; Merrill, A.H., Jr.; Cabot, M.C. Potential role of acid ceramidase in conversion of cytostatic to cytotoxic end-point in pancreatic cancer cells. Cancer Chemoth. Pharm. 2013, 71, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.; Fabrias, G.; Delgado, A.; Casas, J.; Abad, J.L.; Cabot, M.C. C6-ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res. Treat. 2012, 133, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, Y.; Liu, X.; Li, Z.; Xu, W.; He, S.; Huang, Y.; Zhang, H. Acid sphingomyelinase contributes to evodiamine-induced apoptosis in human gastric cancer SGC-7901 cells. DNA Cell Biol. 2011, 30, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kester, M.; Heakal, Y.; Fox, T.; Sharma, A.; Robertson, G.P.; Morgan, T.T.; Altinoglu, E.I.; Tabakovic, A.; Parette, M.R.; Rouse, S.M.; et al. Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells. Nano Lett. 2008, 8, 4116–4121. [Google Scholar] [CrossRef]
- Ganta, S.; Singh, A.; Patel, N.R.; Cacaccio, J.; Rawal, Y.H.; Davis, B.J.; Amiji, M.M.; Coleman, T.P. Development of EGFR-targeted nanoemulsion for imaging and novel platinum therapy of ovarian cancer. Pharm. Res. 2014, 31, 2490–2502. [Google Scholar] [CrossRef]
- Stover, T.; Kester, M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J. Pharmacol. Exp. Ther. 2003, 307, 468–475. [Google Scholar] [CrossRef]
- Chen, L.; Alrbyawi, H.; Poudel, I.; Arnold, R.D.; Babu, R.J. Co-delivery of doxorubicin and ceramide in a liposomal formulation enhances cytotoxicity in murine B16Bl6 melanoma cell lines. AAPS PharmSciTech 2019, 20, 99. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Kimchi, E.T.; Kaifi, J.T.; Qi, X.; Manjunath, Y.; Liu, X.; Deering, T.; Avella, D.M.; Fox, T.; et al. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology 2018, 154, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Li, J.; Hui, S. C6 ceramide potentiates curcumin-induced cell death and apoptosis in melanoma cell lines in vitro. Cancer Chemother. Pharmacol. 2010, 66, 999–1003. [Google Scholar]
- Jiang, Y.; DiVittore, N.A.; Kaiser, J.M.; Shanmugavelandy, S.S.; Fritz, J.L.; Heakal, Y.; Tagaram, H.R.; Cheng, H.; Cabot, M.C.; Staveley-O’Carroll, K.F.; et al. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol. Ther. 2011, 12, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Medatwal, N.; Kumar, S.; Kar, A.; Komalla, V.; Yavvari, P.S.; Mishra, D.; Rizvi, Z.A.; Nandan, S.; Malakar, D.; et al. A localized chimeric hydrogel therapy combats tumor progression through alteration of sphingolipid metabolism. ACS Cent. Sci. 2019, 5, 1648–1662. [Google Scholar] [CrossRef]
- Medatwal, N.; Ansari, M.N.; Kumar, S.; Pal, S.; Jha, S.K.; Verma, P.; Rana, K.; Dasgupta, U.; Bajaj, A. Hydrogel-mediated delivery of celastrol and doxorubicin induces a synergistic effect on tumor regression via upregulation of ceramides. Nanoscale 2020, 12, 18463–18475. [Google Scholar] [CrossRef]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting glioblastoma signaling and metabolism with a re-purposed brain-penetrant drug. Cell. Rep. 2021, 37, 109957. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, J.; Tang, J.; Li, B.; Zhang, Y.; Gu, N.; Yang, F. Sphingosine 1-phosphate liposomes for targeted nitric oxide delivery to mediate anticancer effects against brain glioma tumors. Adv. Mater. 2021, 33, e2101701. [Google Scholar] [CrossRef]
- Saddoughi, S.A.; Gencer, S.; Peterson, Y.K.; Ward, K.E.; Mukhopadhyay, A.; Oaks, J.; Bielawski, J.; Szulc, Z.M.; Thomas, R.J.; Selvam, S.P.; et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 2013, 5, 105–121. [Google Scholar] [CrossRef]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; ME, L.L. Targeting Sphingolipids for Cancer Therapy. Front. Oncol. 2021, 11, 745092. [Google Scholar] [CrossRef]
- Meng, Q.; Zhao, B.; Xu, Q.; Xu, X.; Deng, G.; Li, C.; Luan, L.; Ren, F.; Wang, H.; Xu, H. Indole-propionic acid derivatives as potent, S1P3-sparing and EAE efficacious sphingosine-1-phosphate 1 (S1P1) receptor agonists. Bioorg. Med. Chem. Lett. 2012, 22, 2794–2797. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Guo, Z.; Zhao, X.; Wu, L.; Liu, X.; Zhang, Y.; Zhang, Y.; Deng, Z.; Qu, X.; Cui, S.; et al. Novel 5-fluorouracil sensitizers for colorectal cancer therapy: Design and synthesis of S1P receptor 2 (S1PR2) antagonists. Eur. J. Med. Chem. 2021, 227, 113923. [Google Scholar] [CrossRef] [PubMed]
- Beljanski, V.; Knaak, C.; Smith, C.D. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J. Pharmacol. Exp. Ther. 2010, 333, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Taeb, S.; Jahangiri, S.; Emmenegger, U.; Tran, E.; Bruce, J.; Mesci, A.; Korpela, E.; Vesprini, D.; Wong, C.S.; et al. miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013, 73, 6972–6986. [Google Scholar] [CrossRef]
- Billich, A.; Bornancin, F.; Mechtcheriakova, D.; Natt, F.; Huesken, D.; Baumruker, T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: Function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell. Signal. 2005, 17, 1203–1217. [Google Scholar] [CrossRef]
- Leroux, M.E.; Auzenne, E.; Evans, R.; Hail, N., Jr.; Spohn, W.; Ghosh, S.C.; Farquhar, D.; McDonnell, T.; Klostergaard, J. Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce BCL-2-independent apoptosis in human prostatic adenocarcinoma cells. Prostate 2007, 67, 1699–1717. [Google Scholar] [CrossRef]
- Liu, H.; Kong, Y.; Liu, Z.; Guo, X.; Yang, B.; Yin, T.; He, H.; Gou, J.; Zhang, Y.; Tang, X. Sphingomyelin-based PEGylation Cu (DDC)(2) liposomes prepared via the dual function of Cu(2+) for cancer therapy: Facilitating DDC loading and exerting synergistic antitumor effects. Int. J. Pharm. 2022, 621, 121788. [Google Scholar] [CrossRef]
- Bidan, N.; Lores, S.; Vanhecke, A.; Nicolas, V.; Domenichini, S.; Lopez, R.; de la Fuente, M.; Mura, S. Before in vivo studies: In vitro screening of sphingomyelin nanosystems using a relevant 3D multicellular pancreatic tumor spheroid model. Int. J. Pharm. 2022, 617, 121577. [Google Scholar] [CrossRef]
- Massiot, J.; Rosilio, V.; Ibrahim, N.; Yamamoto, A.; Nicolas, V.; Konovalov, O.; Tanaka, M.; Makky, A. Newly synthesized lipid-porphyrin conjugates: Evaluation of their self-assembling properties, their miscibility with phospholipids and their photodynamic activity in vitro. Chemistry 2018, 24, 19179–19194. [Google Scholar] [CrossRef]
- Lim, E.B.; Haam, S.; Lee, S.W. Sphingomyelin-based liposomes with different cholesterol contents and polydopamine coating as a controlled delivery system. Colloid Surface A 2021, 618, 126447. [Google Scholar] [CrossRef]
- Zembruski, N.C.; Nguyen, C.D.; Theile, D.; Ali, R.M.; Herzog, M.; Hofhaus, G.; Heintz, U.; Burhenne, J.; Haefeli, W.E.; Weiss, J. Liposomal sphingomyelin influences the cellular lipid profile of human lymphoblastic leukemia cells without effect on P-glycoprotein activity. Mol. Pharm. 2013, 10, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kitatani, K.; Toyoshima, M.; Ishibashi, M.; Usui, T.; Minato, J.; Egiz, M.; Shigeta, S.; Fox, T.; Deering, T.; et al. Ceramide nanoliposomes as a MLKL-dependent, necroptosis-inducing, chemotherapeutic reagent in ovarian cancer. Mol. Cancer Ther. 2018, 17, 50–59. [Google Scholar] [CrossRef] [PubMed]

| Active Molecule | Pathway | Function or Activity | Cancer | Refs. |
|---|---|---|---|---|
| Sphingosine kinase | Akt/NF-κB | Cancer progression and chemoresistance | Colon | [74] |
| S1P/S1PR1 | Inflammation and angiogenesis | Breast | [75] | |
| PI3k/Akt/FOXO3a | Apoptosis resistance | Breast | [76] | |
| S1P/Stat3/AKT | Proliferation | Colon | [77] | |
| E-cad | Tumorigenesis and metastasis | Breast | [78] | |
| S1P/ERK/CD44 | Chemoresistance | Colon | [79] | |
| S1P/AKT/GSK-3β | HIF-1α stabilization | Glioblastoma | [80] | |
| Sphingomyelinase | ↑ Sensitivity | Glioblastoma | [81] | |
| ↑ Resistance | Melanoma | [82] | ||
| Induce apoptosis | Colon | [83] | ||
| Induce apoptosis and resistance | Ovarian | [84] | ||
| Acid ceramidase | ↑ Proliferative ↓ Sensitivity | Melanoma | [85,86] | |
| ↓ Sensitivity | Prostate | [87] | ||
| ↑ Radioresistant | Glioblastoma | [87] | ||
| Sphingosine kinase 2 | Mcl-1 | ↑ Cell survival | Leukemia | [88] |
| Sphingosine-1-phosphate lyase | p53 and p38 | ↑ Apoptosis | Colon | [89] |
| Sphingomyelin synthase | TGF- b1 | ↑ Migratory, invasion | Breast | [90] |
| Overexpression of SMS1 | ↓ Cell death | Lymphatic | [91] | |
| Sphingomyelinase | CD95 | ↑ Apoptosis | Lymphatic | [92] |
| p53 | ↑ Apoptosis | Lung | [93] | |
| Sphingosine | Cdk4 | ↓ Cell proliferation | Intestinal adenoma | [94] |
| Ceramide | CerS6/C16-ceramide activated | ↑ Apoptosis | Lung | [95] |
| High cytotoxicity in p53 | ↑ Apoptosis | Breast | [96] |
| Materials | Size (d. nm) | Therapeutics | Cancer Cell Type | Refs. |
|---|---|---|---|---|
| SM-CSS-CPT | 93.1 ± 7.63 | Intravenous injection | CT26, b16, mc38 | [129] |
| UroGm-SNs | 131 ± 12 | SW620 | [130] | |
| DOTAP (DSN) | 142 ± 2 | HCT-116 | [133] | |
| PEGylated Cu (DDC)2 liposomes | 121.5 ± 0.57 | Intravenous injection | 4T1 | [159] |
| SNs–ST | 131 ± 8 | SW480 | [134] | |
| SNs_PEG | 77 ± 3 | PANC-1, | [160] | |
| Lipid–porphyrin conjugates | 180 ± 10 | Kyse-30 | [161] | |
| SMLs@PDA | 229.5 ± 26.3 | [162] | ||
| C6-NBD-SM Liposomes | 71 ± 3 | CCRF-CEM | [163] | |
| CPNPs | 20 | MCF-7 | [142] | |
| C6 ceramide | ~80 | B16, WM-115 | [145] | |
| TRI-Gel | Subcutaneous injection | Lewis lung carcinoma | [147] | |
| Celastrol | Subcutaneous injection | CT26, HCT-8, HCT-116, DLD-1 | [148] | |
| LipC6 | 90 | Intra-splenic injections | liver tumors | [144] |
| Thirty molar percent C6-ceramide in a twelve molar percent pegylated | 80 | Intraperitoneal injection | SKOV3, TOV112D, A2780, A2780CP, PE01, PE04 | [164] |
| S1P/JS-K/Lipo | 189 | Intravenous injection | U87MG | [150] |
| PP2A | A549 | [151] | ||
| 3-[4-(5-aryl-1, 2, 4-oxadiazol-3-yl)-1H-indol-1-yl]propanoic acid series | Intravenous injection | peripheral lymphocyte | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Chen, H.-J.; Wen, H.-Y.; Wang, Z.-G.; Liu, S.-L. Engineered Lipidic Nanomaterials Inspired by Sphingomyelin Metabolism for Cancer Therapy. Molecules 2023, 28, 5366. https://doi.org/10.3390/molecules28145366
Zhu H, Chen H-J, Wen H-Y, Wang Z-G, Liu S-L. Engineered Lipidic Nanomaterials Inspired by Sphingomyelin Metabolism for Cancer Therapy. Molecules. 2023; 28(14):5366. https://doi.org/10.3390/molecules28145366
Chicago/Turabian StyleZhu, Han, Hua-Jie Chen, Hai-Yan Wen, Zhi-Gang Wang, and Shu-Lin Liu. 2023. "Engineered Lipidic Nanomaterials Inspired by Sphingomyelin Metabolism for Cancer Therapy" Molecules 28, no. 14: 5366. https://doi.org/10.3390/molecules28145366
APA StyleZhu, H., Chen, H.-J., Wen, H.-Y., Wang, Z.-G., & Liu, S.-L. (2023). Engineered Lipidic Nanomaterials Inspired by Sphingomyelin Metabolism for Cancer Therapy. Molecules, 28(14), 5366. https://doi.org/10.3390/molecules28145366








