Electronic Nose and Head Space GC–IMS Provide Insights into the Dynamic Changes and Regularity of Volatile Compounds in Zangju (Citrus reticulata cv. Manau Gan) Peel at Different Maturation Stages
Abstract
1. Introduction
2. Results and Discussion
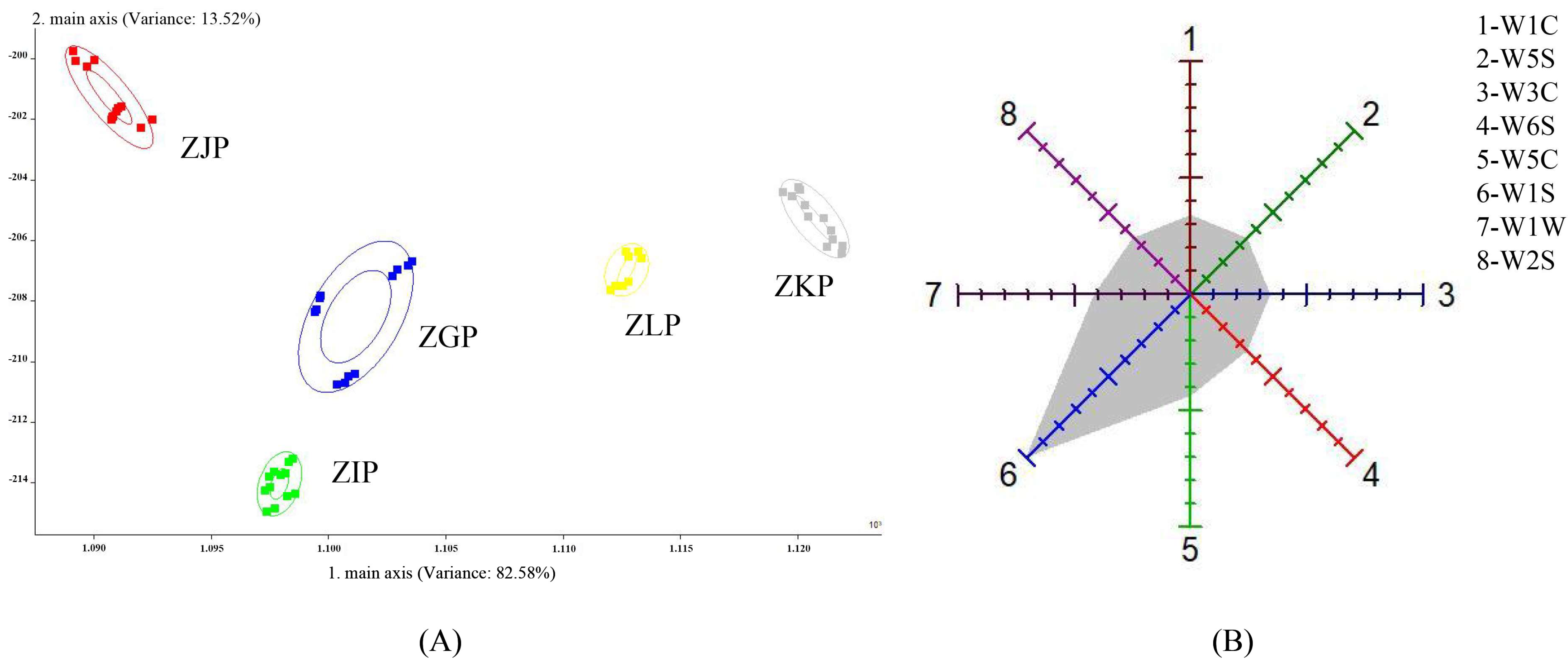
2.1. Results of E-Nose
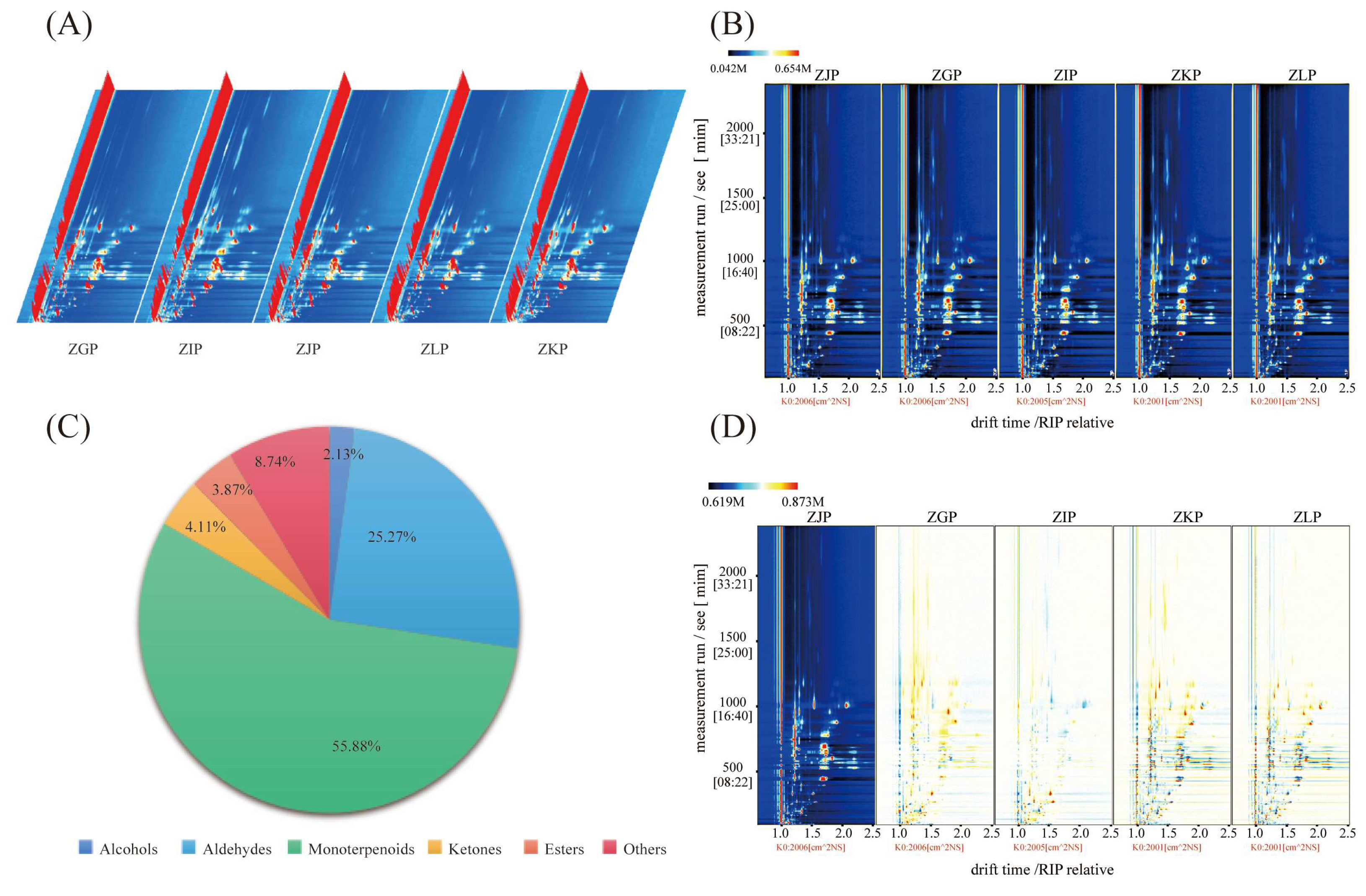
2.2. HS-GC–IMS Visual Topographic Plot Comparison
2.3. Identification of Substances
2.3.1. Alcohols
2.3.2. Aldehydes
2.3.3. Monoterpenoids
2.3.4. Ketones, Esters and Others
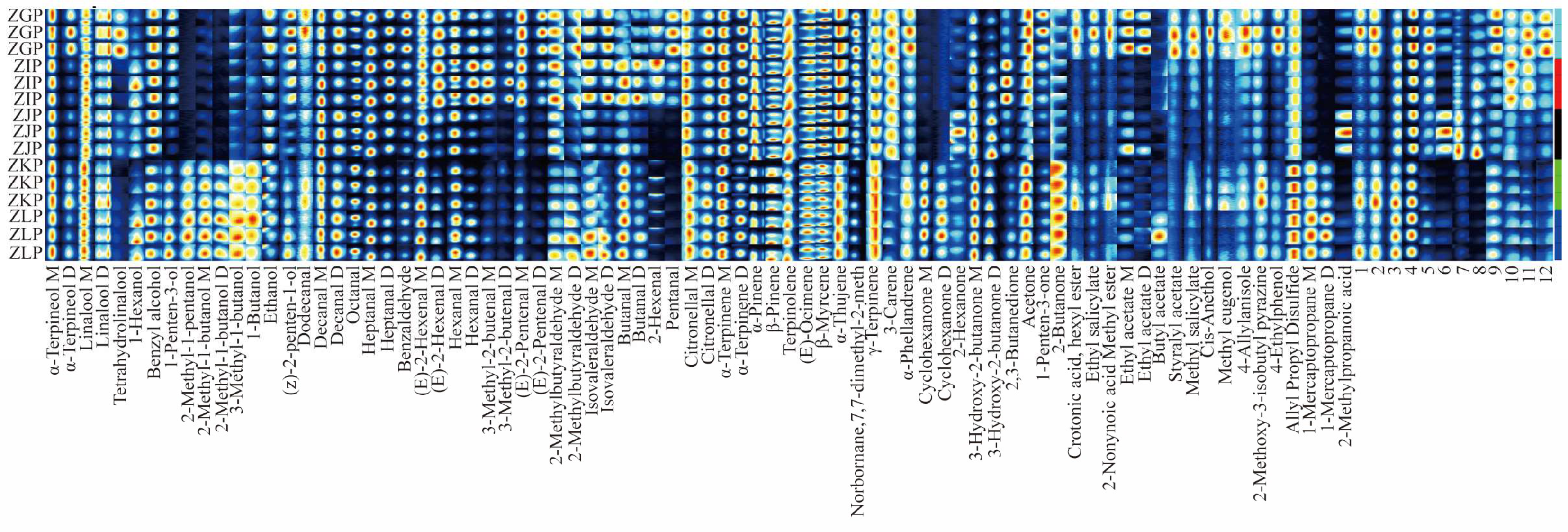
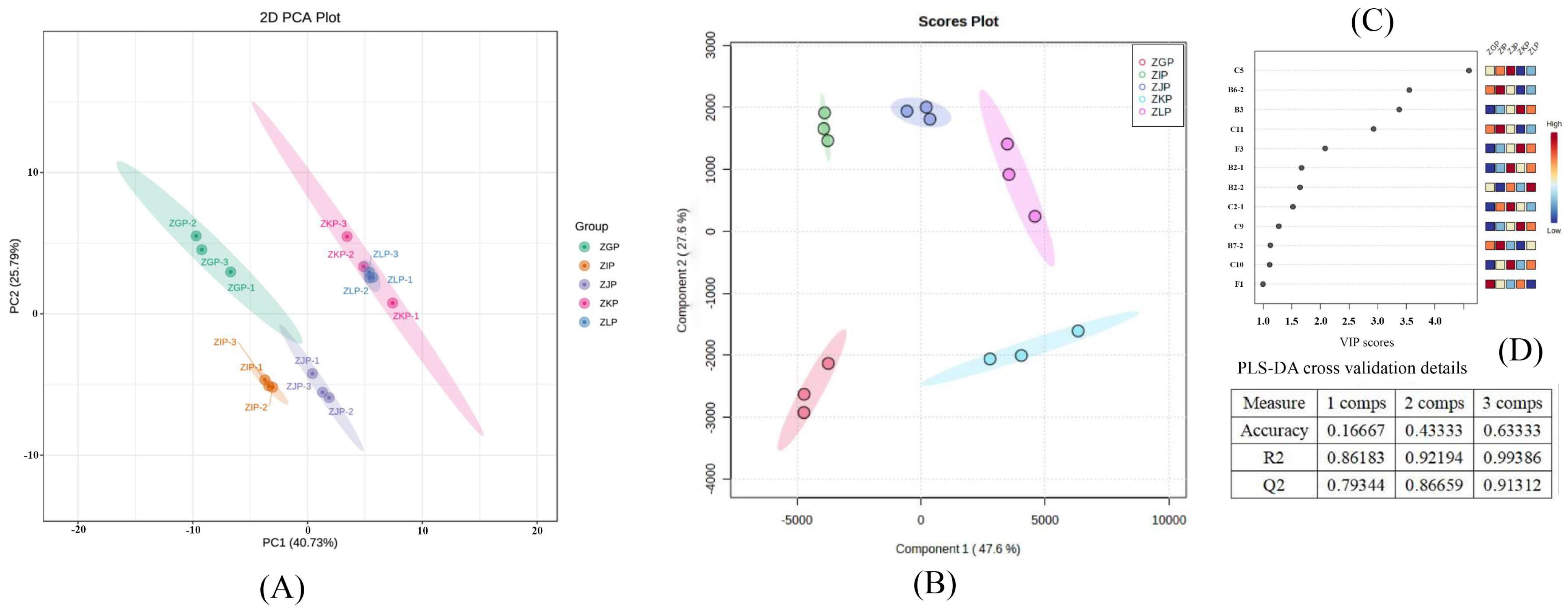
2.4. Analysis of VOC Fingerprints
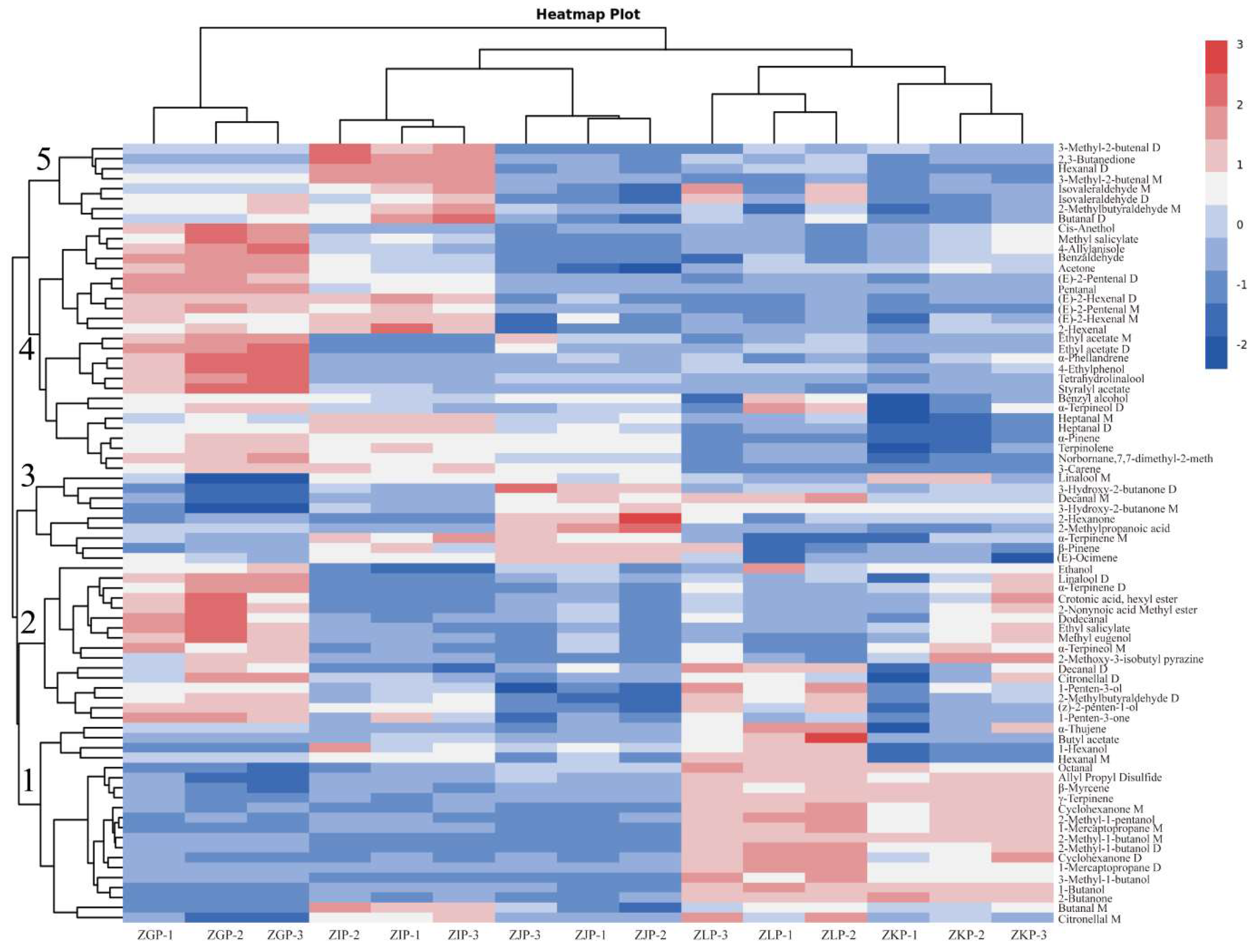
2.5. Analysis of Dynamic Changes and Formation Regularity of VOCs
3. Materials and Methods
3.1. Plant Materials and Preparation
3.2. E-Nose Analysis
3.3. HS-GC–IMS Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Food and Agriculture Organization of the United Nations, FAO. Available online: https://www.fao.org/statistics/en/ (accessed on 20 April 2023).
- Ye, Y.M.; Zhou, K.L. Records of Chinese Fruit Trees (Citrus Roll); China Forestry Publishing House: Beijing, China, 2010. [Google Scholar]
- China Rural Special Technology Association. Available online: https://www.nongjixie.org/Library/Nmap/view?nmap_id=1516 (accessed on 21 March 2023).
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Grauwet, T.; Kebede, B.T.; Hendrickx, M.; Van Loey, A. Study of chemical changes in pasteurised orange juice during shelf-life: A fingerprinting-kinetics evaluation of the volatile fraction. Food Res. Int. 2015, 75, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liang, L.; Wang, Y. Volatile composition changes in navel orange at different growth stages by HS-SPME-GC-MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef]
- Navarra, M.; Mannucci, C.; Delbo, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharm. 2015, 6, 36. [Google Scholar] [CrossRef]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food. Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and Anticancer Activities of Essential Oil from Gannan Navel Orange Peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef]
- Tian, L.; Hu, T.; Zhang, S.; Zhang, H.; Yang, C.; Chen, G.; Pan, S. A Comparative Study on Relieving Exercise-Induced Fatigue by Inhalation of Different Citrus Essential Oils. Molecules 2022, 27, 3239. [Google Scholar] [CrossRef]
- Kwangjai, J.; Cheaha, D.; Manor, R.; Sa-Ih, N.; Samerphob, N.; Issuriya, A.; Wattanapiromsakul, C.; Kumarnsit, E. Modification of brain waves and sleep parameters by Citrus reticulata Blanco. cv. Sai-Nam-Phueng essential oil. Biomed. J. 2021, 44, 727–738. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Garza, G.E.; Antonyan, N.; Hernández, I.L. Orange Peel Dehydration and Creation of New Edible Products. Int. J. Food Eng. 2018, 4, 327–331. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Li, Y.; Li, L. Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods 2022, 11, 2662. [Google Scholar] [CrossRef]
- Zhou, F.F.; Xiao, G.S.; Lin, X.; Xu, Y.J.; Wu, J.J.; Chen, Y.L.; Fu, M.Q. Effects of drying conditions on flavonoids and essential oil components of citrus reticulata ‘Chachi’ peel. Food Ind. Sci. Technol. 2015, 36, 287–291. [Google Scholar]
- Zhang, W.; Chen, T.; Tang, J.; Sundararajan, B.; Zhou, Z. Tracing the production area of citrus fruits using aroma-active compounds and their quality evaluation models. J. Sci. Food. Agric. 2020, 100, 517–526. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, H.J.; Park, K.J.; Kang, S.B.; Park, Y.; Han, S.-G.; Kim, M.; Song, Y.H.; Kim, D.-S. Metabolomic Profiling of Citrus unshiu during Different Stages of Fruit Development. Plants 2022, 11, 967. [Google Scholar] [CrossRef]
- Lehner, T.B.; Siegmund, B. The impact of ventilation during postharvest ripening on the development of flavour compounds and sensory quality of mangoes (Mangifera indica L.) cv. Kent. Food Chem. 2020, 320, 126608. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, L. Tea quality evaluation by applying E-nose combined with chemometrics methods. J. Food Sci. Technol. 2021, 58, 1549–1561. [Google Scholar] [CrossRef]
- Liu, H.; Wen, J.; Xu, Y.; Wu, J.; Yu, Y.; Yang, J.; Liu, H.; Fu, M. Evaluation of dynamic changes and formation regularity in volatile flavor compounds in Citrus reticulata ‘chachi’ peel at different collection periods using gas chromatography-ion mobility spectrometry. LWT 2022, 171, 114126. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, Y.; Zou, B.; Yu, Y.; Yang, J.; Xu, Y.; Chen, X.; Yang, F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC-IMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, X.; Ma, Y.; Wang, Y.; Wang, D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chem. 2022, 369, 130939. [Google Scholar] [CrossRef] [PubMed]
- Bel-Rhlid, R.; Berger, R.G.; Blank, I. Bio-mediated generation of food flavors–Towards sustainable flavor production inspired by nature. Trends Food Sci. Technol. 2018, 78, 134–143. [Google Scholar] [CrossRef]
- Ayseli, M.T.; İpek Ayseli, Y. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci. Technol. 2016, 48, 69–77. [Google Scholar] [CrossRef]
- Find Drugs & Conditions. Available online: https://www.drugs.com/benzyl-alcohol.html (accessed on 24 April 2023).
- Asikin, Y.; Taira, I.; Inafuku, S.; Sumi, H.; Sawamura, M.; Takara, K.; Wada, K. Volatile aroma components and antioxidant activities of the flavedo peel extract of unripe Shiikuwasha (Citrus depressa Hayata). J. Food Sci. 2012, 77, C469–C475. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Zhao, H.; Li, L.; Li, Y.; Tu, Y.; Jiang, L.; Zhao, G. Lipidomic profiling using GC and LC-MS/MS revealed the improved milk quality and lipid composition in dairy cows supplemented with citrus peel extract. Food Res. Int. 2022, 161, 111767. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambla, J.L.; Lopez-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant. Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138 Pt A, 109717. [Google Scholar] [CrossRef]
- Liang, S.; Wen, Z.; Tang, T.; Liu, Y.; Dang, F.; Xie, T.; Wu, H. Study on flavonoid and bioactivity features of the pericarp of Citrus reticulatae ‘chachi’ during storage. Arab. J. Chem. 2022, 15, 103653. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, P.; Fang, Z. Modern technologies for extraction of aroma compounds from fruit peels: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1284–1307. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.I.N.A.T.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Villatoro, C.; Altisent, R.; Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of ‘Pink Lady®’ apples. Postharvest Biol. Technol. 2008, 47, 286–295. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhu, H.; Zhang, H.; Xu, J.; Fu, Q.; Bao, M.; Zhang, J. Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types. Molecules 2021, 26, 7256. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, X.; Qian, C.; He, K.; Xiao, X. Determination of volatile flavors in fresh navel orange by multidimensional gas chromatography quadrupole time-of-flight mass spectrometry. Anal. Lett. 2020, 53, 614–626. [Google Scholar] [CrossRef]
- An, K.; Liu, H.; Fu, M.; Qian, M.C.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Identification of the cooked off-flavor in heat-sterilized lychee (Litchi chinensis Sonn.) juice by means of molecular sensory science. Food Chem. 2019, 301, 125282. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, C.; Li, W.; Qu, Z.; Zeng, M.; Xi, W. Transcriptional regulatory networks controlling taste and aroma quality of apricot (Prunus armeniaca L.) fruit during ripening. BMC Genom. 2019, 20, 45. [Google Scholar] [CrossRef]
- Zheng, G.; Chao, Y.; Liu, M.; Yang, Y.; Zhang, D.; Wang, K.; Tao, Y.; Zhang, J.; Li, Y.; Wei, M. Evaluation of dynamic changes in the bioactive components in Citri reticulatae Pericarpium (Citrus reticulata ‘Chachi’) under different harvesting and drying conditions. J. Sci. Food Agric. 2021, 101, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Wen, J.; An, K.; Wu, J.; Yu, Y.; Zou, B.; Guo, M. A comparative study of aromatic characterization of Yingde Black Tea infusions in different steeping temperatures. LWT 2021, 143, 110860. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8 (Suppl. S1), 3–16. [Google Scholar] [CrossRef]

| Count | Compounds | CAS# | Formula | MW | RI a | Rt b | Dt c | Identification Approach |
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| A1 | 1-Hexanol | 111-27-3 | C6H14O | 102.2 | 876.9 | 356.1 | 1.3275 | RI, Dt |
| A2 | Benzyl alcohol | 100-51-6 | C7H8O | 108.1 | 1026.5 | 618.156 | 1.1718 | RI, Dt |
| A3 | 1-Penten-3-ol | 616-25-1 | C5H10O | 86.1 | 689.1 | 176.263 | 0.9482 | RI, Dt |
| A4 | 2-Methyl-1-pentanol | 105-30-6 | C6H14O | 102.2 | 825.6 | 294.843 | 1.2882 | RI, Dt |
| A5 | 2-Methyl-1-butanol M d | 137-32-6 | C5H12O | 88.1 | 739.9 | 213.899 | 1.2352 | RI, Dt |
| A6 | 2-Methyl-1-butanol D e | 137-32-6 | C5H12O | 88.1 | 738.8 | 212.957 | 1.4757 | RI, Dt |
| A7 | 3-Methyl-1-butanol | 123-51-3 | C5H12O | 88.1 | 732.9 | 208.251 | 1.2467 | RI, Dt |
| A8 | 1-Butanol | 71-36-3 | C4H10O | 74.1 | 670.4 | 167.432 | 1.1837 | RI, Dt |
| A9 | Ethanol | 64-17-5 | C2H6O | 46.1 | 495.1 | 104.055 | 1.1338 | RI, Dt |
| A10 | (z)-2-penten-1-ol | 1576-95-0 | C5H10O | 86.1 | 774.8 | 244.291 | 0.9459 | RI, Dt |
| Aldehydes | ||||||||
| B1 | Dodecanal | 112-54-9 | C12H24O | 184.3 | 1441.7 | 2148.465 | 1.6582 | RI, Dt |
| B2-1 | Decanal M | 112-31-2 | C10H20O | 156.3 | 1189.3 | 1007.691 | 1.5362 | RI, Dt |
| B2-2 | Decanal D | 112-31-2 | C10H20O | 156.3 | 1187.3 | 1001.477 | 2.0588 | RI, Dt |
| B3 | Octanal | 124-13-0 | C8H16O | 128.2 | 1012.7 | 593.035 | 1.8277 | RI, Dt |
| B4-1 | Heptanal M | 111-71-7 | C7H14O | 114.2 | 906.5 | 398.247 | 1.3308 | RI, Dt |
| B4-2 | Heptanal D | 111-71-7 | C7H14O | 114.2 | 903.2 | 393.121 | 1.7000 | RI, Dt |
| B5 | Benzaldehyde | 100-52-7 | C7H6O | 106.1 | 966.1 | 501.991 | 1.1542 | RI, Dt |
| B6-1 | (E)-2-Hexenal M | 6728-26-3 | C6H10O | 98.1 | 860.6 | 335.357 | 1.1867 | RI, Dt |
| B6-2 | (E)-2-Hexenal D | 6728-26-3 | C6H10O | 98.1 | 854.8 | 328.236 | 1.5249 | RI, Dt |
| B7-1 | Hexanal M | 66-25-1 | C6H12O | 100.2 | 797.5 | 265.933 | 1.2560 | RI, Dt |
| B7-2 | Hexanal D | 66-25-1 | C6H12O | 100.2 | 798.4 | 266.823 | 1.5683 | RI, Dt |
| B8-1 | 3-Methyl-2-butenal M | 107-86-8 | C5H8O | 84.1 | 786.4 | 255.252 | 1.0934 | RI, Dt |
| B8-2 | 3-Methyl-2-butenal D | 107-86-8 | C5H8O | 84.1 | 786.5 | 255.335 | 1.3647 | RI, Dt |
| B9-1 | (E)-2-Pentenal M | 1576-87-0 | C5H8O | 84.1 | 756.5 | 227.877 | 1.1106 | RI, Dt |
| B9-2 | (E)-2-Pentenal D | 1576-87-0 | C5H8O | 84.1 | 756.5 | 227.877 | 1.3650 | RI, Dt |
| B10-1 | 2-Methylbutyraldehyde M | 96-17-3 | C5H10O | 86.1 | 670.6 | 167.536 | 1.1586 | RI, Dt |
| B10-2 | 2-Methylbutyraldehyde D | 96-17-3 | C5H10O | 86.1 | 670.8 | 167.61 | 1.4006 | RI, Dt |
| B11-1 | Isovaleraldehyde M | 590-86-3 | C5H10O | 86.1 | 657.5 | 161.68 | 1.1744 | RI, Dt |
| B11-2 | Isovaleraldehyde D | 590-86-3 | C5H10O | 86.1 | 658.7 | 162.188 | 1.4138 | RI, Dt |
| B12-1 | Butanal M | 123-72-8 | C4H8O | 72.1 | 559.5 | 123.921 | 1.1102 | RI, Dt |
| B12-2 | Butanal D | 123-72-8 | C4H8O | 72.1 | 560.3 | 124.194 | 1.2772 | RI, Dt |
| B13 | Cis-2-Hexenal | 505-57-7 | C6H10O | 98.1 | 846.1 | 317.917 | 1.5202 | RI, Dt |
| B14 | Pentanal | 110-62-3 | C5H10O | 86.1 | 704.2 | 186.665 | 1.4317 | RI, Dt |
| Monoterpenoids | ||||||||
| C1-1 | α-Terpineol M | 98-55-5 | C10H18O | 154.3 | 1170.8 | 953.005 | 1.2955 | RI, Dt |
| C1-2 | α-Terpineol D | 98-55-5 | C10H18O | 154.3 | 1169.6 | 949.706 | 1.7928 | RI, Dt |
| C2-1 | Linalool M | 78-70-6 | C10H18O | 154.3 | 1104.5 | 781.237 | 1.2254 | RI, Dt |
| C2-2 | Linalool D | 78-70-6 | C10H18O | 154.3 | 1101.8 | 774.947 | 1.7674 | RI, Dt |
| C3 | Tetrahydrolinalool | 78-69-3 | C10H22O | 158.3 | 1135.6 | 857.495 | 1.2787 | RI, Dt |
| C4-1 | α-Terpinene M | 99-86-5 | C10H16 | 136.2 | 1026.9 | 619 | 1.2185 | RI, Dt |
| C4-2 | α-Terpinene D | 99-86-5 | C10H16 | 136.2 | 1027.5 | 620.072 | 1.7329 | RI, Dt |
| C5 | α-Pinene | 80-56-8 | C10H16 | 136.2 | 939.9 | 453.351 | 1.7396 | RI, Dt |
| C6 | β-Pinene | 127-91-3 | C10H16 | 136.2 | 978.5 | 526.824 | 1.6425 | RI, Dt |
| C7 | Terpinolene | 586-62-9 | C10H16 | 136.2 | 1077.3 | 719.981 | 1.2254 | RI, Dt |
| C8 | (E)-Ocimene | 3779-61-1 | C10H16 | 136.2 | 1045.9 | 655.274 | 1.7067 | RI, Dt |
| C9 | β-Myrcene | 123-35-3 | C10H16 | 136.2 | 995.7 | 563.133 | 1.7204 | RI, Dt |
| C10 | α-Thujene | 2867-05-2 | C10H16 | 136.2 | 928.5 | 433.702 | 1.6680 | RI, Dt |
| C11 | Norbornane,7,7-dimethyl-2-meth | 471-84-1 | C10H16 | 136.2 | 953.1 | 477.273 | 1.2235 | RI, Dt |
| C11 | α-Phellandrene | 99-83-2 | C10H16 | 136.2 | 1155 | 909.13 | 1.2225 | RI, Dt |
| C12 | γ-Terpinene | 99-85-4 | C10H16 | 136.2 | 1058.5 | 680.426 | 1.7029 | RI, Dt |
| C13 | 3-Carene | 13466-78-9 | C10H16 | 136.2 | 1015.2 | 597.656 | 1.2188 | RI, Dt |
| C14-1 | Citronellal M | 106-23-0 | C10H18O | 154.3 | 1144.3 | 880.222 | 1.3530 | RI, Dt |
| C14-2 | Citronellal D | 106-23-0 | C10H18O | 154.3 | 1142.3 | 875.113 | 1.9004 | RI, Dt |
| Ketones | ||||||||
| D1-1 | Cyclohexanone M | 108-94-1 | C6H10O | 98.1 | 896.2 | 382.595 | 1.1568 | RI, Dt |
| D1-2 | Cyclohexanone D | 108-94-1 | C6H10O | 98.1 | 895.1 | 380.964 | 1.4568 | RI, Dt |
| D2 | 2-Hexanone | 591-78-6 | C6H12O | 100.2 | 789.8 | 258.447 | 1.1900 | RI, Dt |
| D3-1 | 3-Hydroxy-2-butanone M | 513-86-0 | C4H8O2 | 88.1 | 720.6 | 198.749 | 1.0570 | RI, Dt |
| D3-2 | 3-Hydroxy-2-butanone D | 513-86-0 | C4H8O2 | 88.1 | 719.4 | 197.846 | 1.3359 | RI, Dt |
| D4 | 2,3-Butanedione | 431-03-8 | C4H6O2 | 86.1 | 585 | 132.781 | 1.1734 | RI, Dt |
| D5 | Acetone | 67-64-1 | C3H6O | 58.1 | 518.5 | 110.878 | 1.1178 | RI, Dt |
| D6 | 1-Penten-3-one | 1629-58-9 | C5H8O | 84.1 | 692.3 | 178.449 | 1.0764 | RI, Dt |
| D7 | 2-Butanone | 78-93-3 | C4H8O | 72.1 | 594.3 | 136.207 | 1.0606 | RI, Dt |
| Esters | ||||||||
| E1 | Crotonic acid, hexyl ester | 19089-92-0 | C10H18O2 | 170.3 | 1374.5 | 1756.51 | 1.4579 | RI, Dt |
| E2 | Ethyl salicylate | 118-61-6 | C9H10O3 | 166.2 | 1283.7 | 1337.581 | 1.2676 | RI, Dt |
| E3 | 2-Nonynoic acid Methyl ester | 111-80-8 | C10H16O2 | 168.2 | 1352.3 | 1642.908 | 1.4606 | RI, Dt |
| E4-1 | Ethyl acetate M | 141-78-6 | C4H8O2 | 88.1 | 613.2 | 143.383 | 1.0996 | RI, Dt |
| E4-2 | Ethyl acetate D | 141-78-6 | C4H8O2 | 88.1 | 611.6 | 142.749 | 1.3408 | RI, Dt |
| E5 | Butyl acetate | 123-86-4 | C6H12O2 | 116.2 | 810 | 278.372 | 1.2387 | RI, Dt |
| E6 | Styralyl acetate | 93-92-5 | C10H12O2 | 164.2 | 1220.4 | 1106.023 | 1.0544 | RI, Dt |
| E7 | Methyl salicylate | 119-36-8 | C8H8O3 | 152.1 | 1200.2 | 1040.893 | 1.2011 | RI, Dt |
| Others | ||||||||
| F1 | Cis-Anethol | 104-46-1 | C10H12O | 148.2 | 1371.5 | 1740.328 | 1.2241 | RI, Dt |
| F2 | Methyl eugenol | 93-15-2 | C11H14O2 | 178.2 | 1404 | 1919.024 | 1.4437 | RI, Dt |
| F3 | 4-Allylanisole | 140-67-0 | C10H12O | 148.2 | 1239.3 | 1170.509 | 1.2348 | RI, Dt |
| F4 | 2-Methoxy-3-isobutyl pyrazine | 24683-00-9 | C9H14N2O | 166.2 | 1212.8 | 1081.021 | 1.2998 | RI, Dt |
| F5 | 4-Ethylphenol | 123-07-9 | C8H10O | 122.2 | 1157.2 | 914.964 | 1.1964 | RI, Dt |
| F6 | Allyl Propyl Disulfide | 2179-59-1 | C6H12S2 | 148.3 | 1068.8 | 701.854 | 1.7138 | RI, Dt |
| F7-1 | 1-Mercaptopropane M | 107-03-9 | C3H8S | 76.2 | 626.5 | 148.635 | 1.1690 | RI, Dt |
| F7-2 | 1-Mercaptopropane D | 107-03-9 | C3H8S | 76.2 | 624.1 | 147.654 | 1.3602 | RI, Dt |
| F8 | 2-Methylpropanoic acid | 79-31-2 | C4H8O2 | 88.1 | 780.7 | 249.895 | 1.1613 | RI, Dt |
| unidentified | ||||||||
| U1 | 1 | unidentified | * | 0 | 1289.5 | 1360.959 | 1.6034 | RI, Dt |
| U2 | 2 | unidentified | * | 0 | 1240 | 1172.995 | 1.3670 | RI, Dt |
| U3 | 3 | unidentified | * | 0 | 1124.8 | 830.153 | 1.1964 | RI, Dt |
| U4 | 4 | unidentified | * | 0 | 1087 | 741.255 | 1.1907 | RI, Dt |
| U5 | 5 | unidentified | * | 0 | 908.3 | 401.022 | 1.2154 | RI, Dt |
| U6 | 6 | unidentified | * | 0 | 782.6 | 251.715 | 1.1745 | RI, Dt |
| U7 | 7 | unidentified | * | 0 | 695.9 | 180.852 | 1.1052 | RI, Dt |
| U8 | 8 | unidentified | * | 0 | 691.9 | 178.146 | 1.3284 | RI, Dt |
| U9 | 9 | unidentified | * | 0 | 586.8 | 133.443 | 1.0226 | RI, Dt |
| U10 | 10 | unidentified | * | 0 | 598.2 | 137.635 | 1.1155 | RI, Dt |
| U11 | 11 | unidentified | * | 0 | 597.9 | 137.522 | 1.2236 | RI, Dt |
| U12 | 12 | unidentified | * | 0 | 563 | 125.098 | 1.2115 | RI, Dt |
| Sample | Alcohols | Aldehydes | Monoterpenoids | Ketones | Esters | Others | Total |
|---|---|---|---|---|---|---|---|
| Peak Intensity | |||||||
| ZGP | 4057.40 ± 85.36 2.13 ± 0.0531% | 48,216.08 ± 669.70 25.27 ± 0.3588% | 106,640.88 ± 1889.00 55.88 ± 0.6799% | 7848.07 ± 414.90 4.12 ± 0.3356% | 7385.46 ± 663.58 3.87 ± 0.3571% | 16,678.18 ± 652.85 8.74 ± 0.2549% | 190,826.08 ± 2979.08 100% |
| ZIP | 3503.93 ± 223.57 1.99 ± 0.1516% | 48,588.76 ± 436.03 27.62 ± 0.3455% | 97,652.15 ± 136.62 55.50 ± 0.1949% | 9587.01 ± 508.14 5.45 ± 0.5158% | 3536.75 ± 78.47 2.01 ± 0.0462% | 13,072.33 ± 143.86 7.43 ± 0.0735% | 175,940.95 ± 606.68 100% |
| ZJP | 3316.51 ± 174.06 1.98 ± 0.0819% | 40,396.04 ± 2093.32 24.15 ± 0.8953% | 96,535.55 ± 917.79 57.77 ± 0.9334% | 10,376.60 ± 654.61 6.21 ± 0.5158% | 3662.04 ± 355.46 2.19 ± 0.2065% | 12,854.95 ± 287.34 7.69 ± 0.1105% | 167,141.68 ± 3621.46 100% |
| ZKP | 4553.94 ± 241.48 2.78 ± 0.1011% | 39,014.17 ± 2260.32 23.83 ± 0.4442% | 89,086.27 ± 6168.29 54.37 ± 0.3045% | 9727.70 ± 268.59 5.95 ± 0.3045% | 5098.73 ± 914.90 3.09 ± 0.4344% | 16,364.86 ± 1310.06 9.98 ± 0.1752% | 163,845.67 ± 11,056.22 100% |
| ZLP | 5734.82 ± 316.98 3.33 ± 0.2417% | 45,457.91 ± 926.48 26.37 ± 0.4901% | 92,519.90 ± 172.36 53.67 ± 0.3186% | 9727.17 ± 285.91 5.64 ± 0.2818% | 3918.24 ± 189.80 2.27 ± 0.1268% | 15,027.25 ± 412.60 8.72 ± 0.2762% | 172,385.29 ± 911.82 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, H.; Zou, J.; Chen, L.; Chen, H.; Hu, Y.; Wang, F.; Liu, Y. Electronic Nose and Head Space GC–IMS Provide Insights into the Dynamic Changes and Regularity of Volatile Compounds in Zangju (Citrus reticulata cv. Manau Gan) Peel at Different Maturation Stages. Molecules 2023, 28, 5326. https://doi.org/10.3390/molecules28145326
Wang P, Wang H, Zou J, Chen L, Chen H, Hu Y, Wang F, Liu Y. Electronic Nose and Head Space GC–IMS Provide Insights into the Dynamic Changes and Regularity of Volatile Compounds in Zangju (Citrus reticulata cv. Manau Gan) Peel at Different Maturation Stages. Molecules. 2023; 28(14):5326. https://doi.org/10.3390/molecules28145326
Chicago/Turabian StyleWang, Peng, Haifan Wang, Jialiang Zou, Lin Chen, Hongping Chen, Yuan Hu, Fu Wang, and Youping Liu. 2023. "Electronic Nose and Head Space GC–IMS Provide Insights into the Dynamic Changes and Regularity of Volatile Compounds in Zangju (Citrus reticulata cv. Manau Gan) Peel at Different Maturation Stages" Molecules 28, no. 14: 5326. https://doi.org/10.3390/molecules28145326
APA StyleWang, P., Wang, H., Zou, J., Chen, L., Chen, H., Hu, Y., Wang, F., & Liu, Y. (2023). Electronic Nose and Head Space GC–IMS Provide Insights into the Dynamic Changes and Regularity of Volatile Compounds in Zangju (Citrus reticulata cv. Manau Gan) Peel at Different Maturation Stages. Molecules, 28(14), 5326. https://doi.org/10.3390/molecules28145326






