Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review
Abstract
1. Introduction
2. Epidemiology of UC
3. Pharmacological Treatment of UC
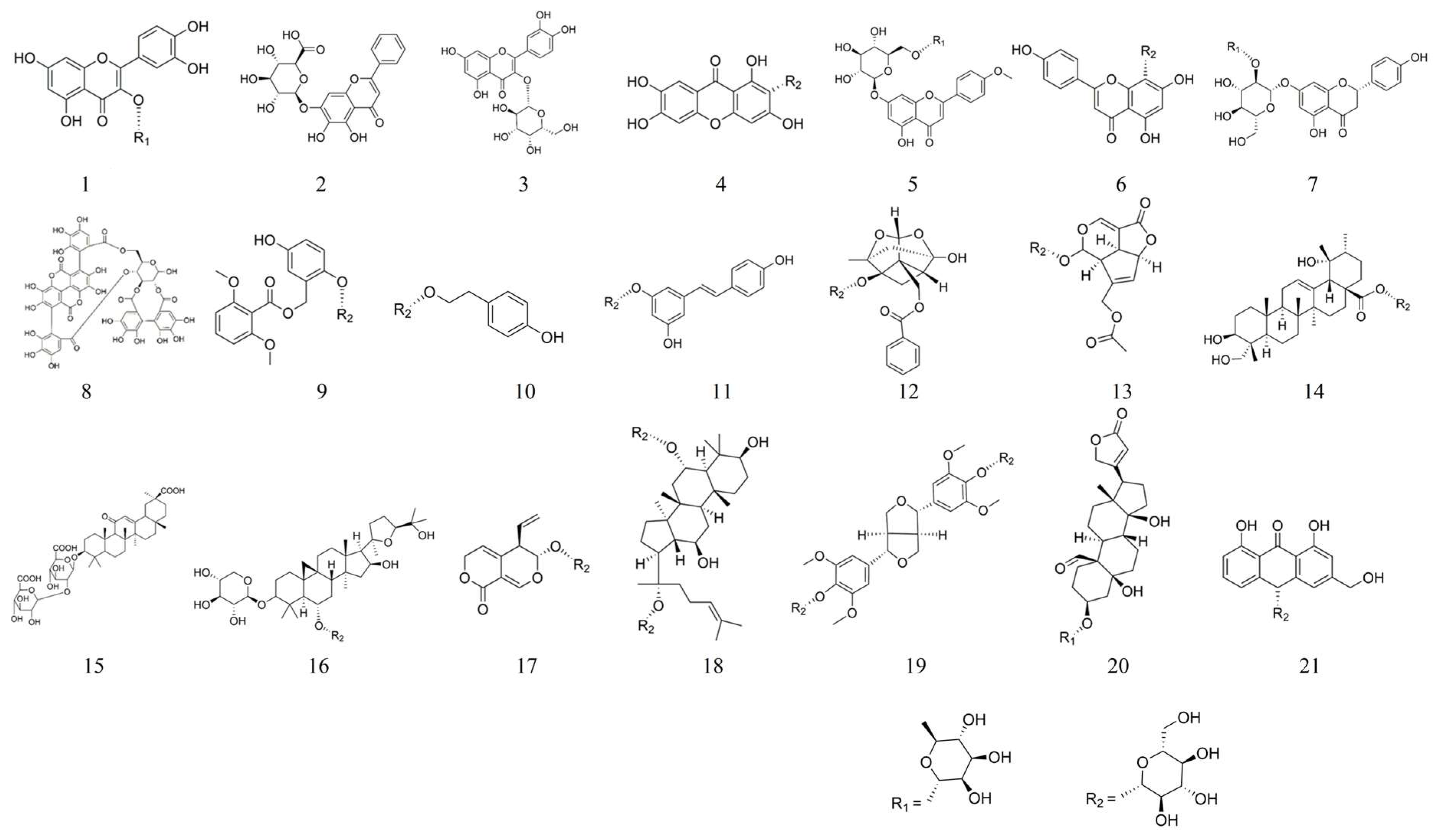
4. Synopsis of Glycosides
5. Anti-Inflammatory Effects of Glycosides in a Model of UC
5.1. Quercitrin 1
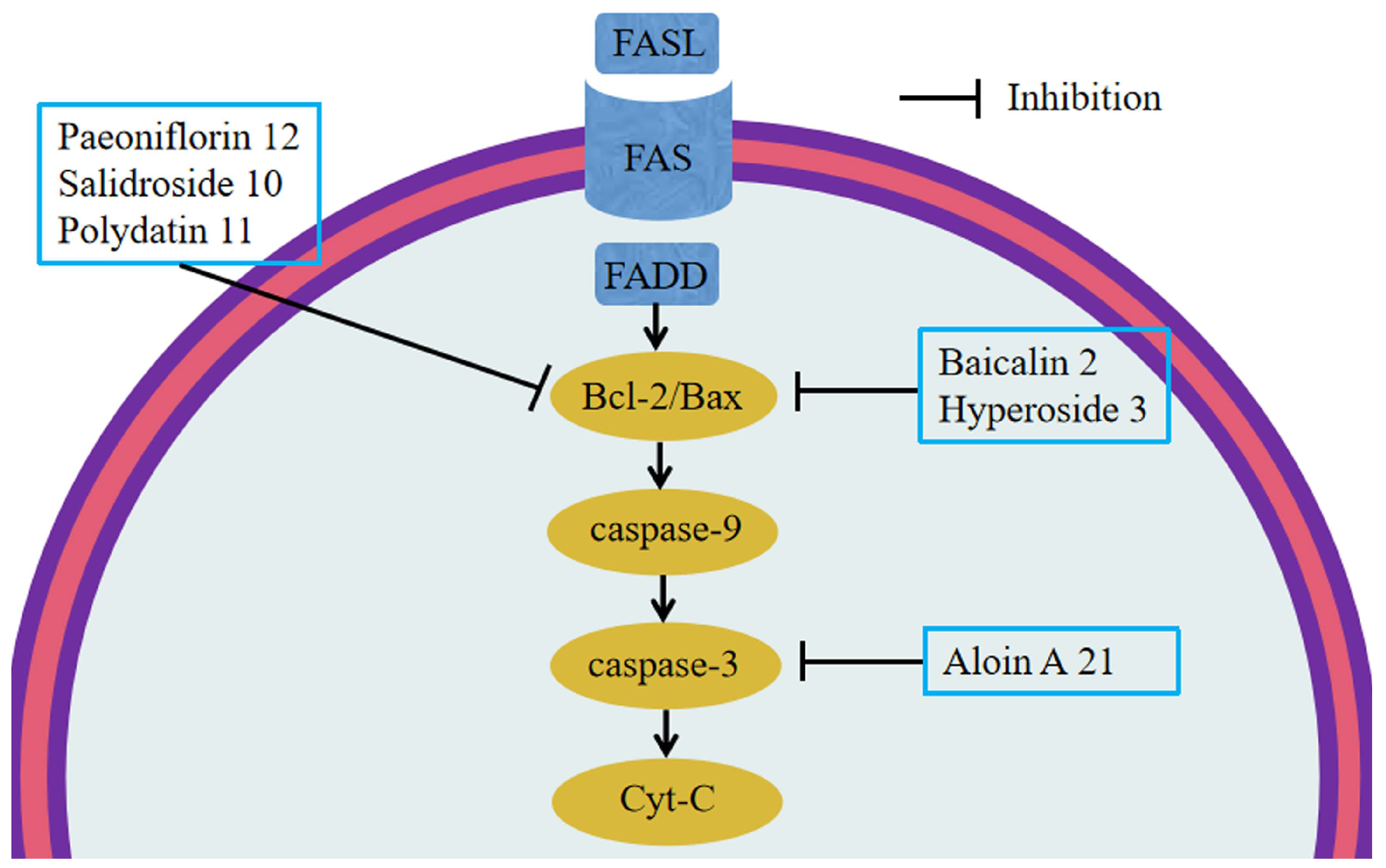
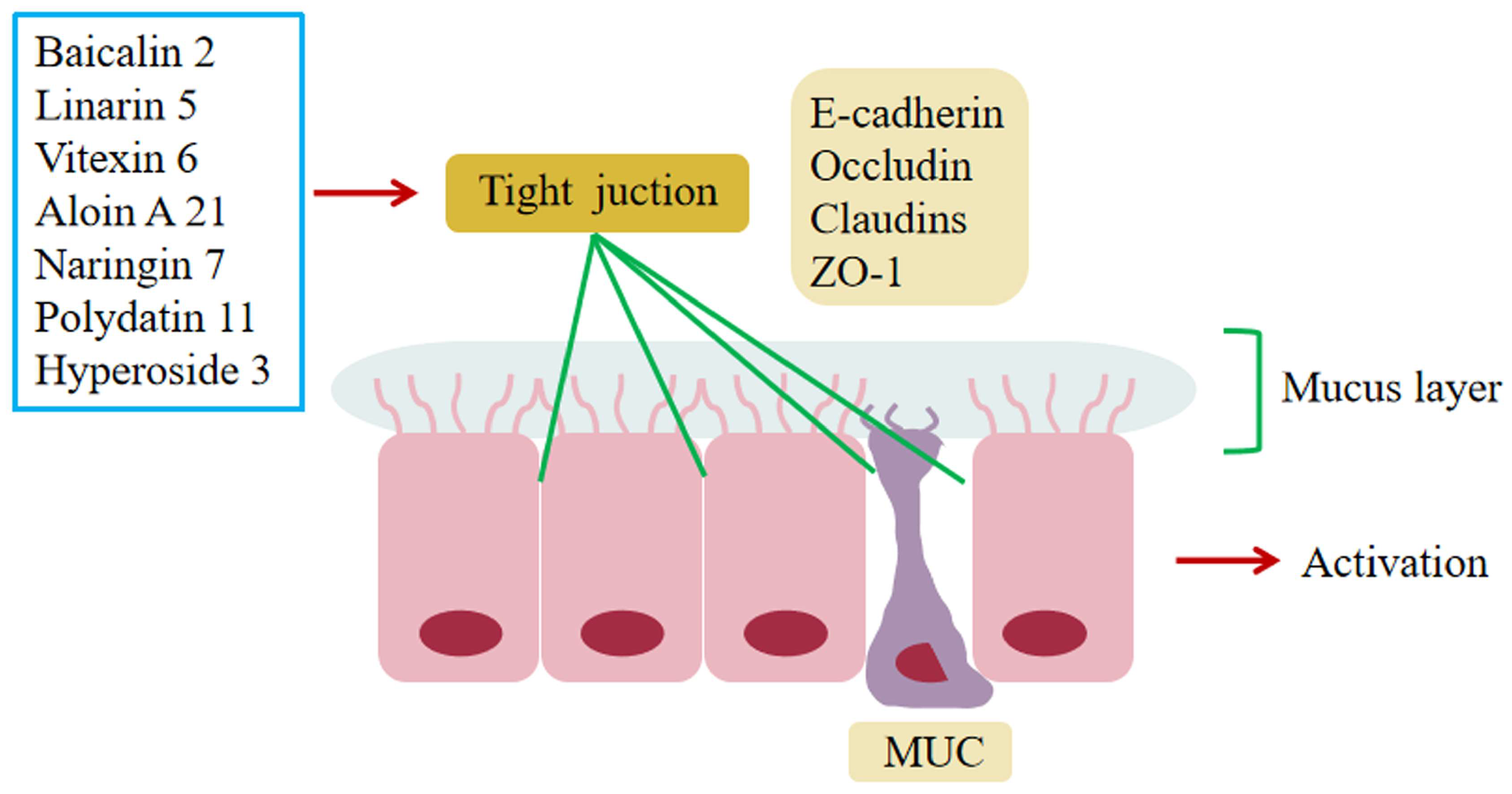
5.2. Baicalin 2
5.3. Hyperoside 3
5.4. Mangiferin 4
5.5. Linarin 5
5.6. Vitexin 6
5.7. Naringin 7
5.8. Punicalagin 8
5.9. Curculigoside 9
5.10. Salidroside 10
5.11. Polydatin 11
5.12. Paeoniflorin 12
5.13. Asperuloside 13
5.14. Pedunculoside 14
5.15. Glycyrrhizin 15
5.16. Astragaloside Ⅳ 16
5.17. Gentiopicroside 17
5.18. Ginsenoside Rg1 18
5.19. Liriodendrin 19
5.20. Convallatoxin 20
5.21. Aloin A 21
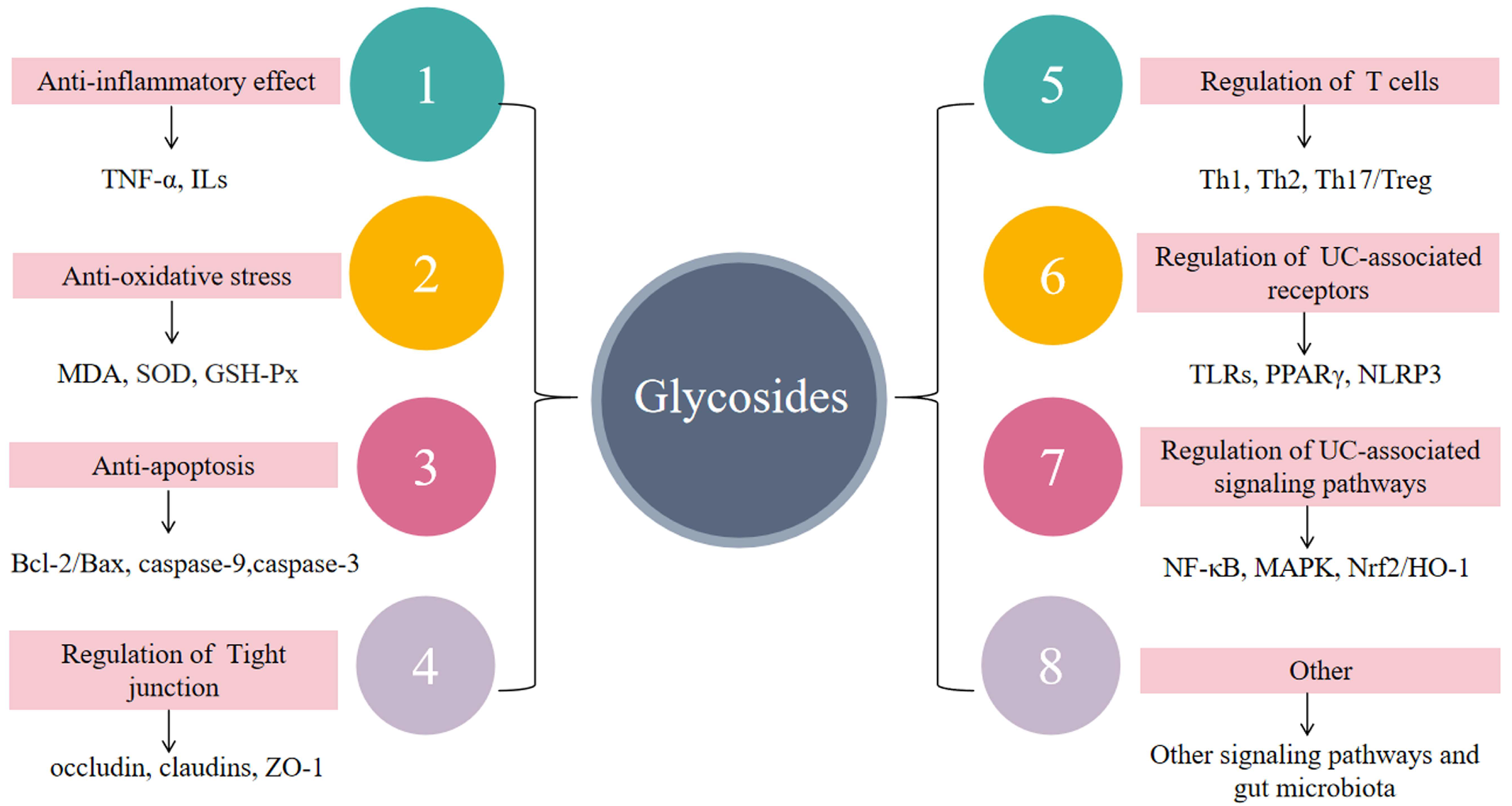
6. Anti-Inflammatory Mechanisms of Glycosides in UC
6.1. Suppressing Inflammatory Responses
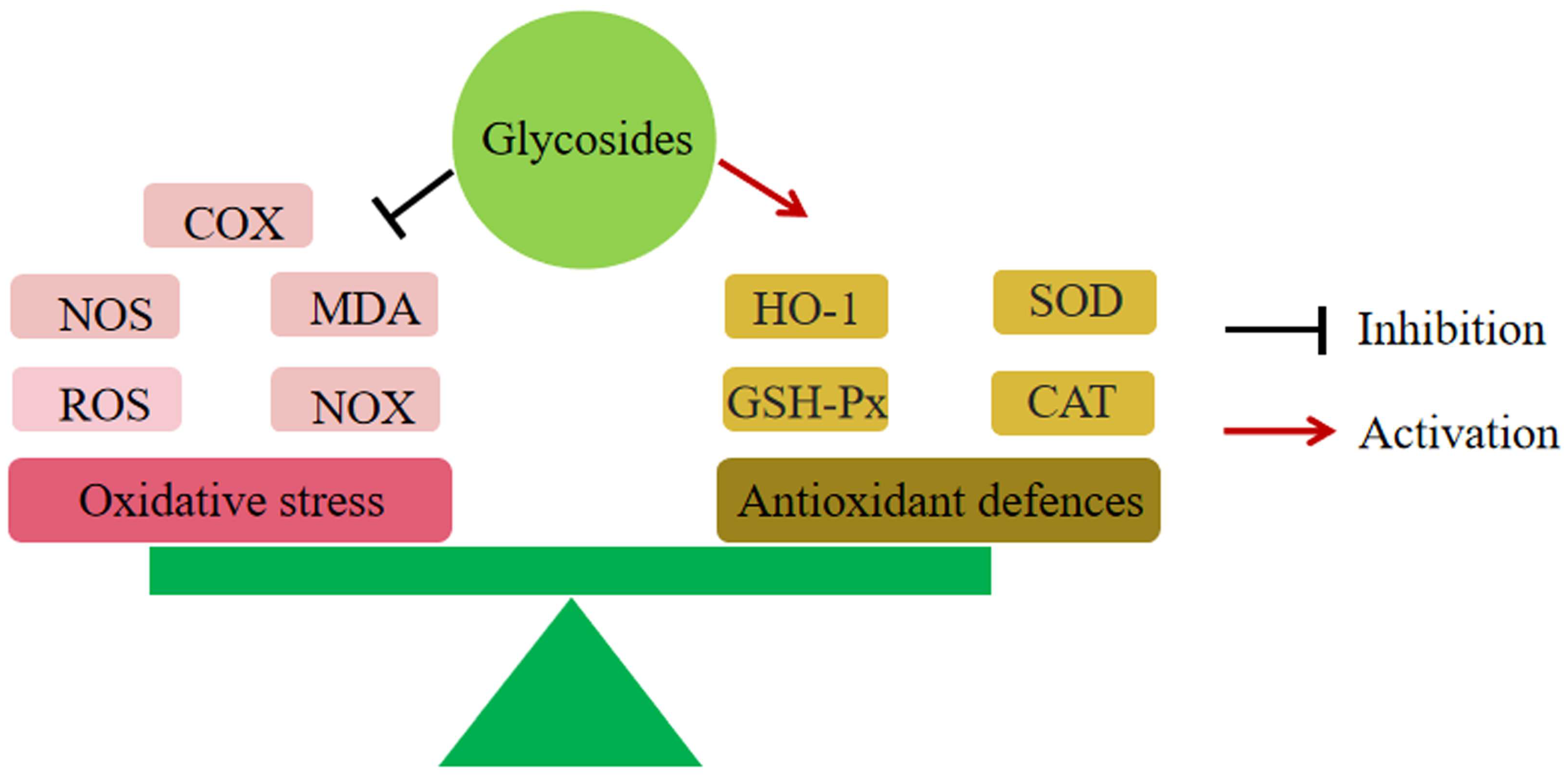
6.2. Reduction of Oxidative Stress
6.3. Anti-Apoptosis
6.4. Regulation of Impaired Intestinal Epithelial Barrier Function
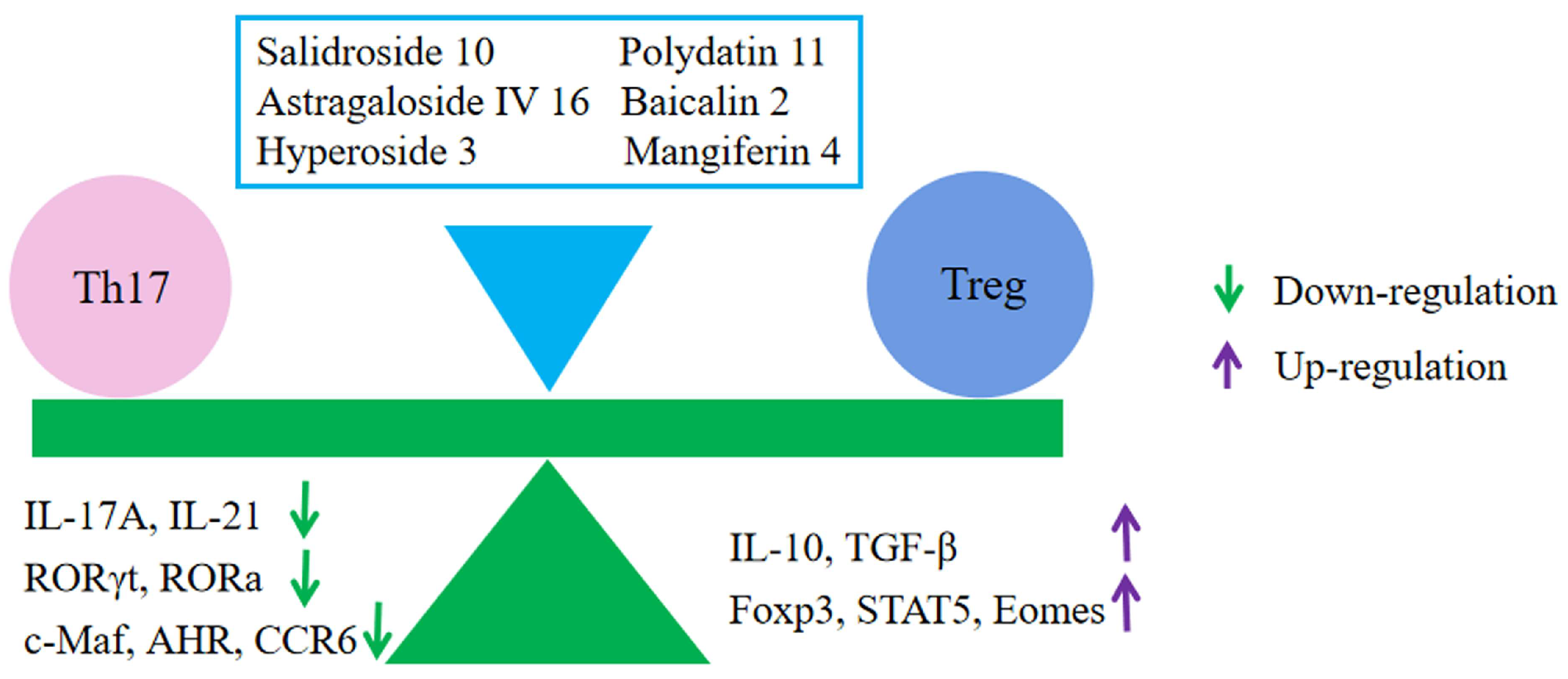
6.5. Regulation of Immune Cells
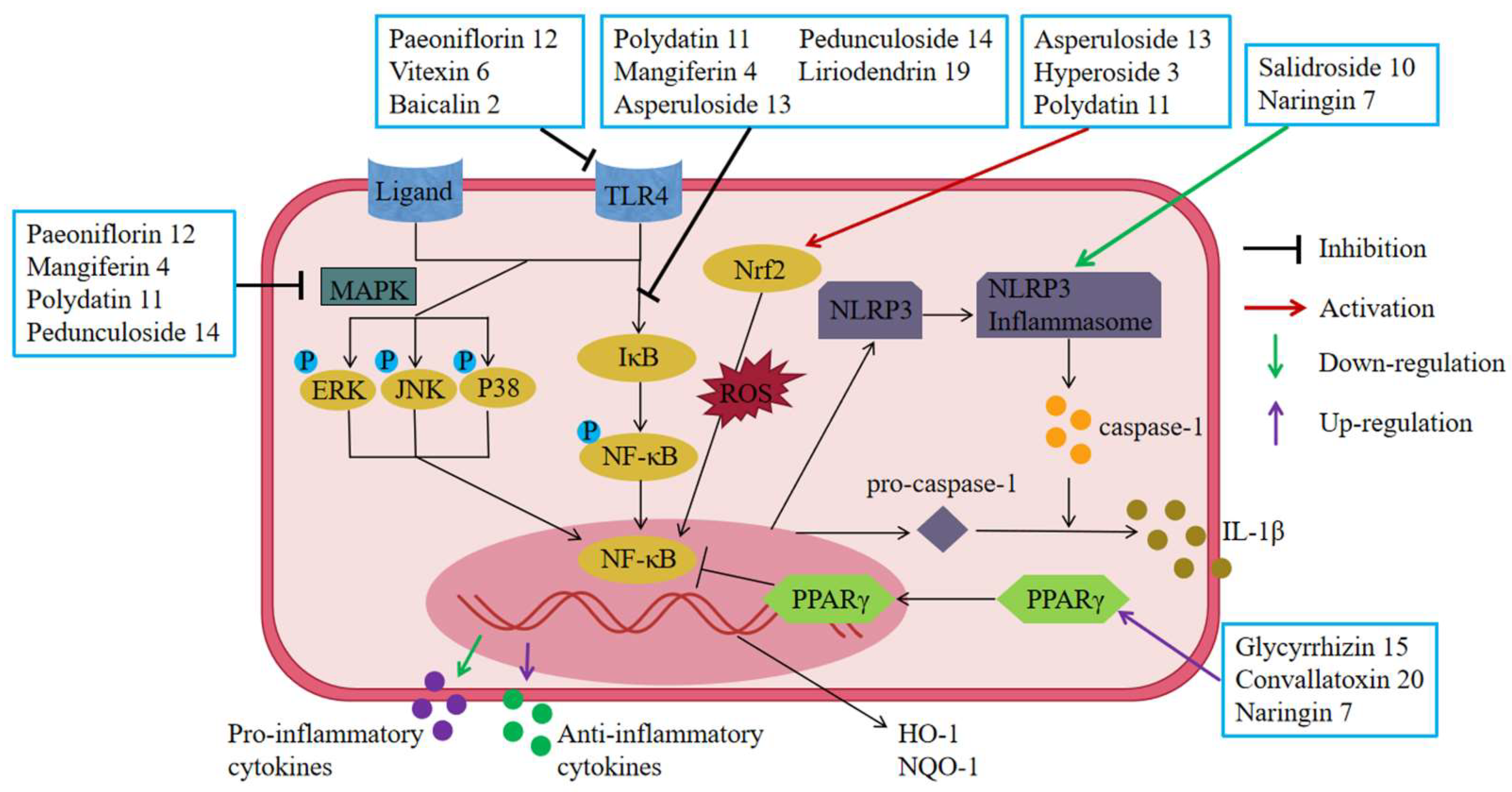
6.6. Regulation of UC-Related Receptors
6.6.1. Inhibition of Toll-like Receptors (TLRs)
6.6.2. Up-Regulation of Peroxisome Proliferator-Activated Receptor (PPARγ)
6.6.3. Inhibition of Nucleotide-Binding Oligomerization Domain (NOD)-Like Receptors (NLRs)
6.7. Regulating Signal Transduction
6.7.1. Inhibition of the NF-κB Pathway
6.7.2. Inhibition of the MAPK Pathway
6.7.3. Inhibition of the Nrf2/HO-1 Pathway
6.7.4. Inhibition of Other Related Pathways
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| IBD | Inflammatory bowel disease |
| TNF-α | Tumor necrosis factor-alpha |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-B |
| STAT | Signal transducer and activator of transcription |
| Treg | Regulatory T cells |
| IFN-γ | Interferon-γ |
| ERK | Extracellular signal-regulated kinase |
| JNK | C-Jun N-terminal kinase |
| TLR4 | Toll-like receptor4 |
| EGFL7 | Epidermal growth factor-like domain 7 |
| MPO | Myeloperoxidase |
| MDA | Malondialdehyde |
| GSH-Px | Glutathione peroxidase |
| GPX4 | Anti-oxidant enzyme glutathione peroxidase 4 |
| HO-1 | Heme oxygenase-1 |
| NQO1 | Quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| COX-2 | Cyclooxygenase-2 |
| INOs | Inducible nitric oxide synthase |
| ERβ | Estrogen receptor-β |
| ICAM-1 | Intercellular cell adhesion molecule-1 |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| HMGB1 | High-mobility group box 1 |
| DCs | Dendritic cells |
| IFN-γ | Interferon γ |
| NOX1 | Nicotinamide adenine dinucleotide phosphate oxidase 1 |
| Shh | Sonic hedgehog |
| Ptch1 | Patched1 |
| Smo | Smoothened |
| Gli1 | Glioma-associated oncogene homolog 1 |
| ERK | Extracellular signal-regulated kinase |
| JNK | C-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MUC | Mucin |
| Th | T-helper |
| Tregs | Regulatory T cells |
| c-Maf | c-Musculoaponeurotic fibrosarcoma |
| AhR | Aryl hydrocarbon receptor |
| EOMES | Recombinant eomesodermin |
| FOXP3 | Forkhead box P3 |
| CCR6 | Chemokine receptor 6 |
| TGF-β1 | Transforming growth factor-β1 |
| CAT | Catalase |
| DLL3 | Delta-like protein 3 |
| PI3K | Phosphoinositide 3-kinase |
| AKT | Protein kinase B |
| PTEN | Phosphatase and tensin homologue deleted on chromosome ten |
| ROS | Reactive oxygen species |
| RORγt | Retinoic acid-related orphan receptor gamma t |
| ZO | Zonula occludens |
| IEC | Intestinal epithelial cell |
| ASC | Amino acid transporter 1 |
| NLRP6 | Nod-like receptor pyrin domain-containing protein 6 |
| MKRN1 | Makorin ring finger protein 1 |
| OCLN | Occludin |
| ATOH1 | Recombinant human atonal homolog 1 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IκBα | Inhibitory κB-α |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| TC | Total cholesterol |
| TG | Triglyceride |
| ASC | Apoptosis-associated particulate protein |
| SGOT | Serum glutamic-oxaloacetic transaminase |
| SGPT | Serum glutamic pyruvic transaminase |
| ALP | Alkaline phosphatase |
| TJ | Tight junction |
| Cyt-C | Cytochrome c |
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Høivik, M.L.; Moum, B.; Solberg, I.C.; Henriksen, M.; Cvancarova, M.; Bernklev, T. IBSEN Group Work disability in inflammatory bowel disease patients 10 years after disease onset: Results from the IBSEN Study. Gut 2013, 62, 368–375. [Google Scholar] [CrossRef]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef]
- Anzai, H.; Hata, K.; Kishikawa, J.; Ishii, H.; Nishikawa, T.; Tanaka, T.; Tanaka, J.; Kiyomatsu, T.; Kawai, K.; Nozawa, H.; et al. Clinical pattern and progression of ulcerative proctitis in the Japanese population: A retrospective study of incidence and risk factors influencing progression. Color. Dis. 2016, 18, O97–O102. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Q.; Sha, S.; Xu, B.; Liang, S. Prevalence of colorectal cancer in patients with ulcerative colitis: A retrospective, monocenter study in China. J. Cancer Res. Ther. 2015, 11, 899–903. [Google Scholar] [CrossRef]
- Rosenzwajg, M.; Lorenzon, R.; Cacoub, P.; Pham, H.P.; Pitoiset, F.; El Soufi, K.; Ribet, C.; Bernard, C.; Aractingi, S.; Banneville, B.; et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019, 78, 209–217. [Google Scholar] [CrossRef]
- Park, J.H.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun. Rev. 2017, 16, 416–426. [Google Scholar] [CrossRef]
- Park, S.C.; Jeen, Y.T. Anti-integrin therapy for inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1868–1880. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Bjerrum, J.T.; Herfarth, H.; Rogler, G. Recent Advances Using Immunomodulators for Inflammatory Bowel Disease. J. Clin. Pharmacol. 2013, 53, 575–588. [Google Scholar] [CrossRef]
- Poitras, P.; Gougeon, A.; Binn, M.; Bouin, M. Extra digestive manifestations of irritable bowel syndrome: Intolerance to drugs? Dig. Dis. Sci. 2008, 53, 2168–2176. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Merikas, E.; Georgopoulos, F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Dev. Ther. 2011, 5, 185–210. [Google Scholar] [CrossRef]
- Xue, J.-C.; Yuan, S.; Meng, H.; Hou, X.-T.; Li, J.; Zhang, H.-M.; Chen, L.-L.; Zhang, C.-H.; Zhang, Q.-G. The role and mechanism of flavonoid herbal natural products in ulcerative colitis. Biomed. Pharmacother. 2023, 158, 114086. [Google Scholar] [CrossRef]
- Araruna, M.E.; Serafim, C.; Alves Júnior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models (2010–2020): A Review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef]
- Santos, J.A.M.; Santos, C.L.A.A.; Freitas Filho, J.R.; Menezes, P.H.; Freitas, J.C.R. Polyacetylene Glycosides: Isolation, Biological Activities and Synthesis. Chem. Rec. 2022, 22, e202100176. [Google Scholar] [CrossRef]
- Tian, X.Y.; Li, M.X.; Lin, T.; Qiu, Y.; Zhu, Y.T.; Li, X.L.; Tao, W.D.; Wang, P.; Ren, X.X.; Chen, L.P. A review on the structure and pharmaco-logical activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef]
- Khan, H.; Pervaiz, A.; Intagliata, S.; Das, N.; Venkata, K.C.N.; Atanasov, A.G.; Najda, A.; Nabavi, S.M.; Wang, D.; Pittalà, V.; et al. The analgesic potential of glycosides derived from medicinal plants. DARU J. Pharm. Sci. 2020, 28, 387–401. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42; quiz e30. [Google Scholar] [CrossRef]
- Torres, J.; Billioud, V.; Sachar, D.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis as A Progressive Disease: The Forgotten Evidence. Inflamm. Bowel Dis. 2012, 18, 1356–1363. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Cohen, R.D.; Yu, A.P.; Wu, E.Q.; Xie, J.; Mulani, P.M.; Chao, J. Systematic review: The costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 2010, 31, 693–707. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Danese, S.; Kupcinskas, L.; Alexeeva, O.; D’Haens, G.; Gibson, P.R.; Moro, L.; Jones, R.; Ballard, E.D.; Masure, J.; et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: Results from the randomised CORE II study. Gut 2014, 63, 433–441. [Google Scholar] [CrossRef]
- Damião, A.O.M.C.; De Azevedo, M.F.C.; Carlos, A.D.S.; Wada, M.Y.; Silva, T.V.M.; Feitosa, F.D.C. Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review. World J. Gastroenterol. 2019, 25, 1142–1157. [Google Scholar] [CrossRef]
- Sultan, K.S.; Berkowitz, J.C.; Khan, S. Combination therapy for inflammatory bowel disease. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 103–113. [Google Scholar] [CrossRef]
- Broekman, M.M.T.J.; Coenen, M.J.H.; van Marrewijk, C.J.; Wanten, G.J.A.; Wong, D.R.; Verbeek, A.L.M.; TOPIC Recruitment Team. More Dose-dependent Side Effects with Mercaptopurine over Azathioprine in IBD Treatment Due to Relatively Higher Dosing. Inflamm. Bowel Dis. 2017, 23, 1873–1881. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Meyers, S.; Shih, J.; Neher, J.O.; Safranek, S. Clinical Inquiries: How effective and safe is fecal microbial transplant in preventing C difficile recurrence? J. Fam. Pract. 2018, 67, 386–388. [Google Scholar]
- Cottone, M.; Kohn, A.; Daperno, M.; Armuzzi, A.; Guidi, L.; D’Inca, R.; Bossa, F.; Angelucci, E.; Biancone, L.; Gionchetti, P.; et al. Advanced Age Is an Independent Risk Factor for Severe Infections and Mortality in Patients Given Anti–Tumor Necrosis Factor Therapy for Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 30–35. [Google Scholar] [CrossRef]
- Suzuki, Y.; Motoya, S.; Hanai, H.; Hibi, T.; Nakamura, S.; Lazar, A.; Robinson, A.M.; Skup, M.; Mostafa, N.M.; Huang, B.; et al. Four-year maintenance treatment with adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J. Gastroenterol. 2017, 52, 1031–1040. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Feagan, B.G.; Marano, C.W.; Padgett, L.; Strauss, R.; Johanns, J.; PURSUIT-IV Study Group. Randomised clinical trial: A placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment. Pharmacol. Ther. 2015, 42, 504–514. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Litaudon, M.; Touboul, D.; Queiroz, E.F. Innovative omics-based approaches for prioritisation and targeted isolation of natural products–new strategies for drug discovery. Nat. Prod. Rep. 2019, 36, 855–868. [Google Scholar] [CrossRef]
- Khan, H.; Saeedi, M.; Nabavi, S.M.; Mubarak, M.S.; Bishayee, A. Glycosides from Medicinal Plants as Potential Anticancer Agents: Emerging Trends Towards Future Drugs. Curr. Med. Chem. 2019, 26, 2389–2406. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Broszczak, D.; Prasad, S.S.; Ekanayake, C.P.; Strappe, P.; Valeris, P.; Naiker, M. Hitting the sweet spot: A systematic review of the bioactivity and health benefits of phenolic glycosides from medicinally used plants. Phytother. Res. 2021, 35, 3484–3508. [Google Scholar] [CrossRef]
- Grubb, C.D.; Zipp, B.J.; Ludwig-Müller, J.; Masuno, M.N.; Molinski, T.F.; Abel, S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004, 40, 893–908. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.-M.; Kojima, M.; Sakakibara, H.; Hou, B.-K. N-Glucosyltransferase UGT76C2 is Involved in Cytokinin Homeostasis and Cytokinin Response in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 2200–2213. [Google Scholar] [CrossRef]
- Hirade, Y.; Kotoku, N.; Terasaka, K.; Saijo-Hamano, Y.; Fukumoto, A.; Mizukami, H. Identification and functional analysis of 2-hydroxyflavanoneC-glucosyltransferase in soybean (Glycine max). FEBS Lett. 2015, 589, 1778–1786. [Google Scholar] [CrossRef]
- Qian, Z.M.; Wan, J.B.; Zhang, Q.W.; Li, S.P. Simultaneous determination of nucleobases, nucleosides and saponins in Panax noto-ginseng using multiple columns high performance liquid chromatography. J. Pharm. Biomed. Anal. 2008, 48, 1361–1367. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Zhang, Y.-Y.; Han, H.; Fan, R.-P.; Hu, Y.; Zhong, L.; Kou, J.-P.; Yu, B.-Y. Advances in the antitumor activities and mechanisms of action of steroidal saponins. Chin. J. Nat. Med. 2018, 16, 732–748. [Google Scholar] [CrossRef]
- Xue, H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Review of the Ethnopharmacology, Phytochemistry, and Pharmacology of the Ge-nus Veronica. Am. J. Chin. Med. 2019, 47, 1193–1221. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef]
- Li, Y.C.; Qiao, J.Y.; Wang, B.Y.; Bai, M.; Shen, J.D.; Cheng, Y.X. Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways. Nutrients 2018, 10, 1024. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Wang, H.; Wang, Y.; Wu, Y.; Xu, H.; Su, C. Paeoniflorin inhibits proliferation of endometrial cancer cells via acti-vating MAPK and NF-κB signaling pathways. Exp. Ther. Med. 2017, 14, 5445–5451. [Google Scholar]
- Zhao, Y.; Zhou, G.; Wang, J.; Jia, L.; Zhang, P.; Li, R.; Shan, L.; Liu, B.; Song, X.; Liu, S.; et al. Paeoniflorin protects against ANIT-induced cholestasis by ameliorating oxidative stress in rats. Food Chem. Toxicol. 2013, 58, 242–248. [Google Scholar] [CrossRef]
- Tu, J.; Guo, Y.; Hong, W.; Fang, Y.; Han, D.; Zhang, P.; Wang, X.; Körner, H.; Wei, W. The Regulatory Effects of Paeoniflorin and Its Derivative Paeoniflorin-6′-O-Benzene Sulfonate CP-25 on Inflammation and Immune Diseases. Front. Pharmacol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Lv, T.; Shen, L.; Yang, L.; Diao, W.; Yang, Z.; Zhang, Y.; Yu, S.; Li, Y. Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via Sonic hedgehog signaling pathway. Int. Immunopharmacol. 2018, 64, 256–263. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Huang, W.; Wang, W.; Wen, G.; Gao, L.; Fu, X.; Wang, M.; Liang, W.; Kwan, H.Y.; et al. Paeoniflorin ameliorates interferon-alpha-induced neuroinflammation and depressive-like behaviors in mice. Oncotarget 2017, 8, 8264–8282. [Google Scholar] [CrossRef]
- Kong, X.; Leng, D.; Liang, G.; Zheng, H.; Wang, Q.; Shen, Y.; Lu, G.; Zhang, H.; Shi, D.; Liu, W. Paeoniflorin augments systemic Candida albicans infection through inhibiting Th1 and Th17 cell expression in a mouse model. Int. Immunopharmacol. 2018, 60, 76–83. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wang, J.; Wu, C.; Dong, L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immun. Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Lin, H.; Chen, H.; Wang, S. Paeoniflorin Inhibits the Proliferation and Metastasis of Ulcerative Colitis-Associated Colon Cancer by Targeting EGFL7. J. Oncol. 2022, 2022, 7498771. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Degly-cosylation by intestinal bacteria and esterification with fatty acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Li, Y.-H.; Xiao, H.-T.; Hu, D.-D.; Fatima, S.; Lin, C.-Y.; Mu, H.-X.; Lee, N.P.; Bian, Z.-X. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol. Res. 2016, 110, 227–239. [Google Scholar] [CrossRef]
- Osman, N.; Adawi, D.; Ahrné, S.; Jeppsson, B.; Molin, G. Probiotics and Blueberry Attenuate the Severity of Dextran Sulfate Sodium (DSS)-Induced Colitis. Dig. Dis. Sci. 2008, 53, 2464–2473. [Google Scholar] [CrossRef]
- Young, Y.; Abreu, M.T. Advances in the pathogenesis of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2006, 8, 470–477. [Google Scholar] [CrossRef]
- Perše, M.; Cerar, A. Dextran Sodium Sulphate Colitis Mouse Model: Traps and Tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef]
- Dönder, Y.; Arikan, T.B.; Baykan, M.; Akyüz, M.; Öz, A.B. Effects of quercitrin on bacterial translocation in a rat model of experimental colitis. Asian J. Surg. 2018, 41, 543–550. [Google Scholar] [CrossRef]
- Romero, M.; Vera, B.; Galisteo, M.; Toral, M.; Gálvez, J.; Perez-Vizcaino, F.; Duarte, J. Protective vascular effects of quercitrin in acute TNBS-colitis in rats: The role of nitric oxide. Food Funct. 2017, 8, 2702–2711. [Google Scholar] [CrossRef]
- Feng, J.; Guo, C.; Zhu, Y.; Pang, L.; Yang, Z.; Zou, Y.; Zheng, X. Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int. J. Clin. Exp. Med. 2014, 7, 4063–4072. [Google Scholar]
- Zhu, L.; Shen, H.; Gu, P.; Liu, Y.; Zhang, L.; Cheng, J. Baicalin alleviates TNBS-induced colitis by inhibiting PI3K/AKT pathway activation. Exp. Ther. Med. 2020, 20, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Wang, J.-Y.; Yao, J.; Cao, X.; Zhang, R.; Li, Y.-X.; Xu, Z.-L.; Zhang, D.-G. Protective effect of baicalin against experimental colitis via suppression of oxidant stress and apoptosis. Pharmacogn. Mag. 2016, 12, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, J.; Cheng, C.; Xu, F.; Au, R.; Zhu, L.; Shen, H. Baicalin Ameliorates DSS-Induced Colitis by Protecting Goblet Cells through Activating NLRP6 Inflammasomes. Evid. Based Complement. Altern. Med. 2022, 2022, 2818136. [Google Scholar] [CrossRef]
- Zou, Y.; Dai, S.X.; Chi, H.G.; Li, T.; He, Z.W.; Wang, J.; Ye, C.G.; Huang, G.L.; Zhao, B.; Li, W.Y.; et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch. Pharmacal Res. 2015, 38, 1873–1887. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Li, Y.; Li, Y.; Yu, S.; Wang, S. Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway. J. Inflamm. 2017, 14, 25. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, W.; Zhang, C.; Ji, P.; Wu, X.; Sha, Z.; Chen, X.; Wang, Y.; Chen, Y.; Cheng, H.; et al. Hyperoside Ameliorates DSS-Induced Colitis through MKRN1-Mediated Regulation of PPARγ Signaling and Th17/Treg Balance. J. Agric. Food Chem. 2021, 69, 15240–15251. [Google Scholar] [CrossRef]
- Szandruk, M.; Merwid-Ląd, A.; Szeląg, A. The impact of mangiferin from Belamcanda chinensis on experimental colitis in rats. Inflammopharmacology 2018, 26, 571–581. [Google Scholar] [CrossRef]
- Lim, S.M.; Jeong, J.J.; Choi, H.S.; Chang, H.B.; Kim, D.H. Mangiferin corrects the imbalance of Th17/Treg cells in mice with TNBS-induced colitis. Int. Immunopharmacol. 2016, 34, 220–228. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Ren, G.; Ding, L.; Sun, A.; Deng, C.; Wu, X.; Wei, X.; Mani, S.; Wang, Z. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int. Immunopharmacol. 2014, 23, 170–178. [Google Scholar] [CrossRef]
- Jin, C.; Liu, J.; Jin, R.; Yao, Y.; He, S.; Lei, M.; Peng, X. Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier, suppression of inflammatory responses and modulation of gut microbiota. Food Funct. 2022, 13, 10574–10586. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, F.; Chen, Z.; Chen, Y.; Yuan, J.; Xiong, Q.; Hou, S.; Huang, S.; Liu, C.; Liang, J. Vitexin Protects against Dextran Sodium Sulfate-Induced Colitis in Mice and Its Potential Mechanisms. J. Agric. Food Chem. 2022, 70, 12041–12054. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef]
- Hambardikar, V.R.; Mandlik, D.S. Protective effect of naringin ameliorates TNBS-induced colitis in rats via improving antioxidant status and pro-inflammatory cytokines. Immunopharmacol. Immunotoxicol. 2022, 44, 373–386. [Google Scholar] [CrossRef]
- Shah, T.A.; Parikh, M.; Patel, K.V.; Patel, K.G.; Joshi, C.G.; Gandhi, T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol. Cell. Biochem. 2016, 419, 65–74. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Wang, J.; Bai, X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 2020, 259, 118356. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Lv, T.; Wang, R.; Zhang, N.; Peng, H.; Diao, W. Salidroside attenuates dextran sulfate sodium-induced colitis in mice via SIRT1/FoxOs signaling pathway. Eur. J. Pharmacol. 2019, 861, 172591. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; Dai, Z.; Luo, S.; Shi, Y.; He, Z.; Chen, Y. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother. Res. 2023, 37, 367–382. [Google Scholar] [CrossRef]
- Ebrahim, H.A.; Elsherbini, D.M.A. Renovation of Intestinal Barrier by Polydatin in Experimentally Induced Ulcerative Colitis: Comparative Ultrastructural Study with L-Carnosine. Cells Tissues Organs 2021, 210, 275–292. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xu, W.H.; Fan, L.M.; Zhang, Y.Q.; Xu, W.; Chen, Y.P.; Chen, L.L.; Chen, L.; Xu, W.; Wang, Y.; et al. Polydatin alleviates DSS- and TNBS-induced colitis by suppressing Th17 cell differentiation via directly inhibiting STAT3. Phytother. Res. 2022, 36, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dou, W.; Zhang, E.; Sun, A.; Ding, L.; Wei, X.; Chou, G.; Mani, S.; Wang, Z. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol. Liver Physiol. 2014, 306, G27–G36. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Zhu, L.; Liu, Y.; Zhang, L.; Liu, J.; Shen, H. Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through in-hibiting NF-kappaB pathway and apoptosis in mice. Int. Immunopharmacol. 2017, 50, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, S.; Li, M.; Bi, J.; Yang, G.; Li, E. Paeoniflorin protects against dextran sulfate sodium (DSS)-induced colitis in mice through inhibition of inflammation and eosinophil infiltration. Int. Immunopharmacol. 2021, 97, 107667. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Xu, S.J.; Lu, Y.Y.; Chen, S.X.; Du, X.H.; Hou, S.Z.; Huang, H.Y.; Liang, J. Asperuloside suppressing oxidative stress and in-flammation in DSS-induced chronic colitis and RAW 264.7 macrophages via Nrf2/HO-1 and NF-κB pathways. Chem. Biol. Interact. 2021, 344, 109512. [Google Scholar] [CrossRef]
- Liu, K.; Li, G.; Guo, W.; Zhang, J. The protective effect and mechanism of pedunculoside on DSS (dextran sulfate sodium) induced ulcerative colitis in mice. Int. Immunopharmacol. 2020, 88, 107017. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.-S.; Wu, K.-X.; Jiao, J.-X.; Sun, L.-H.; Feng, Y.-T. Anti-inflammatory effect of Diammonium Glycyrrhizinate in a rat model of ulcerative colitis. World J. Gastroenterol. 2006, 12, 4578–4581. [Google Scholar] [CrossRef]
- Sethuraman, S.N.; Swaminathan, S.; Nelson, S.B.; Palaninathan, P.S.; Gopalan, T.K.; Velayudham, P. Modulation of PPARγ and TNFα by emu oil and glycyrrhizin in ulcerative colitis. Inflammopharmacology 2015, 23, 47–56. [Google Scholar] [CrossRef]
- Chen, X.; Fang, D.; Li, L.; Chen, L.; Li, Q.; Gong, F.; Fang, M. Glycyrrhizin ameliorates experimental colitis through attenuating inter-leukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol. Res. 2017, 65, 666–680. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, W.; Xiong, Y.; Li, Y.; Wan, Q.; Zhou, W.; Zhao, H.; Xiao, Q.; Liu, D. Astragaloside Ⅳ alleviates ulcerative colitis by regu-lating the balance of Th17/Treg cells. Phytomedicine 2022, 104, 154287. [Google Scholar] [CrossRef]
- Niu, Y.-T.; Zhao, Y.-P.; Jiao, Y.-F.; Zheng, J.; Yang, W.-L.; Zhou, R.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Protective effect of gentiopicroside against dextran sodium sulfate induced colitis in mice. Int. Immunopharmacol. 2016, 39, 16–22. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Zhang, D.; Wang, J.; Tan, Y.; Feng, W.; Peng, C. Ginsenoside Rg1 Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Microbial Tryptophan Metabolism. Front. Immunol. 2022, 13, 817600. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Wang, B.; Zhang, L.; Zhang, Q.; Li, D.; Zhang, S.; Gao, H.; Wang, X. Protective role of liriodendrin in mice with dextran sulphate sodium-induced ulcerative colitis. Int. Immunopharmacol. 2017, 52, 203–210. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhang, Z.H.; Wang, Z.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Ma, J.; et al. Convallatoxin protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB signaling through activation of PPARγ. Pharmacol. Res. 2019, 147, 104355. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, G.-F.; Fang, Y.-X.; Liu, Y.-Q.; Wang, Q.; Zheng, X.; Zhang, D.-J.; Zhang, J.; Yin, Z.-Q. Aloin A prevents ulcerative colitis in mice by enhancing the intestinal barrier function via suppressing the Notch signaling pathway. Phytomedicine 2022, 106, 154403. [Google Scholar] [CrossRef]
- Stavric, B. Role of chemopreventers in human diet. Clin. Biochem. 1994, 27, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. [36] Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef]
- Skaper, S.D.; Fabris, M.; Ferrari, V.; Carbonare, M.D.; Leon, A. Quercetin Protects Cutaneous Tissue-Associated Cell Types Including Sensory Neurons From Oxidative Stress Induced By Glutathione Depletion: Cooperative Effects of Ascorbic Acid. Free. Radic. Biol. Med. 1997, 22, 669–678. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart dis-ease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1711. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Korbut, R.; Robak, J.; Świȩs, J. On the mechanism of antithrombotic action of flavonoids. Biochem. Pharmacol. 1987, 36, 317–322. [Google Scholar] [CrossRef]
- Dou, W.; Mukherjee, S.; Li, H.; Venkatesh, M.; Wang, H.; Kortagere, S.; Peleg, A.; Chilimuri, S.S.; Wang, Z.T.; Feng, Y.; et al. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS ONE 2012, 7, e36075. [Google Scholar] [CrossRef]
- Hong, T.; Jin, G.-B.; Cho, S.; Cyong, J.-C. Evaluation of the Anti-Inflammatory Effect of Baicalein on Dextran Sulfate Sodium-Induced Colitis in Mice. Planta Medica 2002, 68, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics In Vitro and In Vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Yuan, Y.; Shuai, L.; Chen, S.; Huang, L.; Qin, S.; Yang, Z. Flavonoids and antioxidative enzymes in temperature-challenged roots of Scutellaria baicalensis Georgi. Z. Für Nat. C J. Biosci. 2012, 67, 77–85. [Google Scholar]
- Chen, D.; Wu, Y.-X.; Qiu, Y.-B.; Wan, B.-B.; Liu, G.; Chen, J.-L.; Lu, M.-D.; Pang, Q.-F. Hyperoside suppresses hypoxia-induced A549 survival and proliferation through ferrous accumulation via AMPK/HO-1 axis. Phytomedicine 2020, 67, 153138. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, F.; Feng, W.; Qiu, X.; Liu, Y.; Yang, B.; Chen, Y.; Xia, P. Hyperoside inhibits biofilm formation of Pseudomonas aeruginosa. Exp. Ther. Med. 2017, 14, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Kuś, P.; Góralska, E.; Woźniak, D. Mangiferin—A bioactive xanthonoid, not only from mango and not just antiox-idant. Mini Rev. Med. Chem. 2013, 13, 439–455. [Google Scholar] [PubMed]
- Jung, K.; Lee, B.; Han, S.J.; Ryu, J.H.; Kim, D.-H. Mangiferin Ameliorates Scopolamine-Induced Learning Deficits in Mice. Biol. Pharm. Bull. 2009, 32, 242–246. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity of south African herbal teas: Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother. Res. 2007, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Márquez, L.; García-Bueno, B.; Madrigal, J.L.M.; Leza, J.C. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur. J. Nutr. 2012, 51, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Taghrir, H.; Dehsheikh, A.B.; Zomorodian, K.; Irajie, C.; Sourestani, M.M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharmaceuticals 2021, 14, 1104. [Google Scholar] [CrossRef]
- Chengyu, Y.; Long, Z.; Bin, Z.; Hong, L.; Xuefei, S.; Congjuan, L.; Caixia, C.; Yan, X. Linarin Protects the Kidney against Ische-mia/Reperfusion Injury via the Inhibition of Bioactive ETS2/IL-12. Biol. Pharm. Bull. 2021, 44, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, Y.; Sun, S.; Xu, X.; Zhan, J.; Yan, Z.; Shang, P.; Pan, X.; Liu, H. Inhibiting TLR4 signaling by linarin for preventing in-flammatory response in osteoarthritis. Aging 2021, 13, 5369–5382. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.C.; Meng, M.; Sun, Q.S.; Gao, S.M.; Sun, H. Linarin prevents LPS induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NF κB pathways. Int. J. Mol. Med. 2018, 42, 1460–1472. [Google Scholar] [CrossRef]
- Gadioli, I.L.; da Cunha, M.S.B.; de Carvalho, M.V.O.; Costa, A.M.; Pineli, L.L.O. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Rivoira, M.A.; Rodriguez, V.; Talamoni, G.; de Talamoni, N.T. New Perspectives in the Pharmacological Potential of Naringin in Medicine. Curr. Med. Chem. 2021, 28, 1987–2007. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Lv, F.; Ge, X.; Li, G. Naringin protects against bone loss in steroid-treated inflammatory bowel disease in a rat model. Arch. Biochem. Biophys. 2018, 650, 22–29. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS- Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Hou, X.; Li, D.; He, S.; Wan, C.; Yin, P.; Liu, M.; Liu, F.; Xu, J. Punicalagin Induces Nrf2/HO-1 Expression via Upregulation of PI3K/AKT Pathway and Inhibits LPS-Induced Oxidative Stress in RAW264.7 Macrophages. Mediat. Inflamm. 2015, 2015, 380218. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Chen, Y.; Liu, S.; Wu, N.; Jia, D. Curculigoside attenuates myocardial ischemia-reperfusion injury by inhibiting the opening of the mitochondrial permeability transition pore. Int. J. Mol. Med. 2020, 45, 1514–1524. [Google Scholar] [CrossRef]
- Tan, S.; Xu, J.; Lai, A.; Cui, R.; Bai, R.; Li, S.; Liang, W.; Zhang, G.; Jiang, S.; Liu, S.; et al. Curculigoside exerts significant anti arthritic effects in vivo and in vitro via regulation of the JAK/STAT/NF κB signaling pathway. Mol. Med. Rep. 2019, 19, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fu, D.X.; Hou, A.J.; Lei, G.Q.; Liu, Z.J.; Chen, J.K.; Zhou, T.S. Antioxidative phenols and phenolic glycosides from Curculigo or-chioides. Chem. Pharm. Bull. 2005, 53, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, Y.-J.; Han, T.; Zhao, L.; Lv, L.; He, Y.-Q.; Zhang, Q.-Y.; Xin, H.-L. Metabolites of curculigoside in rats and their antiosteoporotic activities in osteoblastic MC3T3-E1 cells. Fitoterapia 2017, 117, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Cheng, Q.; Ding, F. Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol. Cell. Biochem. 2009, 332, 85–93. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, Y.P.; Wu, D.; Ji, Y.J.; Wang, X.; Chen, H.L.; Wu, S.S.; Huang, D.J.; Jiang, W. Salidroside protects against hydrogen perox-ide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway. DNA Cell Biol. 2011, 30, 809–819. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, R.; You, X.; Luo, F.; He, H.; Chang, X.; Zhu, L.; Ding, X.; Yan, T. Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer’s disease via SIRT1/NF-κB pathway. Metab. Brain Dis. 2016, 31, 771–778. [Google Scholar] [CrossRef]
- Ahmad, P.; Alvi, S.S.; Iqbal, D.; Khan, M.S. Insights into pharmacological mechanisms of polydatin in targeting risk factors-mediated atherosclerosis. Life Sci. 2020, 254, 117756. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, L.; Sun, S.; Zhou, Q.; Zeng, Z.; Zehua, Z.; Hussain, M.; Lu, C.; Du, H. High-throughput screening suggests glutathione synthetase as an anti-tumor target of polydatin using human proteome chip. Int. J. Biol. Macromol. 2020, 161, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X. Protective effects of polydatin on multiple organ ischemia-reperfusion injury. Bioorg. Chem. 2020, 94, 103485. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dai, X.; Li, Y.; Li, G.; Lin, X.; Ai, C.; Cao, Y.; Li, T.; Lin, B. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020, 18, 114. [Google Scholar] [CrossRef]
- Chen, X.; Chan, H.; Zhang, L.; Liu, X.; Ho, I.H.T.; Zhang, X.; Ho, J.; Hu, W.; Tian, Y.; Kou, S.; et al. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and au-tophagic flux. J. Cell. Mol. Med. 2019, 23, 4290–4300. [Google Scholar] [CrossRef]
- Huang, Q.H.; Xu, L.Q.; Liu, Y.H.; Wu, J.Z.; Wu, X.; Lai, X.P.; Li, Y.C.; Su, Z.R.; Chen, J.N.; Xie, Y.L. Polydatin Protects Rat Liver against Etha-nol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 7953850. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Meng, T.; Li, Y.; He, D.; Ran, X.; Chen, G.; Guo, W.; Kan, X.; Fu, S.; et al. Polydatin Prevents Lipopolysaccharide (LPS)-Induced Parkinson’s Disease via Regulation of the AKT/GSK3β-Nrf2/NF-κB Signaling Axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Zeng, Y.; Chen, L.; Le Xu, X. Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine 2019, 62, 152935. [Google Scholar] [CrossRef]
- Bheereddy, P.; Yerra, V.G.; Kalvala, A.K.; Sherkhane, B.; Kumar, A. SIRT1 Activation by Polydatin Alleviates Oxidative Damage and Elevates Mitochondrial Biogenesis in Experimental Diabetic Neuropathy. Cell. Mol. Neurobiol. 2021, 41, 1563–1577. [Google Scholar] [CrossRef]
- Xiao, H.-B.; Liang, L.; Luo, Z.-F.; Sun, Z.-L. Paeoniflorin regulates GALNT2-ANGPTL3-LPL pathway to attenuate dyslipidemia in mice. Eur. J. Pharmacol. 2018, 836, 122–128. [Google Scholar] [CrossRef]
- Lal, R.; Dhaliwal, J.; Dhaliwal, N.; Dharavath, R.N.; Chopra, K. Activation of the Nrf2/HO-1 signaling pathway by dimethyl fumarate ameliorates complete Freund’s adjuvant-induced arthritis in rats. Eur. J. Pharmacol. 2021, 899, 174044. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Yang, H.; Sun, W.; Gong, X.; Zhao, J.; Sun, Y.; Diao, G. A Water-Soluble Inclusion Complex of Pedunculoside with the Polymer β-Cyclodextrin: A Novel Anti-Inflammation Agent with Low Toxicity. PLoS ONE 2014, 9, e101761. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, Y.-J.; Tu, Q.-B.; Zhao, Y.-R.; Guo, H.; Wang, J.; Zhang, L.; Shi, H.-W.; Sun, Y. Pedunculoside, a novel triterpene saponin extracted from Ilex rotunda, ameliorates high-fat diet induced hyperlipidemia in rats. Biomed. Pharmacother. 2018, 101, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, G.; Wang, J.; Xu, J.; Zhao, F.; Hu, M.; Xu, Z.; Yang, B.; Guo, J.; Sun, S.; et al. Pedunculoside attenuates pathological phe-notypes of fibroblast-like synoviocytes and protects against collagen-induced arthritis. Scand. J. Rheumatol. 2019, 48, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Tang, H.; He, H.; Liu, J.; Mao, J.; Ji, H.; Lin, H.; Wu, T. Glycyrrhizic acid alleviates bleomycin-induced pulmonary fibrosis in rats. Front. Pharmacol. 2015, 6, 215. [Google Scholar] [CrossRef]
- Deng, Q.-P.; Wang, M.-J.; Zeng, X.; Chen, G.G.; Huang, R.-Y. Effects of Glycyrrhizin in a Mouse Model of Lung Adenocarcinoma. Cell. Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Liu, R.; Wang, Z.; Li, Y.; Zhang, Y.; Hao, X.; Huang, Y.; Xie, W.; Wei, H. Amelioration of concanavalin A-induced au-toimmune hepatitis by magnesium isoglycyrrhizinate through inhibition of CD4(+)CD25(-)CD69(+) subset proliferation. Drug Des. Dev. Ther. 2016, 10, 443–453. [Google Scholar]
- Sui, Y.-B.; Zhang, K.-K.; Ren, Y.-K.; Liu, L.; Liu, Y. The role of Nrf2 in astragaloside IV-mediated antioxidative protection on heart failure. Pharm. Biol. 2020, 58, 1201–1207. [Google Scholar] [CrossRef]
- Chen, J.K.; Guo, M.K.; Bai, X.H.; Chen, L.Q.; Su, S.M.; Li, L.; Li, J.Q. Astragaloside IV ameliorates intermittent hypoxia-induced inflam-matory dysfunction by suppressing MAPK/NF-κB signalling pathways in Beas-2B cells. Sleep Breath. 2020, 24, 1237–1245. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Cheng, H.; Tan, X.; Liu, Y.; Wei, C.; Song, E. Astragaloside IV Protects Against Oxidative Stress in Calf Small Intestine Epithelial Cells via NFE2L2-Antioxidant Response Element Signaling. Int. J. Mol. Sci. 2019, 20, 6131. [Google Scholar] [CrossRef]
- Hase, K.; Li, J.; Basnet, P.; Xiong, Q.; Takamura, S.; Namba, T.; Kadota, S. Hepatoprotective Principles of Swertia japonica Makino on D-Galactosamine/Lipopolysaccharide-Induced Liver Injury in Mice. Chem. Pharm. Bull. 1997, 45, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, N.; Korkmaz, S.; Oztürk, Y.; Başer, K.H. Effects of gentiopicroside, sweroside and swertiamarine, secoiridoids from gentian (Gentiana lutea ssp. symphyandra), on cultured chicken embryonic fibroblasts. Planta Med. 2006, 72, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Liu, N.; Yao, M.; Zhang, Y.; Yao, Z.; Feng, Y.; Liu, J.; Zhou, G. A Review of Neuroprotective Effects and Mechanisms of Gin-senosides From Panax Ginseng in Treating Ischemic Stroke. Front. Pharmacol. 2022, 13, 946752. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Li, M.; Du, Y.; Duan, S.; Huang, Y.; Lu, Y.; Zhang, J.; Wang, T.; Fu, F. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget 2017, 8, 55384–55393. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Y.; Yue, R.; Wang, X.; Shi, Y.; Xu, J.; Wu, B.; Li, Y. Ginsenoside Rg1 Alleviates Podocyte Injury Induced by Hyperlipidemia via Targeting the mTOR/NF-κB/NLRP3 Axis. Evid. Based Complement. Altern. Med. 2020, 2020, 2735714. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Liu, M.; Zhao, H.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J. Antiobesity Effects of Ginsenoside Rg1 on 3T3-L1 Preadipocytes and High Fat Diet-Induced Obese Mice Mediated by AMPK. Nutrients 2018, 10, 830. [Google Scholar] [CrossRef]
- Alolga, R.N.; Nuer-Allornuvor, G.F.; Kuugbee, E.D.; Yin, X.; Ma, G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol. Res. 2020, 152, 104630. [Google Scholar] [CrossRef]
- Li, D.H.; Wang, Y.; Lv, Y.S.; Liu, J.H.; Yang, L.; Zhang, S.K.; Zhuo, Y.Z. Preparative Purification of Liriodendrin from Sargentodoxa cu-neata by Macroporous Resin. BioMed Res. Int. 2015, 2015, 861256. [Google Scholar]
- Jin, C.M.; Lee, J.J.; Yang, Y.J.; Kim, Y.M.; Kim, Y.K.; Ryu, S.Y.; Lee, M.K. Liriodenine inhibits dopamine biosynthesis and L-DOPA-induced dopamine content in PC12 cells. Arch. Pharmacal Res. 2007, 30, 984–990. [Google Scholar] [CrossRef]
- Clark, A.M.; Watson, E.S.; Ashfaq, M.K.; Hufford, C.D. In vivo efficacy of antifungal oxoaporphine alkaloids in experimental dis-seminated candidiasis. Pharm. Res. 1987, 4, 495–498. [Google Scholar] [CrossRef]
- Chen, K.-S.; Wu, Y.-C.; Teng, C.-M.; Ko, F.-N.; Wu, T.-S. Bioactive Alkaloids from Illigera luzonensis. J. Nat. Prod. 1997, 60, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Yamagishi, T.; Lee, K.H. Cytotoxic isoquinoline alkaloids from Xanthorhiza simplicissima. Gaoxiong Yi Xue Ke Xue Za Zhi 1989, 5, 409–411. [Google Scholar] [PubMed]
- Shang, X.; Miao, X.; Yang, F.; Wang, C.; Li, B.; Wang, W.; Pan, H.; Guo, X.; Zhang, Y.; Zhang, J. The Genus Adonis as an Important Cardiac Folk Medicine: A Review of the Ethnobotany, Phytochemistry and Pharmacology. Front. Pharmacol. 2019, 10, 25. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, M.Y.; Wang, Z.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Jin, C.; Xu, G.; Piao, L.; Piao, H.; et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine 2020, 68, 153172. [Google Scholar] [CrossRef]
- Anderson, S.E.; Barton, C.E. The cardiac glycoside convallatoxin inhibits the growth of colorectal cancer cells in a p53-independent manner. Mol. Genet. Metab. Rep. 2017, 13, 42–45. [Google Scholar] [CrossRef]
- Lee, J.; Kang, J.S.; Nam, L.B.; Yoo, O.K.; Keum, Y.S. Suppression of NRF2/ARE by convallatoxin sensitises A549 cells to 5-FU-mediated apoptosis. Free Radic. Res. 2018, 52, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-W.; Zhang, W.-J.; Wang, Y.; Tan, L.-P.; Bao, Y.-L.; Song, Z.-B.; Yu, C.-L.; Wang, S.-Y.; Liu, L.; Li, Y.-X. Convallatoxin induces HaCaT cell necroptosis and ameliorates skin lesions in psoriasis-like mouse models. Biomed. Pharmacother. 2020, 121, 109615. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Carty, E.; Macey, M.; Rampton, D.S. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2000, 14, 1169–1179. [Google Scholar] [CrossRef]
- Biasi, F.; Leonarduzzi, G.M.; Oteiza, P.I.; Poli, G. Inflammatory Bowel Disease: Mechanisms, Redox Considerations, and Therapeutic Targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef]
- Siegmund, B.; Zeitz, M. Innate and adaptive immunity in inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 3178–3183. [Google Scholar] [PubMed]
- Xu, C.-M.; Li, X.-M.; Qin, B.-Z.; Liu, B. Effect of tight junction protein of intestinal epithelium and permeability of colonic mucosa in pathogenesis of injured colonic barrier during chronic recovery stage of rats with inflammatory bowel disease. Asian Pac. J. Trop. Med. 2016, 9, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Van Dieren, J.M.; Kuipers, E.J.; Samsom, J.N.; Nieuwenhuis, E.E.; Van Der Woude, J.C. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm. Bowel Dis. 2006, 12, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ Regulatory T Cells, Th17 Effector Cells, and Cytokine Environment in Inflammatory Bowel Disease. J. Clin. Immunol. 2010, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Nakamura, K.; Ihara, E.; Akiho, H.; Takayanagi, R. CD4+CD25+ regulatory T cells suppress Th17-responses in an ex-perimental colitis model. Dig. Dis. Sci. 2011, 56, 376–386. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mukasa, R.; Hatton, R.D.; Weaver, C.T. Developmental plasticity of Th17 and Treg cells. Curr. Opin. Immunol. 2009, 21, 274–280. [Google Scholar] [CrossRef]
- Hollenbach, E.; Neumann, M.; Vieth, M.; Roessner, A.; Malfertheiner, P.; Naumann, M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004, 18, 1550–1552. [Google Scholar] [CrossRef]
- Roy, P.K.; Rashid, F.; Bragg, J.; Ibdah, J.A. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 200–202. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, W.; Liu, Y.; Sun, D.; Li, H.; Chen, L. A review on natural products with cage-like structure. Bioorganic Chem. 2022, 128, 106106. [Google Scholar] [CrossRef]
- Low, Z.; Lani, R.; Tiong, V.; Poh, C.; AbuBakar, S.; Hassandarvish, P. COVID-19 Therapeutic Potential of Natural Products. Int. J. Mol. Sci. 2023, 24, 9589. [Google Scholar] [CrossRef]
- Ooi, S.L.; Pak, S.C. Editorial: A Feasible Approach for Natural Products to Treatment of Diseases. Molecules 2023, 28, 3791. [Google Scholar] [CrossRef]
- Lu, Q.; Tan, D.; Luo, J.; Ye, Y.; Zuo, M.; Wang, S.; Li, C. Potential of natural products in the treatment of irritable bowel syndrome. Phytomedicine 2022, 106, 154419. [Google Scholar] [CrossRef]
- Li, J.H. The prospect of development and application of natural drugs. Med. Rev. 2002, 8, 472–475. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Jacobs, D.; Gaudier, E.; van Duynhoven, J.; Vaughan, E. Non-Digestible Food Ingredients, Colonic Microbiota and the Impact on Gut Health and Immunity: A Role for Metabolomics. Curr. Drug Metab. 2009, 10, 41–54. [Google Scholar] [CrossRef]
| Types | Drugs | Subjects Treated | Side Effects | References |
|---|---|---|---|---|
| Aminosalicylates | Salazopyridine 5-Aminosalicylic acid Olsalazine Mesalazine | First-line drugs used to treat mild and moderate UC. | Long-term use can lead to drug resistance and may cause adverse effects such as damage to the blood, liver, kidney, and digestive tract and folic acid deficiency. | [22,23] |
| Glucocorticoids | Prednisone Budesonide Beclomethasone Fluticasone propionate | For acute and severe UC and mild patients who are intolerant or refractory to aminosalicylates. | Causes metabolic disorders, osteoporosis, etc. Long-term use can lead to drug dependence and irreversible complications. | [24,25] |
| Immunosuppressants | Azathioprine Methotrexate Tacrolimus | For the palliative treatment of hormone-dependent UC patients and severe cases that do not respond to steroids. | Hepatotoxic and nephrotoxic, may increase the risk of infection, and generally used clinically only as an adjunct. | [26,27] |
| Microbiological agents | Lactobacillus rhamnosus GG | For improving the symptoms of mild to moderate UC relapses. | Risk of bacterial translocation and subsequent bacteremia. | [28,29] |
| Biological agents | Infliximab for Injection Tofacitinib | For patients with acute severe UC; for patients with severe UC where immunosuppressive drugs are ineffective or active UC with severe extraintestinal manifestations. | It is very effective for severe patients, but its use is limited due to its high price and side effects such as leukopenia, neutropenia, and allergy | [30,31,32] |
| No. | Glycosides | Classifications | CAS | Molecular Formulas |
|---|---|---|---|---|
| 1 | Quercitrin | Flavonoids | 522-12-3 | C21H20O11 |
| 2 | Baicalin | Flavonoids | 21967-41-9 | C21H18O11 |
| 3 | Hyperoside | Flavonoids | 482-36-0 | C21H20O12 |
| 4 | Mangiferin | Flavonoids | 4773-96-0 | C19H18O11 |
| 5 | Linarin | Flavonoids | 480-36-4 | C28H32O14 |
| 6 | Vitexin | Flavonoids | 3681-93-4 | C21H20O10 |
| 7 | Naringin | Flavonoids | 10236-47-2 | C27H32O14 |
| 8 | Punicalagin | Phenolics | 65995-63-3 | C48H28O30 |
| 9 | Curculigoside | Phenolics | 85643-19-2 | C22H26O11 |
| 10 | Salidroside | Phenolics | 10338-51-9 | C14H20O7 |
| 11 | Polydatin | Phenolics | 65914-17-2 | C20H22O8 |
| 12 | Paeoniflorin | Terpenoids | 23180-57-6 | C23H28O11 |
| 13 | Asperuloside | Terpenoids | 14259-45-1 | C18H22O11 |
| 14 | Pedunculoside | Terpenoids | 42719-32-4 | C36H58O10 |
| 15 | Glycyrrhizin | Terpenoids | 1405-86-3 | C42H62O16 |
| 16 | Astragaloside Ⅳ | Terpenoids | 84687-43-4 | C41H68O14 |
| 17 | Gentiopicroside | Terpenoids | 20831-76-9 | C16H20O9 |
| 18 | Ginsenoside Rg1 | Terpenoids | 22427-39-0 | C42H72O14 |
| 19 | Liriodendrin | Lignans | 573-44-4 | C34H46O18 |
| 20 | Convallatoxin | Steroids | 508-75-8 | C29H42O10 |
| 21 | Aloin A | Anthraquinones | 1415-73-2 | C21H22O9 |
| Glycosides | Animal | Model | Dose | Effects/Mechanisms of Action | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Behavioral Evaluation | Colon Length | Histopathological Evaluation | Biochemical/Molecular Parameters/mRNA | Related Molecular Mechanisms | |||||
| Quercitrin 1 | Male Wistar–Albino rats | DDS- induced colitis | 1 and 5 mg/kg i.g. 10 days | ↓ Colon tissue damage | ↓ MPO and TNF-α | [59] | |||
| Female Wistar rats | TNBS- induced colitis | 1 and 5 mg/kg p.o. once | ↓ Colon tissue damage | ↓ iNOS, COX-2, NOX1, TNF-α, and IL1β | [60] | ||||
| Baicalin 2 | Female C57BL/6 (B6) mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | 100 mg/kg i.g. twice daily for 7 days | ↓ DAI | ↓ Histological score | ↓ TNF-α, IL-6, and IL-13 ↓ MyD88, NF-κB p65 ↓ TLR2, TLR4, and TLR9 ↑ IL-10 | ↓ TLR4/NF-κB-p65 pathway | [61] | |
| Sprague–Dawley rats | TNBS- induced colitis | 100 mg/kg i.g. 14 days | ↓ p-PI3K/PI3K, p-AKT/AKT, TNF-α,IL-6, and IL-1β ↑ IL-10 and ZO-1 | ↓ PI3K/AKT pathway | [62] | ||||
| Male Sprague–Dawley rats/ RAW264.7 macrophages | TNBS- induced colitis/LPS-induced inflammatory macrophage model | 30, 60 and 120 mg/kg i.g. 14 days | ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ MDA ↓ TGF-β1, Bax, and ROS ↓ Caspase-3, cleaved caspase-3, caspase-9, cleaved caspase-9, Fas, and FasL ↑ CAT, GSH-Px, and SOD ↑ Bcl-2 | ↓ Oxidant stress and apoptosis | [63] | |
| Male C57BL/6J mice | DDS- induced colitis | 20, 50 and 100 mg/kg i.g. 7 days | ↓ DAI | ↓ Histological score | ↓ TNF-α, IL-6, and IL-1β ↓ caspase-1 and claudin-2 ↑ IL-10 ↑ ZO-1, NLRP6, MUC2, ASC, and IL-18 ↑ E-cadherin, claudin-4, and claudin-5 | ↑ NLRP6/IL-18 pathway | [64] | ||
| SD rats | TNBS- induced colitis | 10 mL/kg i.g. twice daily for 7 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ MPO ↓ TNF-α, IL-1β, IL-6, IL-17, and IL-12 ↓ RORγt and Th17/Treg ↑ TGF-β, IL-10, and Foxp3 | [65] | ||
| Hyperoside 3 | Male C57BL/6 mice | DDS- induced colitis | 80 and 120 mg/kg i.g. 14 days | ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ TNF-α, IL-6, COX-2, and NF-κB p65 ↓ MDA ↓ Caspase-3 and Bax ↑ IL-10 ↑ Bcl2 ↑ Nrf2, HO-1, and SOD | ↑ Nrf2 pathway | [66] |
| Male C57BL/6 mice | DDS- induced colitis | 3, 10 and 30 mg/kg p.o. 7 days/3, 10, and 30 μM | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ TNF-α, IL-1β, IL-6, IL-17, and IL-22 ↓ MKRN1, RORγt, and Th17/Treg ↑ ZO-1, claudin-5, and MUC2 ↑ Foxp3, IL-10, and TGF-β | [67] | ||
| Mangiferin 4 | Male Wistar rats | TNBS- induced colitis | 10, 30, and 200 mg/kg i.g. 16 days | ↓ Weight loss | ↓ Structural distortion of crypts, desquamated areas or loss of epithelium, and goblet cell depletion | ↓ TNF-a, IL-17, MDA, and SOD | [68] | ||
| Male C57BL/6 mice | TNBS- induced colitis | 10 and 20 mg/kg p.o. 3 days | ↓ Weight loss | ↓ Colonic shortening | ↓ MPO ↓ TNF-α, IL-17, NF-κB, iNOS, and COX-2 ↓ Th17, IL-17, RORγt, and STAT3 ↑ Treg ↑ Foxp3, IL-10, and STAT5 | [69] | |||
| Female C57BL/6 mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | 50 mg/kg p.o. 13 days | ↓ Weight loss | ↓ Colonic shortening | ↓ Histological score | ↓ MPO ↓ TNF-α, IκBα, p-IκBα, p-p65NF-κB, iNOS, ICAM-1, IL-1β, IL-6, p-ERK1/2, ERK1/2, p-JNK, JNK, p-p38MAPK, and p38MAPK | ↓ NF-κB and MAPK pathways | [70] | |
| Linarin 5 | Male C57BL/6J mice | DDS- induced colitis | 25 and 50 mg/kg i.g. 14 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ MPO ↓ IL-6, TNF-α, IFN-γ, and IL-1β ↑ IL-10 ↑ ZO-1, Occludin, and Claudin-1 | [71] | |
| Vitexin 6 | Male BALB/c mice | DDS- induced colitis | 20 and 80 mg/kg i.g. 7 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ IL-1β, IL-6, TNF-α, p-p65/p65, pIκB/IκB, and p-STAT1/STAT1 ↑ IL-10 ↑ MUC2, ZO-1, and Occludin | [72] | |
| Male BALB/C mice | DDS- induced colitis | 40 and 80 mg/kg p.o. 7 days | ↓ Histological scores of liver | ↓ TNF-α, IL-6, and IL-1β ↓ ALT, TC, AST, and TG ↓ TLR4, NF-κB p65, p-p65, IκBα, and p-IκBα | ↓ TLR4/NF-κB pathway | [73] | |||
| Naringin 7 | Male C57BL/6 mice | DDS- induced colitis | 25, 50, and 100 mg/kg p.o. 7 days | ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ TNF-α, IL-1β, and IL-6 ↓ p-p65NF-κB, p-IκBα, p-p38MAPK, p-ERK, and p-JNK ↓ NLRP3, ASC, and Caspase-1 ↑ PPARγ and ZO-1 | [74] | |
| Male Wistar rats | TNBS- induced colitis | 20, 40, and 80 mg/kg p.o. 14 days | ↓ Weight loss ↓ Rectal bleeding ↓ The ratio of colon weight/colon length ↓ Diarrhea score | ↓ Histological score | ↓ MDA and MPO ↓ TNF-a and IL-12 ↓ SGPT, SGOT, and ALP ↑ SOD, GSH-Px, and CAT | [75] | |||
| Punicalagin 8 | Male SD rats | DNBS- induced colitis | 4 mg/kg p.o. 18 days | ↓ DAI | ↓ CMDI | ↓ MPO, MDA, and NO ↓ TNF-α, IL-1β, IL-18, and NF-κB | [76] | ||
| Curculigoside 9 | Male C57BL/6J mice | DDS- induced colitis | 50 and 100 mg/kg p.o. 7 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ Iron overload ↓ ROS and MDA ↑ GSH, GPX4, and SOD | [77] | |
| Salidroside 10 | Male C57BL/6 mice | DDS- induced colitis | 20 and 40 mg/kg i.g. 7 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Colon tissue damage | ↓ Bax, caspase-3, and cleaved-caspase-3 ↑ SOD, GSH-Px, and CAT ↑ Bcl-2 ↑ SIRT1, FoxO1, FoxO3a, and FoxO4 | ↑ SIRT1/FoxOs pathway | [78] |
| Male C57BL/6 mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | 7.5, 10, and 15 mg/kg i.g. 7 days/10, 20, 40, and 80 μM | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Colonic mucosal erosion, crypt loss, and extensive lymphocyte infiltration | ↓ MPO ↓ IL-1β, IL-6, IFN-γ, and IL-17A ↓ NLRP3, caspase-1, TREM1, DAP12, and GSDMD p30 ↓ Th17 ↑ Treg | ↓ TREM1 signal cascade ↓ Th17/Treg imbalance | [79] | |
| Polydatin 11 | Male C57BL/6 mice | DDS- induced colitis | 15, 30, and 45 mg/kg i.p. 7 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ MDA ↓ Caspase 3, cleaved caspase 3, and Bax ↑ SOD and GSH-Px ↑ Bcl-2 ↑ Shh, Ptc, Smo, and Gli1 | ↓ Oxidative stress and apoptosis ↑ Shh pathway | [47] |
| Male Wistar rats | Acetic-acid- induced colitis | 45 mg/kg p.o. 10 days | ↓ DAI ↓ Adhesion score | ↓ Histological score | ↓ MPO, IL-1β, TNF-α, and IL-6 ↑ SOD and GSH-Px ↓ Caspase 3 | ↓ Oxidative stress and apoptosis partially | [80] | ||
| C57BL/6 mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | p.o. 11 days /100, 200, 300, and 400 μM | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ TNF-α, IL-6, IL-4, iNOS, and COX-2 ↓ ERK1/2, JNK1/2, and p38 ↑ IL-10 ↑ Claudin-1, Occludin, ZO-1, MUC2, and MUC3A ↑ AKT, Nrf2, HO-1, and NQO-1 | ↓ Oxidative stress ↓ NF-κB and MAPK pathways ↑ AKT/NF-κB/NQO-2/HO-1pathway | [50] | |
| Male C57BL/6J mice | DDS- induced colitis/ TNBS- induced colitis | 30 and 60 mg/kg i.g. 10 days/5 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ TNF-α and IL-17A ↓ Th17/ Treg cells ↑ Occludin | ↓ JAK/STAT pathway | [81] | |
| Paeoniflorin 12 | Female C57BL/6 mice | DDS- induced colitis | 50 mg/kg p.o. 10 days | ↓ MPO, TNF-α, and IL-6 ↓ NF-κB, ERK1/2, JNK, and p38 MAPKs | ↓ MAPK/NF-κB pathway | [82] | |||
| Female C57BL/6 mice | AOM/DSS-induced CAC model | 3 g/kg p.o. 28 days | ↓ TNF-α, IL-1β, IL-6, IL-13, NF-κB, TLR4, and EGFL7 | ↓ TLR4/NF-κB pathway | [51] | ||||
| Male Balb/c mice | TNBS-induced colitis | 15, 30, and 45 mg/kg p.o. 7 days | ↓ Weight loss | ↓ Colonic shortening | ↓ Colonic damage of macroscopic scores | ↓ MPO, IL-2, IL-6, IL-10, IL-12, IL-1β, TNF-α, and IFN-γ ↓ Bax, cytochrome c, caspase 3, and caspase 9 ↓ p-JNK/JNK ↑ p-P38/P38, p-ERK/ERK, p-NF-κB/NF-κB, and p-IκBα/IκBα ↑ Bcl-2 | ↓ MAPK/NF-κB pathway ↓ Apoptosis | [83] | |
| Male C57BL/6 mice | DDS- induced colitis | 20 mg/kg p.o. 7 days | ↓ Weight loss | ↓ Colonic shortening ↓ Increased spleen weight | ↓ Eosinophil infiltration | ↓ Eosinophil infiltration ↓ Inflammatory cytokines ↑ Treg, p-STAT3, and CCR3 ↑ Eotaxin | ↓ NF-κB pathway | [84] | |
| Asperuloside 13 | Male KM mice / RAW264.7 macrophages | DDS- induced colitis/ LPS-induced inflammatory macrophage model | 0.125 0.5 mg/kg p.o. 38 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening and increased colon thickness | ↓ Inflammatory cell infiltration, epithelial cell destruction, mucosal thickening, and lower microscopic score | ↓ MPO and MDA ↓ TNF-α, IL-6, and NF-κB ↑ SOD and GSH-Px ↑ Nrf2, HO-1, and NQO-1 | ↓ Oxidative stress and NF-κB activation ↑ Nrf2/HO-1 pathway | [85] |
| Pedunculoside 14 | Male C57BL/6 mice/ RAW264.7 macrophages | DDS- induced colitis/ LPS-induced inflammatory macrophage model | 5, 15, and 30 mg/kg p.o. 7 days | ↓ Colonic shortening | ↓ Loss of goblet cells and crypts, increased inflammatory tissue infiltration, and severe destruction of colon structure | ↓ MPO ↓ AKT, ERK1/2, JNK1/2, p65, and p38 ↓ IL-1β, IL-6, TNF- α, COX-2, iNOS, and NF-κB | ↓ MAPK and AKT/NF-κB pathways | [86] | |
| Glycyrrhizin 15 | Female SD rats | Acetic-acid- induced colitis | 40 mg/kg i.p. 7 days | ↓ DAI | ↓ Morphologic injury and histological changes | ↓ MPO ↓ NF-κB, TNF-α, and ICAM-1 | [87] | ||
| Albino Wistar rats | Acetic-acid- induced colitis | 100 mg/kg p.o. 8 days | ↓ Colonic tissue injury | ↓ MPO ↓ TNF-α ↑ SOD, GSH-Px, and CAT ↑ PPARγ | [88] | ||||
| Male BABL/c mice | TNBS- induced colitis | 50 mg/kg i.p. once every 2 days for 5 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ HMGB1, IFN-γ, IL-6, and TNF-α ↓ Th17, Th1, CDs | [89] | ||
| Astragaloside Ⅳ 16 | Male C57BL/6 mice | DDS- induced colitis | 50 and 100 mg/kg i.g. 7 days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening ↓ Colon weight | ↓ Histological score | ↓ AHR, c-Maf, RORa, and RORrt ↓ CCR6 ↓ IL-17A, IL-21 ↓ Eomes, Foxp3, and STAT5 ↓ MDA ↓ DLL3, Jagged1, Jagged2, Notch2, Notch3, Hes1, and Hes2 ↑ IL-10 and TGF-β1 ↑ CAT, SOD, and GSH-Px | ↓ Oxidative stress ↓ Th17/Treg ↓ Notch pathway | [90] |
| Gentiopicroside 17 | Male ICR mice | DDS- induced colitis | 50, 100, and 200 mg/kg i.g. 7 days | ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ MPO ↓ TNF-α, IL-1β, IL-6, iNOS, and COX-2 | [91] | |
| Ginsenoside Rg1 18 | Male C57BL/6 mice | DDS- induced colitis | 200 mg/kg p.o. 10 days | ↓ Weight loss | ↓ Colonic shortening | ↓ Histological score | ↓ IL-2 and TNF-α | [92] | |
| Liriodendrin 19 | Male BALB/c mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | 100 mg/kg i.g. 10 days | ↓ DAI | ↓ Colonic shortening | ↓ Histological damage | ↓ MPO and MDA ↓ TNF-α IL-1β and IL-6 ↑ SOD and GSH-Px ↑ ERβ | ↓ Akt and NF-κB pathways | [93] |
| Convallatoxin 20 | Female C57BL/6 mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | 50 and 150 μg/kg | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ TNF-α, IL-1β, and IL-6 ↓ NF-κB-p65 and IκBα ↓ COX-2 and iNOS ↑ PPARγ | [94] | ||
| Aloin A 21 | Male C57BL/6J mice/ RAW264.7 macrophages | DDS- induced colitis/LPS-induced inflammatory macrophage model | 25 and 50mg/kg i.g. 7days | ↓ Weight loss ↓ DAI | ↓ Colonic shortening | ↓ Histological score | ↓ MPO ↓ IL-1β, TNF-α, and IL-6 ↓ Cleaved caspase-3 ↓ Notch1 and Hes1 ↑ IL-10 ↑ Ki-67 ↑ MUC2, ATOH1, ZO-1, and Occludin | ↓ Notch pathway | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Zhang, J.; Shi, D.; Zang, W.; Niu, J. Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review. Molecules 2023, 28, 5210. https://doi.org/10.3390/molecules28135210
Niu Y, Zhang J, Shi D, Zang W, Niu J. Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review. Molecules. 2023; 28(13):5210. https://doi.org/10.3390/molecules28135210
Chicago/Turabian StyleNiu, Yating, Jun Zhang, Dianhua Shi, Weibiao Zang, and Jianguo Niu. 2023. "Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review" Molecules 28, no. 13: 5210. https://doi.org/10.3390/molecules28135210
APA StyleNiu, Y., Zhang, J., Shi, D., Zang, W., & Niu, J. (2023). Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review. Molecules, 28(13), 5210. https://doi.org/10.3390/molecules28135210






