Efficient D-π-π-A-Type Dye Sensitizer Based on a Benzothiadiazole Moiety: A Computational Study
Abstract
1. Introduction
2. Results
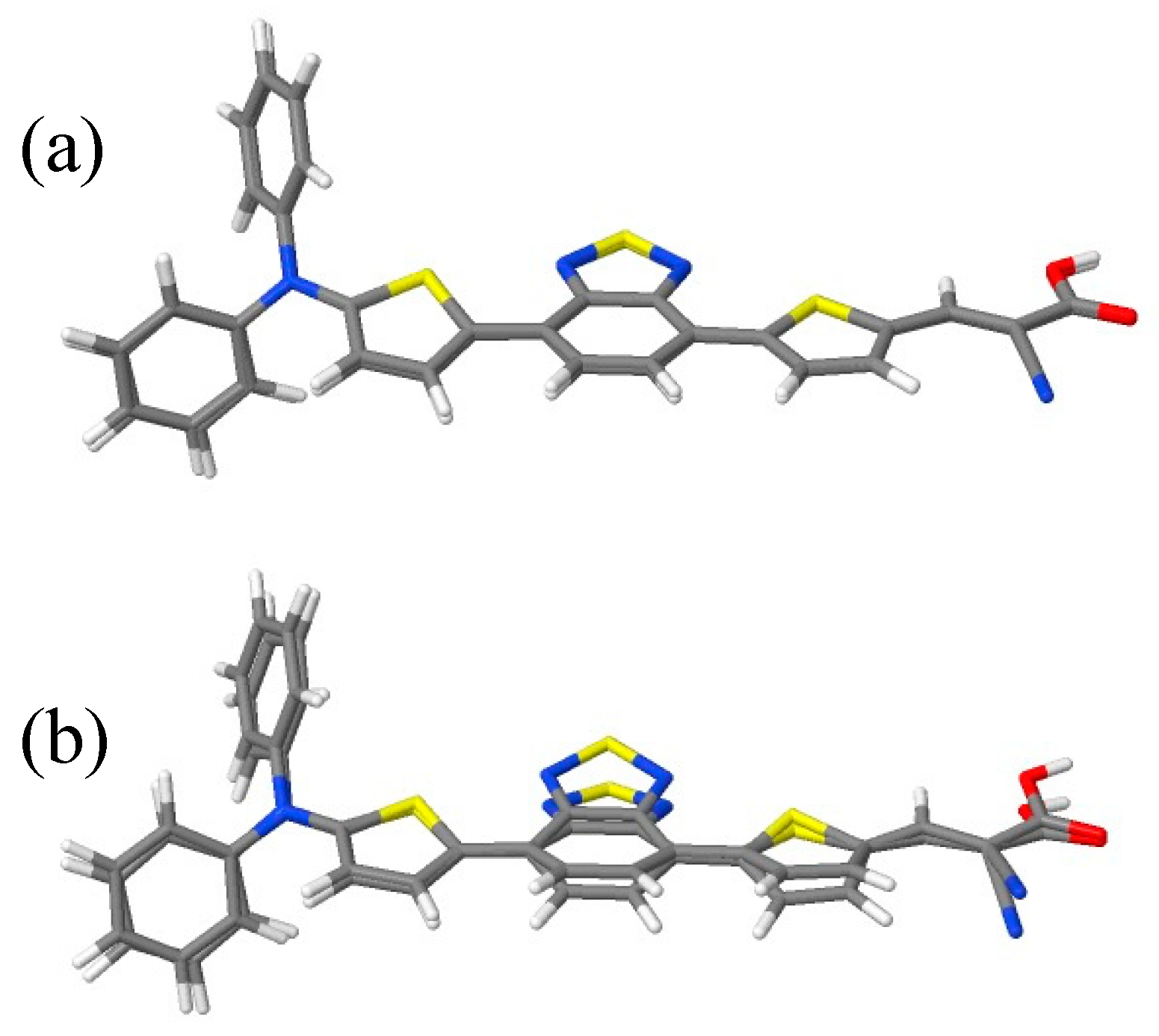
2.1. Geometries
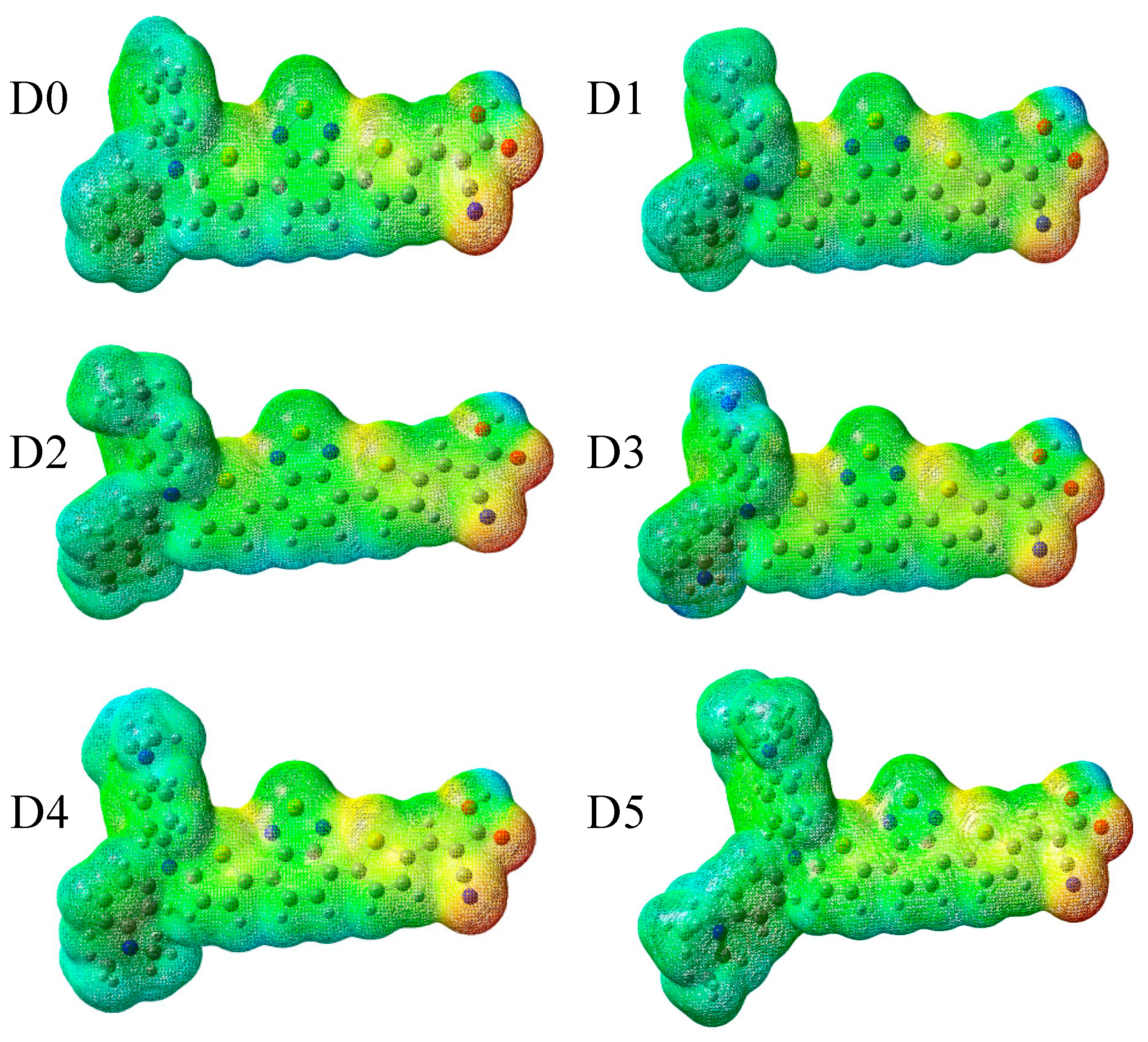
2.2. Electrostatic Potentials
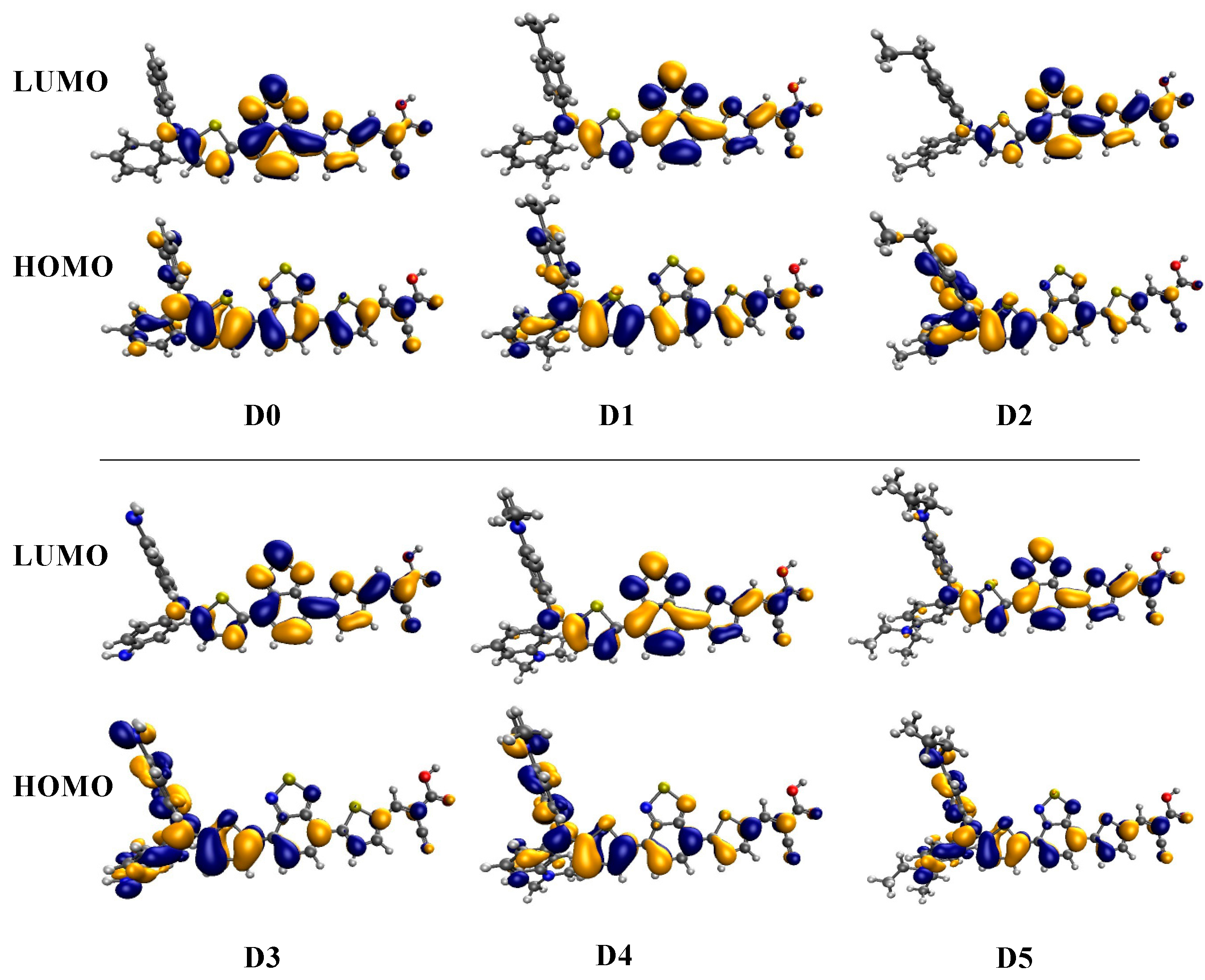
2.3. Frontier Molecular Orbitals and Chemical Reactivity
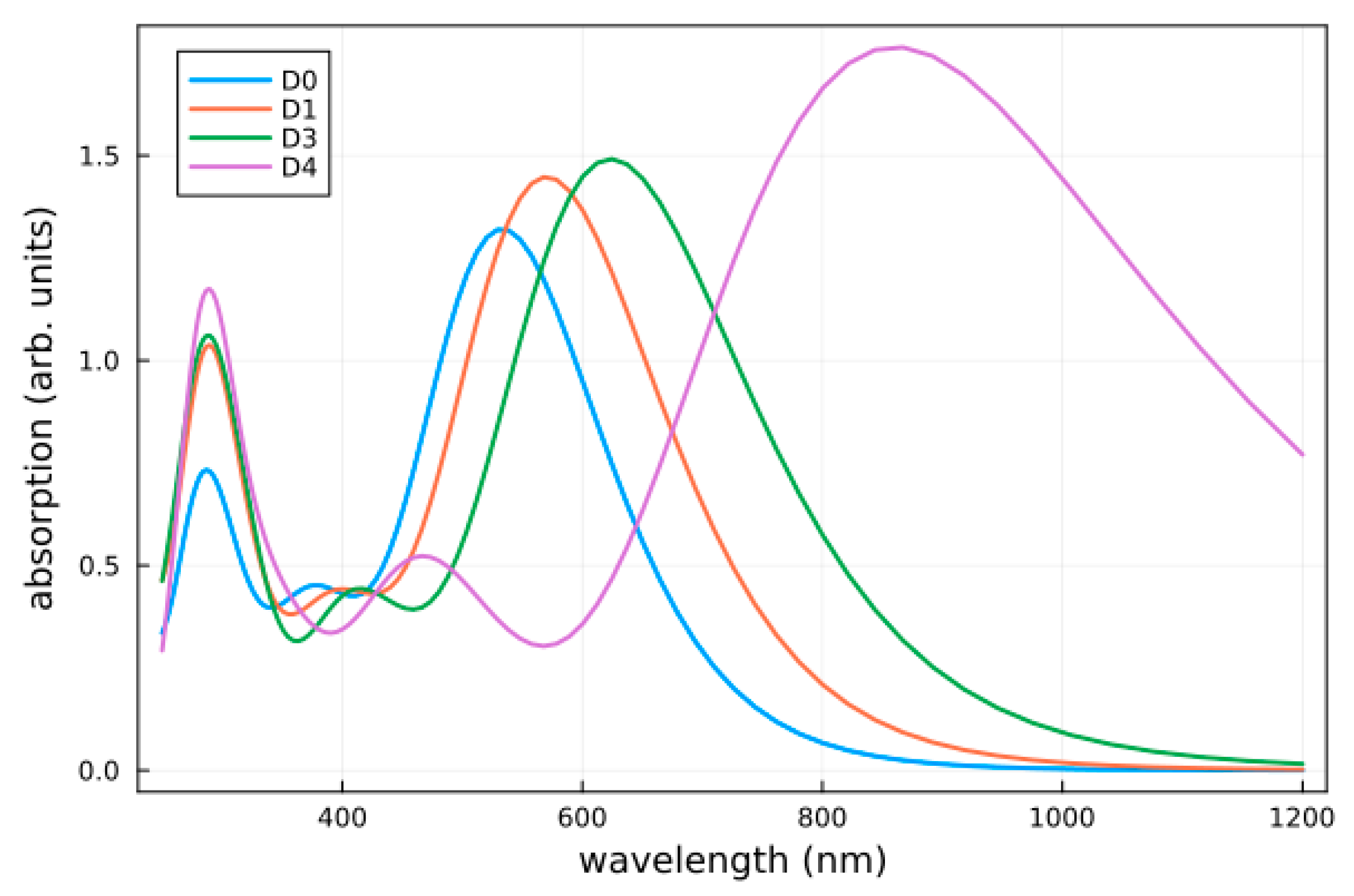
2.4. Optical Absorption
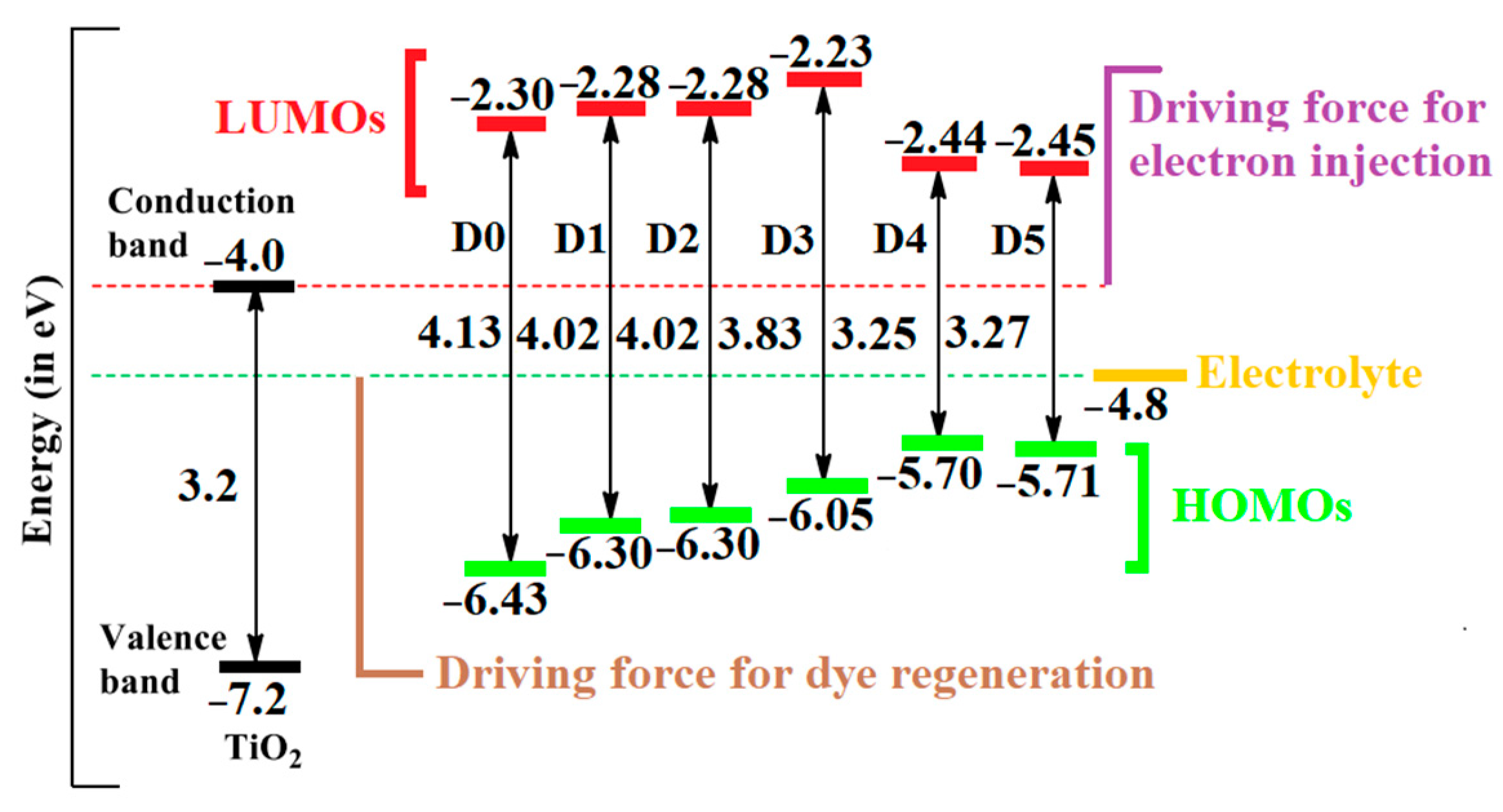
2.5. Electron Injection, Dye Regeneration, and Open-Circuit Voltage
3. Discussion
4. Materials and Methods
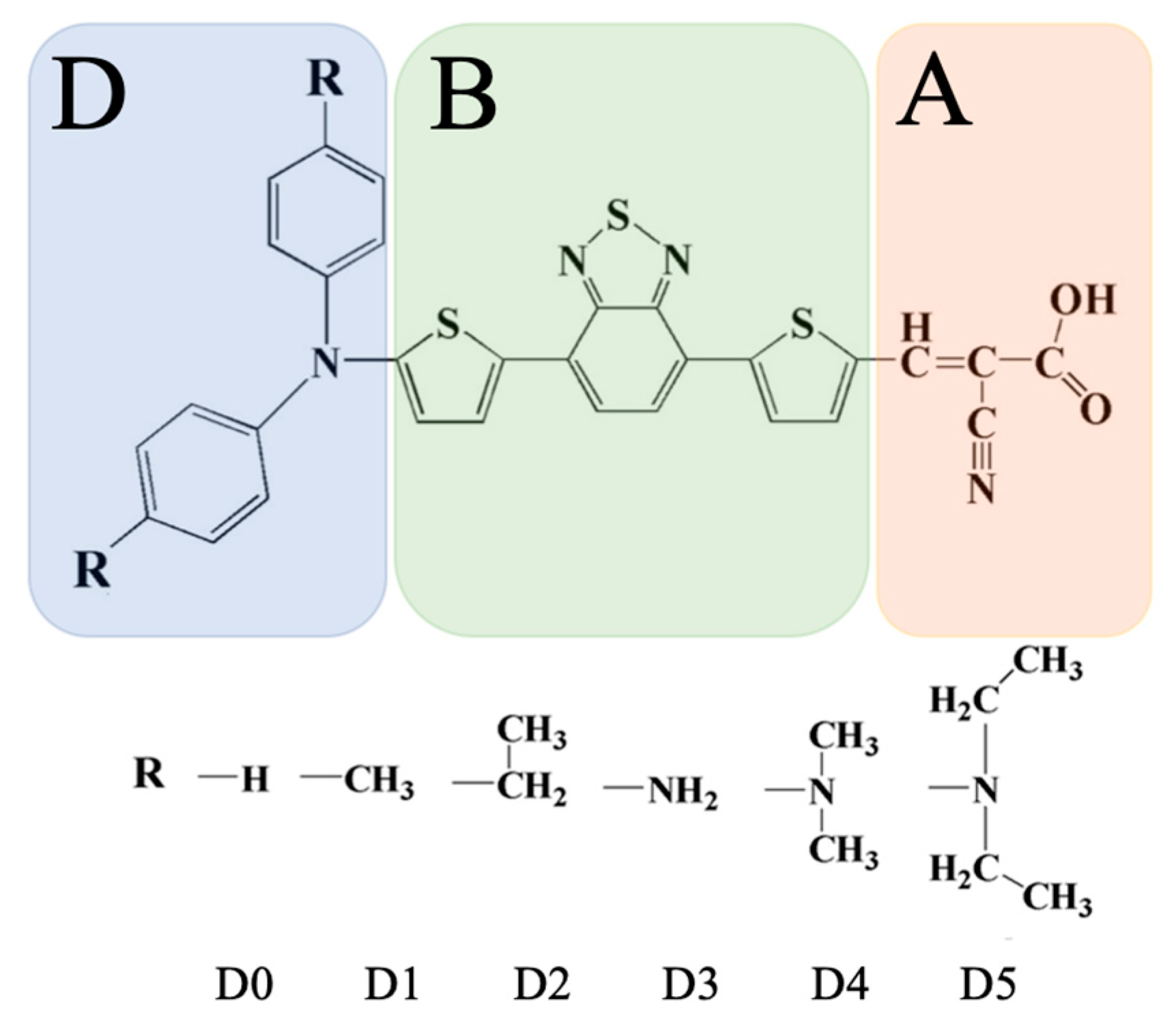
4.1. Characterization of Dye Molecules
4.2. Computational Chemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-Sensitized Solar Cells Strike Back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced Research Trends in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bae, E.; Lee, J.-J.; Park, J.; Choi, W. Effect of the Anchoring Group in Ru−Bipyridyl Sensitizers on the Photoelectrochemical Behavior of Dye-Sensitized TiO2 Electrodes: Carboxylate versus Phosphonate Linkages. J. Phys. Chem. B 2006, 110, 8740–8749. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-P.; Lin, R.Y.-Y.; Lin, L.-Y.; Li, C.-T.; Chu, T.-C.; Sun, S.-S.; Lin, J.T.; Ho, K.-C. Recent Progress in Organic Sensitizers for Dye-Sensitized Solar Cells. RSC Adv. 2015, 5, 23810–23825. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, M.; Li, J.-Y.; Pootrakulchote, N.; Alibabaei, L.; Ngoc-le, C.; Decoppet, J.-D.; Tsai, J.-H.; Grätzel, C.; Wu, C.-G.; et al. Highly Efficient Light-Harvesting Ruthenium Sensitizer for Thin-Film Dye-Sensitized Solar Cells. ACS Nano 2009, 3, 3103–3109. [Google Scholar] [CrossRef]
- Ahmad, S.; Guillén, E.; Kavan, L.; Grätzel, M.; Nazeeruddin, M.K. Metal Free Sensitizer and Catalyst for Dye Sensitized Solar Cells. Energy Environ. Sci. 2013, 6, 3439–3466. [Google Scholar] [CrossRef]
- Liu, B.; Wang, R.; Mi, W.; Li, X.; Yu, H. Novel Branched Coumarin Dyes for Dye-Sensitized Solar Cells: Significant Improvement in Photovoltaic Performance by Simple Structure Modification. J. Mater. Chem. 2012, 22, 15379–15387. [Google Scholar] [CrossRef]
- Teng, C.; Yang, X.; Yang, C.; Tian, H.; Li, S.; Wang, X.; Hagfeldt, A.; Sun, L. Influence of Triple Bonds as π-Spacer Units in Metal-Free Organic Dyes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 11305–11313. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, M.J.; Song, H.M.; Song, B.J.; Seo, K.D.; Pastore, M.; Anselmi, C.; Fantacci, S.; De Angelis, F.; Nazeeruddin, M.K.; et al. Organic Dyes Incorporating Low-Band-Gap Chromophores Based on π-Extended Benzothiadiazole for Dye-Sensitized Solar Cells. Dye. Pigment. 2011, 91, 192–198. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-Functionalized Carbazole Dyes for Efficient Molecular Photovoltaics: Tuning of Solar-Cell Performance by Structural Modification. Chem. Mater. 2008, 20, 3993–4003. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A.; Khan, M.U.; Khalid, M.; Ullah, N.; Kalgaonkar, R.; Alnoaimi, K.; Baqader, N.; Jamil, S. Theoretical and Conceptual Framework to Design Efficient Dye-Sensitized Solar Cells (DSSCs): Molecular Engineering by DFT Method. J. Clust. Sci. 2021, 32, 243–253. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Li, W.; Wang, Z.-S.; Tian, H.; Zhu, W. Hexylthiophene-Featured D–A–π–A Structural Indoline Chromophores for Coadsorbent-Free and Panchromatic Dye-Sensitized Solar Cells. Adv. Energy Mater. 2012, 2, 149–156. [Google Scholar] [CrossRef]
- Pounraj, P.; Mohankumar, V.; Pandian, M.S.; Ramasamy, P. Donor Functionalized Quinoline Based Organic Sensitizers for Dye Sensitized Solar Cell (DSSC) Applications: DFT and TD-DFT Investigations. J. Mol. Model. 2018, 24, 343. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Kurashige, M.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Design of New Coumarin Dyes Having Thiophene Moieties for Highly Efficient Organic-Dye-Sensitized Solar Cells. New J. Chem. 2003, 27, 783–785. [Google Scholar] [CrossRef]
- Ali Siddique, S.; Arshad, M.; Naveed, S.; Yasir Mehboob, M.; Adnan, M.; Hussain, R.; Ali, B.; Ahmed Siddique, M.B.; Liu, X. Efficient Tuning of Zinc Phthalocyanine-Based Dyes for Dye-Sensitized Solar Cells: A Detailed DFT Study. RSC Adv. 2021, 11, 27570–27582. [Google Scholar] [CrossRef]
- Akram, M.; Siddique, S.A.; Iqbal, J.; Hussain, R.; Mehboob, M.Y.; Siddique, M.B.A.; Naveed, S.; Ali, B.; Hanif, A.; Sajid, M.; et al. End-Capped Engineering of Bipolar Diketopyrrolopyrrole Based Small Electron Acceptor Molecules for High Performance Organic Solar Cells. Comput. Theor. Chem. 2021, 1201, 113242. [Google Scholar] [CrossRef]
- Siddique, S.A.; Naveed, S.; Alvi, M.U.; Mehboob, M.Y.; Ali, B.; Rauf, A.; Siddique, M.B.A.; Hussain, R.; Arshad, M.; Liu, X. Deciphering the Role of End-Capped Acceptor Units for Amplifying the Photovoltaic Properties of Donor Materials for High-Performance Organic Solar Cell Applications. Comput. Theor. Chem. 2021, 1205, 113454. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine Organic Dyes for Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef]
- Joly, D.; Pellejà, L.; Narbey, S.; Oswald, F.; Chiron, J.; Clifford, J.N.; Palomares, E.; Demadrille, R. A Robust Organic Dye for Dye Sensitized Solar Cells Based on Iodine/Iodide Electrolytes Combining High Efficiency and Outstanding Stability. Sci. Rep. 2014, 4, 4033. [Google Scholar] [CrossRef]
- Xu, L.; Aumaitre, C.; Kervella, Y.; Lapertot, G.; Rodríguez-Seco, C.; Palomares, E.; Demadrille, R.; Reiss, P. Increasing the Efficiency of Organic Dye-Sensitized Solar Cells over 10.3% Using Locally Ordered Inverse Opal Nanostructures in the Photoelectrode. Adv. Funct. Mater. 2018, 28, 1706291. [Google Scholar] [CrossRef]
- Godfroy, M.; Liotier, J.; Mwalukuku, V.M.; Joly, D.; Huaulmé, Q.; Cabau, L.; Aumaitre, C.; Kervella, Y.; Narbey, S.; Oswald, F.; et al. Benzothiadiazole-Based Photosensitizers for Efficient and Stable Dye-Sensitized Solar Cells and 8.7% Efficiency Semi-Transparent Mini-Modules. Sustain. Energy Fuels 2021, 5, 144–153. [Google Scholar] [CrossRef]
- Velusamy, M.; Justin Thomas, K.R.; Lin, J.T.; Hsu, Y.-C.; Ho, K.-C. Organic Dyes Incorporating Low-Band-Gap Chromophores for Dye-Sensitized Solar Cells. Org. Lett. 2005, 7, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Ganjoo, A.; Chetti, P. Influence of Internal Acceptor and Thiophene Based π-Spacer in D-A-π-A System on Photophysical and Charge Transport Properties for Efficient DSSCs: A DFT Insight. Sol. Energy 2020, 209, 194–205. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, D.; Li, X.; Xu, Y. D-A-π-A Based Organic Dyes for Efficient DSSCs: A Theoretical Study on the Role of π-Spacer. Comput. Mater. Sci. 2019, 161, 163–176. [Google Scholar] [CrossRef]
- Hailu, Y.M.; Nguyen, M.T.; Jiang, J.-C. Effects of the Terminal Donor Unit in Dyes with D–D–π–A Architecture on the Regeneration Mechanism in DSSCs: A Computational Study. Phys. Chem. Chem. Phys. 2018, 20, 23564–23577. [Google Scholar] [CrossRef]
- Wazzan, N.A. A DFT/TDDFT Investigation on the Efficiency of Novel Dyes with Ortho-Fluorophenyl Units (A1) and Incorporating Benzotriazole/Benzothiadiazole/Phthalimide Units (A2) as Organic Photosensitizers with D–A2–π–A1 Configuration for Solar Cell Applications. J. Comput. Electron. 2019, 18, 375–395. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.-B.; Sun, S.-L.; Geng, Y.; Wu, Y.; Su, Z.-M. Density Functional Theory Characterization and Design of High-Performance Diarylamine-Fluorene Dyes with Different π Spacers for Dye-Sensitized Solar Cells. J. Mater. Chem. 2011, 22, 568–576. [Google Scholar] [CrossRef]
- Fan, W.; Tan, D.; Deng, W.-Q. Acene-Modified Triphenylamine Dyes for Dye-Sensitized Solar Cells: A Computational Study. ChemPhysChem 2012, 13, 2051–2060. [Google Scholar] [CrossRef]
- Haid, S.; Marszalek, M.; Mishra, A.; Wielopolski, M.; Teuscher, J.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M.; Bäuerle, P. Significant Improvement of Dye-Sensitized Solar Cell Performance by Small Structural Modification in π-Conjugated Donor–Acceptor Dyes. Adv. Funct. Mater. 2012, 22, 1291–1302. [Google Scholar] [CrossRef]
- Feng, J.; Jiao, Y.; Ma, W.; Nazeeruddin, M.K.; Grätzel, M.; Meng, S. First Principles Design of Dye Molecules with Ullazine Donor for Dye Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 3772–3778. [Google Scholar] [CrossRef]
- Ma, W.; Jiao, Y.; Meng, S. Modeling Charge Recombination in Dye-Sensitized Solar Cells Using First-Principles Electron Dynamics: Effects of Structural Modification. Phys. Chem. Chem. Phys. 2013, 15, 17187–17194. [Google Scholar] [CrossRef]
- Kar, S.; Roy, J.K.; Leszczynski, J. In Silico Designing of Power Conversion Efficient Organic Lead Dyes for Solar Cells Using Todays Innovative Approaches to Assure Renewable Energy for Future. NPJ Comput. Mater. 2017, 3, 22. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Song, P.; Ma, F.; Sun, M. D–A−π–A System: Light Harvesting, Charge Transfer, and Molecular Designing. J. Phys. Chem. C 2017, 121, 12546–12561. [Google Scholar] [CrossRef]
- Manzoor, T.; Pandith, A.H. Theoretical Studies on the Structure, Optoelectronic and Photosensitizer Applications of NKX Class of Coumarin Dye Molecules. ChemistrySelect 2018, 3, 2376–2385. [Google Scholar] [CrossRef]
- Manzoor, T.; Niaz, S.; Pandith, A.H. Exploring the Effect of Different Coumarin Donors on the Optical and Photovoltaic Properties of Azo-Bridged Push-Pull Systems: A Theoretical Approach. Int. J. Quantum Chem. 2019, 119, e25979. [Google Scholar] [CrossRef]
- Bokareva, O.S.; Grell, G.; Bokarev, S.I.; Kühn, O. Tuning Range-Separated Density Functional Theory for Photocatalytic Water Splitting Systems. J. Chem. Theory Comput. 2015, 11, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of Light to Electricity by Cis-X2bis(2,2′-Bipyridyl-4,4′-Dicarboxylate)Ruthenium(II) Charge-Transfer Sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingfort, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
| Dye | IP (eV) | EA (eV) | η (eV) | μ (eV) | ω (eV) | ω+ (eV) | ω− (eV) |
|---|---|---|---|---|---|---|---|
| D0 | 6.43 (5.45) | 2.31 | 2.06 | −4.37 | 9.26 | 2.70 | 10.41 |
| D1 | 6.31 (5.31) | 2.28 | 2.01 | −4.30 | 9.17 | 2.69 | 10.32 |
| D2 | 6.30 (5.31) | 2.28 | 2.01 | −4.29 | 9.18 | 2.69 | 10.32 |
| D3 | 6.06 (5.07) | 2.23 | 1.91 | −4.14 | 8.98 | 2.66 | 10.10 |
| D4 | 5.70 (4.75) | 2.45 | 1.63 | −4.07 | 10.20 | 3.27 | 11.48 |
| D5 | 5.72 (4.76) | 2.45 | 1.63 | −4.09 | 10.23 | 3.28 | 11.51 |
| Dye | Emax (eV) | λmax (nm) | f | LHE |
|---|---|---|---|---|
| D0 | 2.32 (1.88) | 534.2 (658.3) | 1.31 (1.04) | 0.95 (0.91) |
| D1 | 2.17 (2.32) | 570.7 (533.0) | 1.43 (1.10) | 0.96 (0.92) |
| D2 | 2.16 (2.32) | 571.2 (534.0) | 1.44 (1.11) | 0.96 (0.92) |
| D3 | 1.98 (2.18) | 624.3 (566.8) | 1.48 (1.14) | 0.97 (0.93) |
| D4 | 1.44 (1.74) | 860.8 (709.3) | 1.76 (1.32) | 0.98 (0.95) |
| D5 | 1.44 (1.75) | 857.1 (710.2) | 1.75 (1.33) | 0.98 (0.95) |
| Dye | ΔGinj | ΔGreg | Voc | PCE |
|---|---|---|---|---|
| D0 | −0.87 | 0.65 | 1.69 | 100 |
| D1 | −0.86 | 0.51 | 1.72 | 101 |
| D2 | −0.85 | 0.51 | 1.77 | 100 |
| D3 | −0.91 | 0.27 | 1.77 | 111 |
| D4 | −0.69 | −0.05 | 1.55 | 75 |
| D5 | −0.68 | −0.04 | 1.55 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, F.M.; Abdel-Latif, M.K.; Abdel-Khalek, A.A.; Kühn, O. Efficient D-π-π-A-Type Dye Sensitizer Based on a Benzothiadiazole Moiety: A Computational Study. Molecules 2023, 28, 5185. https://doi.org/10.3390/molecules28135185
Mustafa FM, Abdel-Latif MK, Abdel-Khalek AA, Kühn O. Efficient D-π-π-A-Type Dye Sensitizer Based on a Benzothiadiazole Moiety: A Computational Study. Molecules. 2023; 28(13):5185. https://doi.org/10.3390/molecules28135185
Chicago/Turabian StyleMustafa, Fatma M., Mahmoud K. Abdel-Latif, Ahmed A. Abdel-Khalek, and Oliver Kühn. 2023. "Efficient D-π-π-A-Type Dye Sensitizer Based on a Benzothiadiazole Moiety: A Computational Study" Molecules 28, no. 13: 5185. https://doi.org/10.3390/molecules28135185
APA StyleMustafa, F. M., Abdel-Latif, M. K., Abdel-Khalek, A. A., & Kühn, O. (2023). Efficient D-π-π-A-Type Dye Sensitizer Based on a Benzothiadiazole Moiety: A Computational Study. Molecules, 28(13), 5185. https://doi.org/10.3390/molecules28135185







