Investigating the Vital Role of the Identified Abietic Acid from Helianthus annuus L. Calathide Extract against Hyperuricemia via Human Embryonic Kidney 293T Cell Model
Abstract
1. Introduction
2. Results
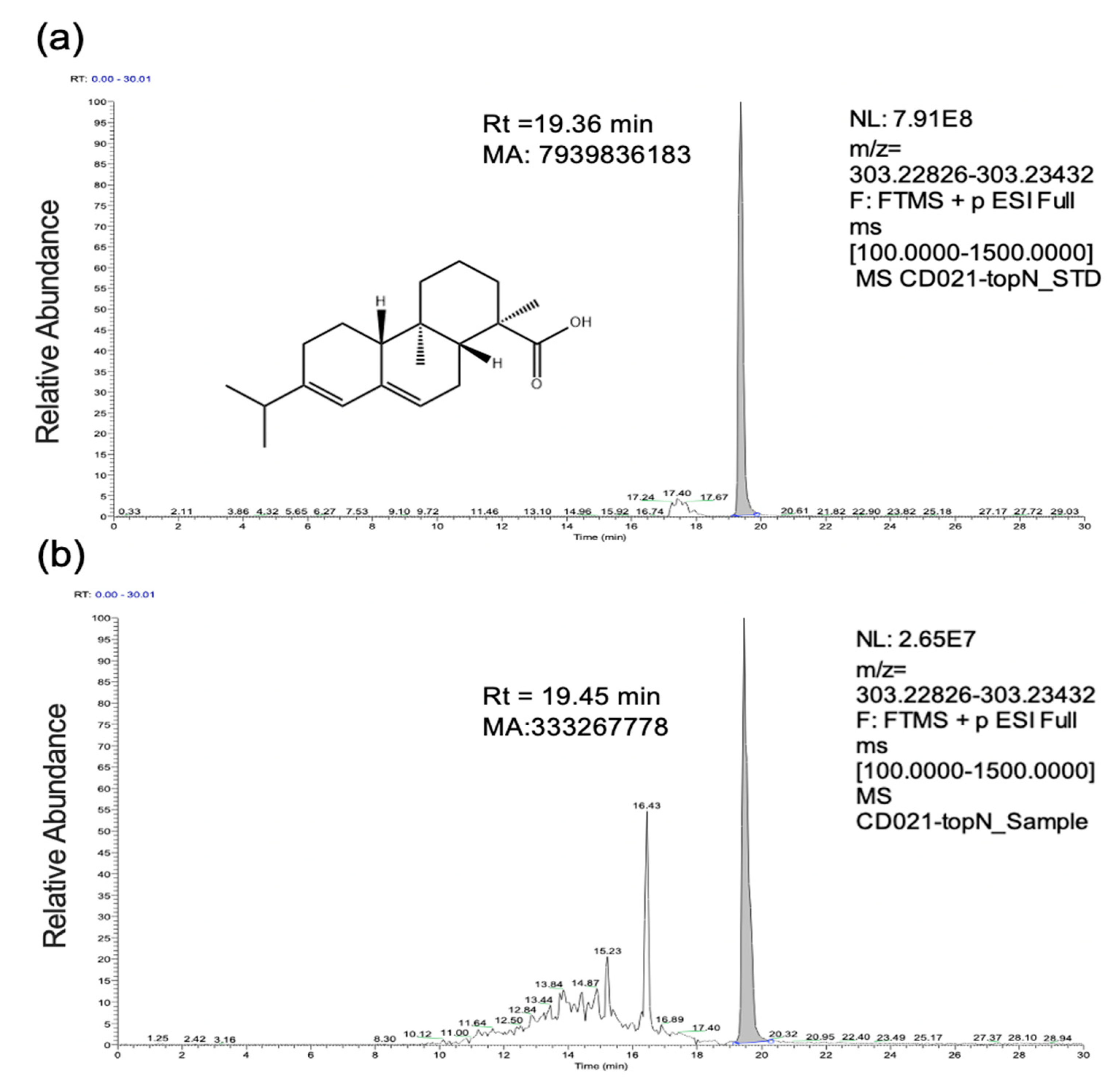
2.1. Identification of Abietic Acid
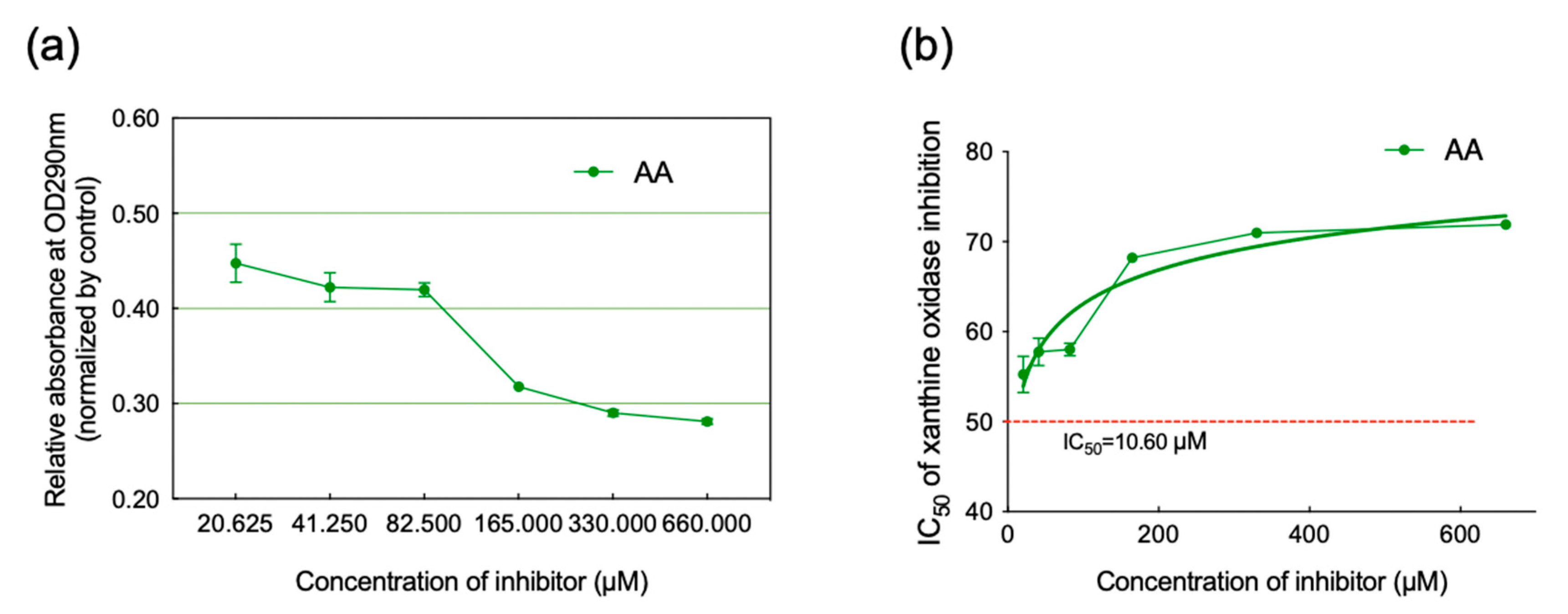
2.2. Inhibition of XO Activity
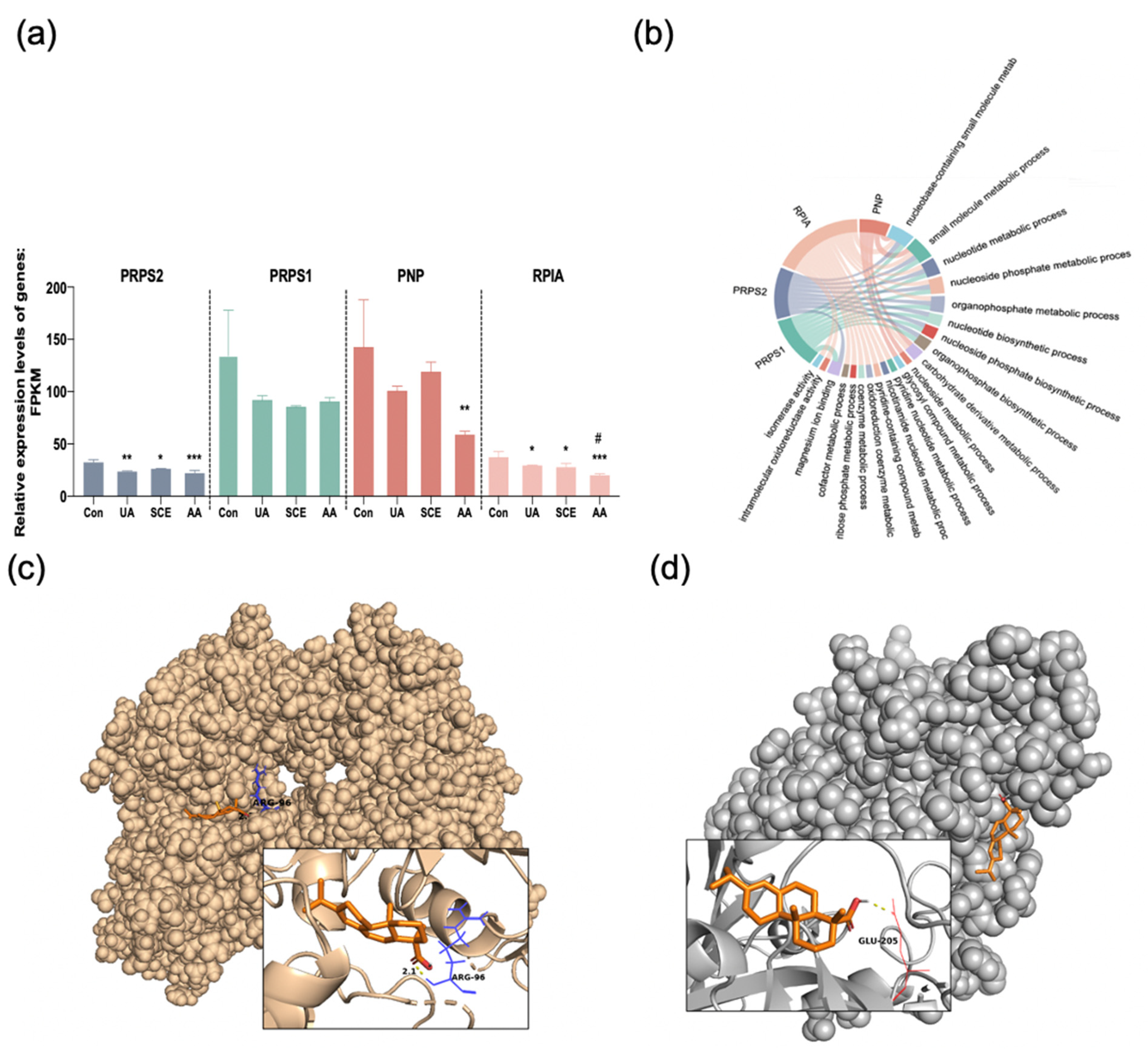
2.3. Docking Analysis
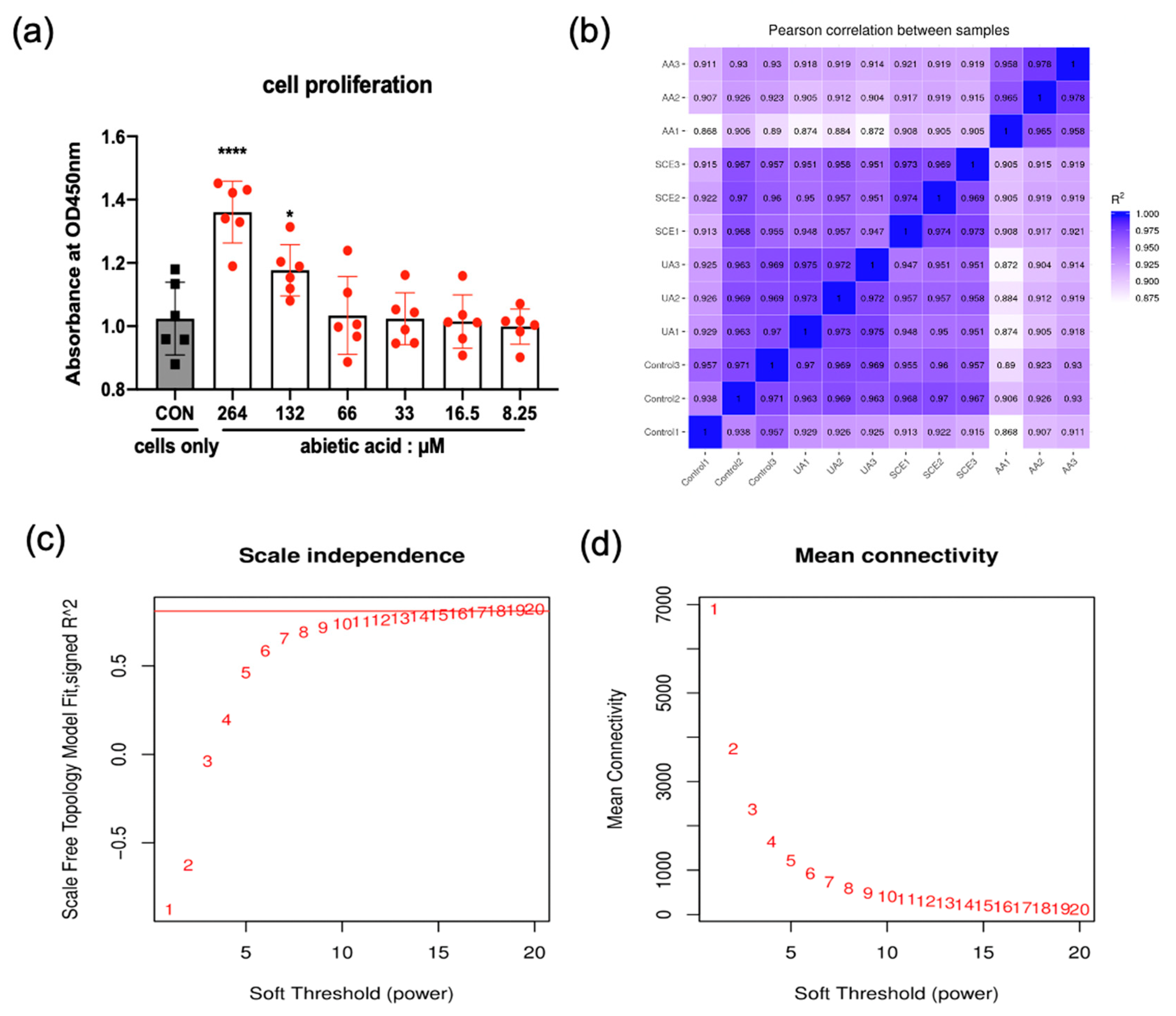
2.4. Cell Proliferation
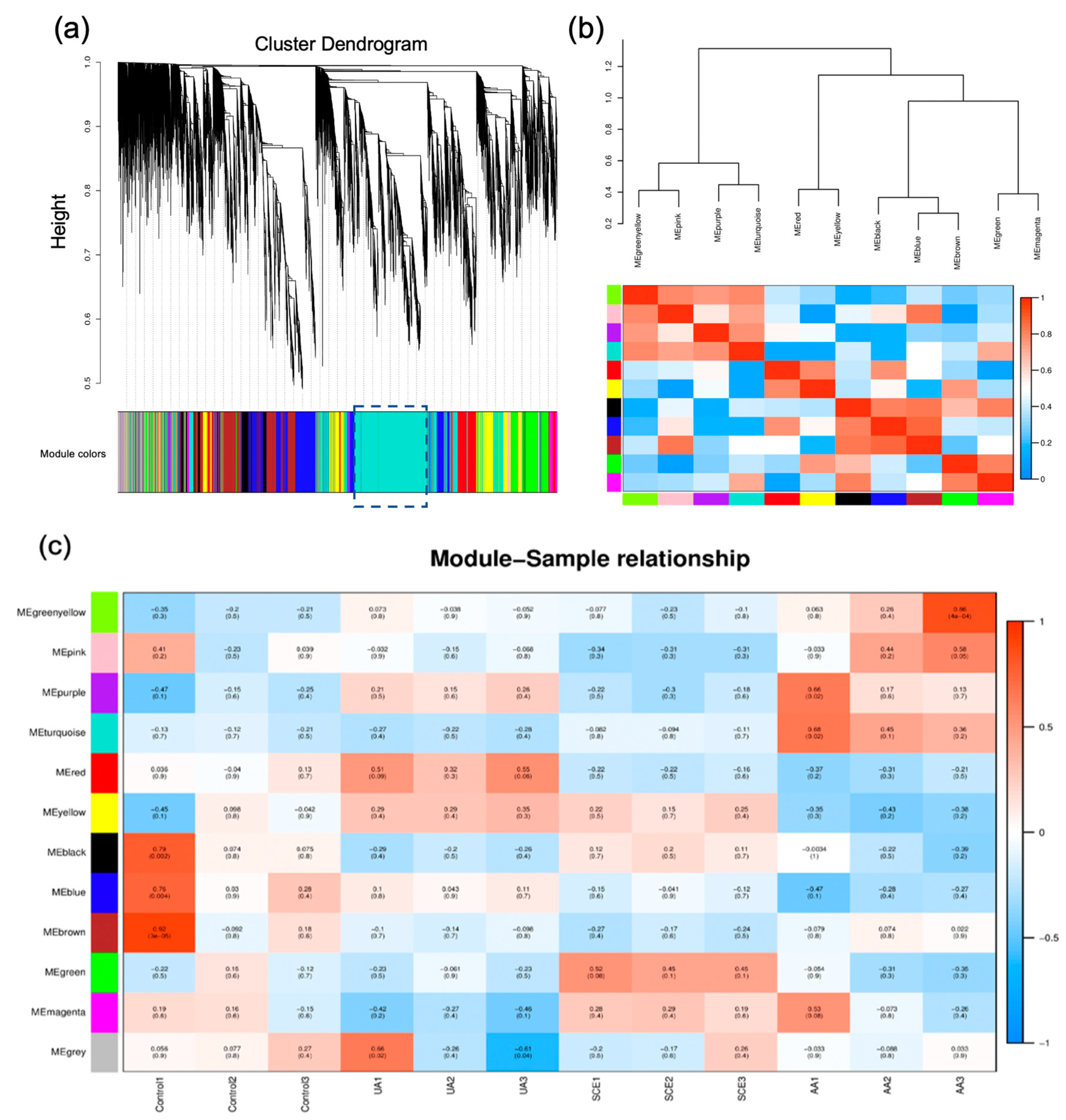
2.5. Pearson’s Correlation Coefficient and Soft Threshold
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
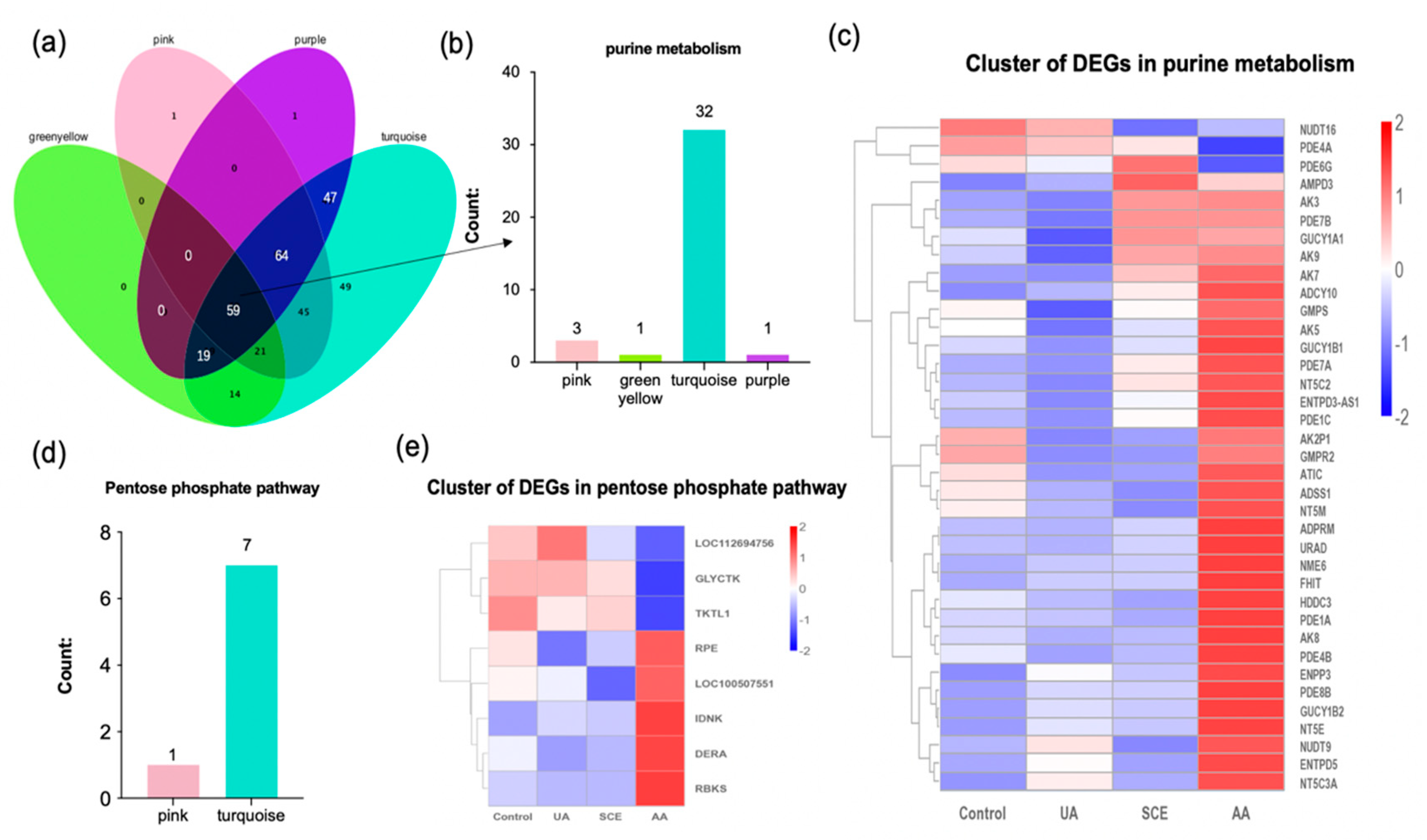
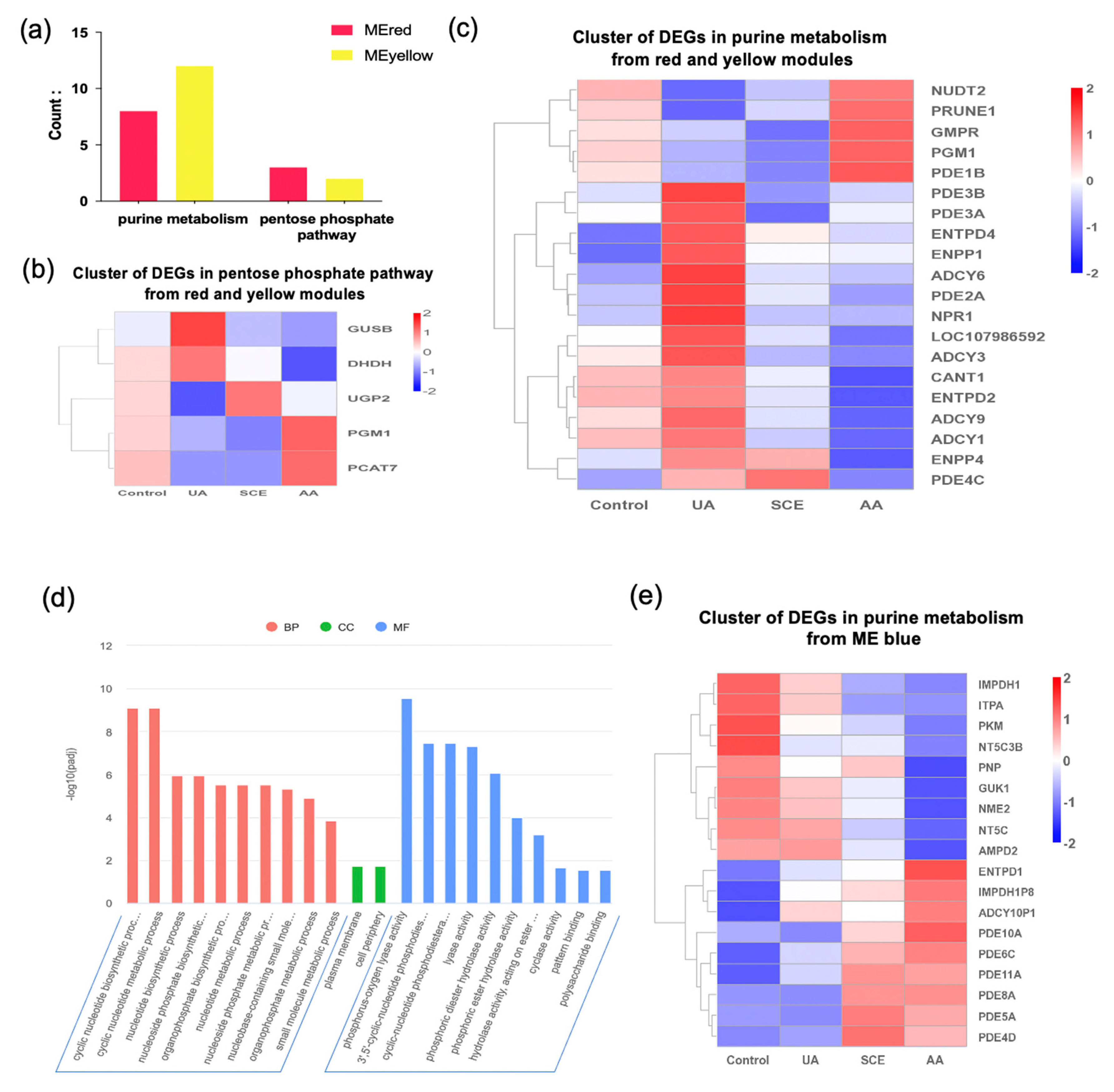
2.7. Bioinformatics Analysis
3. Materials and Methods
3.1. Preparation of Sunflower Calathide Hydrolyzed Extract (SCE)
3.2. Characterization of Extract via UPLC-Q-Orbitrap HRMS
3.3. Spectrophotometer-Based Determination of Enzyme Activity
3.4. Molecular Docking
3.5. Cell Culture
3.6. Construction of cDNA Library and Transcriptome Sequencing
3.7. Datasets
3.8. Bioinformatic Analysis
3.9. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yamaoka, T.; Itakura, M. Metabolism of purine nucleotides and the production of uric acid. Nihon Rinsho Jpn. J. Clin. Med. 1996, 54, 3188–3194. [Google Scholar]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.W.; Chiu, H.T.; Tsai, C.W.; Ting, I.W.; Yeh, H.C.; Huang, H.C.; Kuo, C.C.; Group, C.K.R. Comparative effectiveness of allopurinol, febuxostat and benzbromarone on renal function in chronic kidney disease patients with hyperuricemia: A 13-year inception cohort study. Nephrol. Dial. Transplant. 2018, 33, 1620–1627. [Google Scholar] [CrossRef]
- Mendez Landa, C.E. Renal Effects of Hyperuricemia. Contrib. Nephrol. 2018, 192, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, X.F.; Lu, X.; Zhao, J.Y.; Li, X.L. Sunflower head enzymatic hydrolysate relives hyperuricemia by inhibiting crucial proteins (xanthine oxidase, adenosine deaminase, uric acid transporter1) and restoring gut microbiota in mice. J. Funct. Foods 2020, 72, 104055. [Google Scholar] [CrossRef]
- Li, L.; Teng, M.; Liu, Y.; Qu, Y.; Zhang, Y.; Lin, F.; Wang, D. Anti-Gouty Arthritis and Antihyperuricemia Effects of Sunflower (Helianthus annuus) Head Extract in Gouty and Hyperuricemia Animal Models. Biomed. Res. Int. 2017, 2017, 5852076. [Google Scholar] [CrossRef]
- Dai, H.; Lv, S.; Qiao, Z.A.; Wang, K.; Zhou, X.; Bao, C.; Zhang, S.; Fu, X.; Li, W. The Active Components of Sunflower (Helianthus annuus L.) Calathide and the Effects on Urate Nephropathy Based on COX-2/PGE2 Signaling Pathway and the Urate Transporter URAT1, ABCG2, and GLUT9. Front. Nutr. 2022, 8, 769555. [Google Scholar] [CrossRef]
- Dai, H.; Lv, S.; Fu, X.; Li, W. Identification of scopoletin and chlorogenic acid as potential active components in sunflower calathide enzymatically hydrolyzed extract towards hyperuricemia. Appl. Sci. 2021, 11, 10306. [Google Scholar] [CrossRef]
- Thummuri, D.; Guntuku, L.; Challa, V.S.; Ramavat, R.N.; Naidu, V.G.M. Abietic acid attenuates RANKL induced osteoclastogenesis and inflammation associated osteolysis by inhibiting the NF-KB and MAPK signaling. J. Cell. Physiol. 2019, 234, 443–453. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y.; Dai, G.C.; Zhou, B.B.; Yan, X.N.; Tan, R.X. Abietic acid ameliorates psoriasis-like inflammation and modulates gut microbiota in mice. J. Ethnopharmacol. 2021, 272, 113934. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Liu, Q.; Dai, J. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKβ/NF-κB signaling. OncoTargets Ther. 2019, 12, 4825–4837. [Google Scholar] [CrossRef]
- Wen, H.; Mo, Q.; Cui, Y.; Tangg, J. Inhibition of Cancer Cell Growth by Abietic Acid in Cisplatin-Resistant Human Nasopharyngeal Cancer Cells Is Mediated via G2/M Cell Cycle Arrest, Blocking the PI3K/AKT/mTOR Signalling Pathway, and Suppression of Cell Migration and Invasion. Med. Sci. Monit. 2019, 25, 4122–4129. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.; Kushnazarova, R.; Pavlov, R.; Valeeva, F.; Lenina, O.; Bushmeleva, K.; Kuryashov, D.; Vyshtakalyuk, A.; Gaynanova, G.; Petrov, K.; et al. Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels. Molecules 2022, 27, 6447. [Google Scholar] [CrossRef]
- Ulusu, N.N.; Ercil, D.; Sakar, M.K.; Tezcan, E.F. Abietic acid inhibits lipoxygenase activity. Phytother. Res. PTR 2002, 16, 88–90. [Google Scholar] [CrossRef]
- Li, X.; Liao, B.; Liu, R.; Ma, Y. Abietic acid attenuates LPS-induced acute lung injury by restoring Th17/Treg balance. Trop. J. Pharm. Res. 2022, 21, 1879–1884. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.E.; Jin, Y.J.; Roh, Y.J.; Song, H.J.; Seol, A.; Park, S.H.; Seo, S.; Lee, H.; Hwang, D.Y. Anti-Atopic Dermatitis Effects of Abietic Acid Isolated from Rosin under Condition Optimized by Response Surface Methodology in DNCB-Spread BALB/c Mice. Pharmaceuticals 2023, 16, 407. [Google Scholar] [CrossRef]
- Kratzer, J.T.; Lanaspa, M.A.; Murphy, M.N.; Cicerchi, C.; Graves, C.L.; Tipton, P.A.; Ortlund, E.A.; Johnson, R.J.; Gaucher, E.A. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. USA 2014, 111, 3763–3768. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.-H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Li, Y.; Li, L.; Jiang, Y.; Cao, Y.; Pang, J. Characterizations of the Urate Transporter, GLUT9, and Its Potent Inhibitors by Patch-Clamp Technique. SLAS Discov. Adv. Life Sci. R D 2020, 26, 450–459. [Google Scholar] [CrossRef]
- Shin, H.J.; Takeda, M.; Enomoto, A.; Fujimura, M.; Miyazaki, H.; Anzai, N.; Endou, H. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology 2011, 16, 156–162. [Google Scholar] [CrossRef]
- Zhao, Z.-A.; Jiang, Y.; Chen, Y.-Y.; Wu, T.; Lan, Q.-S.; Li, Y.-M.; Li, L.; Yang, Y.; Lin, C.-T.; Cao, Y.; et al. CDER167, a dual inhibitor of URAT1 and GLUT9, is a novel and potent uricosuric candidate for the treatment of hyperuricemia. Acta Pharmacol. Sin. 2021, 43, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Tang, J.; Han, Y.; Wang, D. Co-expression modules construction by WGCNA and identify potential prognostic markers of uveal melanoma. Exp. Eye Res. 2018, 166, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, A.X.; Zhao, J.L.; Chen, F.L. Identification of Key Gene Modules in Human Osteosarcoma by Co-Expression Analysis Weighted Gene Co-Expression Network Analysis (WGCNA). J. Cell. Biochem. 2017, 118, 3953–3959. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Pauff, J.; Hille, R. Substrate orientation and the origin of catalytic power in xanthine oxidoreductase. Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. 2011, 50, 355–362. [Google Scholar]
- Norazmi, N.; Abdul Rasad, Z.R.; Mohamad, M.; Manap, H. Uric acid detection using uv-vis spectrometer. IOP Conf. Series. Mater. Sci. Eng. 2017, 257, 12031. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Timofeev, V.I.; Abramchik, Y.A.; Zhukhlistova, N.E.; Muravieva, T.I.; Esipov, R.S.; Kuranova, I.P. Three-dimensional structure of E. Coli purine nucleoside phosphorylase at 0.99 Å resolution. Crystallogr. Rep. 2016, 61, 249–257. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Bai, Y.; Kirschner-Schwabe, R.; Yang, J.J.; Chen, Y.; Lu, G.; Tzoneva, G.; Ma, X.; Wu, T.; et al. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat. Med. 2015, 21, 563–571. [Google Scholar] [CrossRef]
- Cunningham, J.T.; Moreno, M.V.; Lodi, A.; Ronen, S.M.; Ruggero, D. Protein and Nucleotide Biosynthesis Are Coupled by a Single Rate-Limiting Enzyme, PRPS2, to Drive Cancer. Cell 2014, 157, 1088–1103. [Google Scholar] [CrossRef]
- Yu-Ting, C.; Jiang, J.-K.; Yang, M.-H.; Jeng-Wei, L.; Hua-Kuo, L.; Wang, H.-D.; Yuh, C.-H. Identification of a noncanonical function for ribose-5-phosphate isomerase A promotes colorectal cancer formation by stabilizing and activating [Beta]-catenin via a novel C-terminal domain. PLoS Biol. 2018, 16, e2003714. [Google Scholar] [CrossRef]
- Becker, M.A.; Taylor, W.; Smith, P.R.; Ahmed, M. Increased rate of PRPS1 gene transcription in PRPP synthetase catalytic superactivity. Clin. Biochem. 1997, 30, 246. [Google Scholar] [CrossRef]
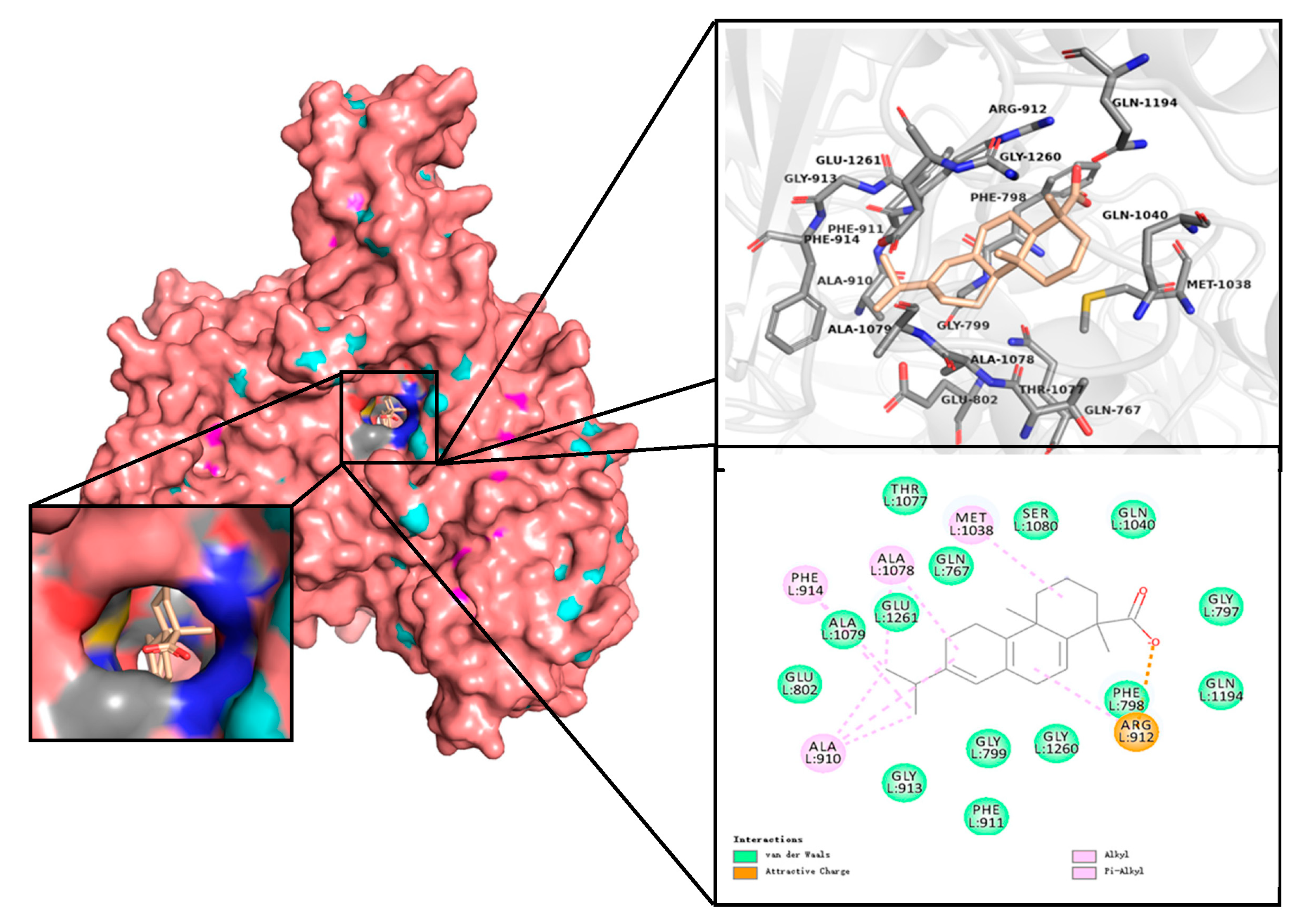
| Sub-Rank | Run | Binding Energy: kcal/mol | Inhibition Constant: Ki, nM | Cluster RMSD |
|---|---|---|---|---|
| 1 | 3 | −9.16 | 193.65 | 0.00 |
| 2 | 10 | −9.15 | 195.27 | 0.03 |
| 3 | 4 | −9.15 | 195.36 | 0.02 |
| 4 | 6 | −9.15 | 195.48 | 0.04 |
| 5 | 1 | −9.15 | 197.34 | 0.03 |
| 6 | 9 | −9.15 | 197.40 | 0.03 |
| 7 | 5 | −9.15 | 197.94 | 0.03 |
| 8 | 8 | −9.14 | 198.22 | 0.03 |
| 9 | 7 | −9.13 | 202.29 | 0.15 |
| 10 | 2 | −9.13 | 203.26 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Xu, X.; Li, W.; Fu, X.; Han, W.; Li, G. Investigating the Vital Role of the Identified Abietic Acid from Helianthus annuus L. Calathide Extract against Hyperuricemia via Human Embryonic Kidney 293T Cell Model. Molecules 2023, 28, 5141. https://doi.org/10.3390/molecules28135141
Dai H, Xu X, Li W, Fu X, Han W, Li G. Investigating the Vital Role of the Identified Abietic Acid from Helianthus annuus L. Calathide Extract against Hyperuricemia via Human Embryonic Kidney 293T Cell Model. Molecules. 2023; 28(13):5141. https://doi.org/10.3390/molecules28135141
Chicago/Turabian StyleDai, Huining, Xiao Xu, Wannan Li, Xueqi Fu, Weiwei Han, and Guodong Li. 2023. "Investigating the Vital Role of the Identified Abietic Acid from Helianthus annuus L. Calathide Extract against Hyperuricemia via Human Embryonic Kidney 293T Cell Model" Molecules 28, no. 13: 5141. https://doi.org/10.3390/molecules28135141
APA StyleDai, H., Xu, X., Li, W., Fu, X., Han, W., & Li, G. (2023). Investigating the Vital Role of the Identified Abietic Acid from Helianthus annuus L. Calathide Extract against Hyperuricemia via Human Embryonic Kidney 293T Cell Model. Molecules, 28(13), 5141. https://doi.org/10.3390/molecules28135141







